Abstract
During entry into stationary phase, many free-living, gram-negative bacteria express genes that impart cellular resistance to environmental stresses, such as oxidative stress and osmotic stress. Many genes that are required for stationary-phase adaptation are controlled by RpoS, a conserved alternative sigma factor, whose expression is, in turn, controlled by many factors. To better understand the numbers and types of genes dependent upon RpoS, we employed a genetic screen to isolate more than 100 independent RpoS-dependent gene fusions from a bank of several thousand mutants harboring random, independent promoter-lacZ operon fusion mutations. Dependence on RpoS varied from 2-fold to over 100-fold. The expression of all fusion mutations was normal in an rpoS/rpoS+ merodiploid (rpoS background transformed with an rpoS-containing plasmid). Surprisingly, the expression of many RpoS-dependent genes was growth phase dependent, albeit at lower levels, even in an rpoS background, suggesting that other growth-phase-dependent regulatory mechanisms, in addition to RpoS, may control postexponential gene expression. These results are consistent with the idea that many growth-phase-regulated functions in Escherichia coli do not require RpoS for expression. The identities of the 10 most highly RpoS-dependent fusions identified in this study were determined by DNA sequence analysis. Three of the mutations mapped to otsA, katE, ecnB, and osmY—genes that have been previously shown by others to be highly RpoS dependent. The six remaining highly-RpoS-dependent fusion mutations were located in other genes, namely, gabP, yhiUV, o371, o381, f186, and o215.
Like many other free-living bacteria, Escherichia coli lives in environments that may change rapidly with respect to both nutrients and physical conditions. To survive stresses associated with starvation, E. coli expresses many stationary-phase-specific genes whose expression depends largely on an alternative sigma factor, ςs, encoded by rpoS (27, 30). Inactivation of this gene renders the cell sensitive to heat shock (25, 29), oxidative stress (25, 29), osmotic challenge (29), and near-UV light (40). Proteins that depend on RpoS include catalase HPII (33, 39, 42) and catalase HPI (32), exonuclease III (39), penicillin-binding proteins (15), and osmoprotective proteins (21, 22, 53). RpoS is required for virulence (17) and acid tolerance (6) in Salmonella typhimurium. Although the signal(s) giving rise to increased expression of RpoS itself is not completely understood, homoserine lactone (23), UDP-6-glucose (10), and weak acids, such as acetate (42), have been shown to be inducers of RpoS.
Several approaches have been used to enumerate and identify RpoS-regulated functions. Many of these genes, however, are probably still unidentified. Two-dimensional gel electrophoresis studies of proteins expressed in wild-type and rpoS strains have revealed that the RpoS regulon is quite large (30). Mutagenesis with random lacZ (16, 51) or lux insertions (46), coupled with screening for RpoS-related characteristic phenotypes, has also been successfully employed to identify new RpoS-regulated genes (51). However, unlike other regulons, the RpoS regulon does not have a single unifying characteristic or differentiating phenotype that all members share. These factors, in addition to its suspected large size, have delayed complete characterization of the regulon. To circumvent the problems associated with the characteristics described above, we have employed a mutant identification scheme in which an rpoS null allele is introduced into strains containing random promoter-lacZ fusions to directly identify RpoS dependency. Since this procedure does not rely on a phenotype specific for the regulon (e.g., carbon starvation), this method should be of general use in the identification of members of any regulon for which a null allele of a positive-acting regulator is available.
MATERIALS AND METHODS
Bacterial strains, phage, and plasmid.
The bacterial strains, phage, and plasmid used in this study are listed in Table 1.
TABLE 1.
E. coli strains, plasmid, and bacteriophage used in this study
| Strain, plasmid, or bacteriophage | Genotype | Source or reference |
|---|---|---|
| Strains | ||
| MC4100 | Δ(argF-lacZ)205 araD139 flbB5301 relA1 rpsL150 thi ptsF25 | 42 |
| GC4468 | ΔlacU169 rpsL | 42 |
| KL16 | Hfr (PO45) relA1 spoT1 thi-1 | K. B. Low |
| NC4468 | As GC4468, but φ(katE::lacZ+)131 | 41 |
| NC122 | As NC4468, but rpoS13::Tn10 | 41 |
| HS180 | Like KL16, but rpoS13::Tn10 | P1vir (NC122) × KL16→Tetr |
| GC122 | As GC4468, but φ(rpoS13::Tn10) | 41 |
| GC202 | As GC4468, but φ(katG::Tn10) | 42 |
| HS1001–HS1105 | As GC4468, but carrying RpoS dependent promoter-lacZ fusions | This study |
| HS1001T–HS1105T | As HS1001-HS1105, but rpoS13::Tn10 | This study |
| 13C10 | As GC4468, but carrying a growth-phase-dependent, RpoS-independent promoter-lacZ fusion | This study |
| Plasmid; pMMkatF3 | Carries rpoS (katF) gene | 33 |
| Bacteriophage; P1vir | Generalized transducing phage | Laboratory collection |
Chemicals and media.
All chemicals were supplied by either Fisher Scientific, Ltd. (Toronto, Ontario, Canada), Sigma Chemical Co. (St. Louis, Mo.), or Gibco BRL (Burlington, Ontario, Canada). Antibiotics and other nonautoclavable stock solutions were filter sterilized with Gelman Sciences (Ann Arbor, Mich.) Acrodisc sterile filters (pore size, 0.45 μm). Liquid and solid media were prepared as described by Miller (31). Cultures were routinely grown in Luria-Bertani (LB) rich broth. The concentrations of antibiotics used were as follows: kanamycin, 50 μg/ml; streptomycin, 50 μg/ml; tetracycline, 15 μg/ml; and ampicillin, 100 μg/ml. X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) was used at a concentration of 50 μg/ml.
Growth conditions.
All growth and survival assays were performed with GC4468 derivatives. Cultures were grown overnight in LB medium containing the appropriate antibiotics. Cell growth was monitored spectrophotometrically (UV-VIS spectrophotometer, model UV-1201; Shimadzu Corporation, Kyoto, Japan) by optical density at 600 nm (OD600). For expression studies, bacterial cultures were maintained in the early exponential phase (OD600 of <0.2) for at least 8 generations prior to the start of the experiment. Cultures were grown in flasks at 37°C at 200 rpm, sampled, and assayed for β-galactosidase activity at the times indicated.
For survival assays, bacterial cultures were incubated for 10 days in LB broth at 37°C in sealed microtiter plates (to minimize evaporation). Following this period, cultures were diluted in M9 salts buffer, plated on LB medium, and enumerated after 24 h of incubation.
Enzyme assays.
β-Galactosidase activity was assayed as described by Miller (31). Units of activity were calculated as [1,000 × OD420]/[time of incubation (min) × volume (ml) × OD600] and were expressed as Miller units. Catalase activity was measured spectrophotometrically by monitoring hydrogen peroxide decomposition at OD240 as described previously (42).
Genetic methods.
