Abstract
Prognostic factors and standards of care for astrocytoma, isocitrate dehydrogenase (IDH)-mutant, CNS WHO grade 4, remain poorly defined. Here we sought to explore disease characteristics, prognostic markers, and outcome in patients with this newly defined tumor type. We determined molecular biomarkers and assembled clinical and outcome data in patients with IDH-mutant astrocytomas confirmed by central pathology review. Patients were identified in the German Glioma Network cohort study; additional cohorts of patients with CNS WHO grade 4 tumors were identified retrospectively at two sites. In total, 258 patients with IDH-mutant astrocytomas (114 CNS WHO grade 2, 73 CNS WHO grade 3, 71 CNS WHO grade 4) were studied. The median age at diagnosis was similar for all grades. Karnofsky performance status at diagnosis inversely correlated with CNS WHO grade (p < 0.001). Despite more intensive treatment upfront with higher grade, CNS WHO grade was strongly prognostic: median overall survival was not reached for grade 2 (median follow-up 10.4 years), 8.1 years (95% CI 5.4–10.8) for grade 3, and 4.7 years (95% CI 3.4–6.0) for grade 4. Among patients with CNS WHO grade 4 astrocytoma, median overall survival was 5.5 years (95% CI 4.3–6.7) without (n = 58) versus 1.8 years (95% CI 0–4.1) with (n = 12) homozygous CDKN2A deletion. Lower levels of global DNA methylation as detected by LINE-1 methylation analysis were strongly associated with CNS WHO grade 4 (p < 0.001) and poor outcome. MGMT promoter methylation status was not prognostic for overall survival. Histomolecular stratification based on CNS WHO grade, LINE-1 methylation level, and CDKN2A status revealed four subgroups of patients with significantly different outcomes. In conclusion, CNS WHO grade, global DNA methylation status, and CDKN2A homozygous deletion are prognostic in patients with IDH-mutant astrocytoma. Combination of these parameters allows for improved prediction of outcome. These data aid in designing upcoming trials using IDH inhibitors.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00401-023-02662-1.
Keywords: Brain, CDKN2A, CNS WHO grade, IDH, LINE-1, Molecular
Introduction
The 2016 revision of the World Health Organization (WHO) classification of tumors of the central nervous system (CNS) had placed major emphasis on the isocitrate dehydrogenase (IDH) mutation status when classifying diffuse gliomas in adults [29]. Patients with diffuse gliomas with seemingly similar histology had very different outcomes when stratified for IDH mutation status [2–4, 8, 24, 52]. However, the diagnostic separation of adult-type diffuse astrocytic gliomas into IDH-mutant and IDH-wildtype tumors has generated new challenges regarding the role of grading and molecular prognosticators within these newly defined tumor types [7]. Detection of an IDH mutation in a diffuse astrocytic glioma with microvascular proliferation or necrosis is no longer compatible with a glioblastoma diagnosis, i.e., such tumors are now diagnosed as astrocytoma, IDH-mutant, CNS WHO grade 4 [7, 30]. Moreover, homozygous deletion of the CDKN2A/CDKN2B tumor suppressor gene locus has been introduced as a molecular biomarker for CNS WHO grade 4 in an IDH-mutant astrocytoma [30, 41]. Other molecular alterations that have been associated with aggressive behavior and shorter survival include high tumor mutational burden and increased copy number variation load, as well as various aberrations affecting single genes or chromosomes, such as type of IDH mutation, PIK3R1 mutation, PDGFRA amplification, copy number neutral loss of 17p, loss of 19q, and others [42]. In addition, reduced global DNA methylation, referred to as glioma CpG island methylator phenotype low (gCIMPlow), has been associated with worse outcome [11, 41]. Detection of the global DNA methylation status can be accomplished by microarray- or sequencing-based methylome analyses [9, 11, 31], with focused methylation analysis of the LINE-1 repetitive element being reported as a valuable surrogate marker for global DNA methylation level assessment [57].
Histological grading of IDH-mutant astrocytomas is subject to inter-observer variability [29] and its prognostic relevance is a matter of ongoing discussion. While some studies questioned the prognostic role of histological grading, others showed distinct outcomes according to tumor grade (for review see [7]). Further, whether patients diagnosed with IDH-mutant astrocytoma, CNS WHO grade 4, should be treated like IDH-wildtype glioblastoma patients or rather like patients with IDH-mutant astrocytoma, CNS WHO grade 3, remains controversial [47]. The IDH mutation has recently gained clinical importance as a therapeutic target since vorasidenib, an oral brain-penetrant inhibitor of mutant IDH1 and IDH2 enzymes, significantly improved progression-free survival in patients with CNS WHO grade 2 IDH-mutant gliomas [32].
To further define the prognostic roles of clinical features, CNS WHO grade, and selected molecular biomarkers in IDH-mutant astrocytoma patients, we assembled a large, clinically well documented patient cohort with long-term follow-up data from the German Glioma Network (GGN) and two institutional cohorts.
Patients and methods
Patients
Patients were enrolled in the GGN (n = 212) or followed at the University Hospitals of Lille, France (n = 32) or Zurich, Switzerland (n = 14). The GGN is a prospective, non-interventional cohort study that comprised eight University Hospitals in Germany. All GGN patients gave written informed consent for participation in the GGN and its translational research projects. Local ethics approvals were in place in Lille and Zurich. Patient characteristics, treatment, and outcome data were collected prospectively within the GGN and assembled retrospectively following a similar data capture scheme for patients from Lille and Zurich.
