Abstract
Purpose of review:
Hearing loss is the most common sensory deficit and in young children sensorineural hearing loss is most frequently genetic in etiology. Hearing aids and cochlear implant do not restore normal hearing. There is significant research and commercial interest in directly addressing the root cause of hearing loss through gene therapies. This article provides an overview of major barriers to cochlear gene therapy and recent advances in preclinical development of precision treatments of genetic deafness.
Recent findings:
Several investigators have recently described successful gene therapies in many common forms of genetic hearing loss in animal models. Elegant strategies that do not target a specific pathogenic variant, such as mini gene replacement and mutation-agnostic RNA interference with engineered replacement, facilitate translation of these findings to development of human therapeutics. Clinical trials for human gene therapies are in active recruitment.
Summary:
Gene therapies for hearing loss are expected to enter clinical trials in the immediate future. To provide referral for appropriate trials and counseling regarding benefits of genetic hearing loss evaluation, specialists serving children with hearing loss such as pediatricians, geneticists, genetic counselors, and otolaryngologists should be acquainted with ongoing developments in precision therapies.
Keywords: gene therapy, genetic hearing loss, RNAi, CRISPR/Cas9
1. INTRODUCTION
Deafness is the most common congenital sensory deficit, affecting approximately 1 in 500 at birth, approximately 80% of which has a genetic etiology[1–3]. The prevalence increases with age, with 15% of children aged 6–19 having at least a mild hearing loss [4]. Hearing aids (HAs) and cochlear implants (CIs) are the mainstay in rehabilitation of sensorineural hearing loss (SNHL). However, such strategies are symptomatic in nature. HAs increase gain of input sound in order to maximize the listener’s ability to discriminate signal from noise, while CIs directly stimulate the cochlear nerve, bypassing a dysfunctional inner ear. Although sophisticated algorithms have been developed to improve the quality of hearing with both strategies, neither strategy restores natural hearing. Spurred by advances in our understanding of the molecular biology of hearing, the past two decades have seen substantial progress in the development of preclinical therapies for genetic deafness as summarized in Table 1 [5–29]. Two critical barriers must be overcome for the deployment of any gene therapy in hearing loss:
Table 1.
Summary of previously described preclinical gene therapy studies using murine models. For genes with multiple prior studies, only studies published since 2021 are included. Green: gene therapy delivered after onset of murine hearing (P14-P15, roughly equivalent to approximately the 18th week gestational week in humans). Orange: gene therapy delivered prior to onset of hearing.
| Gene (locus) | Animal model | Age | Strategy | Vector | Delivery | Results | Reference |
|---|---|---|---|---|---|---|---|
| Autosomal recessive | |||||||
| CLRN1 (USH3A) | TgAC1/Clrn1 −/− | P1 | replacement | AAV-S | RWM | preservation of ABR, hair bundle morphology, and auditory nerve fibers | Invanchenko et al. 2021 (5) |
| GJB2 (DFNB1) | Sox10iCreERT/Gjb2 (CKO) | P28 | replacement | AAV2/Anc80 | RWM+CF | restoration of supporting cell Cx26 expression without hearing restoration | Guo et al. 2021 (6) |
| KCNE1 (JLNS2) | Kcne1 −/− | P1 | replacement | AAV1-CB7 | canalostomy | partial preservation of hair cells and ABR, restoration of gross semicircular canal morphologic development | Wu et al. 2021 (7) |
| KCNQ1 (JLNS1) | Kcnq1 −/− | P1 | replacement | AAV2/1 | RWM | restoration of ABR and Kcnq1 expression only with scala media injection | Chang et al. 2015 (8) |
| scala media inoculation | |||||||
| LHFPL5 (DFNB67) | Lhfpl5 −/− | P1 | replacement | exo-AAV2/1 | RWM | partial ABR restoration | Gyorgy et al. 2017 (9) |
| MSRB3 (DFNB74) | Msrb3 −/− | E12.5 | replacement | AAV2/1 | transuterine otocyst | restoration of near-normal ABR and hair bundle morphology | Kim et al. 2016 (10) |
| OTOF (DFNB22) | Otof −/− | P10, P17, P30 | replacement | dual AAV2/2-quadY-F | RWM | restoration of near-normal ABR | Akil et al. 2019 (11) |
| PCDH15 (DFNB23/USH1F) | Pcdh15 av-3J/av-3J | P1 | CRISPR-Cas9 frame restoration | AAV2/9 | scala media inoculation | partial restoration of ABR, reduced vestibular dysfunction | Liu et al. 2022 (12) |
| Pchd15fl/fl (CKO) | P1 | mini gene replacement | AAV2/9-PHP.B | RWM | partial restoration of ABR dependent on deletion timing | Ivanchenko et al. 2023 (13) | |
| Pchd15 R245X/R245X | |||||||
| PJVK (DFNB59) | Pjvk G292R/G292R | P1 | replacement | AAV2/Anc80 | RWM | ABR, hair cell, and balance preservation | Lu et al. 2022 (14) |
| SLC26A4 (DFNB4/PDS) | Slc26a4 −/− | E12.5 | replacement | AAV2/1 | otocyst | partial hearing restoration and prevention of enlargement of membranous labyrinth, without restoration of vestibular function | Kim et al. 2019 (15) |
