Abstract
Iron and manganese oxides or oxyhydroxides are abundant transition metals, and in aquatic environments they serve as terminal electron acceptors for a large number of bacterial species. The molecular mechanisms of anaerobic metal reduction, however, are not understood. Shewanella putrefaciens is a facultative anaerobe that uses Fe(III) and Mn(IV) as terminal electron acceptors during anaerobic respiration. Transposon mutagenesis was used to generate mutants of S. putrefaciens, and one such mutant, SR-21, was analyzed in detail. Growth and enzyme assays indicated that the mutation in SR-21 resulted in loss of Fe(III) and Mn(IV) reduction but did not affect its ability to reduce other electron acceptors used by the wild type. This deficiency was due to Tn5 inactivation of an open reading frame (ORF) designated mtrB. mtrB encodes a protein of 679 amino acids and contains a signal sequence characteristic of secreted proteins. Analysis of membrane fractions of the mutant, SR-21, and wild-type cells indicated that MtrB is located on the outer membrane of S. putrefaciens. A 5.2-kb DNA fragment that contains mtrB was isolated and completely sequenced. A second ORF, designated mtrA, was found directly upstream of mtrB. The two ORFs appear to be arranged in an operon. mtrA encodes a putative 10-heme c-type cytochrome of 333 amino acids. The N-terminal sequence of MtrA contains a potential signal sequence for secretion across the cell membrane. The amino acid sequence of MtrA exhibited 34% identity to NrfB from Escherichia coli, which is involved in formate-dependent nitrite reduction. To our knowledge, this is the first report of genes encoding proteins involved in metal reduction.
Bacterial Fe(III) and Mn(IV) reduction constitute major respiratory processes in aquatic environments and in some instances account for the oxidation of 80 to 90% of the available organic carbon (7, 33). The soluble reduced metals diffuse upward in the water column, where they get oxidized to form insoluble particles. These particles settle down into the suboxic zone to be used again as terminal electron acceptors during anaerobic respiration. In addition to playing a role in the carbon cycle, iron and manganese contribute to the removal of toxic metals and phosphates from the aerobic zones into the anaerobic sediments (for details see reference 33). Due to its environmental significance, anaerobic metal reduction has been the focus of intense investigation in the past decade. This has led to the isolation and identification of a large number of metal-reducing bacteria. Many of these bacteria can couple metal reduction to the anaerobic oxidation of aromatic hydrocarbons, such as benzene and toluene (19, 21). Bacterial species identified as dissimilatory metal reducers include facultative anaerobes such as Shewanella putrefaciens and Shewanella alga as well as strict anaerobes such as Geobacter metallireducens and Desulfovibrio sp. (17, 20, 33).
In spite of the attention bacterial metal reduction has received, very little is known about the biochemical or molecular mechanisms underlying this process. S. putrefaciens, a gram-negative organism that is widely distributed in freshwater and marine environments (32, 33, 37, 41), serves as a good model system to study metal reduction. S. putrefaciens is a strict respirer that can grow both aerobically and anaerobically. Under anaerobic conditions, S. putrefaciens can use a wide range of electron acceptors. These include fumarate, trimethylamine N-oxide (TMAO), dimethyl sulfoxide (DMSO), nitrate, nitrite, thiosulfate, and sulfite, as well as insoluble acceptors, such as metal oxides or oxyhydroxides (24, 28). Direct contact of bacterial cells with metal oxides appears to be required for reduction to occur (31). Reduction is not observed upon separation of cells from the oxide particles by dialysis membranes (4). Cell fractionation studies of S. putrefaciens MR-1 revealed the presence of ferric reductase activity in the outer membranes of anaerobically grown cells (25). It was suggested that association of ferric reductase with the outer membrane may be necessary for the bacteria to use insoluble terminal electron acceptors (25). Fe(III) reductase activity, however, was also found in the inner membrane fraction of S. putrefaciens MR-1 (25). It is not clear why both membrane fractions exhibited Fe(III) reductase activity. It is possible that S. putrefaciens MR-1 has multiple ferric reductases. Two such enzymes are thought to be present in S. putrefaciens sp200 (3), although the location of these enzymes is yet to be determined. Experimental evidence suggests the involvement of c-type cytochromes in metal reduction. S. putrefaciens cells, which are orange in color, lose this pigmentation and the ability to reduce Fe(III) when starved for iron (36). Reduced-minus-oxidized spectra of iron-starved cells indicate the absence of c-type cytochromes (36). The cellular distribution of c-type cytochromes in S. putrefaciens MR-1 is atypical. While some are soluble periplasmic proteins as in other bacteria, others are associated with the outer membrane (26, 27). The role of outer membrane c-type cytochromes in metal reduction is not clear. To date, the genes or proteins involved in metal reduction have not been identified. In this paper, we describe the isolation and analysis of mtrB, which encodes an outer membrane protein involved in Fe(III) and Mn(IV) reduction.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