Central neuropathology review
Representative tumor specimens from all patients were subjected to central pathology review at the Brain Tumor Reference Center of the German Society for Neuropathology and Neuroanatomy (DGNN) in Bonn (TP) and Düsseldorf (GR). In addition to histological confirmation of a diffuse astrocytic glioma, the tumors were histologically graded according to the World Health Organization (WHO) classification of central nervous system (CNS) tumors [30]. Accordingly, CNS WHO grade 3 tumors were distinguished from CNS WHO grade 2 tumors by the presence of focal or dispersed anaplasia and significant mitotic activity, while CNS WHO grade 4 tumors were distinguished from the CNS WHO grade 2 and 3 tumors by the presence of microvascular proliferation and/or necrosis and/or homozygous CDKN2A/CDKN2B deletion [30]. All tumors were screened for the IDH1-R132H mutation using immunohistochemistry with a mutation-specific monoclonal antibody (clone H09, Dianova, Hamburg, Germany) [10]. Tumors negative for IDH1-R132H by immunohistochemistry were assessed for non-canonical IDH1 or IDH2 mutations using Sanger sequencing or pyrosequencing [15, 21, 22]. For the molecular analyses, DNA was extracted from frozen tissue samples using the PureLink™ Genomic DNA Mini Kit (Life Technologies, Carlsbad, CA) or ultracentrifugation [23]. Alternatively, DNA was extracted from formalin-fixed and paraffin-embedded tissue samples using the QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany), the GeneRead DNA FFPE Kit (Qiagen), or the Maxwell® RSC FFPE Plus DNA Kit together with the Maxwell® RSC instrument (Promega, Mannheim, Germany). Tumor tissue samples used for DNA extraction were histologically evaluated to contain a sufficient tumor cell content of usually more than 80%. In four tumors (1 CNS WHO grade 2 and 3 CNS WHO grade 4 tumors), classification as IDH-mutant astrocytoma was based only on array-based DNA methylome analysis using the Heidelberg classifier version v.12.5 (https://www.molecularneuropathology.org/mnp/) without further specification of the specific IDH1 or IDH2 mutation by DNA sequencing (Table 1).
Table 1.
Patient and disease characteristics by CNS WHO grade
| Astrocytoma, IDH-mutant, CNS WHO grade 2 n = 114 |
Astrocytoma, IDH-mutant, CNS WHO grade 3 n = 73 |
Astrocytoma, IDH-mutant, CNS WHO grade 4 n = 71 |
|
|---|---|---|---|
| Age at diagnosis | |||
| Median (years) | 36 | 39 | 37 |
| Range (years) | 19–69 | 21–80 | 23–79 |
| Sex | |||
| Male | 78 (68.4%) | 46 (63.0%) | 47 (66.2%) |
| Female | 36 (31.6%) | 27 (37.0%) | 24 (33.8%) |
| KPS at diagnosis | |||
| 90–100 | 71 (86.6%) | 39 (70.9%) | 33 (47.1%) |
| 70–80 | 11 (13.4%) | 14 (25.5%) | 25 (35.7%) |
| <70 | 0 | 2 (3.6%) | 12 (17.1%) |
| No data | 32 | 18 | 1 |
| Tumor location | |||
| Frontal | 41 (36.0%) | 27 (37.0%) | 36 (50.7%) |
| Temporal | 25 (21.9%) | 16 (21.9%) | 5 (7.0%) |
| Parietal | 9 (7.9%) | 4 (5.5%) | 7 (9.9%) |
| Cerebellar, brain stem | 0 | 0 | 2 (2.8%) |
| Not localized to one site | 25 (21.9%) | 18 (24.7%) | 20 (28.2%) |
| Multifocal | 1 (0.9%) | 0 | 0 |
| Others | 12 (10.5%) | 8 (11.0%) | 1 (1.4%) |
| Unknown | 1 (0.9%) | 0 | 0 |
| IDH mutation status | |||
| IDH1R132H mutation | 101 (88.6%) | 66 (90.4%) | 62 (87.3%) |
| Other IDH1 mutations | 11 (9.6%)a | 6 (8.2%)b | 5 (7.0%)c |
| IDH2R172K mutation | 1 (0.9%) | 1 (1.4%) | 1 (1.4%) |
| IDH mutation type not determinedd | 1 (0.9%) | 0 | 3 (4.2%) |
| MGMT promoter methylation status | |||
| Methylated | 72 (65.5%) | 58 (84.1%) | 53 (74.6%) |
| Unmethylated | 38 (34.5%) | 11 (15.9%) | 18 (25.4%) |
| No data | 4 | 4 | 0 |
| CDKN2A deletion status | |||
| Homozygous deletion | 0 | 0 | 12 (17.1%) |
| No homozygous deletion | 66 (100%) | 61 (100%) | 58 (82.9%) |
| No data | 48 | 12 | 1 |
| LINE-1 methylation status | |||
| Methylated alleles (%, median) | 83.0 | 82.7 | 72.8 |
| Methylated alleles (%, range) | 74.2–86.0 | 71.2–86.3 | 60.0–79.1 |
| No data | 62 | 15 | 1 |
CI confidence interval, KPS Karnofsky performance status
aOther IDH1 mutations are R132C (n = 4), R132G (n = 3), R132L (n = 2), R132S (n = 2)
bOther IDH1 mutations are R132C (n = 3), R132G (n = 2), R132L (n = 1)
cOther IDH1 mutations are R132C (n = 2), R132G (n = 1), R132S (n = 2)
dAssignment to methylation class astrocytoma, IDH-mutant based on DNA methylome analysis
In addition to IDH mutation testing, tumors were investigated for 1p/19q codeletion status using either microsatellite-based loss of heterozygosity (LOH) analysis [13, 58] or comparative genomic hybridization [51]. In individual cases, the 1p/19q codeletion status was determined based on copy number profiles obtained by array-based DNA methylome analysis. None of the 258 tumors demonstrated a 1p/19q codeletion.
The MGMT promoter methylation status was determined by methylation-specific PCR or DNA pyrosequencing [14, 46] or, in individual cases, by DNA methylome analysis using the STP27 algorithm [1]. A total of 173 of the 212 GGN cases had been included in previous GGN studies [5, 6, 19, 20, 22, 27, 36, 37, 39, 40, 48–51, 53].
Detection of homozygous deletion of CDKN2A by droplet digital PCR (ddPCR)
We used a commercially available ddPCR assay (Bio-Rad Laboratories) for the detection of CDKN2A homozygous deletions on 9p21 [55]. The loci NCKAP5 and KCNS3 (2p24.2) served as reference loci. The threshold for detection of a homozygous deletion was set to a calculated relative CDKN2A copy number value of <0.5 which was experimentally demonstrated to reliably detect a homozygous deletion when the tumor cell content in the tissue sample used for DNA extraction was ≥75% [55].