| STRC (DFNB16/DIS) | Strc −/− | P1 | replacement | dual AAV9/PHP.B | utricle | preservation of ABR and OAE in 50% of animals | Shubina-Oleinik et al. 2021 (16) |
| SYNE4 (DFNB76) | Syne4 −/− | P1 | replacement | AAV2/9-PHP.B | canalostomy | preservation of ABR and hair cells | Taiber et al. 2021 (17) |
| TMC1 (DFNB7/11) | Tmc1−/−, Tmc1N193I/N193I | P1 | replacement | AAV2/9-PHP.B | utricle | preservation of ABR and hair cells | Marcovich et al. 2021 (18) |
| Tmc1−/−, Tmc1Y182C/Y182C | P1, P7 | replacement | AAV2/9-PHP.B | utricle | preservation of ABR and hair cells | Wu et al. 2021 (19) | |
| Tmc1 −/− | P1 | CRISPR-Cas9 base correction | AAV2/Anc80 | intracochlear | preservation of ABR and hair cells, restoration of mechanotransduction | Yeh et al. 2021 (20) | |
| USH1C (USH1C) | Ush1c c.216G>A/c.216G>A | P1 | splice-switching ASO | none | RWM | partial restoration of ABR, reduced vestibular dysfunction | Lentz et al. 2020 (21) |
| P5 | TM topical | slight improvement of ABR, reduced vestibular dysfunction | |||||
| P20 | TM injection | reduced vestibular dysfunction | |||||
| USH1G (USH1G) | Ush1g −/− | P2 | replacement | AAV2/8 | RWM | partial restoration of ABR, reduced vestibular dysfunction | Emptoz et al. 2017 (22) |
| WHRN (USH2D) | Whrn wi/wi | neonatal | replacement | AAV2/9 | canalostomy | slight improvement of ABR, reduced vestibular dysfunction | Isgrig et al. 2017 (23) |
| Autosomal dominant | |||||||
| KCNQ4 (DFNA2A) | Kcnq4 G229D/+ | P1 | CRISPR-Cas9 disruption | AAV-PHP.eB | cochleostomy | slight preservation of ABR and DPOAE thresholds | Cui et al. 2022 (24) |
| Kcnq4 W276S/+ | P1 | CRISPR-Cas9 disruption | AAV2/Anc80, RNP | RWM, cochleostomy, canalostomy, utricle | slight preservation of ABR and DPOAE thresholds | Noh et al. 2022 (25) | |
| MYO6 (DFNA22) | Myo6C442/+, Myo6C442Y/C442Y | P1 | CRISPR-Cas9 disruption | AAV-PHP.eB | cochleostomy/scala media | partial preservation of ABR, DPOAE, and hair cells | Xue et al. 2022 (26) |
| Myo6C442/+, Myo6C442Y/C442Y | P1 | Cas13 RNA base editing | AAV-PHP.eB | cochleostomy/scala media | partial preservation of ABR and hair cells | Xiao et al. 2022 (27) | |
| SLC17A8 (DFNA25) | Slc17a8 −/− | P1 | replacement | AAV2/1 | RWM | restoration of ABR and synaptic morphology, superior outcome with P1 injection | Akil et al. 2013 (28) |
| P10 | RWM, cochleostomy | ||||||
| TMC1 (DFNA36) | Tmc1 M412K/+ | P1 | CRISPR-Cas9 disruption | dual-vector AAV2/9-PHP.B | utricle | preservation of ABR and hair cells | Wu et al. 2021 (19) |
| P18 | mutation-agnostic RNAi + replacement | AAV2/2 | RWM + CF | - preservation of ABR and hair cells - mutation agnosticism |
Iwasa & Klimara et al. 2023 (29) | ||
ABR: auditory brainstem response; ASO: anti-sense oligonucleotide; CF: canal fenestration; CKO: conditional knockout; DFNA: deafness, autosomal dominant; DFNB: deafness, autosomal recessive; DIS: deafness-infertility syndrome; JLNS, Jervell and Lange-Nielsen syndrome; PDS: Pendred syndrome; RNP: ribonucleoprotein; RWM: round window membrane; TM: tympanic membrane; USH: Usher syndrome.
First, any treatment must be expressed within specialized target cells within the mammalian organ of Corti or spiral ganglion. Atraumatic access to these tissues is challenging.
Second, the mutational spectrum of genetic hearing loss is diverse. Each deafness-associated gene and each pathogenic variant comprises a small fraction of the overall mutational landscape of genetic deafness. This diversity necessitates a wide armamentarium of gene-specific therapies and complicates deployment of variant-specific treatments.
In this review, we summarize recent advances in cochlear gene therapy with a focus on the latest advances which have improved the field’s ability to meet these challenges head on.
II. TEXT OF REVIEW
II.A. DELIVERY OF THERAPIES TO TARGET CELLS
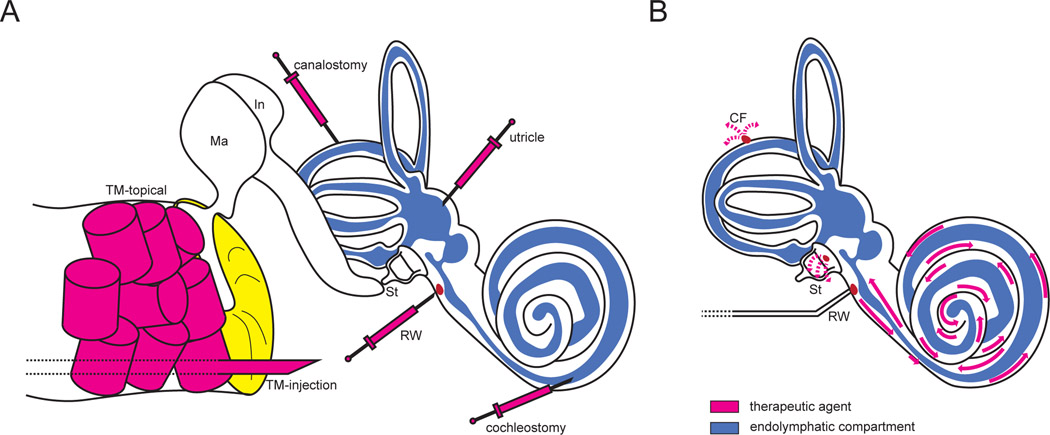
The barriers to delivery of gene therapies to the inner ear are several. First, the otic capsule which surrounds the cochlea is the strongest bone in the mammalian body. It limits access to the membranous labyrinth and restricts the volume of therapeutic that can be safely delivered [30, 31]. Second, the blood-perilymph barrier, composed of endothelial cells with tight junctions similar to the blood-brain barrier, largely precludes efficient systemic delivery of small molecules and viral vectors to the mature inner ear [32]. Lastly, although delivery to the immature, incompletely ossified murine inner ear via round window membrane (RWM), utricle, posterior semicircular canal, or scala media is trivial, the delicate structures of the mature cochlea are highly vulnerable to damage with intralabyrinthine delivery, and the human cochlea is fully ossified and mature at birth [33–37]. To this end, investigators have developed an array of techniques for efficient, atraumatic delivery to the murine inner ear as depicted in Figure 1A.
Figure 1.