The phenotypic screen for RpoS dependence is based on the observation that introduction, by transduction, of rpoS::Tn10 into a strain containing a katE::lacZ fusion abolishes β-galactosidase activity (41). Since conjugation is much more efficient and amenable to large numbers of transfers than P1-mediated transduction, we reasoned that the use of an appropriate Hfr donor containing a null mutation in the rpoS gene close to the point of origin could facilitate simultaneous testing of several thousand colonies for dependence on RpoS. Since the point of origin of transfer in the Hfr strain KL16 is located at 64 min and DNA is transferred during mating in a counterclockwise direction, rpoS, located at 62 min, should be transferred shortly after initiation of conjugation. The rpoS13::Tn10 mutation was introduced into Hfr KL16 from strain NC122 (rpoS13::Tn10; katE::lacZ) by P1-mediated transduction. Transductants were selected on media supplemented with tetracycline (to select against the recipient) and streptomycin (to select against the donor). Transductants were flooded with 30% hydrogen peroxide to confirm transfer of the rpoS13::Tn10 mutation (E. coli colonies normally evolve gas bubbles when flooded with hydrogen peroxide because of the activity of catalase HPII, the catalase encoded by katE). This Hfr donor was confirmed to be HPII negative and was designated HS180. The Hfr transfer capability of HS180 was tested with the control strains NC4468 (katE::lacZ) and MC4100. All transconjugants exhibited reduced catalase levels, and as expected, transconjugants produced by mating HS180 with NC4468 also exhibited reduced β-galactosidase activity.
Plasmid transformations were performed with the TSS (transformation and storage solution) method of transforming recipient cells (13). To select for transformation of the pMMkatF3 plasmid, LB agar plates containing kanamycin, streptomycin, and ampicillin were used. Transformed rpoS transconjugants were selected on the same medium supplemented with tetracycline.
Identification of RpoS-dependent fusions.
To isolate promoter-lacZ fusions that depend on RpoS, we used a previously constructed collection of 5,000 independent transcriptional lacZ+ mutants as F− recipients (42). These LacZ+ mutants, in an MC4100 background, harbor randomly inserted λplacMu53 phage that also confer kanamycin resistance (12). The donor strain (HS180) was grown to the exponential phase (OD600 = 0.3) in LB broth, and 200-μl samples of culture were placed into microtiter plate wells. Recipient strains were grown to saturation in microtiter plate wells containing 200 μl of LB broth. Aliquots (20 μl) of strain HS180 were mated with each F− recipient directly in microtiter wells for 30 min and replica plated onto selective plates containing streptomycin, kanamycin, tetracycline, and X-Gal. Transfer of the rpoS allele was confirmed by testing the resulting transconjugants for catalase activity (see above). Putative RpoS-dependent (rsd) fusions were identified by comparing the levels of β-galactosidase activity of the fusions in rpoS+ and rpoS strains on LB plates containing X-Gal. Recipients were then purified, and one clone from each was tested for RpoS dependency. Complementation tests were done by transforming each transconjugant with pMMkatF3, a plasmid containing the rpoS gene (33). To ensure that strains contained single-copy chromosomal lacZ insertions, the fusions were transduced into GC4468 and retested for RpoS dependence.
Induction of λ lysogens.
Because the phage used to generate the mutant bank was a lambda derivative (12), bacterial DNA proximal to the introduced promoter-lacZ mutation can be isolated by UV induction of the lambdoid prophage from the bacterial mutants (38). A single clone was inoculated into LB medium containing streptomycin and kanamycin and grown overnight at 37°C. Cells were subcultured into 50 ml of fresh medium (1/10) the next morning, grown to an OD600 of 0.4, centrifuged, and resuspended in 10 ml of 10 mM MgSO4. Induction of the lambdoid prophage was performed by irradiating the culture at 25 mW for 7 s (approximately 35 J/m2). Five milliliters of 3xLL (38) medium was added, and the irradiated culture was shaken vigorously in a petri plate until lysis was observed (3 to 5 h). The lysate was transferred to a 30-ml glass Corex tube. Chloroform was added, the phage lysate was mixed vigorously, and cell debris was removed by centrifugation at 10,000 × g for 20 min. DNase (10-μg/ml final concentration) was added to remove traces of chromosomal DNA.
Sequencing of bacterial DNA proximal to the λ fusion junction.
Preparation and sequencing of DNA from UV-induced lysates were performed as previously described (38) with a 25-mer primer (5′-CCCGAATAATCCAATGTCCTCCCGG-3′) located 30 nucleotides from the Mu c end boundary. DNA sequencing was performed by the MOBIX central facility at McMaster University. The amount of DNA used in each sequencing reaction was approximately 0.5 to 1.0 μg. Sequences were compared to those in the GenBank database by using the BLASTN alignment algorithm (1). Bacterial homologs of identified E. coli RpoS-dependent genes were determined by using the gapped BLASTX alignment algorithim (2).
RNA extraction and Northern blot analysis.
Cultures were grown as described above, and aliquots were removed from exponential- and stationary-phase cultures. RNA was extracted with an RNeasy Midi Kit (Qiagen, Inc., Valencia, Calif.). Northern analysis was performed with equal amounts of RNA from the different samples by standard methods (43). Total RNA was blotted onto BIOTRANS nylon membranes (ICN, Montreal, PQ, Canada) as described in reference 43 and fixed by baking at 80°C for 2 h. Prehybridization and hybridization were performed at 42°C with gentle agitation. When necessary, blots were stripped by boiling in 0.1 to 0.5% sodium dodecyl sulfate according to the manufacturer’s (ICN) instructions for reprobing.
Oligonucleotide primers were synthesized and used in PCRs to generate specific probes to five identified rsd genes and to a control non-rsd gene control (rrnA): katE, forward (with respect to the open reading frame [ORF]), 5′-CAAAGCGGATTTCCTCTCAGATC-3′, and reverse, 5′-TGTCAAATGGCGTCTGACTTAG-3′; osmY, forward, 5′-CTGCTGGCTGTAATGTTGACCTC-3′, and reverse, 5′-CATCTACCGCTTTGGCGATACTT-3′; gabD, forward, 5′-GAAAGGCGAAATCAGCTACGC-3′, and reverse, 5′-CTTCGATGCCATACTTCGAACCT-3′; gabP, forward, 5′-CCATCTGGTTATTTTCCCTCG-3′, and reverse, 5′-GGTAATAAAGCCGATGACTAGCCAG-3′; and rrnA, forward, 5′-GTGCCCAGATGGGATTAGCTAGTAG-3′, and reverse, 5′-GTCGAGTTGCAGACTCCAATCC-3′. Each PCR tube contained 1× PCR buffer (500 mM KCl, 200 mM Tris [pH 8.4]), 50 pmol of each of the forward and reverse primers, 0.4 mM each of the four deoxynucleoside triphosphates, 4 mM MgCl2, ∼50 ng of E. coli DNA, and ∼10 U of Taq polymerase in a final volume of 50 μl. Reactions were run for 25 cycles under the following conditions: (i) 96°C for 30 s, (ii) 61°C for 60 s; and (iii) 72°C for 90 s. PCR products were purified with the QIAquick PCR purification kit (Qiagen, Inc.) and radiolabelled with [α-32P]dCTP (NEN Life Science Products, Inc., Boston, Mass.) by random priming. The identity of all PCR products was confirmed by DNA sequencing.
RESULTS
Isolation of ςs-dependent fusion mutants.