Determination of LINE-1 methylation by pyrosequencing
As a surrogate marker for the global DNA methylation status [57], we determined the methylation level of the LINE-1 repetitive element (GenBank accession number X58075) in the tumor DNA using DNA pyrosequencing of sodium bisulfite converted DNA. The primer pair LINE-1-bisu-F1 5ʹ- taggattttttgagttaggtgtg and LINE-1-bisu-R1 5ʹ-[Btn]caaaaaatcaaaaaattccctttcc (biotinylated at the 5ʹ -end) was used for amplification of a 156-bp fragment. Pyrosequencing on the PyroMark Q24 (Qiagen, Hilden, Germany) was performed using the sequencing primer LINE-1-bisu-Seq1 5ʹ -ttaggtgtgggatatagt with the sequence to analyze being “TTYGTGGTGYGTYGTTT”. The three investigated CpG sites correspond to the first three CpGs covered by the PyroMark Q96 CpG LINE-1 kit from Qiagen. After pyrosequencing, we calculated the mean value of the methylated allele percentages at the three investigated CpG sites. A ROC analysis was performed to determine an appropriate cut-off value for the LINE-1 methylation levels, i.e., percentage of methylated alleles that distinguished best between CNS WHO grade 4 tumors as opposed to CNS WHO grade 2 or 3 tumors. Thereby, a cut-off value of ≤77% methylated alleles was calculated with an area under the curve (AUC) of 0.98.
Array-based DNA methylation analyses
Large-scale DNA methylation data obtained by hybridization of tumor DNA to 450 k methylation bead arrays (Illumina, San Diego, CA) were available for 85 patients with IDH-mutant astrocytoma included in this study, comprising 31 CNS WHO grade 2, 31 CNS WHO grade 3, and 23 CNS WHO grade 4 tumors. LINE-1 methylation data were available from 80 of these tumors (29 grade 2, 28 grade 3, and 23 grade 4). 450 k DNA methylation data were generated as described [9] and analyzed with the Heidelberg classifier algorithm version v.12.5 (www.molecularneuropathology.org). Tumors were assigned to the methylation classes “astrocytoma, IDH-mutant, lower grade” or “astrocytoma, IDH-mutant, high-grade” based on calibrated classifier scores of ≥0.9. Principles of the DNA methylation-based classification of central nervous system tumors, the distinction of methylation classes, and the role of calibrated classifier scores have been reported [9].
Statistical analyses
Progression-free survival (PFS) was calculated from the day of first surgery until tumor progression, death, or end of follow-up. Overall survival (OS) was calculated from the day of first surgery until death or end of follow-up. Kaplan–Meier survival curves, Log-rank test, and Cox regression were used for univariate and multivariate analyses of survival. Chi2-test and Fisher’s exact test were used to analyze categorical data. Quantitative data were analyzed by t test and Mann–Whitney U test. A ROC analysis was performed to determine an appropriate cut-off value for the percentage of LINE-1 methylated alleles. Sensitivity and specificity with 95% confidence interval (CI) were calculated.
Results
Patient characteristics
The median age was below 40 years for all CNS WHO grades. Less than half (47.1%) of the patients with CNS WHO grade 4 tumors had a KPS 90 or 100, as opposed to 86.6 and 70.9% of the patients with CNS WHO grade 2 or 3 tumors (p < 0.001). IDH-mutant astrocytomas of CNS WHO grade 4 were numerically more often located in the frontal lobes (p = 0.118) (Table 1), and a gross total resection was numerically more often performed in these patients than in patients with CNS WHO grade 2 or 3 tumors (p = 0.177) (Table S1).
Molecular characteristics
The canonical IDH-R132H mutation was detected in almost 90% of the tumors with equal frequencies across grades. MGMT promoter methylation was detected in more than 70% of all tumors, with the lowest percentage of 65.5% detected in CNS WHO grade 2 tumors (Table 1). CDKN2A homozygous deletions were detected in 12 of 71 patients (16.9%) with CNS WHO grade 4 tumors. Eleven of these tumors also showed histological features of CNS WHO grade 4, i.e., microvascular proliferation or necrosis or both. The LINE-1 methylation levels were lower in CNS WHO grade 4 tumors than in CNS WHO grade 2 or 3 groups (Table 1).
Treatment and outcome
Patients with CNS WHO grade 4 tumors received combined modality treatment upfront more often than patients with CNS WHO grade 2 or 3 tumors. A wait-and-scan strategy was more commonly adopted in patients with CNS WHO grade 2 tumors than in patients with CNS WHO grade 3 or 4 tumors. PFS did not differ between patients with CNS WHO grade 2 versus 3 tumors (p = 0.557), but was significantly lower in patients with CNS WHO grade 4 tumors (p < 0.001) (Fig. 1a, Table S1). At progression, 36 of 50 CNS WHO grade 2 tumors (72%) and 4 of 15 CNS WHO grade 3 tumors (27%) that were subjected to repeated surgery showed histological progression to a higher grade in the recurrent tumor tissue. The rates of documented progression events were similar across the tumor grades. The percentages of patients treated at first progression varied from 90.5% in patients with CNS WHO grade 2 tumors to 81.6 and 82.1% for patients with CNS WHO grade 3 and 4 tumors.
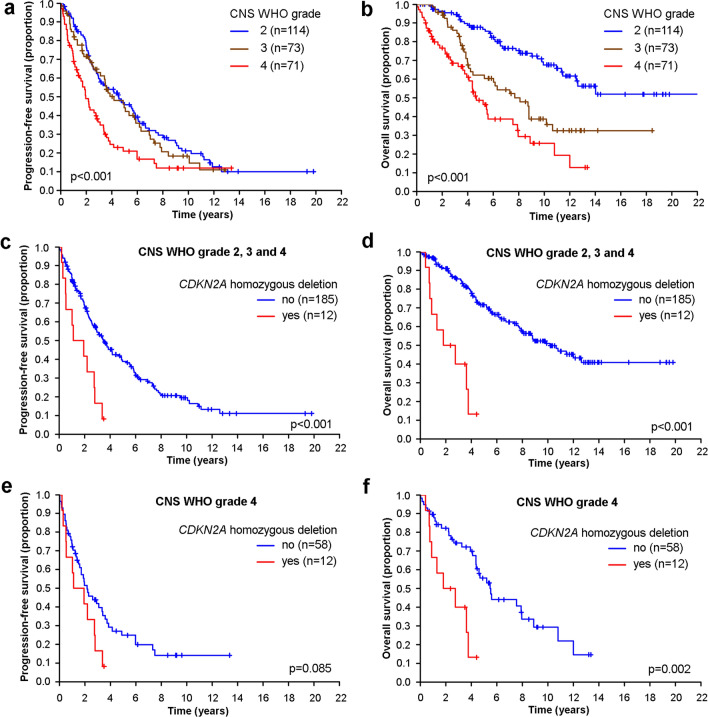
Fig. 1.