Well-described delivery techniques for cochlear gene therapies. (A) A variety of delivery methods have been developed for delivery to the endolymphatic and perilymphatic compartments. Topical transtympanic delivery utilizes absorbable packing saturated in a gene therapy agent and is reliant on diffusion across the tympanic membrane and round window to achieve therapeutic levels of the drug within the cochlea (magenta cylinders). Transtympanic injection bypasses the tympanic membrane to inject within the middle ear space (large magenta needle). The remaining approaches (small magenta needles) offer direct delivery to the endolymphatic compartment, perilymphatic compartment (round window), or both (utricle, canalostomy, cochleostomy, utricle). B) Round window membrane injection combined with posterior or horizontal semicircular canal venting facilitates efficient, atraumatic vector transduction in murine models. In humans and non-human primates, a similar approach is proposed using a stapes footplate/oval window vent, which can be accessed via a transcanal tympanoplasty or mastoidectomy approach.
CF: canal fenestration; In: incus; Ma: malleus; RW: round window; St: stapes; TM: tympanic membrane.
Our laboratory has previously described incorporation of a fenestration in the posterior semicircular canal during round window delivery via microinjection needle in mature mice (Figure 1B) [31]. The addition of a canalostomy provides a perilymph vent, relieving excessive intracochlear pressure and creating a gradient whereby agents are effectively distributed throughout the cochlear duct, vestibule, and semicircular canals. Andres-Mateos and colleagues recently demonstrated the viability of a similar technique in a non-human primate model. Using a rhesus macaque model, a transmastoid facial recess approach (similar to that of CI) allowed simultaneous RWM injection and oval window venting to achieve high transduction of inner and outer hair cells using a synthetic adeno-associated virus (AAV) AAV2/Anc80L65), though the authors did not describe audiologic outcomes of the procedure [38]. Although the described approach requires mastoidectomy, the wider external auditory canal of humans facilitates access to the round and oval windows via a transcanal tympanoplasty approach, eliminating the need for bony dissection and reducing recovery time.
Intratympanic and topical transtympanic delivery are minimally invasive approaches under active investigation and consideration [39]. Unique among local delivery techniques, these do not require trauma to the membranous labyrinth. However, agents must efficiently diffuse across the round window membrane without any pressure gradient, and, if delivered into the middle ear, are subject to drainage by the eustachian tube [34]. Recently, Lentz and colleagues demonstrated partial rescue of a mouse model of Usher syndrome type 1C using topical application of a splice-switching anti-sense oligonucleotide (ASO) [21]. This ASO targets a pathogenic variant in Ush1c that creates a de novo splice site; treatment with ASO blocks aberrant splicing to restore normal RNA splicing and protein expression. Lentz et al. (2020) leveraged a variant-targeted ASO applied directly to the TM using gelfoam packing in P5 mice, with subsequent improvement of auditory brainstem response (ABR) thresholds by up to 25 dB SPL in treated mice. However, earlier delivery via round window membrane injection was more effective, and it remains unclear whether such an approach could be applied successfully to the much larger primate ear, which is completely ossified at birth.
Adeno-associated virus (AAV) has emerged as a preferred vector for gene delivery to the inner ear due to broad cellular tropism, low immunogenicity, inability to establish productive infection in the absence of a helper virus, and well-documented non-ototoxicity [31, 40]. AAV2/2, AAV2/9, and the synthetic AAV2/Anc80L65 and AAV2/9PHP.eB exhibit highly efficient transduction of cochlear hair cells and are now preferred by many groups [31, 33, 38, 41, 42]. Isgrig et al. have additionally demonstrated tropism of AAV8 and AAV8BP2 to cells in the lateral wall, suggesting their utility in addressing the many forms of genetic hearing loss resulting from dysfunction of the stria vascularis [43]. However, the packaging capacity of AAV is only 4.7 kB (and is decreased by addition of any regulatory elements or co-expressed products) precluding use of single-AAV gene replacement strategies for many genes [44]. Described alternatives to single-AAV delivery include dual- or triple-AAV delivery [11, 19, 41, 45] and alternative vectors such as conventional recombinant adenovirus [46, 47], helper-dependent adenovirus [48], and lentivirus [49].
II.B. ADDRESSING THE MUTATIONAL SPECTRUM OF GENETIC DEAFNESS
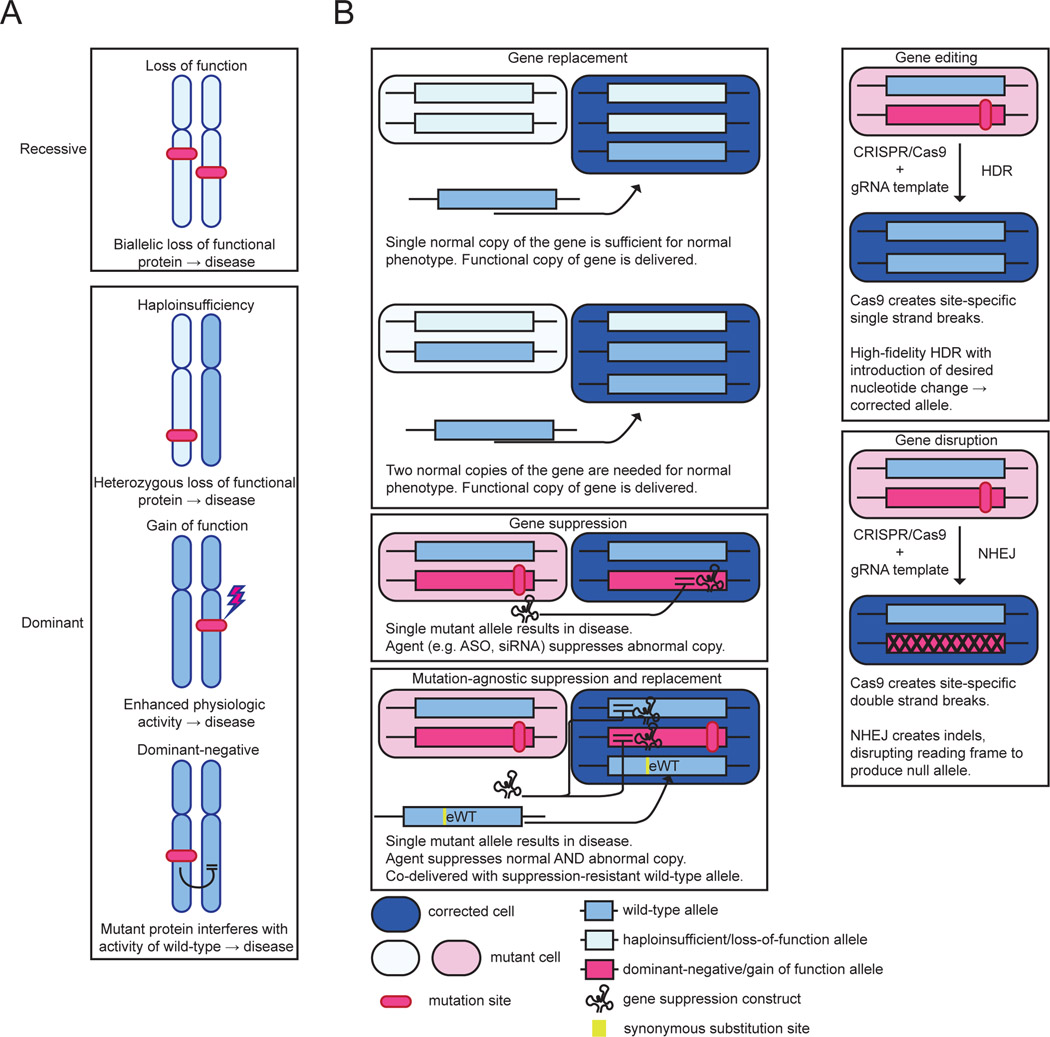