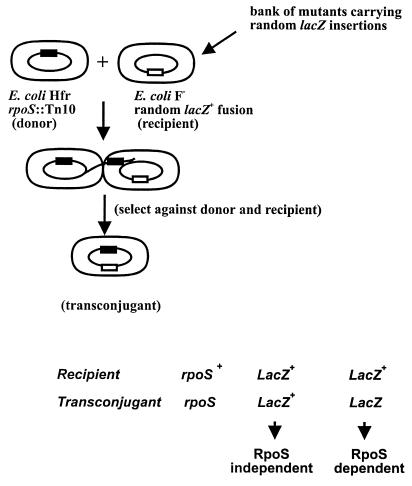
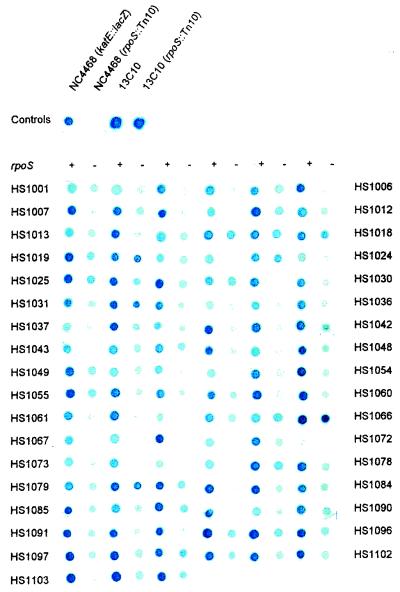
A diagrammatic representation of the screening procedure for the isolation of ςs-dependent promoter-lacZ fusions is shown in Fig. 1. Putative ςs-dependent fusions were identified by comparing the level of β-galactosidase activity of wild-type (with respect to rpoS) recipients to that of rpoS::Tn10 transconjugants on LB plates containing X-Gal. From this screen of 5,000 mutants, 105 rpoS::Tn10 transconjugants were identified that exhibited reduced β-galactosidase activity compared with that of the wild-type recipients. Putative RpoS-dependent (rsd) transcriptional fusions were transduced into GC4468 and retested for ςs dependency. A ςs-dependent katE::lacZ fusion strain, NC4468, served as a positive control, and a strain carrying a ςs-independent fusion, 13C10, was used as a negative control in subsequent mating procedures. The lacZ expression of all 105 transductants was RpoS dependent (Fig. 2).
FIG. 1.
Schematic representation of the strategy used to identify transconjugants harboring RpoS (ςs)-dependent promoter-lacZ fusions.
FIG. 2.
The 105 recipient (rpoS+) and transconjugant (rpoS) pairs in a GC4468 background. Strains were plated on M9 minimal media supplemented with 0.4% glucose. The ςs-dependent and -independent control strains NC4468 (katE::lacZ) and 13C10, respectively, were placed in the top row, with rpoS derivatives placed adjacent to them. rpoS+ and rpoS derivative pairs are adjacent to one another in rows, starting from the top left. The rpoS status of each column is shown on the top (+, rpoS+; −, rpoS).
To confirm that the lower β-galactosidase activity of the transconjugants was due to introduction of the rpoS null mutation and was not the result of a secondary mutation, transconjugants were transformed with pMMkatF3 containing a wild-type rpoS gene. Recipients were transformed in parallel, serving as controls for any variation in β-galactosidase levels due to the presence of the vector. In many cases, the transformed wild-type and rpoS strains exhibited higher levels of β-galactosidase activity than the nontransformed derivatives. This may be due to the increased levels of rpoS expression on multicopy plasmids, an observation reported by other investigators (39). As expected, all 105 mutants were efficiently complemented when transformed with plasmid-borne rpoS (data not shown).
Growth-phase expression of rsd-lacZ fusions.
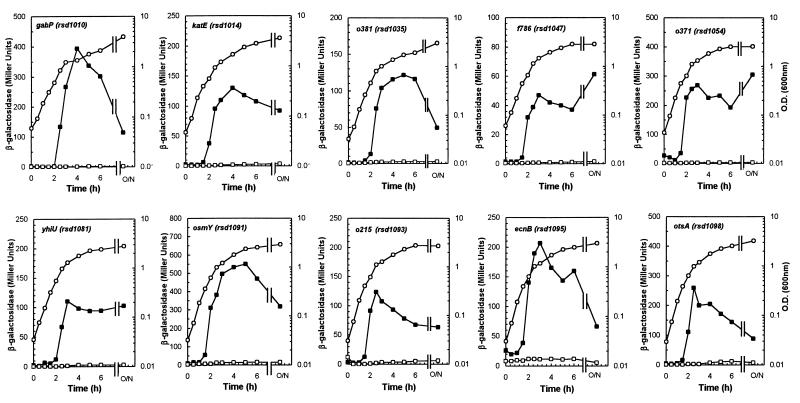
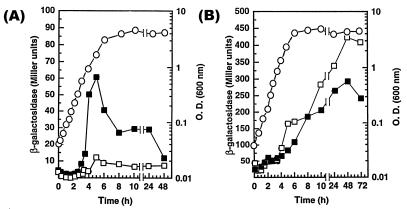
Many known RpoS-regulated genes are expressed at relatively low levels in the exponential phase but are induced as cells enter the stationary phase in rich medium (for review, see reference 19). We tested growth-phase induction of the rsd promoter-lacZ mutations isolated in this study. As expected, all fusions were maximally expressed in the early stationary phase or in 24-h cultures (Fig. 3). In each case, induction began before the cultures reached an OD600 of 0.3, suggesting that the signal(s) required for induction of these genes, whatever its nature, is present in early-exponential-phase cultures. We further examined growth-phase dependence in the other 95 fusion mutants and found that in each case, induction was initiated in the early exponential phase. We have previously observed that transcriptional induction of a single-copy rpoS::lacZ fusion occurs in the early exponential phase (42).
FIG. 3.
Growth-phase-dependent expression of 10 highly-RpoS-dependent fusions in rich medium. Flasks containing LB broth were inoculated with exponentially growing cultures as described in Materials and Methods, sampled periodically as indicated, and assayed for growth (OD600) and β-galactosidase activity. Each panel shows the growth of the culture and the β-galactosidase activity in strains carrying promoter-lacZ fusions to the indicated gene. The levels of growth of the wild-type strain and rpoS derivatives were equivalent, and thus only growth data for the wild-type strain are shown. ○, growth (OD600); ■, β-galactosidase activity in the wild-type strain; □, β-galactosidase activity in the rpoS derivative.
Identification of rsd-lacZ fusion junctions.