Outcome of patients with IDH-mutant astrocytoma stratified according to CNS WHO grade and CDKN2A copy number status. (a, b) PFS (a) and OS (b) of the entire cohort of patients with IDH-mutant astrocytomas stratified by CNS WHO grade 2, 3 or 4. (c–f) PFS (c, e) and OS (d, f) of the entire cohort of patients with IDH-mutant astrocytoma (c, d) or IDH-mutant astrocytoma of CNS WHO grade 4 only (e, f)
Survival was longer for patients with CNS WHO grade 2 tumors than for patients with CNS WHO grade 3 or 4 tumors (p < 0.001) (Table S1, Fig. 1b). Furthermore, patients with CNS WHO grade 3 tumors lived longer than patients with CNS WHO grade 4 tumors (p = 0.023) (Table S1, Fig. 1b). Since CDKN2A loss is a defining feature of CNS WHO grade 4 and since there was insufficient tissue to complete CDKN2A assessment for all CNS WHO grade 2 and 3 tumors, we performed a sensitivity analysis omitting all CNS WHO grade 2 and 3 tumors without CDKN2A assessment. These analyses revealed essentially the same survival curves (Fig. S1a, b), confirming that CDKN2A loss is infrequent in morphologically defined CNS WHO grade 2 and 3 tumors.
Molecular marker profiles and outcome: type of IDH mutation and MGMT promoter methylation
Figure S2 shows survival curves stratified by IDH mutation type, i.e., IDH1-R132H versus all other (non-canonical) IDH1 or IDH2 mutations. In the entire cohort (Fig. S2a) as well as in the grade 2 (Fig. S2b) and grade 4 (Fig. S2d) subcohorts, there were no differences in OS by IDH mutation type. Only in patients with CNS WHO grade 3 tumors, presence of a non-canonical IDH mutation was associated with a better outcome (p = 0.021) (Fig. S2c).
We also compared the outcome by MGMT promoter methylation status across the entire cohort and by CNS WHO grade. In the entire cohort, MGMT promoter methylation was not prognostic for PFS but inversely related to OS (Fig. S3a,b). The latter finding is explained by the overall lower frequency of MGMT promoter methylation in the CNS WHO grade 2 tumors compared with CNS WHO grade 3 and 4 tumors (Table 1). Among patients with CNS WHO grade 4 tumors, MGMT promoter methylation was associated with longer PFS, but not OS (Fig. S3c, d, Table S2). Within the cohorts of patients with CNS WHO grade 2 or 3 tumors, the MGMT promoter methylation status was not related to PFS and OS (Fig. S3e–h). MGMT promoter methylation status was also not related to OS when survival analyses were restricted to patients exposed to alkylating agents (Fig. S4).
Molecular markers associated with CNS WHO grade 4: CDKN2A homozygous deletion and lower levels of LINE-1 methylation
Homozygous deletion of CDKN2A is per definition restricted to IDH-mutant astrocytomas of CNS WHO grade 4 [30] where it has been detected in 20–40% of tumors [26, 41]. We confirmed that homozygous CDKN2A deletion was highly prognostic over the complete dataset encompassing all CNS WHO grades (Fig. 1c,d) and remained prognostic among CNS WHO grade 4 tumors (Fig. 1e,f).
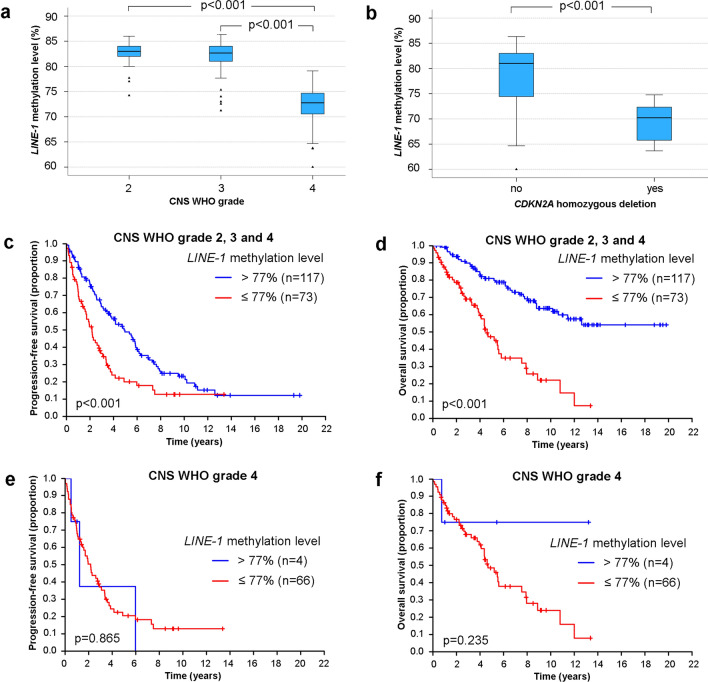
LINE-1 methylation levels were significantly lower in CNS WHO grade 4 compared to lower grade tumors (Fig. 2a). IDH-mutant astrocytomas with homozygous CDKN2A deletion showed lower LINE-1 methylation levels than IDH-mutant astrocytomas without complete CDKN2A loss (Fig. 2b). Correlation of LINE-1 methylation levels with survival using a cut-off of 77% methylated alleles revealed that patients whose tumors had LINE-1 methylation levels of ≤ 77% showed less favorable PFS and OS (Fig. 2c,d). Among patients with CNS WHO grade 4 tumors, LINE-1 methylation levels ≤77% were not associated with shorter PFS or OS, a finding likely related to the low fraction of tumors with LINE-1 methylation level >77% (n = 4) (Fig. 2e,f). We also performed a comparative analysis between LINE-1 methylation levels and the assignment of tumors into the methylation classes “astrocytoma, IDH-mutant, lower grade” or “astrocytoma, IDH-mutant, high-grade” according to the Heidelberg brain tumor classified v.12b5 (www.molecularneuropathology.org) using available 450 k DNA methylome data of 85 IDH-mutant astrocytomas included in our cohort, with LINE-1 methylation data being available for 80 of these cases. Overall, 31 of 31 CNS WHO grade 2 and 29 of 31 CNS WHO grade 3 tumors were assigned to the methylation class “astrocytoma, IDH-mutant, lower grade”, while 17 of 23 CNS WHO grade 4 tumors were assigned to the methylation class “astrocytoma, IDH-mutant, high-grade”. Similar to lower levels of LINE-1 methylation, the DNA methylation class “astrocytoma, IDH-mutant, high-grade” was associated with CNS WHO grade 4 (p < 0.001). Correspondingly, LINE-1 methylation levels were significantly lower in tumors assigned to the methylation class “astrocytoma, IDH-mutant, high-grade” (Fig. S5a). The previously determined LINE-1 cut-off value of ≤77% methylated alleles was applied to assess its value to discriminate the two methylation classes. Overall, sensitivity for detection of the “astrocytoma, IDH mutant, high grade” methylation class by a LINE-1 methylation level of ≤77% was 94.7% (95% CI 74.0–99.9%) and specificity was 90.2% (95% CI 79.8–96.3%). Among 23 CNS WHO grade 4 tumors with available 450 k DNA methylome data, a similar trend of lower LINE-1 methylation levels in “astrocytoma, IDH-mutant, high-grade” tumors was observed, albeit the p value remained insignificant likely due to the low number of tumors assigned to the methylation class “astrocytoma, IDH-mutant, lower grade” among the CNS WHO grade 4 tumors (Fig. S5b).