Nonsyndromic hearing loss (NSHL) accounts for approximately 75–80% of genetic hearing loss [50, 51]. The spectrum of pathogenic variants causing hereditary deafness is staggeringly diverse, with over 150 causative genes cloned [52]. Each pathogenic variant is, with few exceptions, rare in the general population, rendering deployment of variant-specific therapies infeasible [53]. Approximately 80% of NSHL is autosomal recessive in inheritance, and is most often associated with biallelic loss-of-function variants in GJB2, STRC, or MYO15A [51, 54]. Autosomal dominant forms of hearing loss, arising due to haploinsufficiency, dominant-negative, or gain of function variants in genes such as TECTA, KCNQ4, and WFS1, account from approximately 15% of NSHL cases [51, 54]. The mechanism of disease impacts the ideal therapeutic strategy as summarized in Figure 2. Gene replacement, arguably the simplest form of gene therapy, can effectively address haploinsufficiency or loss of function, but not dominant-negative or gain-of-function variants. Gene suppression with RNA interference (RNAi) or anti-sense oligonucleotides (ASO) is in contrast used to knock down mutant alleles in dominant-negative and gain-of-function associated disease. Gene editing strategies may be adapted for use in disease with any mechanism of disease. However, gene editing and suppression strategies are generally variant-specific, hindering application to human clinical care.
Figure 2.
Mechanisms of genetic disease and impacts on therapeutic strategies. (A) Autosomal recessive and autosomal dominant genetic disease arise via distinct mechanisms ranging from biallelic complete loss of protein function in autosomal recessive disease, to deleterious effects on the wild-type protein in dominant-negative disease. (B) General strategies for gene therapy. Gene replacement or gene editing may be used to treat disease mediated by loss of function or haploinsufficiency. In contrast, diseases with dominant-negative and gain-of-function mechanisms require suppression, disruption, or correction of the mutant allele.
ASO: anti-sense oligonucleotide; CRISPR: clustered regularly interspaced short palindromic repeats; eWT: engineered wild-type allele; gRNA: guide RNA; HDR: homology-directed repair; NHEJ: non-homologous end joining; siRNA: small interfering RNA.
Autosomal recessive nonsyndromic hearing loss
GJB2-associated deafness, the most common form of genetic hearing loss, remains an elusive target for preclinical gene therapy trials, hindered by embryonic lethality of homozygous Gjb2 deletion in mouse models, poor tropism of viral vectors specifically targeting supporting cells expressing its gene product, and toxicity of ectopic Gjb2 expression to inner hair cells resulting in hair cell death with vectors tropic to inner hair cells [6, 55]. However, inroads have recently been made in STRC-associated deafness, the second most common cause of genetic hearing impairment. Pathogenic variants include single-nucleotide variants, gene-pseudogene conversions, and large genomic deletions which frequently encompass the nearby CATSPER2, a cation channel protein critical for spermatid development [56, 57]. Biallelic pathogenic variants in STRC result in a non-progressive, mild-to-moderate SNHL termed DFNB16 [56]. Biallelic STRC-CATSPER2 deletions in males additionally result in male-factor infertility, termed deafness-infertility syndrome [56]. Shubina-Oleinik and colleagues recently described successful gene therapy in a murine model of STRC-associated hearing loss [16]. As the coding sequence of Strc is too large for packaging within a single AAV vector, a dual AAV approach was employed with intein-mediated recombination of N- and C-terminal fragments to restore full-length Strc expression. Delivery of this treatment via neonatal utricle injection resulted in partial restoration of ABR, otoacoustic emissions, and stereocilia morphology in 50% of treated animals. Although this represents a critical step forward to realization of human STRC gene therapy, stereocilin is thought to be critical to the development of the outer hair cell and its attachment to the tectorial membrane, which occurs early in utero in primates [58, 59]. It is uncertain whether a similar therapeutic could be delivered at a biologically relevant timepoint in humans.
Two alternative strategies to circumvent the limited capacity of AAV are illustrated by recent studies in mouse models of PCDH15-related hearing loss. PCDH15 is the binding partner of cadherin-23, which comprise the double-stranded tip link complex expressed in cochlear hair cells and responsible for opening mechanotransduction channels. Biallelic missense and loss-of-function variants are associated with autosomal recessive deafness and Usher syndrome, type 1F, with nearly half of pathogenic variants being frameshifts due to small insertions or deletions [53, 60]. The 7.9 kB full-length Pcdh15 transcript far exceeds the maximum delivery capacity of AAV, precluding single-AAV gene replacement. Liu et al. (2022) directly addressed single-nucleotide insertions and deletions using a murine model with autosomal recessive hearing loss arising due to a single-nucleotide insertion in Pcdh15 [12]. The authors developed and validated a guide RNA which preferentially generates single-base insertions and deletions via non-homologous end rejoining in a Cas9-dependent fashion. Induction of a single-nucleotide deletion at the targeted site results in restoration of the reading frame and a full-length coding sequence. When packaged in AAV2/9 vector and delivered via cochleostomy, this resulted in partial restoration of auditory and vestibular function. This frame restoration strategy may be a viable alternative to gene replacement for genes too large to be delivered with a single vector, albeit at the cost of variant specificity.