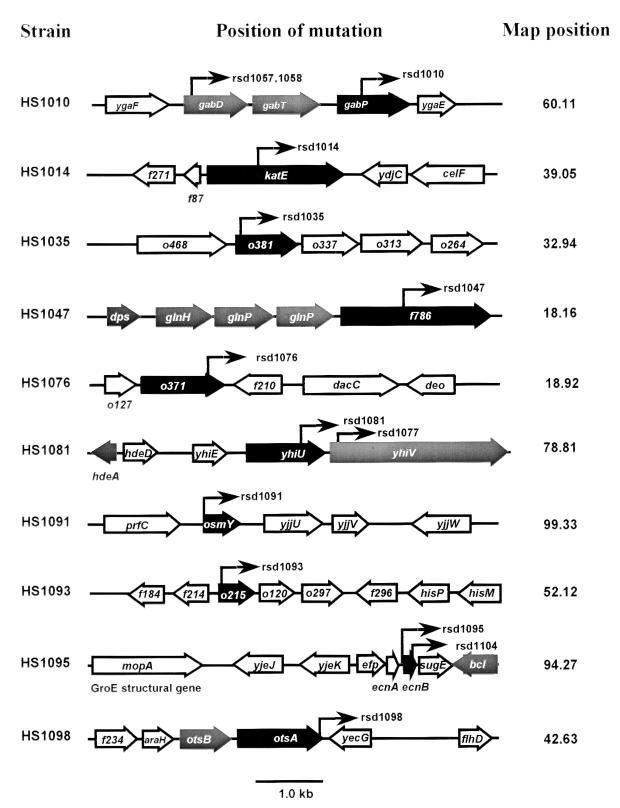
λ DNA was prepared from induced lysogens as described in Materials and Methods. The Mu c end vector sequence (5′-AATACA-3′) was confirmed for all sequences, and the determined DNA sequence proximal to the fusion junction was compared to published E. coli sequences. We have identified 50 of the 105 RpoS-dependent fusions isolated in this study by sequencing DNA prepared from UV-induced phage lysates as previously described (38). It is well established that the promoters of many RpoS-dependent genes can also be recognized by RpoD (45), the main vegetative sigma factor of E. coli. Since we were primarily interested in identifying genes that specifically require RpoS for expression (as opposed to those genes having promoters that can be recognized by both RpoS and RpoD [45]), we initially characterized the 10 fusions which exhibited the highest degree of RpoS dependence. Three of the 10 most-highly-RpoS-dependent mutations mapped to genes previously shown to be RpoS regulated, including katE (rsd1014), the structural gene for HPII catalase; otsA (rsd1098), which encodes trehalose synthase (21); and osmY (rsd1091), a probable lipoprotein of unknown function (54). The other seven mutations mapped to genes not previously known to require RpoS for expression.
The rsd1010 mutation is located in the terminal member of the gab operon (35), gabP, encoding a γ-aminobutyric acid (GABA) permease that can also transport other amine compounds (11). Two other mutations, rsd1057 and rsd1058, are in the first member of the operon, gabD (Fig. 4), which encodes a succinate semialdehyde dehydrogenase. The two gabD fusions were found to be slightly less RpoS dependent than rsd1010-lacZ, which may be due to a repetitive (REP) element that lies between gabT and gabP. This is consistent with the suggestion that these short DNA sequences may have a role in attenuating gene expression (5).
FIG. 4.
Location of points of insertion of transcriptional fusions in RpoS-regulated genes identified in this study. Arrows indicate the direction of transcription of genes. ▪, highly RpoS-dependent genes carrying the promoter-lacZ fusions identified in this study;  , other RpoS-dependent genes (see text); □, genes not known to require RpoS for expression.
, other RpoS-dependent genes (see text); □, genes not known to require RpoS for expression.
Strain HS1035 contains a fusion in o381, encoding a protein that is homologous to PotF, a periplasmic putrescine-binding protein (37). There are two known polyamine transport systems in E. coli. The potABCD and potFGHI operons are involved in transport of putrescine and spermidine, respectively (37). Interestingly, the ORFs downstream of o381 (o337, o313, and o264) (Fig. 4) are homologous to the corresponding members of the potABCD and potFGHI operons, suggesting that o381 is part of a third, conserved polyamine transport operon.
The rsd1047-lacZ mutation (strain HS1047) is located in f786, a gene of unknown function that is conserved in other bacteria matching hypothetical membrane proteins from Synechocystis sp. and S. typhimurium. This gene is immediately downstream of dps and the glutamine uptake operon, glnHPQ (Fig. 4), which are also induced in stationary-phase cultures. Combined with the dps gene (3) and the glnHPQ operon (52), these genes, dps-glnHPQ-f786, may constitute a large stationary-phase-specific operon.
The rsd1076 mutation mapped to o371, a reading frame of unknown function, that is homologous to glucose dehydrogenase B from Acinetobacter sp. (14).
The rsd1095 mutation mapped to the newly described ecnB locus, a gene coding for a bacteriolytic protein that may play a role in the selective elimination of moribund cells in stationary-phase populations (7) (Fig. 4). The expression of this gene is now known to be RpoS dependent (7). The ecnB gene was originally described as part of a longer gene, sugEL, a suppressor of GroEL chaperone function in E. coli (44). The sugEL gene was reported to have two promoters, one of which is induced in stationary-phase cells (44). An adjacent divergently transcribed reading frame encodes the Bcl lipoprotein, whose expression is also RpoS regulated (8).
One mutation (rsd1081) mapped to yhiU (Fig. 4), the first member of an operon encoding a probable two-member drug efflux pump that is homologous to AcrAB and EnvCD. A second fusion (rsd1077) mapped to yhiV, previously shown to be RpoS dependent (4). These membrane-bound complexes coordinate the energy-dependent transport of a wide variety of noxious compounds (for review, see reference 36).
Several of the other identified fusions were located in known RpoS-dependent genes, including rsd1004, which mapped to ldcC, encoding a lysine decarboxylase of E. coli (45, 49), and rsd1082, which mapped to aidB (49). The remaining fusions mapped to genes whose regulation was not previously known to be controlled by RpoS and will be reported elsewhere.
Expression of highly-RpoS-dependent genes in rich and minimal media.
The expression of highly-RpoS-dependent fusions was examined in rich (Table 2) and in minimal (Table 3) media. The growth of the rpoS derivatives tested was similar to that of the rpoS+ strains in both rich and minimal media. As expected, the expression of all promoter fusions was dependent on RpoS in the stationary phase in rich media. In the exponential phase, the expression of most fusions was several-fold higher in a wild-type strain than in an rpoS strain, suggesting that RpoS may be important for the expression of these genes in exponentially growing cells. This was true of cells growing in minimal medium (Table 3) as well as those grown in rich medium (Table 2). The expression of rsd1095 (ecnB) was moderately growth-phase dependent, even in an rpoS background, as previously shown (18).
TABLE 2.