Fig. 2.
LINE-1 methylation levels in IDH-mutant astrocytomas. LINE-1 methylation levels according to CNS WHO grade 2 (n = 62), CNS WHO grade 3 (n = 58), and CNS WHO grade 4 (n = 70) (a). LINE-1 methylation levels according to CDKN2A deletion status based on 11 tumors with homozygous CDKN2A deletion and 178 tumors without this alteration (b). PFS (c) and OS (d) of the entire patient cohort according to LINE-1 methylation level stratified into ≤77% methylated alleles versus >77% methylated alleles. PFS (e) and OS (f) of the patients with CNS WHO grade 4 tumors according to LINE-1 methylation levels
Prognostic factor analyses
Univariate analyses over the entire cohort revealed that CNS WHO grade, age, KPS, extent of resection, CDKN2A deletion, and LINE-1 methylation level were prognostic. MGMT promoter methylation was prognostic, too, but with a better outcome for patients with tumors lacking MGMT promoter methylation (see above). On multivariate analysis, CNS WHO grade, extent of resection, and CDKN2A deletion were retained as prognostic factors (Table 2). Similar results were obtained when the same analyses were restricted to patients with IDH-mutant astrocytoma, CNS WHO grade 4 (Table S3).
Table 2.
Prognostic factors in IDH-mutant astrocytoma: univariate and multivariate analyses
| Entire cohort, univariate analyses | Hazard ratio | p value | 95% CI |
|---|---|---|---|
| CNS WHO grade | |||
| 2 (ref) | – | ||
| 3 | 2.35 | <0.001 | 1.47–3.76 |
| 4 | 4.14 | <0.001 | 2.60–6.57 |
| Age (years) | |||
| > 40 versus ≤ 40 (ref.) | 1.61 | 0.013 | 1.10–2.34 |
| KPS | |||
| <80 versus ≥80 (ref.) | 2.55 | <0.001 | 1.53–4.25 |
| Surgery | |||
| No total versus total (ref.) | 1.73 | 0.017 | 1.10–2.73 |
| MGMT promoter status | |||
| Methylated versus unmethylated (ref.) | 1.64 | 0.043 | 1.01–2.64 |
| IDH mutation status | |||
| IDH1-R132H versus other IDH1 or IDH2 mutations (ref.) | 1.66 | 0.169 | 0.81–3.40 |
| CDKN2A deletion status | |||
| Homozygous versus no homozygous (ref.) | 6.04 | <0.001 | 2.90–12.56 |
| LINE-1 methylation status | |||
| ≤77 versus >77% methylated alleles (ref.) | 3.54 | <0.001 | 2.25–5.56 |
| Entire cohort, multivariate analyses | Hazard ratio | p value | 95% CI |
|---|---|---|---|
| CNS WHO grade | |||
| 2 (ref) | – | ||
| 3 | 3.09 | 0.007 | 1.36–7.00 |
| 4 | 1.42 | 0.577 | 0.42–4.80 |
| Age (years) | |||
| > 40 versus ≤ 40 (ref.) | 1.58 | 0.098 | 0.92–2.72 |
| KPS | |||
| <80 versus ≥80 (ref.) | 1.17 | 0.627 | 0.621–2.21 |
| Surgery | |||
| No total versus total (ref.) | 2.61 | < 0.001 | 1.49–4.58 |
| MGMT promoter status | |||
| Methylated versus unmethylated (ref.) | 1.03 | 0.936 | 0.54–1.96 |
| IDH mutations status | |||
| IDH1-R132H versus other IDH1 or IDH2 mutations (ref.) | 1.85 | 0.165 | 0.78–4.39 |
| CDKN2A deletion status | |||
| Homozygous versus no homozygous (ref.) | 3.74 | 0.008 | 1.41–9.92 |
| LINE-1 methylation status | |||
| ≤77 versus >77% methylated alleles (ref.) | 3.39 | 0.025 | 1.17–9.85 |
Combination of CNS WHO grade and molecular biomarkers for improved prediction of outcome
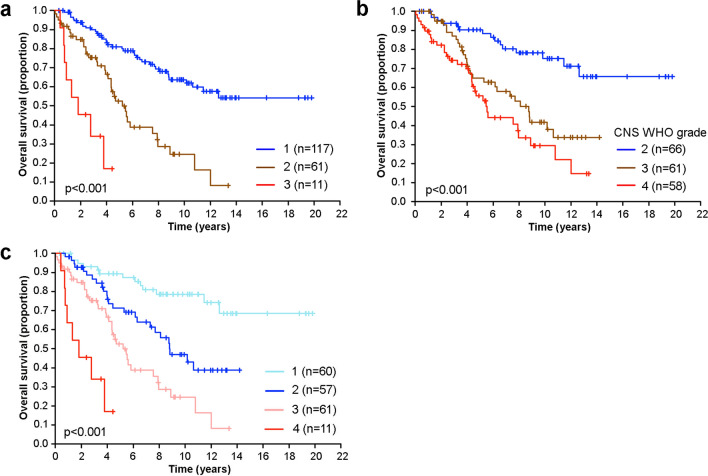
Next we explored whether our findings might provide a new approach for improved prognostic assessment of patients with IDH-mutant astrocytoma. Stratification according to LINE-1 methylation levels and CDKN2A copy number status resulted in three groups of patients with distinct overall survival (Fig. 3a). Group assignment remained highly significant upon adjustment for other prognostic factors (Table S4). Since CDKN2A homozygous deletion was a profound negative prognostic factor in our cohort (Figs. 1c–f), we also explored the prognostic significance of the current WHO classification when patients with CDKN2A homozygously deleted tumors were excluded. This resulted in a less distinct separation of outcome of CNS WHO grade 3 and 4 tumors (Fig. 3b). Yet, in tumors without homozygous CDKN2A deletion, lower LINE-1 methylation levels were highly associated with CNS WHO grade 4 (Figs. S6a, b). Finally, placing LINE-1 methylation levels at the apex of the prognostic stratification delineated four subgroups with relevant significant outcome differences (group 2 versus 1, HR = 2.99, p = 0.011; group 3 versus 2, HR = 1.68, p = 0.095; group 4 versus 3, HR = 3.51, p = 0.010).