Ivanchenko et al. (2023) more recently leveraged advances in understanding of PCDH15’s functional domains and binding partners to design “mini-PCDH15” proteins in which extracellular domains of the protein were eliminated under the hypothesis that some domains were redundant or unnecessary for partial hearing restoration [13]. After extensive evaluation of these mini-PCDH15s’ binding properties in silico and in vitro, three mini-PCDH15 constructs were packaged in AAV-PHP.B. These were delivered to both constitutive knockout and conditional deletion models of Usher syndrome 1F, allowing treatment efficacy to be evaluated with deletion of Pcdh15 at various timepoints. This therapy resulted in partial rescue of ABR, stereocilium bundle morphology, and mechanotransduction with the efficacy of therapy decreasing with earlier deletion. This elegant strategy has the distinct advantage of variant agnosticism and demonstrates that full-length protein expression may not be necessary in all cases. Moreover, a mini-gene approach may also be a viable pathway to treatment of retinitis pigmentosa in Usher syndrome in the future, though the authors do not report attempts to evaluate or target vision loss in this model. Better understanding of hair cell molecular biology may allow further refinement of mini-gene replacement strategies through elimination of redundant or non-functional domains from gene therapy constructs.
Autosomal dominant nonsyndromic hearing loss
Autosomal dominant NSHL frequently arises via dominant-negative or gain of function mechanisms which are not amenable to gene replacement alone, requiring some form of gene editing/disruption or knockdown for effective treatment. Dominant-negative variants in KCNQ4 are a common cause of autosomal dominant NSHL, termed DFNA2A [61, 62]. This gene encodes a homotetrameric potassium channel expressed in the basolateral membrane of cochlear outer hair cells which is responsible for the rectifier current that repolarizes the cell after depolarization in response to activation of the mechanotransduction complex [62, 63]. Dominant-negative variants in KCNQ4 nearly abolish this current, resulting in buildup of intracellular potassium and abolition of membrane repolarization, ultimately resulting in hair cell death manifesting as a predominantly high frequency, progressive hearing loss. Two laboratories recently and contemporaneously leveraged CRISPR/Cas9-based targeted disruption in murine models of DFNA2A [24, 25]. In both cases, intracochlear delivery of sgRNA/SpCas9 resulted in partial preservation of hearing. However, the preservation was incomplete and non-durable, with treated animals exhibiting ABRs improved by only up to 20 dB SPL at 11–12 weeks of age. Substantial optimization is therefore necessary prior to consideration of extending this approach to humans.
The gene TMC1 encodes the pore-forming unit of the hair cell mechanotransduction channel which opens in response to stereocilia deflection and results in cellular depolarization [64]. Pathogenic variants in TMC1 are responsible for approximately 2% of all genetic hearing loss, with loss-of-function variants resulting in autosomal recessive hearing loss at the DFNB7/11 locus and dominant-negative variants causing DFNA36. Since its role in hearing loss was first described [65], several investigators have developed gene therapies in mouse models of both forms of TMC1-associated deafness using gene replacement, targeted gene editing/disruption, or RNA interference. Mutation-specific approaches are infeasible to apply in man due to the need to independently develop constructs for each pathogenic variant. Our laboratory recently validated a mutation-agnostic approach incorporating knockdown of both the wild-type and mutant allele with a single highly efficient miRNA with concomitant co-delivery of a knockdown-resistant, engineered wild-type allele (Figure 2B) [29]. Incorporation of knockdown of the dominant negative allele with simultaneous gene replacement increases the ratio of wild-type:mutant RNA, and facilitates fine-tuning of gene dosage through manipulation of promoter and regulatory elements. Delivery of mutation-agnostic RNAi+replacement construct using an AAV2/2 vector at a mature timepoint resulted in excellent preservation of ABR thresholds in treated animals durable to 20 weeks of age. However, like all prior studies of the mouse model of DNFA36, this therapy failed to rescue hearing at the highest frequencies, suggesting that hair cell damage in the basal turn may be early in onset and irreversible.
II.C. Translation to clinical trials
Two precision medicine pharmaceutical developers, Akouos and Decibel Therapeutics, recently began phase 1/2 clinical trials of gene therapies for OTOF-related hearing loss [66, 67]. OTOF encodes otoferlin, a calcium sensor required for exocytosis of synaptic vesicles at the basolateral membrane of inner hair cells; homozygous loss-of-function results in auditory neuropathy spectrum disorder presenting with congenital severe-to-profound hearing loss [68]. Critical to OTOF’s position as the first target for human clinical trials of gene therapy, cochlear hair cells and outer hair cell function are preserved, allowing for phenotypic rescue at biologically relevant timepoints with gene replacement in multiple prior studies in murine models [11, 69–71]. Previous work established that dual-AAV delivery of Otof via the murine wound window restores near-normal hearing even in mature mice [11], and that delivery of dual-AAV constructs encoding OTOF is non-toxic to the nonhuman primate cochlea [72]. Akouos and Decibel Therapeutics have independently developed dual-AAV constructs for OTOF gene replacement in inner hair cells with delivery via round window injection with perilymph venting, delivery of which is expected to begin by the time of publication of this review.
III. CONCLUSION
After the audiogram, targeted genetic testing is the highest-yield study in the evaluation of bilateral hearing loss in children in order to determine the cause of deafness, identify other indicated tests, and provide prognostic information regarding future progression [73]. To this list, in the near future we will be able to consider disease-specific treatment modalities that may obviate the need for amplification or CI. However, translation from murine models of genetic deafness to primates is challenging due to differences in temporal bone anatomy and development. Further development of variant-agnostic approaches is critically important to the goal of deployment of gene therapies in many forms of genetic hearing loss. The cost of development and validation of variant-specific constructs, with many targeting only a few patients worldwide, would be exorbitant to the point of infeasibility. Moreover, it is likely that the therapeutic window for cochlear gene therapies is disease specific and successful rescue in some forms of hearing loss may require treatments beyond simple gene replacement, disruption, suppression, or editing. Ameliorating forms of hearing loss in which hair cell death occurs early in life, for instance, may require hair cell regeneration in addition to addressing pathogenic variants.