A list of the bacterial strains and plasmids used in this study is given in Table 1. S. putrefaciens and Escherichia coli strains were grown aerobically in Luria-Bertani (LB) medium (39) at 30 and 37°C, respectively. Antibiotics, when needed, were added to growth media at the following final concentrations: kanamycin (Km), 25 μg/ml; tetracycline (Tc), 17 μg/ml; ampicillin (Amp), 100 μg/ml; rifampin (Rif), 10 μg/ml. LML medium (0.02% yeast extract, 0.01% peptone, 20 mM lactate, 10 mM HEPES [pH 7.4]) was used for anaerobic growth of S. putrefaciens.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| E. coli | ||
| DH5αMCR | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 phoA supE44 λ− thi-1 gyrA96 relA1 | BRL |
| S17-1 | Pro (r− m+) contains integrated plasmid RP4-2-Tc::Mu-Km::Tn7, Tpr Smr | 42 |
| S. putrefaciens | ||
| MR-1 | Lake Oneida isolate | 28 |
| MR-1R | Spontaneous mutant of MR-1, Rifr | This work |
| SR-21 | MR-1R but freC::Tn5, Rifr Kmr | This work |
| SRC-21 | SR-21 carrying pSC52, Rifr Kmr Tcr | This work |
| Plasmids | ||
| pUC119 | Cloning and sequencing vector, Ampr | BRL |
| pSPORT1 | Cloning and sequencing vector, Ampr | BRL |
| pRK415 | IncP plamid, Tcr | 15 |
| pSUP1011 | pACYC184 derivative carrying RP4 oriT and Tn5, Cmr Kmr | 42 |
| pSRF212 | pSPORT1 with freB-containing 6.2-kb EcoRI-BamHI fragment | This work |
| pAB52 | pUC119 with freBC-containig 5.8-kb EcoRI-HindIII fragment | This work |
| pSC52 | pRK415 with freBC-containing 5.8-kb EcoRI-HindIII fragment | This work |
Anaerobic reduction of electron acceptors.
S. putrefaciens strains were grown anaerobically in a Coy anaerobic chamber by using LML supplemented with fumarate (10 mM), TMAO (20 mM) or DMSO (3 mM) and the appropriate antibiotics. Growth was monitored spectrophotometrically (A600). Total protein concentrations were measured by using a Bio-Rad Dc Protein kit. Reduction of nitrate and nitrite was tested in LML broth supplemented with 10 mM formate, 0.02% vitamin-free Casamino Acids, and 5 mM NaNO3 or 0.45 mM KNO2. Nitrite concentration was measured as described previously (11). Thiosulfate and sulfite reduction was tested in vials containing LML soft agar (0.8%), 0.05 mM FeSO4, and 5 mM Na2S2O3 or 2 mM Na2SO3. Reduction of these electron acceptors was indicated by the formation of the black precipitate FeS (Ksp of FeS is 10−2.95 [43]). Mn(IV) reduction was tested in LML soft agar supplemented with 2 mM MnO2 (18). The insoluble brown MnO2 is reduced to soluble Mn(II), which is colorless. Fe(III) reduction was measured by using the ferrozine assay as described previously (8).
Isolation of mutants.
A spontaneous Rif-resistant mutant of S. putrefaciens, MR-1R, was used in mating experiments with E. coli S17-1 harboring pSUP1011 (42) (Table 1). Both strains were grown in LB medium overnight, washed, and mixed in a 1:1 ratio before spotting onto LB agar plates. Following a 4-h incubation at room temperature, the cells were scraped off the agar and plated onto LML agar supplemented with 10 mM ferric citrate (pH 7.4), 25 μg of Km/ml, and 10 μg of Rif/ml. Putative Fe(III) reduction-negative mutants were identified by using ferrozine to detect Fe(II) production. Briefly, ferrozine was sprayed on the agar surface as described previously (12). Production of Fe(II) was detected by the observation of a magenta color that formed immediately following ferrozine addition. Of approximately 35,000 colonies, 60 did not result in the formation of the magenta color and were analyzed further. Five mutants were identified as deficient in the reduction of Fe(III) and Mn(IV), and one mutant, SR-21, was selected for further analysis.
Cloning of mtrA and mtrB genes.
DNA isolation, restriction digestion, and other manipulations were performed by standard techniques (39). SR-21 total DNA was digested with BamHI and EcoRI and ligated to pSPORT1 (BRL Life Technologies). The ligated DNA was used to transform E. coli DH5αMCR (Table 1) by electroporation (BRL). Km-resistant colonies were selected, and plasmid DNA was purified. Selection for Km resistance resulted in the isolation of the plasmid pSRF212, which contains part of Tn5 and adjacent S. putrefaciens DNA. We used pSRF212 and a 17-base primer complementary to the 5′ end of Tn5 (Biosynthesis Inc., Lewisville, Tex.), to obtain the S. putrefaciens DNA sequence adjacent to the transposon insertion site. Further sequence data were obtained by primer walking using the dideoxy sequencing procedure (40).