Fig. 3.
Prognostic stratification based on CNS WHO grade, LINE-1 methylation level, and CDKN2A homozygous deletion status. (a) OS stratified by LINE-1 methylation level (77% cut-off) and CDKN2A homozygous deletion status. The three distinct groups of patients correspond to: 1, LINE-1 methylation levels of >77% without homozygous CDKN2A deletion; 2, LINE-1 methylation levels of ≤77% without homozygous CDKN2A deletion; 3, LINE-1 methylation levels of ≤77% with homozygous CDKN2A deletion). (b) OS by CNS WHO grade with omission of patients with CDKN2A homozygously deleted tumors. (c) OS stratified based on LINE-1 methylation levels followed by CNS WHO grade 2 versus CNS WHO grade 3 or 4 in tumors with high LINE-1 methylation levels or followed by CDKN2A homozygous deletion status in tumors with low LINE-1 methylation levels The four distinct groups of patients correspond to: 1, LINE-1 methylation levels of >77% and CNS WHO grade 2; 2, LINE-1 methylation levels of >77% and CNS WHO grade 3 or 4; 3, LINE-1 methylation levels of ≤77% without homozygous CDKN2A deletion; and 4, LINE-1 methylation levels of ≤77% and homozygous CDKN2A deletion
In the group of patients whose tumors showed high LINE-1 methylation levels (>77% methylated alleles), outcome was significantly different for patients with CNS WHO grade 2 tumors versus patients with CNS WHO grade 3 or (rare) grade 4 tumors. In the group of patients whose tumors showed low LINE-1 methylation levels (≤77%), outcome was significantly different for patients with CDKN2A homozygously deleted tumors versus patients whose tumors had no homozygous CDKN2A deletion (Fig. 3c). The differences in outcome between the four groups were significant on univariate and multivariate analysis except for group 3 versus 2 (Table S5).
Discussion
The present study provides contemporary data on the patterns of presentation, treatment, and outcome in the newly defined adult-type diffuse glioma group of IDH-mutant astrocytomas [7, 30] with a particular focus on astrocytoma, IDH-mutant, CNS WHO grade 4. The grading of IDH-mutant astrocytomas remains a matter of ongoing controversy [7, 18, 30, 34, 38]. Here, we report that stratification of these tumors into CNS WHO grades 2, 3, or 4 as recently defined in the WHO 2021 classification [30] is prognostically important (Fig. 1). Currently, the distinction of CNS WHO grade 2 versus 3 according to the WHO classification 2021 relies on the presence of focal or dispersed anaplasia and significant mitotic activity; however, a distinct cut-off for the mitotic count was not established [30]. A recent study based on patients included in the EORTC trials 26053 (CATNON) and 22033–26033 supported a prognostic role of mitotic activity and reported that a cut-off of two mitoses per ten microscopic high power fields was linked to significantly longer PFS and marginally longer OS in patients with IDH-mutant astrocytoma without homozygous CDKN2A/CDKN2B deletion [28]. Another recent study reported that the combination of <6 mitoses per 3 mm2 and a residual tumor volume of <1 cm2 upon postsurgical imaging was indicative of longer time to treatment and overall survival in patients with IDH-mutant astrocytomas of CNS WHO grade 2 or 3 [45].
The majority of IDH-mutant astrocytomas, CNS WHO grade 4, present de novo, rather than arising from a pre-existing lower grade astrocytoma [26]. The similar age at diagnosis across the groups defined by CNS WHO grade reported here (Table 1) supports this notion. A recent analysis of pooled data from clinical trials suggested that the canonical R132H mutation may confer an inferior survival compared with the less common, non-canonical mutations in IDH1 or mutations in IDH2 [44]. This association was confirmed in a cohort from Italy [17] and a recent meta-analysis [12], while data from the French POLA cohort revealed no clear prognostic association by type of IDH mutation [35]. We observed an association of non-canonical IDH mutations with longer OS only in the group of patients with CNS WHO grade 3 tumors (Fig. S2), but the sample size was overall small.
The limited prognostic role of MGMT promoter methylation status despite the broad use of alkylating agents in our patient population was surprising, but may confirm a recent cohort study [25] and is in line with previous data indicating MGMT promoter methylation as a predictive marker of response to alkylating agents in IDH-wildtype glioblastoma but not in IDH-mutant gliomas [53]. We observed MGMT promoter methylation in IDH-mutant astrocytomas of CNS WHO grade 2 less frequently than in CNS WHO grade 3 or 4 tumors, a finding which might contribute to the lack of prognostic association of the MGMT status.
Our study confirms the strong negative prognostic value of CDKN2A homozygous deletion in IDH-mutant astrocytoma patients. As reported before [16, 26, 41], presence of a CDKN2A/CDKN2B homozygous deletion is associated with particularly poor outcome of IDH-mutant astrocytoma patients, even within the group of patients with CNS WHO grade 4 tumors [26]. Thus, our findings lend further support for this molecular alteration as an independent indicator of CNS WHO grade 4 behavior [30]. The WHO classification recommends diagnostic testing for CDKN2A/CDKN2B homozygous in IDH-mutant astrocytomas showing histological features of anaplasia corresponding to CNS WHO grade 3, but not for IDH-mutant astrocytomas with histological features corresponding to CNS WHO grade 2 tumors [30], as the latter generally lack CDKN2A/CDKN2B homozygous deletion [41] (Table 1). However, CDKN2A/CDKN2B homozygous deletion is not very common even in CNS WHO grade 4 tumors, and novel markers of CNS WHO grade 4 that can be easily tested in clinical practice are urgently needed.