IV. KEY POINTS.
Advances in genetic sequencing technologies and knowledge of the molecular biology of hearing loss have facilitated tremendous progress in the preclinical development of gene therapies for hereditary hearing loss. However, the diverse mutational spectrum of genetic deafness and the fragility of the mature organ of Corti pose significant barriers to these efforts.
Novel surgical techniques such as round window membrane injection with semicircular canal or oval window fenestration hold the promise of atraumatic delivery of therapeutics to the inner ear.
Sophisticated construct designs such as mini-gene replacement and mutation-agnostic RNA interference with engineered replacement facilitate treatment of a broad spectrum of pathogenic variants with a single therapeutic. Pediatric gene therapy trials are expected to begin this year for OTOF-related hearing loss.
V. ACKNOWLEDGEMENTS
There are no acknowledgements.
VI. FINANCIAL DISCLOSURE
This project was supported in part by NIH-NIDCD grant 3T32DC000040 (MJK) and NIH-NIDCD R01s DC002842, DC012049, and DC017955 (RJHS).
VII. CONFLICT OF INTEREST
RJHS directs the Molecular Otolaryngology and Renal Research Laboratories, which developed and offers comprehensive genetic testing for patients with hearing loss, and is a cofounder of Akouos, which develops gene therapies for genetic hearing loss.
VIII. REFERENCES AND RECOMMENDED READING
Papers of particular interest published within the period of review, are highlighted as:
* of special interest
** of outstanding interest
- 1.Smith RJ, Bale JF, Jr., White KR. Sensorineural hearing loss in children. Lancet. 2005;365(9462):879–90. [DOI] [PubMed] [Google Scholar]
- 2.Schmuziger N, Veraguth D, Probst R. [Universal newborn hearing screening--a silent revolution]. Praxis (Bern 1994). 2008;97(19):1015–21. [DOI] [PubMed] [Google Scholar]
- 3. Shearer AE, Hildebrand MS, Smith RJH. Hereditary Hearing Loss and Deafness Overview. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Mirzaa G, et al. , editors. GeneReviews((R)). Seattle (WA) 1993. *Interested readers are invited to review this overview of the evaluation, differential diagnosis, and management of hereditary hearing loss.
- 4.Niskar AS, Kieszak SM, Holmes A, et al. Prevalence of hearing loss among children 6 to 19 years of age: the Third National Health and Nutrition Examination Survey. JAMA. 1998;279(14):1071–5. [DOI] [PubMed] [Google Scholar]
- 5.Ivanchenko MV, Hanlon KS, Hathaway DM, et al. AAV-S: A versatile capsid variant for transduction of mouse and primate inner ear. Mol Ther Methods Clin Dev. 2021;21:382–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo J, Ma X, Skidmore JM, et al. GJB2 gene therapy and conditional deletion reveal developmental stage-dependent effects on inner ear structure and function. Mol Ther Methods Clin Dev. 2021;23:319–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu X, Zhang L, Li Y, et al. Gene therapy via canalostomy approach preserves auditory and vestibular functions in a mouse model of Jervell and Lange-Nielsen syndrome type 2. Nat Commun. 2021;12(1):697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang Q, Wang J, Li Q, et al. Virally mediated Kcnq1 gene replacement therapy in the immature scala media restores hearing in a mouse model of human Jervell and Lange-Nielsen deafness syndrome. EMBO Mol Med. 2015;7(8):1077–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gyorgy B, Sage C, Indzhykulian AA, et al. Rescue of Hearing by Gene Delivery to Inner-Ear Hair Cells Using Exosome-Associated AAV. Mol Ther. 2017;25(2):379–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim MA, Cho HJ, Bae SH, et al. Methionine Sulfoxide Reductase B3-Targeted In Utero Gene Therapy Rescues Hearing Function in a Mouse Model of Congenital Sensorineural Hearing Loss. Antioxid Redox Signal. 2016;24(11):590–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Akil O, Dyka F, Calvet C, et al. Dual AAV-mediated gene therapy restores hearing in a DFNB9 mouse model. Proc Natl Acad Sci U S A. 2019;116(10):4496–501. *This study is the first report of the use of dual-AAV delivery of Otof gene replacement for treatment of the murine model of DFNB9 and is the foundation of clinical trials of human hearing loss due to OTOF planned to begin in 2023.
- 12.Liu L, Zou L, Li K, et al. Template-independent genome editing in the Pcdh15(av-3j) mouse, a model of human DFNB23 nonsyndromic deafness. Cell Rep. 2022;40(2):111061. [DOI] [PubMed] [Google Scholar]
- 13. Ivanchenko MV, Hathaway DM, Klein AJ, et al. Mini-PCDH15 gene therapy rescues hearing in a mouse model of Usher syndrome type 1F. Nat Commun. 2023;14(1):2400. **This study reports the successful treatment of the murine model of PCDH15-related hearing loss using a designed mini-PCDH15. As PCDH15 is too large to be packaged in a single AAV vector, this clever approach uses advances in understanding of the molecular biology of PCDH15 and the hair cell tip-link complex to eliminate putative redundant domains and reduce the size of the delivered package.
- 14.Lu YC, Tsai YH, Chan YH, et al. Gene therapy with a synthetic adeno-associated viral vector improves audiovestibular phenotypes in Pjvk-mutant mice. JCI Insight. 2022;7(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim MA, Kim SH, Ryu N, et al. Gene therapy for hereditary hearing loss by SLC26A4 mutations in mice reveals distinct functional roles of pendrin in normal hearing. Theranostics. 2019;9(24):7184–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shubina-Oleinik O, Nist-Lund C, French C, et al. Dual-vector gene therapy restores cochlear amplification and auditory sensitivity in a mouse model of DFNB16 hearing loss. Sci Adv. 2021;7(51):eabi7629. **This study is the first report of successful partial rescue of the murine model of STRC-related hearing loss, which is the second most common form of genetic deafness.