Growth-phase-dependent expression of highly-RpoS-dependent promoter-lacZ fusions in strains grown in rich mediuma
| Mutation | Identified gene | β-Galactosidase activity (Miller units)
|
|||||
|---|---|---|---|---|---|---|---|
| Exponential phase
|
Stationary phase
|
||||||
| Wild type | rpoS13::Tn10 | RpoS dependence | Wild type | rpoS13::Tn10 | RpoS dependence | ||
| rsd1010 | gabP | 6.3 ± 0.7 | 0.4 ± 0.1 | 15 | 116.7 ± 8.8 | 1.3 ± 0.0 | 92.0 |
| rsd1014 | katE | 1.6 ± 0.0 | 0.4 ± 0.0 | 3.6 | 60.0 ± 2.0 | 1.2 ± 0.0 | 52.0 |
| rsd1035 | o381 | 6.1 ± 0.6 | 0.7 ± 0.0 | 8.2 | 52.6 ± 7.7 | 1.6 ± 0.1 | 33.7 |
| rsd1047 | f786 | 2.3 ± 0.1 | 0.3 ± 0.0 | 8.6 | 40.1 ± 1.6 | 0.8 ± 0.0 | 52.4 |
| rsd1076 | o371 | 11.0 ± 0.8 | 1.5 ± 0.3 | 7.5 | 137.4 ± 3.9 | 2.7 ± 0.1 | 50.9 |
| rsd1081 | yhiU | 6.3 ± 0.5 | 1.0 ± 0.2 | 6.2 | 108.0 ± 0.3 | 2.1 ± 0.4 | 50.7 |
| rsd1091 | osmY | 8.6 ± 0.9 | 3.0 ± 0.2 | 2.9 | 408.8 ± 27.8 | 6.0 ± 0.4 | 67.6 |
| rsd1093 | o215 | 4.5 ± 0.1 | 0.4 ± 0.2 | 10.4 | 60.4 ± 1.3 | 1.3 ± 0.3 | 45.0 |
| rsd1095 | ecnB | 11.3 ± 0.9 | 3.1 ± 1.0 | 3.8 | 152.8 ± 12.2 | 7.5 ± 0.1 | 20.4 |
| rsd1098 | otsA | 10.7 ± 0.4 | 1.2 ± 0.3 | 9.1 | 158.3 ± 8.3 | 1.4 ± 0.1 | 115.3 |
Strains were grown overnight in LB broth, subcultured, and maintained in the exponential phase for 8 generations prior to sampling. Cultures were sampled at the exponential phase (OD600 = 0.3) and stationary phase (OD600 = 1.6).
TABLE 3.
Growth-phase-dependent expression of highly-RpoS-dependent promoter-lacZ fusions in strains grown in glucose minimal mediuma
| Mutation | Identified gene | β-Galactosidase activity (Miller units)
|
|||||
|---|---|---|---|---|---|---|---|
| Exponential phase
|
Stationary phase
|
||||||
| Wild type | rpoS13::Tn10 | RpoS dependence | Wild type | rpoS13::Tn10 | RpoS dependence | ||
| rsd1010 | gabP | 8.5 ± 0.2 | 0.9 ± 0.1 | 9.0 | 43.0 ± 9.0 | 1.4 ± 0.0 | 30.5 |
| rsd1014 | katE | 7.9 ± 1.4 | 0.5 ± 0.1 | 15.4 | 53.8 ± 6.8 | 1.3 ± 0.1 | 42.7 |
| rsd1035 | o381 | 14.4 ± 0.9 | 0.6 ± 0.0 | 23.2 | 66.4 ± 3.4 | 0.9 ± 0.1 | 74.2 |
| rsd1047 | f786 | 5.1 ± 1.1 | 0.5 ± 0.1 | 10.6 | 38.3 ± 3.2 | 0.6 ± 0.1 | 63.0 |
| rsd1076 | o371 | 9.8 ± 0.7 | 1.0 ± 0.1 | 9.6 | 72.2 ± 7.4 | 1.9 ± 0.1 | 40.1 |
| rsd1081 | yhiU | 13.2 ± 1.2 | 1.6 ± 0.2 | 8.1 | 53.1 ± 0.3 | 3.0 ± 0.4 | 17.4 |
| rsd1091 | osmY | 12.8 ± 0.1 | 2.1 ± 0.2 | 6.2 | 107.9 ± 7.8 | 2.3 ± 0.1 | 46.3 |
| rsd1093 | o215 | 13.6 ± 1.0 | 0.7 ± 0.1 | 19.2 | 79.7 ± 15.5 | 0.9 ± 0.1 | 86.4 |
| rsd1095 | ecnB | 5.9 ± 0.3 | 2.0 ± 0.1 | 2.9 | 95.6 ± 7.5 | 4.4 ± 0.2 | 21.8 |
| rsd1098 | otsA | 10.1 ± 1.6 | 2.0 ± 0.1 | 4.8 | 98.2 ± 3.6 | 2.9 ± 0.2 | 34.2 |
Strains were grown overnight in minimal medium, subcultured, and maintained for 8 generations in the exponential phase prior to sampling. Cultures were sampled in the exponential phase (OD600 = 0.2) and stationary phase (OD600 = 0.9).
Levels of mRNA of identified genes expressed in the exponential and stationary phases of growth.
To confirm that the identified promoter-lacZ fusions accurately represented RpoS-dependent gene control in a wild-type strain, RNA was prepared from wild-type (GC4468) and rpoS (GC122) strains sampled in the exponential (OD600 = 0.2) and stationary (OD600 = 1.6) phases and hybridized to PCR-amplified probes specific for gabP, osmY, and katE (Fig. 5). katE and osmY, two well-characterized RpoS-dependent genes, were highly expressed in the stationary phase in the wild-type strain, but they were expressed at low levels in the exponential phase and were largely absent in the rpoS strain (Fig. 5). Similarly, the expression of gabP was also growth phase dependent only in the wild-type strain and was expressed at low levels in an rpoS strain (Fig. 5). Since gabP is part of an operon that includes another identified rsd gene, gabD (Fig. 4), we also performed Northern analysis with a probe specific to gabD. As expected, the expression pattern of this gene was similar to that of gabP (data not shown). The expression of the other identified highly-RpoS-dependent genes was also confirmed, by Northern analyses, to be growth phase and RpoS dependent (data not shown).
FIG. 5.
RpoS- and growth-phase-dependent expression of rsd genes. The results of Northern analyses of total RNA isolated from exponential-phase (E) and stationary-phase (S) cultures of wild-type (GC4468) and rpoS (GC122) strains are shown. RNA was hybridized with probes specific for osmY, katE, and gabP. To confirm that equivalent amounts of RNA were extracted and loaded, control blots were probed with rrnA, an RpoS-independent gene (data not shown).
RpoS-independent, growth-phase-dependent gene expression.
Surprisingly, many of the fusions were found to be growth-phase dependent even in an rpoS background. For example, the expression of rsd1004, which mapped to ldcC, was strongly growth-phase dependent in both rpoS+ and rpoS backgrounds (Fig. 6A). Of the 105 RpoS-dependent fusions isolated, 15 strains exhibited greater than fivefold induction of β-galactosidase as cells entered the stationary phase, suggesting that regulation by factors other than RpoS may be important in control of growth-phase-dependent gene expression. If this is true, then the expression of many of the other fusions in the mutant bank that were determined to be RpoS independent in the initial screening should exhibit growth-phase dependence even in an rpoS background. We found this to be the case for many mutants selected at random from our bank of transcriptional mutants. Figure 6B shows one such example of an RpoS-independent, growth-phase-dependent promoter. We then examined expression of 49 RpoS-independent fusions in both the exponential and stationary phases. Eight of the 49 fusions exhibited greater than fivefold induction, a proportion that does not differ significantly (χ1,1 = 0.609, ρ = 0.43) from the proportion of RpoS-dependent fusions that showed a similar degree of growth-phase induction in rpoS derivatives (Table 4). Taken in toto, these results suggest that a large number of nonessential genes of E. coli do not require RpoS for elevated stationary-phase expression and raise the intriguing possibility that RpoS-dependent genes may constitute only a small fraction of stationary-phase genes in E. coli.