Here, we report that the LINE-1 methylation level, a surrogate marker for the global DNA methylation status, is markedly lower in IDH-mutant astrocytomas of CNS WHO grade 4 compared with lower-grade tumors. So far, LINE-1 methylation levels have not been studied in depth in gliomas. One study reported lower LINE-1 methylation levels in glioblastomas compared to low-grade gliomas, and higher LINE-1 methylation levels were associated with MGMT promoter methylation and longer survival of glioblastoma patients [33]. Another study revealed that high levels of LINE-1 methylation and gene-specific hypermethylation of several genes were linked to longer survival of glioma patients [59]. However, both studies were based on histologically classified glioblastomas and lower grade diffuse gliomas, i.e., did not stratify the investigated cohorts according to the IDH mutation status. Here, we found a significantly lower level of LINE-1 methylation in IDH-mutant astrocytomas of CNS WHO grade 4 compared with IDH-mutant astrocytomas of CNS WHO grade 2 or 3. In addition, lower LINE-1 methylation levels were associated with shorter survival in IDH-mutant astrocytoma patients. Our findings confirm large-scale methylome analyses that identified a subset of IDH-mutant astrocytomas with lower levels of global DNA methylation and shorter survival, which were referred to as “glioma-CpG island methylator phenotype (G-CIMP)-low” tumors as opposed to “G-CIMP-high” tumors [11, 31, 42, 43]. Along this line, array-based DNA methylome profiling using the Heidelberg methylation classifier version v.12.5 identifies two distinct DNA methylation classes of IDH-mutant astrocytoma, namely “astrocytoma, IDH-mutant, lower grade” and “astrocytoma, IDH-mutant, high-grade” (www.molecularneuropathology.org) [9], which largely overlap with the “G-CIMP-high” and “G-CIMPlow” groups, respectively [42]. Correlative analysis in relation to these two distinct methylation classes consequently revealed lower levels of LINE-1 methylation in the “astrocytoma, IDH-mutant, high-grade” methylation class. Taken together, our findings, thus, confirm lower levels of global DNA methylation as a prognostically unfavorable molecular alteration in IDH-mutant astrocytomas [42, 43, 56] that can be detected by DNA methylation arrays and other methods like LINE-1 methylation analysis using pyrosequencing, i.e., a method already established in many laboratories for the assessment of the MGMT promoter methylation status [54]. Hence, detection of lower levels of LINE-1 methylation may represent a novel biomarker that may support grading of IDH-mutant astrocytoma by indicating CNS WHO grade 4 behavior. In our study, a LINE-1 methylation level of 77% calculated across three selected LINE-1 CpG sites was employed to distinguish high level versus low-level methylated cases. However, quantitative methylation levels may vary according to specific assays and equipment used; hence, validation and potential adaptation of cut-offs to work-flows used in other laboratories will likely be required, as also indicated by the variable levels of LINE-1 methylation reported in astrocytic gliomas before [33, 59].
LINE-1 methylation levels were an independent prognostic variable for survival upon multivariate analysis (Table 2). However, individual cases of CNS WHO grade 2 and 3 tumors showed LINE-1 methylation levels of ≤77% while individual cases of CNS WHO grade 4 tumors without CDKN2A homozygous deletion had LINE-1 methylation levels of >77% (Fig. S6b). In addition, other authors reported on a prognostically unfavorable association of lower global DNA methylation levels in a cohort of IDH-mutant grade 4 astrocytic gliomas/glioblastomas [56]. Thus, determination of the global DNA methylation level may provide information beyond WHO grading, as supported by other studies [11, 31].
Limitations of our study include the retrospective design, potential bias of enrolling patients with favorable outcome into cohorts like the GGN and lack of standardized approaches to treatment and follow-up. We also did not quantitatively evaluate mitotic count as performed in the recent studies by Kros et al. [28] and Tran et al. [45] that reported on various cut-offs for mitotic counts predicting outcome. In addition, the LINE-1 cut-off used in this study would require independent validation in a distinct patient cohort. Furthermore, potential diagnostic use in individual patients would demand the establishment of a validated assay that is standardized concerning, among others, the definition of CpG sites to be interrogated, the method for calculation of methylated allele frequencies, input amount of DNA and completeness of bisulfite conversion, appropriate control samples, and the actual pyrosequencing protocol. Nevertheless, the present cohort of patients with IDH-mutant astrocytomas is relatively large and may serve as a framework for further efforts aiming at characterizing novel markers for improved prediction of therapy response and outcome that could also guide treatment strategy and clinical trial design, notably with the view to defining the role of IDH inhibitors along the disease trajectory [32]. Furthermore, we provide possible future avenues to improve histomolecular prognostic assessment of IDH-mutant astrocytoma based on CNS WHO grade, global DNA methylation level, and CDKN2A homozygous deletion (Fig. 3).
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the staff at the participating clinical centers of the German Glioma Network as well as in Lille, France and Zurich, Switzerland, and the Tumorothèque du C2RC de Lille for their support. Britta Friedensdorf and Heike Seul (Institute of Neuropathology, Heinrich Heine University and University Hospital Düsseldorf, Germany), as well as Ulrike Schoenwiese (Institute for Medical Informatics, Statistics and Epidemiology, University Leipzig, Germany) are acknowledged for excellent technical assistance. The German Glioma Network was supported by the German Cancer Aid (Deutsche Krebshilfe, grant number 70-3163-Wi 3).
Abbreviations
- CI
Confidence interval
- CNS
Central nervous system
- GGN
German Glioma Network
- HR
Hazard ratio
- IDH
Isocitrate dehydrogenase
- KPS
Karnofsky performance status
- LINE-1
Long interspersed nuclear element 1
- MGMT
O6-methylguanine-DNA methyltransferase
- OS
Overall survival
- PFS
Progression-free survival
- RT
Radiotherapy
- TMZ
Temozolomide
- WHO
World Health Organization
Funding
Open access funding provided by University of Zurich.