- 17.Taiber S, Cohen R, Yizhar-Barnea O, et al. Neonatal AAV gene therapy rescues hearing in a mouse model of SYNE4 deafness. EMBO Mol Med. 2021;13(2):e13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marcovich I, Baer NK, Shubina-Oleinik O, et al. Optimized AAV Vectors for TMC1 Gene Therapy in a Humanized Mouse Model of DFNB7/11. Biomolecules. 2022;12(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu J, Solanes P, Nist-Lund C, et al. Single and Dual Vector Gene Therapy with AAV9-PHP.B Rescues Hearing in Tmc1 Mutant Mice. Mol Ther. 2021;29(3):973–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeh WH, Shubina-Oleinik O, Levy JM, et al. In vivo base editing restores sensory transduction and transiently improves auditory function in a mouse model of recessive deafness. Sci Transl Med. 2020;12(546). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lentz JJ, Pan B, Ponnath A, et al. Direct Delivery of Antisense Oligonucleotides to the Middle and Inner Ear Improves Hearing and Balance in Usher Mice. Mol Ther. 2020;28(12):2662–76. *This study reports the partial rescue of auditory and vestibular phenotypes using local delivery of a splice-switching anti-sense oligonucleotide through minimally invasive transtympanic approaches.
- 22.Emptoz A, Michel V, Lelli A, et al. Local gene therapy durably restores vestibular function in a mouse model of Usher syndrome type 1G. Proc Natl Acad Sci U S A. 2017;114(36):9695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Isgrig K, Shteamer JW, Belyantseva IA, et al. Gene Therapy Restores Balance and Auditory Functions in a Mouse Model of Usher Syndrome. Mol Ther. 2017;25(3):780–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cui C, Wang D, Huang B, et al. Precise detection of CRISPR-Cas9 editing in hair cells in the treatment of autosomal dominant hearing loss. Mol Ther Nucleic Acids. 2022;29:400–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noh B, Rim JH, Gopalappa R, et al. In vivo outer hair cell gene editing ameliorates progressive hearing loss in dominant-negative Kcnq4 murine model. Theranostics. 2022;12(5):2465–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xue Y, Hu X, Wang D, et al. Gene editing in a Myo6 semi-dominant mouse model rescues auditory function. Mol Ther. 2022;30(1):105–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao Q, Xu Z, Xue Y, et al. Rescue of autosomal dominant hearing loss by in vivo delivery of mini dCas13X-derived RNA base editor. Sci Transl Med. 2022;14(654):eabn0449. [DOI] [PubMed] [Google Scholar]
- 28.Akil O, Seal RP, Burke K, et al. Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy. Neuron. 2012;75(2):283–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Iwasa Y, Klimara MJ, Yoshimura H, et al. Mutation-agnostic RNA interference with engineered replacement rescues Tmc1-related hearing loss. Life Sci Alliance. 2023;6(3). *This study is the first report of the use of a mutation-agnostic RNA interference approach for the successful rescue of a murine model of genetic hearing loss associated with a dominant-negative mechanism of disease.
- 30.Anniko M, Wikstrom SO, Wroblewski R. Microanalytic and light microscopic studies on the developing otic capsule. Acta Otolaryngol. 1987;104(5–6):429–38. [DOI] [PubMed] [Google Scholar]
- 31.Yoshimura H, Shibata SB, Ranum PT, et al. Enhanced viral-mediated cochlear gene delivery in adult mice by combining canal fenestration with round window membrane inoculation. Sci Rep. 2018;8(1):2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shibata SB, Yoshimura H, Ranum PT, et al. Intravenous rAAV2/9 injection for murine cochlear gene delivery. Sci Rep. 2017;7(1):9609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee J, Nist-Lund C, Solanes P, et al. Efficient viral transduction in mouse inner ear hair cells with utricle injection and AAV9-PHP.B. Hear Res. 2020;394:107882. [DOI] [PubMed] [Google Scholar]
- 34.Valentini C, Szeto B, Kysar JW, et al. Inner Ear Gene Delivery: Vectors and Routes. Hearing Balance Commun. 2020;18(4):278–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu J, Choi JW, Ishibashi Y, et al. Refining surgical techniques for efficient posterior semicircular canal gene delivery in the adult mammalian inner ear with minimal hearing loss. Sci Rep. 2021;11(1):18856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chien WW, McDougald DS, Roy S, et al. Cochlear gene transfer mediated by adeno-associated virus: Comparison of two surgical approaches. Laryngoscope. 2015;125(11):2557–64. [DOI] [PubMed] [Google Scholar]
- 37.Shu Y, Tao Y, Wang Z, et al. Identification of Adeno-Associated Viral Vectors That Target Neonatal and Adult Mammalian Inner Ear Cell Subtypes. Hum Gene Ther. 2016;27(9):687–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andres-Mateos E, Landegger LD, Unzu C, et al. Choice of vector and surgical approach enables efficient cochlear gene transfer in nonhuman primate. Nat Commun. 2022;13(1):1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoon JY, Yang KJ, Kim DE, et al. Intratympanic delivery of oligoarginine-conjugated nanoparticles as a gene (or drug) carrier to the inner ear. Biomaterials. 2015;73:243–53. [DOI] [PubMed] [Google Scholar]
- 40.Pillay S, Zou W, Cheng F, et al. Adeno-associated Virus (AAV) Serotypes Have Distinctive Interactions with Domains of the Cellular AAV Receptor. J Virol. 2017;91(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Omichi R, Yoshimura H, Shibata SB, et al. Hair Cell Transduction Efficiency of Single- and Dual-AAV Serotypes in Adult Murine Cochleae. Mol Ther Methods Clin Dev. 2020;17:1167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Landegger LD, Pan B, Askew C, et al. A synthetic AAV vector enables safe and efficient gene transfer to the mammalian inner ear. Nat Biotechnol. 2017;35(3):280–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Isgrig K, Ishibashi Y, Lee HJ, et al. AAV8BP2 and AAV8 transduce the mammalian cochlear lateral wall and endolymphatic sac with high efficiency. Mol Ther Methods Clin Dev. 2022;26:371–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marrone L, Marchi PM, Azzouz M. Circumventing the packaging limit of AAV-mediated gene replacement therapy for neurological disorders. Expert Opin Biol Ther. 2022;22(9):1163–76. [DOI] [PubMed] [Google Scholar]
- 45.Chen ZR, Guo JY, He L, et al. Co-transduction of dual-adeno-associated virus vectors in the neonatal and adult mouse utricles. Front Mol Neurosci. 2022;15:1020803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shu Y, Tao Y, Li W, et al. Adenovirus Vectors Target Several Cell Subtypes of Mammalian Inner Ear In Vivo. Neural Plast. 2016;2016:9409846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tarabichi O, Correa T, Kul E, et al. Development and evaluation of helper dependent adenoviral vectors for inner ear gene delivery. Hear Res. 2023;435:108819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wenzel GI, Xia A, Funk E, et al. Helper-dependent adenovirus-mediated gene transfer into the adult mouse cochlea. Otol Neurotol. 2007;28(8):1100–8. [DOI] [PubMed] [Google Scholar]
- 49.Pan S, Wan J, Liu S, et al. Lentivirus carrying the Atoh1 gene infects normal rat cochlea. Neural Regen Res. 2013;8(17):1551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li XC, Friedman RA. Nonsyndromic hereditary hearing loss. Otolaryngol Clin North Am. 2002;35(2):275–85. [DOI] [PubMed] [Google Scholar]
- 51.Klimara MJ, Nishimura C, Wang D, et al. De novo variants are a common cause of genetic hearing loss. Genet Med. 2022;24(12):2555–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith RJVC G. Hereditary Hearing Loss Homepage 2018. [Available from: https://hereditaryhearingloss.org/.