We used primers generated during the course of sequencing to amplify DNA fragments flanking the Tn5 insertion in SR-21 by PCR (39). These fragments were radiolabelled and used to probe a genomic library of S. putrefaciens DNA in λGEM-11 (Promega). Several clones were isolated, and one, λAB3, was used for further work. λAB3 DNA was purified and analyzed by restriction digestion and Southern blotting. A 5.2-kb fragment that hybridized to the PCR-generated probes was purified and cloned into pUC119 (Table 1). The nucleotide sequence of the cloned fragment was obtained either manually (40) or with an ABI automated sequencing apparatus (Biotech Research Laboratories, Inc.). Sequences were analyzed with MacVector 6.0 and AssemblyLign software (Oxford Molecular Group, Campbell, Calif.), and comparisons to database sequences were made using the BLAST and FASTA algorithms (1, 2).
Complementation of SR-21.
The broad-host-range plasmid pRK415 (Tcr [15]) was used for the complementation of SR-21. A 5.2-kb EcoRI-HindIII fragment containing mtrA and mtrB was cloned into pRK415 to generate pSC52 (Table 1). E. coli S17-1 was transformed with pSC52 and then used to transfer the plasmid into SR-21 by conjugation. SR-21(pSC52) recombinant colonies were selected on LB agar plates supplemented with Tc and Km. One such isolate, SR-21C, was tested for Fe(III) and Mn(IV) reduction as described above.
Enzyme assays.
MR-1, SR-21, and SR-21C cells were grown with 10 mM fumarate to mid-log phase. The cells were harvested and washed in 10 mM Tris-HCl (pH 7.5)–50 mM NaCl. Cells were lysed with lysozyme, and the proteins were solubilized with Triton X-100 as described previously (10). Fumarate, DMSO, and TMAO reductase activities were detected on native polyacrylamide gels. Bands of enzyme activity were visualized with reduced benzyl viologen (22) followed by the addition of fumarate (10 mM), TMAO (20 mM), or DMSO (3 mM).
Preparation of outer membrane proteins.
MR-1, SR-21, and SR-21C were grown anaerobically in LB medium supplemented with either ferric citrate (5 mM) or fumarate (10 mM). Following a 2-day incubation, the cells were harvested by centrifugation and lysed by French press. Preparation of inner and outer membrane fractions was performed as described by Leisman et al. (16). Proteins of both inner and outer membrane fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Nucleotide sequence accession number.
The sequence presented in this paper has been deposited in GenBank under accession no. AF083240.
RESULTS
Analysis of the metal-reduction-deficient mutant SR-21.
Fe(III)-reduction-deficient mutants were generated by Tn5 insertional mutagenesis. The mutants were initially isolated based on their inability to produce appreciable amounts of Fe(II) when grown on LML-ferric citrate agar plates. These mutants were then analyzed for their ability to use other terminal electron acceptors. Five of these mutants were found to be deficient in both Fe(III) and Mn(IV) reduction. One mutant, designated SR-21, was analyzed in detail.
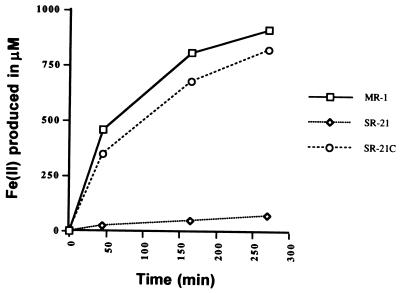
The ability of SR-21 to reduce Fe(III) was measured in liquid cultures supplemented with ferric citrate. The amount of Fe(II) produced was detected by using ferrozine as described previously (8). As shown in Fig. 1, very little Fe(II) was produced in SR-21 cultures compared with the wild-type strain, MR-1. To determine if the mutation in SR-21 was specific for Fe(III) reduction, we tested the ability of this mutant to utilize the different terminal electron acceptors used by the wild type. Mn(IV) reduction was tested in anaerobic vials containing LML soft agar supplemented with 2 mM MnO2 (18). In contrast to MR-1, SR-21 was unable to reduce Mn(IV), as shown by the brownish-black color of the medium in Fig. 2.
FIG. 1.
Levels of Fe(III) reduction in wild-type MR-1, mutant SR-21, and complemented mutant SR-21C. Reduction of Fe(III) to Fe(II) was monitored using ferrozine at 562 nm.
FIG. 2.
Mn(IV) reduction by MR-1, SR-21, and SR-21C. Reduction of Mn(IV) is detected by clearing of brown color in the medium as seen in wild-type MR-1 and the complemented mutant SR-21C but not in the mutant SR-21.