FIG. 6.
Growth-phase-dependent expression of an RpoS-dependent fusion and an RpoS-independent fusion. Flasks containing LB broth were inoculated with exponentially growing cultures as described in Materials and Methods, sampled periodically as indicated, and assayed for growth (OD600) and β-galactosidase activity. Each panel shows growth of the culture and β-galactosidase activity in strains carrying promoter-lacZ fusions to the indicated gene. The levels of growth of the wild-type strain and rpoS derivatives were equivalent, and thus only growth data for the wild-type strain are shown. (A) rsd1004 (ldcC) (RpoS dependent). (B) 13C10 (RpoS independent). ○, growth (OD600); ■, β-galactosidase activity in the rpoS+ strain; □, β-galactosidase activity in the rpoS derivative.
TABLE 4.
Proportion of E. coli mutants whose expression is RpoS and growth-phase dependenta
| Fusion type | No. of mutants with expression:
|
||
|---|---|---|---|
| Growth-phase dependent | Growth-phase independent | Total | |
| RpoS dependent | 15 | 90 | 105 |
| RpoS independent | 8 | 41 | 49 |
Wild-type and rpoS derivatives of rsd strains (105 total) and a randomly selected group of mutants carrying promoter-lacZ fusions whose expression is not RpoS dependent (49 total) were grown in LB broth and assayed in the exponential and stationary phases for β-galactosidase activity. A given promoter-lacZ mutation was classified as growth-phase dependent if the stationary-phase level of β-galactosidase, in an rpoS strain, was more than five times greater than that observed in the exponential-phase cultures.
Stationary-phase survival of rsd mutants.
To test whether deficiency in any of the 10 identified highly-RpoS-dependent functions would impair stationary-phase survival, cultures were grown to saturation in LB broth and incubated for 10 days at 37°C. All of the mutants exhibited a 10-fold reduction in viability, about the same as that of the wild-type strain (GC4468), while the survival of an rpoS strain, GC122, was approximately 0.1% during this time period, consistent with results obtained by others (55).
DISCUSSION
In this paper, we describe a method for identifying members of a gene regulon by comparing expression of lacZ fusions in transconjugants containing a null allele of the putative regulator to that of an isogenic strain carrying the wild-type allele. We have found that this method can be used to reproducibly detect differences in expression between wild-type and rpoS strains that are as low as twofold. We employed this method in the study of the RpoS-controlled stationary-phase response. The probable large size of this regulon, its dependence on a single regulator, RpoS, and the fact that many of the members of this regulon may yet be undiscovered render the study of the RpoS regulon highly amenable to this type of analysis. In theory, this conjugation protocol could, however, be used to characterize any regulon for which null-selectable alleles in a single controlling regulator exist.
The number of RpoS-regulated proteins identified has increased markedly over the past few years. Currently, more than 40 genes are known to be regulated by RpoS (for review, see reference 20). Results of in vitro transcription assays (34) indicate that many RpoS (ςs) promoters are also recognized by RpoD (ς70) (45). Although we do not yet know which promoter determinants contribute to the specificity of RpoS recognition of the fusions identified in this study, many of the fusions identified are highly dependent on RpoS for expression. As such, they should be useful in the identification of factors important in regulation by this alternative sigma factor.
A total of 105 transcriptional fusion mutants were identified in this study that are RpoS dependent. Based on our previous work isolating catalase mutants from this bank (42), we estimate there is probably a twofold redundancy in the number of isolated RpoS-dependent genes. The early-exponential-phase induction of all of the fusions identified was somewhat surprising, since RpoS, subject to complex controls at the transcriptional, translational, and posttranslational levels, is fully active only in the early stationary phase (28). However, expression of rpoS is induced at the transcriptional level early in the exponential phase at an OD600 of 0.2 (42), consistent with the idea that this is the earliest point at which induction of RpoS-dependent functions can occur. Although maximal expression was usually observed in the stationary phase, the expression of all promoter fusions isolated in this study began at an OD600 of 0.3, suggesting that concerted expression of RpoS-regulated genes begins well before the commencement of the stationary phase. This pattern would be consistent with the idea that adaptive proteins required for survival during periods of nutrient deprivation must be produced while the cell is capable of robust gene expression. Other identified RpoS-regulated genes exhibit a similar pattern of expression. For example, induction of dnaN begins in the exponential phase but is maximally expressed in stationary-phase cultures (48). Similar patterns of expression have been observed for bolA (9, 23), another highly-RpoS-dependent gene.
The fact that several of the genes identified were not previously known to be regulated by RpoS may be explained by several factors. First, the gene product may be masked by another compensatory functional activity with the cell. For example, the physiological function of the ecnB/sugE gene product is probably masked in cells that produce GroEL, the major chaperonin in E. coli. A second possible explanation is that some proteins are expressed at levels too low to measure—LdcC, a second lysine decarboxylase in E. coli, is detectable only when expressed on a multicopy plasmid (24). Finally, the gene of interest may be one of the many ORFs (currently more than half of all ORFs) in E. coli that have not been assigned any function and thus not been previously studied (e.g., o371).
The fact that a large proportion of fusions in the mutant bank were found to be growth-phase regulated (both RpoS dependent and RpoS independent) cannot readily be explained by current models of growth-phase regulation. We estimate that almost 20% of the mutants in the bank (∼1,000) carry growth-phase-inducible fusions, a relatively small fraction (105/1,000 [∼10%]) of which are RpoS dependent. This suggests that a large proportion of the bacterium’s genetic repertoire is involved in adaptation to nutrient deprivation or to some other growth-phase-related stimulus. The non-RpoS-dependent component of this response has thus far received little attention, but its characterization is undoubtedly critical in understanding how bacteria adapt to suboptimal conditions. There are probably other transcriptional factors besides RpoS that lead to increased expression of certain genes during the stationary phase. Transcriptional control of “gearbox” promoters (9, 47) is tightly coupled to growth rate, and one of these promoters is known to be RpoS independent (9, 26). Additional sequence analysis and primer extension studies are required to determine if the promoters of the rsd-lacZ fusions are homologous to the proposed gearbox consensus promoter sequence (47). Factors affecting posttranslational stationary-phase expression have been described and include alterations in ribosome assembly (50) and differential protein degradation (28). These are, however, unlikely to be involved in the regulation of the fusions isolated in this study, since the mutagen employed (λplacMu) generates transcriptional promoter fusions (12).
The characterization of other fusion mutations isolated in this study should aid in the identification of genes that are expressed specifically in the stationary phase and may provide additional clues regarding the regulation and physiological function of the RpoS regulon.
ACKNOWLEDGMENTS
We thank J. H. Weiner for communicating information regarding ecnB prior to publication. We thank Suzana Gligorjevic and Xiaoli Zhao for capable technical assistance and R. A. Morton and T. M. Finan for helpful discussion and suggestions. We gratefully acknowledge the help of B. Allore and Dinsdale Gooden of the MOBIX central facility for their help with the synthesis of DNA primers and DNA sequencing.