- 53.Azaiez H, Booth KT, Ephraim SS, et al. Genomic Landscape and Mutational Signatures of Deafness-Associated Genes. Am J Hum Genet. 2018;103(4):484–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sloan-Heggen CM, Bierer AO, Shearer AE, et al. Comprehensive genetic testing in the clinical evaluation of 1119 patients with hearing loss. Hum Genet. 2016;135(4):441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gabriel HD, Jung D, Butzler C, et al. Transplacental uptake of glucose is decreased in embryonic lethal connexin26-deficient mice. J Cell Biol. 1998;140(6):1453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y, Malekpour M, Al-Madani N, et al. Sensorineural deafness and male infertility: a contiguous gene deletion syndrome. J Med Genet. 2007;44(4):233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shearer AE, Kolbe DL, Azaiez H, et al. Copy number variants are a common cause of non-syndromic hearing loss. Genome Med. 2014;6(5):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verpy E, Leibovici M, Michalski N, et al. Stereocilin connects outer hair cell stereocilia to one another and to the tectorial membrane. J Comp Neurol. 2011;519(2):194–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kelly MC, Chen P. Development of form and function in the mammalian cochlea. Curr Opin Neurobiol. 2009;19(4):395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahmed ZM, Riazuddin S, Ahmad J, et al. PCDH15 is expressed in the neurosensory epithelium of the eye and ear and mutant alleles are responsible for both USH1F and DFNB23. Hum Mol Genet. 2003;12(24):3215–23. [DOI] [PubMed] [Google Scholar]
- 61.Coucke PJ, Van Hauwe P, Kelley PM, et al. Mutations in the KCNQ4 gene are responsible for autosomal dominant deafness in four DFNA2 families. Hum Mol Genet. 1999;8(7):1321–8. [DOI] [PubMed] [Google Scholar]
- 62.Kubisch C, Schroeder BC, Friedrich T, et al. KCNQ4, a novel potassium channel expressed in sensory outer hair cells, is mutated in dominant deafness. Cell. 1999;96(3):437–46. [DOI] [PubMed] [Google Scholar]
- 63.Kharkovets T, Dedek K, Maier H, et al. Mice with altered KCNQ4 K+ channels implicate sensory outer hair cells in human progressive deafness. EMBO J. 2006;25(3):642–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Holt JR, Tobin M, Elferich J, et al. Putting the Pieces Together: the Hair Cell Transduction Complex. J Assoc Res Otolaryngol. 2021;22(6):601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kurima K, Peters LM, Yang Y, et al. Dominant and recessive deafness caused by mutations of a novel gene, TMC1, required for cochlear hair-cell function. Nat Genet. 2002;30(3):277–84. [DOI] [PubMed] [Google Scholar]
- 66. Reape K, Inc. A. (2023 May - ). A Phase 1/2 Trial of AAVAnc80-hOTOF Gene Therapy in Individuals With Sensorineural Hearing Loss Due to Biallelic Otoferlin Gene Mutations. Identifier NCT05821959. https://clinicaltrials.gov/ct2/show/study/NCT05821959. *This is one of two planned human clinical trials of OTOF gene replacement. This clinical trials page provides inclusion and exclusion criteria as well as contact information for interested parties.
- 67. Bance M, Therapeutics D. (2023. May - ) A Phase 1/2 Open-Label Multicenter Trial with a Single Ascending Dose Cohort with Unilateral Cochlear Injection Followed by a Bilateral Injection Expansion Cohort to Evaluate the Safety, Tolerability, and Efficacy of DB-OTO in Children and Infants with Biallelic hOTOF Mutations. Identifier NCT05788536. https://clinicaltrials.gov/ct2/show/NCT05788536. *This is one of two planned human clinical trials of OTOF gene replacement. This clinical trials page provides inclusion and exclusion criteria as well as contact information for interested parties.
- 68.Azaiez H, Thorpe RK, Smith RJH. OTOF-Related Deafness. In: Adam MP, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, et al. , editors. GeneReviews((R)). Seattle (WA)1993. [PubMed] [Google Scholar]
- 69.Thorpe RK, Azaiez H, Wu P, et al. The natural history of OTOF-related auditory neuropathy spectrum disorders: a multicenter study. Hum Genet. 2022;141(3–4):853–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tang H, Wang H, Wang S, et al. Hearing of Otof-deficient mice restored by trans-splicing of N- and C-terminal otoferlin. Hum Genet. 2023;142(2):289–304. [DOI] [PubMed] [Google Scholar]
- 71.Rankovic V, Vogl C, Dorje NM, et al. Overloaded Adeno-Associated Virus as a Novel Gene Therapeutic Tool for Otoferlin-Related Deafness. Front Mol Neurosci. 2020;13:600051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hickox AE, Gao Y, Francis SP, et al. Nonclinical In Vivo Expression, Durability of Effect, Biodistribution/Shedding, and Safety Evaluations Support Planned Clinical Development of AK-OTOF (AAVAnc80-hOTOF Vector) for OTOF-mediated Hearing Loss. American Society of Gene and Cell Therapy 25th Annual Meeting; May 19, 2022; Washington, D.C. United States.2022. [Google Scholar]
- 73.Liming BJ, Carter J, Cheng A, et al. International Pediatric Otolaryngology Group (IPOG) consensus recommendations: Hearing loss in the pediatric patient. Int J Pediatr Otorhinolaryngol. 2016;90:251–8. [DOI] [PubMed] [Google Scholar]