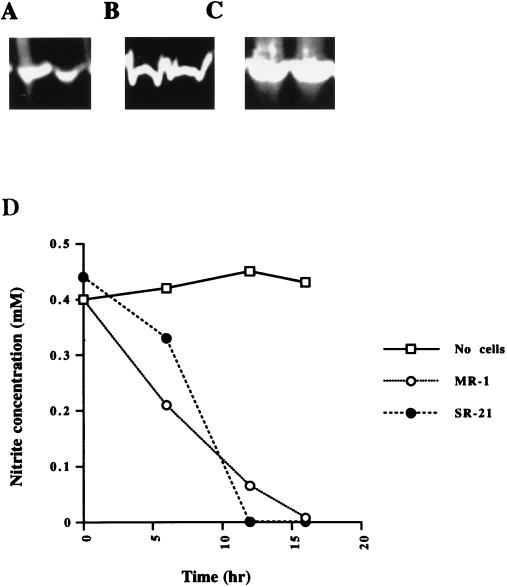
Reduction of fumarate, TMAO, and DMSO by SR-21 was tested by using benzyl viologen as an artificial electron donor (22). Cell extracts from both S. putrefaciens MR-1 and SR-21 exhibited bands of activities corresponding to all three reductases, as shown in Fig. 3. To determine if SR-21 was still able to couple the reduction of TMAO, DMSO, and fumarate to carbon oxidation, we monitored the growth of SR-21 in the presence of these electron acceptors. SR-21 and MR-1 exhibited similar growth patterns when supplemented with the electron acceptors mentioned above (data not shown).
FIG. 3.
Reduction of fumarate, DMSO, TMAO, and nitrite by MR-1 and SR-21. Cell extracts of MR-1 and SR-21 were separated by native PAGE and assayed for fumarate (A), DMSO (B), and TMAO (C) reductase activities. Benzyl viologen was used as the artificial electron donor. Clear bands in all samples indicate the ability of MR-1 and SR-21 to reduce these electron acceptors. (D) Nitrite reduction by MR-1 and SR-21 was assayed by monitoring the decrease in nitrite concentration in the medium. No differences were observed in the ability of SR-21 to use nitrite compared to the wild type.
Simple colorimetric assays were used to detect the reduction of sulfite, thiosulfate, NO3−, and NO2−. SR-21 was able to reduce nitrite at a rate similar to that of MR-1, as shown in Fig. 3. SR-21 was also able to reduce sulfite, thiosulfate, and NO3− (Table 2). Our results indicate that the mutation in SR-21 disrupted genes that are specific for Fe(III) and Mn(IV) reduction but are not required for the reduction of other electron acceptors used by the wild type.
TABLE 2.
Anaerobic reduction of various electron acceptors by wild-type MR-1 and the mutant SR-21a
| Electron acceptor | Reduction by:
|
|
|---|---|---|
| MR-1 | SR-21 | |
| Fe(III) | + | − |
| Mn(IV) | + | − |
| Nitrate | + | + |
| Nitrite | + | + |
| Fumarate | + | + |
| DMSO | + | + |
| TMAO | + | + |
| Thiosulfate | + | + |
| Sulfite | + | + |
−, no reduction; +, reduction.
Sequence analysis of mtrA and mtrB.
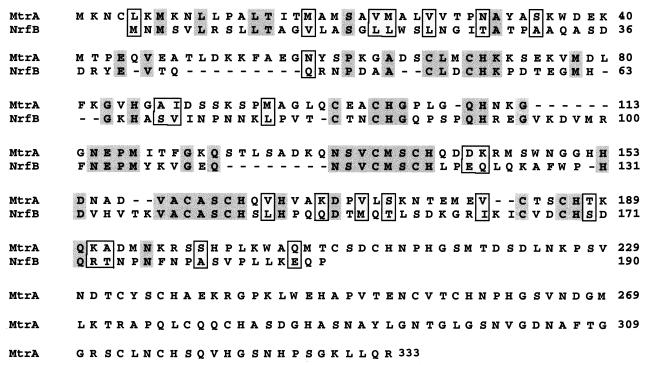
To identify the genes involved in Fe(III) and Mn(IV) reduction, we used a probe corresponding to the Tn5 insertion site in SR-21 to isolate a 5.2-kb EcoRI-HindIII fragment from λAB3 (Table 1). The complete nucleotide sequence of this DNA fragment was obtained. We identified two open reading frames (ORFs) that we designated mtrA (metal reduction) and mtrB. mtrA is proposed to start with an ATG initiation codon at position 1376. A potential ribosome binding site is located 5 nucleotides upstream of the ATG codon. The deduced amino acid sequence of MtrA consists of 333 amino acids with a putative signal sequence characteristic of secreted proteins. Cleavage of this signal sequence is predicted to be between amino acids 34 and 35 (35). MtrA contains 10 putative heme-binding sites (CXXCH) characteristic of c-type cytochromes. Lack of hydrophobic regions within MtrA, with the exception of the signal sequence, suggests that MtrA is not likely to be associated with the inner membrane. Using the PSORT algorithm (30), MtrA was predicted to be a periplasmic protein. Comparison of MtrA to sequences in the databases revealed a high degree of similarity to NrfB from E. coli (34% identity and 49% similarity [Fig. 4]). NrfB is a c cytochrome involved in formate-dependent nitrite reduction (14). MtrA also had similarities with other c-type cytochromes from several bacterial species, mostly due to the highly conserved nature of the heme-binding sites.