This work was funded from an operating grant to H.E.S. from the Natural Sciences and Engineering Council (NSERC) of Canada.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1998;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altuvia S, Almiron M, Huisman G, Kolter R, Storz G. The dps promoter is activated by OxyR during growth and by IHF and sigma S in stationary phase. Mol Microbiol. 1994;13:265–272. doi: 10.1111/j.1365-2958.1994.tb00421.x. [DOI] [PubMed] [Google Scholar]
- 4.Altuvia S, Weinstein-Fischer D, Zhang A, Postow L, Storz G. A small, stable RNA induced by oxidative stress: role as a pleiotropic regulator and antimutator. Cell. 1997;90:43–53. doi: 10.1016/s0092-8674(00)80312-8. [DOI] [PubMed] [Google Scholar]
- 5.Bachellier S, Gilson E, Hofnung M, Hill C W. Repeated sequences. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 2. Washington, D.C: ASM Press; 1996. pp. 2012–2040. [Google Scholar]
- 6.Baik H S, Bearson S, Dunbar S, Foster J W. The acid tolerance response of Salmonella typhimurium provides protection against organic acids. Microbiology. 1996;142:3195–3200. doi: 10.1099/13500872-142-11-3195. [DOI] [PubMed] [Google Scholar]
- 7.Bishop R E, Leskiw B K, Hodges R S, Kay C M, Weiner J H. The entericidin locus of Escherichia coli and its implications for programmed bacterial cell death. J Mol Biol. 1998;280:583–596. doi: 10.1006/jmbi.1998.1894. [DOI] [PubMed] [Google Scholar]
- 8.Bishop R E, Penfold S S, Frost L S, Holtje J V, Weiner J H. Stationary phase expression of a novel Escherichia coli outer membrane lipoprotein and its relationship with mammalian apolipoprotein D. Implications for the origin of lipocalins. J Biol Chem. 1995;270:23097–23103. doi: 10.1074/jbc.270.39.23097. [DOI] [PubMed] [Google Scholar]
- 9.Bohannon D E, Connell N, Keener J, Tormo A, Espinosa-Urgel M, Zambrano M M, Kolter R. Stationary-phase-inducible “gearbox” promoters: differential effects of katF mutations and role of ς70. J Bacteriol. 1991;173:4482–4492. doi: 10.1128/jb.173.14.4482-4492.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Böhringer J, Fischer D, Mosler G, Hengge-Aronis R. UDP-glucose is a potential intracellular signal molecule in the control of expression of ςs and ςs-dependent genes in Escherichia coli. J Bacteriol. 1995;177:413–422. doi: 10.1128/jb.177.2.413-422.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brechtel C E, Hu L, King S C. Substrate specificity of the Escherichia coli 4-aminobutyrate carrier encoded by gabP. Uptake and counterflow of structurally diverse molecules. J Biol Chem. 1996;271:783–788. doi: 10.1074/jbc.271.2.783. [DOI] [PubMed] [Google Scholar]
- 12.Bremer E, Silhavy T J, Weinstock G M. Transposable λ placMu bacteriophages for creating lacZ operon fusions and kanamycin resistance insertions in Escherichia coli. J Bacteriol. 1985;162:1092–1099. doi: 10.1128/jb.162.3.1092-1099.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung C T, Niemela S L, Miller R H. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci USA. 1989;86:2172–2175. doi: 10.1073/pnas.86.7.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cleton-Jansen A M, Goosen N, Vink K, van de Putte P. Cloning, characterization and DNA sequencing of the gene encoding the Mr 50,000 quinoprotein glucose dehydrogenase from Acinetobacter calcoaceticus. Mol Gen Genet. 1989;217:430–436. doi: 10.1007/BF02464914. [DOI] [PubMed] [Google Scholar]
- 15.Dougherty T J, Pucci M J. Penicillin-binding proteins are regulated by rpoS during transitions in growth states of Escherichia coli. Antimicrob Agents Chemother. 1994;38:205–210. doi: 10.1128/aac.38.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang F C, Chen C-Y, Guiney D G, Xu Y. Identification of ςs-regulated genes in Salmonella typhimurium: complementary regulatory interactions between ςs and cyclic AMP receptor protein. J Bacteriol. 1996;178:5112–5120. doi: 10.1128/jb.178.17.5112-5120.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang F C, Libby S J, Buchmeier N A, Loewen P C, Switala J, Harwood J. The alternative sigma factor katF (rpoS) regulates Salmonella virulence. Proc Natl Acad Sci USA. 1992;89:11978–11982. doi: 10.1073/pnas.89.24.11978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greener T, Govezensky D, Zamir A. A novel multicopy suppressor of a groEL mutation includes two nested open reading frames transcribed from different promoters. EMBO J. 1993;12:889–896. doi: 10.1002/j.1460-2075.1993.tb05729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hengge-Aronis R. Back to log phase: sigma S as a global regulator in the osmotic control of gene expression in Escherichia coli. Mol Microbiol. 1996;21:887–893. doi: 10.1046/j.1365-2958.1996.511405.x. [DOI] [PubMed] [Google Scholar]
- 20.Hengge-Aronis R. Regulation of gene expression during entry into stationary phase. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C: ASM Press; 1996. pp. 1497–1512. [Google Scholar]
- 21.Hengge-Aronis R, Klein W, Lange R, Rimmele M, Boos W. Trehalose synthesis genes are controlled by the putative sigma factor encoded by rpoS and are involved in stationary-phase thermotolerance in Escherichia coli. J Bacteriol. 1991;173:7918–7924. doi: 10.1128/jb.173.24.7918-7924.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hengge-Aronis R, Lange R, Henneberg N, Fischer D. Osmotic regulation of rpoS-dependent genes in Escherichia coli. J Bacteriol. 1993;175:259–265. doi: 10.1128/jb.175.1.259-265.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huisman G W, Kolter R. Sensing starvation: a homoserine lactone-dependent signaling pathway in Escherichia coli. Science. 1994;265:537–539. doi: 10.1126/science.7545940. [DOI] [PubMed] [Google Scholar]
- 24.Kikuchi Y, Kojima H, Tanaka T, Takatsuka Y, Kamio Y. Characterization of a second lysine decarboxylase isolated from Escherichia coli. J Bacteriol. 1998;179:4486–4492. doi: 10.1128/jb.179.14.4486-4492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lange R, Fischer D, Hengge-Aronis R. Identification of transcriptional start sites and the role of ppGpp in the expression of rpoS, the structural gene for the ςs subunit of RNA polymerase in Escherichia coli. J Bacteriol. 1995;177:4676–4680. doi: 10.1128/jb.177.16.4676-4680.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lange R, Hengge-Aronis R. Growth phase-regulated expression of bolA and morphology of stationary-phase Escherichia coli cells are controlled by the novel sigma factor ςs. J Bacteriol. 1991;173:4474–4481. doi: 10.1128/jb.173.14.4474-4481.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lange R, Hengge-Aronis R. Identification of a central regulator of stationary-phase gene expression in Escherichia coli. Mol Microbiol. 1991;5:49–59. doi: 10.1111/j.1365-2958.1991.tb01825.x. [DOI] [PubMed] [Google Scholar]
- 28.Lange R, Hengge-Aronis R. The cellular concentration of the sigma S subunit of RNA polymerase in Escherichia coli is controlled at the levels of transcription, translation, and protein stability. Genes Dev. 1994;8:1600–1612. doi: 10.1101/gad.8.13.1600. [DOI] [PubMed] [Google Scholar]