FIG. 4.
Amino acid sequence alignment of NrfB from E. coli and the deduced amino acid sequence of MtrA. Identical amino acids are shaded and similar amino acids are boxed.
The second ORF, mtrB, starts at position 2390, with an ATG initiation codon located 13 nucleotides downstream of the mtrA stop codon. This putative initiation codon is preceded by a ribosome binding site 7 nucleotides upstream. The loss of Fe(III) and Mn(IV) reduction in SR-21 was due to the insertional inactivation of mtrB, which encodes a protein of 679 amino acids. MtrB contains a stretch of hydrophobic amino acids characteristic of signal peptides, with a predicted cleavage site between amino acids 21 and 22 (35), suggesting that it is a secreted protein. Based on hydrophobicity profiles, MtrB is not likely to be located in the inner membrane. Comparison of the amino acid sequence of MtrB to sequences in the databases revealed similarities to outer membrane or surface proteins from several bacteria. It was 27% identical and 41% similar (over 235 amino acids) to OmpC, an outer membrane protein from Salmonella typhimurium (34), and 21% identical and 53% similar (over 351 amino acids) to AltE from Staphylococcus epidermidis (13). MtrB was also similar to the E. coli siderophore receptor FepA (23) with 19% identity and 40% similarity over 252 amino acids. Similar identity scores were obtained with other outer membrane proteins, such as rOmpA from Rickettsia conorii (9) and Eae from E. coli (44). These similarities suggested that MtrB may be an outer membrane protein. Using the PSORT algorithm, the location of MtrB was also predicted to be in the outer membrane (30). This prediction was confirmed by analyzing membrane fractions of wild-type and mutant strains of S. putrefaciens MR-1 (see below).
A potential rho-independent termination signal was found downstream of mtrB. Another such signal was also found upstream of mtrA. This suggests that mtrA and mtrB constitute the operon. This was further supported by complementation studies. The recombinant strain, SR-21C, which contains pRK415 carrying the 5.2-kb DNA fragment, was able to reduce both Mn(IV) and Fe(III), as shown in Fig. 1 and 2.
Localization of MtrB to the outer membrane.
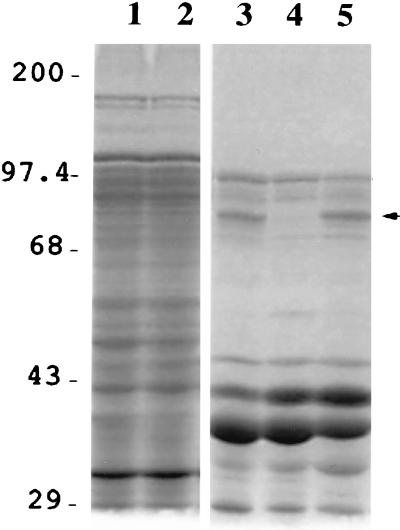
The cellular location of MtrB was determined by using S. putrefaciens MR-1, SR-21, and SR-21C cells grown anaerobically with either fumarate or Fe(III) as terminal electron acceptors. Inner and outer membrane fractions were prepared as described in Materials and Methods, and the proteins were analyzed by SDS-PAGE. Since MtrB was similar to outer membrane proteins from other bacteria, we expected to see the loss of this protein in outer membrane fractions of the mutant SR-21. The results shown in Fig. 5 confirm this prediction. Outer membrane fractions of SR-21 appear to lack a protein band of 75 kDa in size. This corresponds to the predicted molecular mass of MtrB (75.5 kDa) following cleavage of the signal sequence. MtrB was not detected in outer membrane fractions of SR-21 cells grown anaerobically with either fumarate or Fe(III) as terminal electron acceptors, but was present in wild-type and SR-21C cells grown under both conditions. These results suggest that mtrB expression is not dependent on the presence of Fe(III) in the medium.
FIG. 5.
SDS-PAGE of inner and outer membrane proteins of anaerobically grown MR-1, SR-21, and SR-21C. Lanes: 1 and 2, cell membrane fractions from MR-1 and SR-21, respectively; 3, 4, and 5, outer membrane fractions of MR-1, SR-21, and SR-21C, respectively. The band that corresponds to MtrB, which is not detected in the outer membrane fraction of the mutant SR-21, is indicated by an arrow. Numbers correspond to protein molecular mass markers (in kilodaltons).
DISCUSSION
How bacteria use insoluble metal oxides as terminal electron acceptors is an intriguing problem that is currently under intense investigation. In order to identify the components involved in this process of anaerobic respiration, we have isolated mutants of the metal reducer S. putrefaciens MR-1 that are incapable of utilizing Fe(III) or Mn(IV) as terminal electron acceptors. To date, we have not identified mutants that are deficient solely in either Fe(III) or Mn(IV) reduction, although the isolation of such mutants has been described (5, 12). The mutants we have isolated were generated by transposon mutagenesis, while the mutants described by DiChristina and colleagues (5, 12) were generated by using ethyl methane sulfonate. This may explain the differences in the types of mutants isolated by each group.
We have chosen one of our transposon-generated mutants, SR-21, for further analyses. SR-21 can use all of the electron acceptors utilized by the wild-type strain with the exceptions of Fe(III) and Mn(IV). Fe(III) reduction by SR-21, although extremely low, was not completely abolished. It has been suggested that S. putrefaciens sp200 possesses two ferric reductases that differ markedly in their activities (3). The background levels of Fe(III) reduction that were observed in SR-21 cultures may be due to the presence of a second ferric reductase in S. putrefaciens MR-1, whose activity is still intact in the mutant.
Fe(III) and Mn(IV) reduction deficiency in SR-21 was due to Tn5 insertion into mtrB. This gene encodes a protein of 679 amino acids that contains a putative signal sequence and is therefore predicted to be transported across the cell membrane. The location of MtrB in S. putrefaciens was determined following membrane fraction analysis of both wild-type and SR-21 cells. Our results indicate that MtrB is located in the outer membrane fraction of S. putrefaciens MR-1. MtrB was detected in outer membrane preparations of MR-1 cells grown anaerobically with either fumarate or Fe(III) as terminal electron acceptors. This suggests that the expression of MtrB is not regulated by Fe(III). The role of MtrB in metal reduction is not yet clear. It has been suggested that the ferric reductase of S. putrefaciens, in contrast to other known terminal reductases, is an outer membrane-associated enzyme. Although the nature of this enzyme and its subunits have not been identified, it is possible that MtrB may be one of these subunits. The identification of MtrB in the outer membrane fraction of S. putrefaciens cells, and the inability of SR-21 cell extracts to reduce Fe(III) (data not shown), support this idea. MtrB may play a role in the binding of metals during reduction. It contains the sequence CXXC (amino acids 42 to 45), which in other proteins is postulated to be a metal binding site (6).
mtrA encodes a putative c-type cytochrome that contains 10 heme-binding sites. The large number of heme-binding sites in this protein is unusual. Most c-type cytochromes studied to date contain one to five heme-binding sites. Exceptions include cytochrome c3 from Desulfovibrio vulgaris which has 16 heme-binding sites (38) and a recently identified deca-heme c-type cytochrome from S. putrefaciens (27, 29). Since mtrA is located in the same operon as mtrB, we suspect that MtrA also plays a role in Fe(III) and Mn(IV) reduction. MtrA may be another subunit of the metal reductase that is located in the outer membrane. The presence of c-type cytochromes in the outer membrane of S. putrefaciens has been reported (27, 29). Alternatively, MtrA may be located in the periplasmic space, perhaps loosely attached to other components of the metal reductase in the outer membrane.
To our knowledge, this is the first report of genes involved specifically in Fe(III) and Mn(IV) reduction. In addition to the genes described above, we have identified an ORF upstream of mtrA that encodes a c-type cytochrome and appears to be involved in Fe(III) and Mn(IV) reduction (unpublished results). Experiments are in progress to determine the role of each of these proteins in metal reduction.
ACKNOWLEDGMENTS
This work was supported by National Science Foundation grant MCB 9604298 and a University of Massachusetts Faculty Research grant.
We thank M. McBride for helpful comments and critical reading of the manuscript. We also thank D. Lies for MnO2 preparations and J. McLaughlin for help with membrane fractionation studies.