- 29.McCann M P, Fraley C D, Matin A. The putative ς factor KatF is regulated posttranscriptionally during carbon starvation. J Bacteriol. 1993;175:2143–2149. doi: 10.1128/jb.175.7.2143-2149.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCann M P, Kidwell J P, Matin A. The putative ς factor KatF has a central role in development of starvation-mediated general resistance in Escherichia coli. J Bacteriol. 1991;173:4188–4194. doi: 10.1128/jb.173.13.4188-4194.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller J H. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1992. [Google Scholar]
- 32.Mukhopadhyay S, Schellhorn H E. Induction of Escherichia coli hydroperoxidase I by acetate and other weak acids. J Bacteriol. 1994;176:2300–2307. doi: 10.1128/jb.176.8.2300-2307.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mulvey M R, Sorby P A, Triggs-Raine B L, Loewen P C. Cloning and physical characterization of katE and katF required for catalase HPII expression in Escherichia coli. Gene. 1988;73:337–345. doi: 10.1016/0378-1119(88)90498-2. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen L H, Jensen D B, Thompson N E, Gentry D R, Burgess R R. In vitro functional characterization of overproduced Escherichia coli katF/rpoS gene product. Biochemistry. 1993;32:11112–11117. doi: 10.1021/bi00092a021. [DOI] [PubMed] [Google Scholar]
- 35.Niegemann E, Schulz A, Bartsch K. Molecular organization of the Escherichia coli gab cluster: nucleotide sequence of the structural genes gabD and gabP and expression of the GABA permease gene. Arch Microbiol. 1998;160:454–460. doi: 10.1007/BF00245306. [DOI] [PubMed] [Google Scholar]
- 36.Paulsen I T, Brown M H, Skurray R A. Proton-dependent multidrug efflux systems. Microbiol Rev. 1996;60:575–608. doi: 10.1128/mr.60.4.575-608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pistocchi R, Kashiwagi K, Miyamoto S, Nukui E, Sadakata Y, Kobayashi H, Igarashi K. Characteristics of the operon for a putrescine transport system that maps at 19 minutes on the Escherichia coli chromosome. J Biol Chem. 1993;268:146–152. [PubMed] [Google Scholar]
- 38.Roy R N, Mukhopadhyay S, Wei L I, Schellhorn H E. Isolation and sequencing of gene fusions carried by lambda placMu specialized transducing phage. Nucleic Acids Res. 1995;23:3076–3078. doi: 10.1093/nar/23.15.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sak B D, Eisenstark A, Touati D. Exonuclease III and the catalase hydroperoxidase II in Escherichia coli are both regulated by the Escherichia coli katF gene product. Proc Natl Acad Sci USA. 1989;86:3271–3275. doi: 10.1073/pnas.86.9.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sammartano L J, Tuveson R W, Davenport R. Control of sensitivity to inactivation by H2O2 and broad-spectrum near-UV radiation by the Escherichia coli katF locus. J Bacteriol. 1986;168:13–21. doi: 10.1128/jb.168.1.13-21.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schellhorn H E, Hassan H M. Transcriptional regulation of katE in Escherichia coli K-12. J Bacteriol. 1988;170:4286–4292. doi: 10.1128/jb.170.9.4286-4292.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schellhorn H E, Stones V L. Regulation of katF and katE in Escherichia coli K-12 by weak acids. J Bacteriol. 1992;174:4769–4776. doi: 10.1128/jb.174.14.4769-4776.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seldon R F. Analysis of RNA by northern hybridization. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates and John Wiley and Sons; 1989. pp. 4.9.1–4.9.8. [Google Scholar]
- 44.Srikumar R, Kon T, Gotoh N, Poole K. Expression of Pseudomonas aeruginosa multidrug efflux pumps MexA-MexB-OprM and MexC-MexD-OprJ in a multidrug-sensitive Escherichia coli strain. Antimicrob Agents Chemother. 1998;42:65–71. doi: 10.1128/aac.42.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanaka K, Takayanagi Y, Fujita N, Ishihama A, Takahashi H. Heterogeneity of the principal sigma factor in Escherichia coli: the rpoS gene product, sigma 38, is a second principal sigma factor of RNA polymerase in stationary-phase Escherichia coli. Proc Natl Acad Sci USA. 1993;90:3511–3515. doi: 10.1073/pnas.90.8.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Dyk T K, Ayers B L, Morgan R W, LaRossa R A. Constricted flux through the branched-chain amino acid biosynthetic enzyme acetolactate synthase triggers elevated expression of genes regulated by rpoS and internal acidification. J Bacteriol. 1998;180:785–792. doi: 10.1128/jb.180.4.785-792.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vicente M, Kushner S R, Garrido T, Aldea M. The role of the ‘gearbox’ in the transcription of essential genes. Mol Microbiol. 1991;5:2085–2091. doi: 10.1111/j.1365-2958.1991.tb02137.x. [DOI] [PubMed] [Google Scholar]
- 48.Villarroya M, Perez-Roger I, Macian F, Armengod M E. Stationary phase induction of dnaN and recF, two genes of Escherichia coli involved in DNA replication and repair. EMBO J. 1998;17:1829–1837. doi: 10.1093/emboj/17.6.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Volkert M R, Hajec L I, Matijasevic Z, Fang F C, Prince R. Induction of the Escherichia coli aidB gene under oxygen-limiting conditions requires a functional rpoS (katF) gene. J Bacteriol. 1994;176:7638–7645. doi: 10.1128/jb.176.24.7638-7645.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wada A, Yamazaki Y, Fujita N, Ishihama A. Structure and probable genetic location of a “ribosome modulation factor” associated with 100S ribosomes in stationary-phase Escherichia coli cells. Proc Natl Acad Sci USA. 1990;87:2657–2661. doi: 10.1073/pnas.87.7.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weichart D, Lange R, Henneberg N, Hengge-Aronis R. Identification and characterization of stationary phase-inducible genes in Escherichia coli. Mol Microbiol. 1993;10:407–420. [PubMed] [Google Scholar]
- 52.Xu J, Johnson R C. Identification of genes negatively regulated by Fis: Fis and RpoS comodulate growth-phase-dependent gene expression in Escherichia coli. J Bacteriol. 1995;177:938–947. doi: 10.1128/jb.177.4.938-947.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yim H H, Brems R L, Villarejo M. Molecular characterization of the promoter of osmY, an rpoS-dependent gene. J Bacteriol. 1994;176:100–107. doi: 10.1128/jb.176.1.100-107.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yim H H, Villarejo M. osmY, a new hyperosmotically inducible gene, encodes a periplasmic protein in Escherichia coli. J Bacteriol. 1992;174:3637–3644. doi: 10.1128/jb.174.11.3637-3644.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zambrano M M, Siegele D A, Almiron M, Tormo A, Kolter R. Microbial competition: Escherichia coli mutants that take over stationary phase cultures. Science. 1993;259:1757–1760. doi: 10.1126/science.7681219. [DOI] [PubMed] [Google Scholar]