REFERENCES
- 1.Altschul S, Gish W, Miller W, Myers E, Lipman D. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S, Madden T, Schaffer A, Zhang J, Zhang Z, Miller W, Lipman D. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold R G, DiChristina T J, Hoffman M R. Inhibitor studies of dissimilative Fe(III) reduction by Pseudomonas sp. strain 200 (“Pseudomonas ferrireductans”) Appl Environ Microbiol. 1986;52:281–289. doi: 10.1128/aem.52.2.281-289.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold R, DiChristina T, Hoffman M. Reductive dissolution of Fe(III) oxides by Pseudomonas sp. 200. Biotechnol Bioeng. 1988;32:1081–1096. doi: 10.1002/bit.260320902. [DOI] [PubMed] [Google Scholar]
- 5.Burnes B, Mulberry M, DiChristina T. Design and application of two rapid screening techniques for isolation of Mn(IV) reduction-deficient mutants of Shewanella putrefaciens. Appl Environ Microbiol. 1998;64:2716–2720. doi: 10.1128/aem.64.7.2716-2720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calder K M, Horwitz M A. Identification of iron-regulated proteins of Mycobacterium tuberculosis and cloning of tandem genes encoding a low iron-induced protein and a metal transporting ATPase with similarities to two-component metal transport systems. Microb Pathog. 1998;24:133–143. doi: 10.1006/mpat.1997.9999. [DOI] [PubMed] [Google Scholar]
- 7.Canfield D E, Thamdrup B, Hansen J W. The anaerobic degradation of organic matter in Danish coastal sediments: iron reduction, manganese reduction and sulfate reduction. Geochim Cosmochim Acta. 1993;57:3867–3885. doi: 10.1016/0016-7037(93)90340-3. [DOI] [PubMed] [Google Scholar]
- 8.Carter P. Spectrophotometric determination of serum iron at the submicrogram level with a new reagent (ferrozine) Anal Biochem. 1971;40:450–458. doi: 10.1016/0003-2697(71)90405-2. [DOI] [PubMed] [Google Scholar]
- 9.Crocquet-Valdes P, Weiss K, Walker D. Sequence analysis of the 190-kDa antigen-encoding gene of Rickettsia conorii (Malish 7 strain) Gene. 1994;140:115–119. doi: 10.1016/0378-1119(94)90740-4. [DOI] [PubMed] [Google Scholar]
- 10.Cull M, McHenry C. Preparation of extracts from prokaryotes. In: Deutscher M P, editor. Guide to protein purification. Vol. 182. San Diego, Calif: Academic Press; 1990. pp. 147–153. [DOI] [PubMed] [Google Scholar]
- 11.Daniels L, Hanson R S, Phillips J A. Methods for general and molecular bacteriology. Washington, D.C: American Society for Microbiology; 1994. [Google Scholar]
- 12.DiChristina T J, DeLong E F. Isolation of anaerobic respiratory mutants of Shewanella putrefaciens and genetic analysis of mutants deficient in anaerobic growth on Fe3+ J Bacteriol. 1994;176:1468–1474. doi: 10.1128/jb.176.5.1468-1474.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heilmann C, Hussain M, Peters G, Gotz F. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol Microbiol. 1997;24:1013–1024. doi: 10.1046/j.1365-2958.1997.4101774.x. [DOI] [PubMed] [Google Scholar]
- 14.Hussain H, Grove J, Griffiths L, Busby S, Cole J. A seven-gene operon essential for formate-dependent nitrite reduction to ammonia by enteric bacteria. Mol Microbiol. 1994;12:153–163. doi: 10.1111/j.1365-2958.1994.tb01004.x. [DOI] [PubMed] [Google Scholar]
- 15.Keen N T, Tamaki S, Kobayashi D, Trolliger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988;70:191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 16.Leisman G B, Waukau J, Forst S A. Characterization and environmental regulation of outer membrane proteins in Xenorhabdus nematophilus. Appl Environ Microbiol. 1995;61:200–204. doi: 10.1128/aem.61.1.200-204.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lovley D. Dissimilatory Fe (III) and Mn (IV) reduction. Microbiol Rev. 1991;55:259–287. doi: 10.1128/mr.55.2.259-287.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lovley D, Phillips E. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reuction of iron or manganese. Appl Environ Microbiol. 1988;51:683–689. doi: 10.1128/aem.54.6.1472-1480.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lovley D, Baedecker M, Lonergan D, Cozzarelli I, Phillips E, Siegel D. Oxidation of aromatic contaminants coupled to microbial iron reduction. Nature. 1989;339:297–299. [Google Scholar]
- 20.Lovley D, Coates J, Saffarini D, Lonergan D. Dissimilatory iron reduction. In: Winkelmann G, Carrano C, editors. Transition metals in microbial metabolism. Amsterdam, The Netherlands: Harwood Academic Publishers; 1997. pp. 187–215. [Google Scholar]
- 21.Lovley D R, Lonergan D J. Anaerobic oxidation of toluene, phenol, and p-cresol by the dissimilatory iron-reducing organism, GS-15. Appl Environ Microbiol. 1990;56:1858–1864. doi: 10.1128/aem.56.6.1858-1864.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lund K, DeMoss J. Association-dissociation behavior and subunit structure of heat-released nitrate reductase from Escherichia coli. J Biol Chem. 1976;251:2207–2216. [PubMed] [Google Scholar]
- 23.Lundrigan M D, Kadner R J. Nucleotide sequence of the gene for the ferrienterochelin receptor FepA in Escherichia coli. Homology among outer membrane receptors that interact with TonB. J Biol Chem. 1986;261:10797–10801. [PubMed] [Google Scholar]
- 24.Moser D, Nealson K. Growth of the facultative anaerobe Shewanella putrefaciens by elemental sulfur reduction. Appl Environ Microbiol. 1996;62:2100–2105. doi: 10.1128/aem.62.6.2100-2105.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myers C, Myers J. Ferric reductase is associated with the membranes of anaerobically grown Shewanella putrefaciens MR-1. FEMS Microbiol Lett. 1993;108:15–22. [Google Scholar]
- 26.Myers C, Myers J. Localization of cytochromes to the outer membrane of anaerobically grown Shewanella putrefaciens MR-1. J Bacteriol. 1992;174:3429–3438. doi: 10.1128/jb.174.11.3429-3438.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myers C, Myers J. Outer membrane cytochromes of Shewanella putrefaciens MR-1: spectral analysis, and purification of the 83-kDa c-type cytochrome. Biochim Biophys Acta. 1997;1326:307–318. doi: 10.1016/s0005-2736(97)00034-5. [DOI] [PubMed] [Google Scholar]
- 28.Myers C, Nealson K. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science. 1988;240:1319–1321. doi: 10.1126/science.240.4857.1319. [DOI] [PubMed] [Google Scholar]
- 29.Myers J, Myers C. Isolation and sequence of omcA, a gene encoding a decaheme outer membrane cytochrome c of Shewanella putrefaciens MR-1, and detection of omcA homologs in other strains of S. putrefaciens. Biochim Biophys Acta. 1998;1373:237–251. doi: 10.1016/s0005-2736(98)00111-4. [DOI] [PubMed] [Google Scholar]
- 30.Nakai K, Kanehisa M. Expert system for predicting protein localization sites in gram-negative bacteria. Proteins Struct Funct Genet. 1991;11:95–110. doi: 10.1002/prot.340110203. [DOI] [PubMed] [Google Scholar]
- 31.Nealson K, Rosson R, Myers C. Mechanisms of oxidation and reduction of manganese. In: Beveridge T, Doyle R, editors. Metal ions and bacteria. New York, N.Y: John Wiley and Sons, Inc.; 1989. pp. 383–411. [Google Scholar]
- 32.Nealson K H, Myers C R, Wimpee B. Isolation and identification of manganese reducing bacteria, and estimates of microbial manganese reducing potential in the Black Sea. Deep-Sea Res. 1991;38:S907–S920. [Google Scholar]
- 33.Nealson K, Saffarini D. Iron and manganese in anaerobic respiration: environmental significance, physiology, and regulation. Annu Rev Microbiol. 1994;48:311–343. doi: 10.1146/annurev.mi.48.100194.001523. [DOI] [PubMed] [Google Scholar]
- 34.Negm R, Pistole T. OmpC of Salmonella typhimurium mediates adherence to macrophages. 1998. GenBank submission AF039309. [PubMed] [Google Scholar]
- 35.Nielson H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 36.Obuekwe C, Westlake D. Effects of medium composition on cell pigmentation, cytochrome content and ferric iron reduction in a Pseudomonas sp. isolated from crude oil. Can J Microbiol. 1982;28:989–992. doi: 10.1139/m82-148. [DOI] [PubMed] [Google Scholar]
- 37.Obuekwe C, Westlake D, Plambeck J, Cook F. Corrosion of mild steel in cultures of ferric iron reducing bacteria isolated from crude oil. II. Mechanism of anodic depolarization. Corrosion (Houston) 1981;37:632–637. [Google Scholar]
- 38.Pollock W B, Loutfi M, Bruschi M, Rapp-Giles B J, Wall J D, Voordouw G. Cloning, sequencing, and expression of the gene encoding the high-molecular-weight cytochrome c from Desulfovibrio vulgaris Hildenborough. J Bacteriol. 1991;173:220–228. doi: 10.1128/jb.173.1.220-228.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 40.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Semple K, Westlake D. Characterization of iron-reducing Alteromonas putrefaciens strains from oil field fluids. Can J Microbiol. 1987;33:366–371. [Google Scholar]
- 42.Simon R, Priefer U, Puhler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 43.Theberge S M, Luther G W., III Determination of the electrochemical properties of a soluble aqueous FeS species present in sulfidic solutions. Aquat Geochem. 1997;3:191–211. [Google Scholar]
- 44.Yu J, Kaper J B. Cloning and characterization of the eae gene of enterohaemorrhagic Escherichia coli O157:H7. Mol Microbiol. 1992;6:411–417. doi: 10.1111/j.1365-2958.1992.tb01484.x. [DOI] [PubMed] [Google Scholar]