Data availability
Anonymized data may be shared upon appropriate request of qualified investigators for purposes of replicating procedures and results.
Declarations
Conflict of interest
MiW has received research grants from Quercis and Versameb, and honoraria for lectures or advisory board participation or consulting from Bayer, Curevac, Medac, Novartis, Novocure, Orbus, Philogen, Roche and Sandoz. PR has received honoraria for lectures or advisory board participation from Alexion, Bristol-Myers Squibb, Boehringer Ingelheim, Debiopharm, Midatech Pharma, Novocure, QED, and Roche and research support from Merck Sharp and Dohme and Novocure. DK received honoraria for advisory board participation from Novocure and Bayer and for lectures from Brainlab. UH has received lecture and/or advisory board honoraria from Medac and Bayer. MaW received honoraria for an advisory board from DoubleBond. JCT has received research grants from novocure and Munich Surgical Imaging and honoraria for lectures or consulting from Seagen and AAA-Novartis. ELR has received research grants from Bristol Meyers Squibb (BMS), and honoraria for lectures or advisory board participation or consulting from Bayer, Janssen, Leo Pharma, Pierre Fabre, Roche, Seattle Genetics, and Servier. JF, BH, DG, NK, MWo, MR, LR, CAM, AvD, TP, and GR declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bady P, Delorenzi M, Hegi ME. Sensitivity analysis of the MGMT-STP27 model and impact of genetic and epigenetic context to predict the MGMT methylation status in gliomas and other tumors. J Mol Diagn. 2016;18:350–361. doi: 10.1016/j.jmoldx.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Baumert BG, Hegi ME, van den Bent MJ, von Deimling A, Gorlia T, Hoang-Xuan K, Brandes AA, Kantor G, Taphoorn MJB, Hassel MB, Hartmann C, Ryan G, Capper D, Kros JM, Kurscheid S, Wick W, Enting R, Reni M, Thiessen B, Dhermain F, Bromberg JE, Feuvret L, Reijneveld JC, Chinot O, Gijtenbeek JMM, Rossiter JP, Dif N, Balana C, Bravo-Marques J, Clement PM, Marosi C, Tzuk-Shina T, Nordal RA, Rees J, Lacombe D, Mason WP, Stupp R. Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033–26033): a randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2016;17:1521–1532. doi: 10.1016/S1470-2045(16)30313-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van den Bent MJ, Baumert B, Erridge SC, Vogelbaum MA, Nowak AK, Sanson M, Brandes AA, Clement PM, Baurain JF, Mason WP, Wheeler H, Chinot OL, Gill S, Griffin M, Brachman DG, Taal W, Rudà R, Weller M, McBain C, Reijneveld J, Enting RH, Weber DC, Lesimple T, Clenton S, Gijtenbeek A, Pascoe S, Herrlinger U, Hau P, Dhermain F, van Heuvel I, Stupp R, Aldape K, Jenkins RB, Dubbink HJ, Dinjens WNM, Wesseling P, Nuyens S, Golfinopoulos V, Gorlia T, Wick W, Kros JM. Interim results from the CATNON trial (EORTC study 26053–22054) of treatment with concurrent and adjuvant temozolomide for 1p/19q non-co-deleted anaplastic glioma: a phase 3, randomised, open-label intergroup study. Lancet. 2017 doi: 10.1016/S0140-6736(17)31442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van den Bent MJ, Carpentier AF, Brandes AA, Sanson M, Taphoorn MJB, Bernsen HJJA, Frenay M, Tijssen CC, Grisold W, Sipos L, Haaxma-Reiche H, Kros JM, van Kouwenhoven MCM, Vecht CJ, Allgeier A, Lacombe D, Gorlia T. Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: a randomized European Organisation for Research and Treatment of Cancer phase III trial. J Clin Oncol. 2006;24:2715–2722. doi: 10.1200/JCO.2005.04.6078. [DOI] [PubMed] [Google Scholar]
- 5.Binder H, Willscher E, Loeffler-Wirth H, Hopp L, Jones DTW, Pfister SM, Kreuz M, Gramatzki D, Fortenbacher E, Hentschel B, Tatagiba M, Herrlinger U, Vatter H, Matschke J, Westphal M, Krex D, Schackert G, Tonn JC, Schlegel U, Steiger H-J, Wick W, Weber RG, Weller M, Loeffler M. DNA methylation, transcriptome and genetic copy number signatures of diffuse cerebral WHO grade II/III gliomas resolve cancer heterogeneity and development. Acta Neuropathol Commun. 2019;7:59. doi: 10.1186/s40478-019-0704-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brand F, Förster A, Christians A, Bucher M, Thomé CM, Raab MS, Westphal M, Pietsch T, von Deimling A, Reifenberger G, Claus P, Hentschel B, Weller M, Weber RG. FOCAD loss impacts microtubule assembly, G2/M progression and patient survival in astrocytic gliomas. Acta Neuropathol. 2020;139:175–192. doi: 10.1007/s00401-019-02067-z. [DOI] [PubMed] [Google Scholar]
- 7.Brat DJ, Aldape K, Colman H, Figrarella-Branger D, Fuller GN, Giannini C, Holland EC, Jenkins RB, Kleinschmidt-DeMasters B, Komori T, Kros JM, Louis DN, McLean C, Perry A, Reifenberger G, Sarkar C, Stupp R, van den Bent MJ, von Deimling A, Weller M. cIMPACT-NOW update 5: recommended grading criteria and terminologies for IDH-mutant astrocytomas. Acta Neuropathol. 2020;139:603–608. doi: 10.1007/s00401-020-02127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckner JC, Shaw EG, Pugh SL, Chakravarti A, Gilbert MR, Barger GR, Coons S, Ricci P, Bullard D, Brown PD, Stelzer K, Brachman D, Suh JH, Schultz CJ, Bahary J-P, Fisher BJ, Kim H, Murtha AD, Bell EH, Won M, Mehta MP, Curran WJ. Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. 2016;374:1344–1355. doi: 10.1056/NEJMoa1500925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capper D, Jones DTW, Sill M, Hovestadt V, Schrimpf D, Sturm D, Koelsche C, Sahm F, Chavez L, Reuss DE, Kratz A, Wefers AK, Huang K, Pajtler KW, Schweizer L, Stichel D, Olar A, Engel NW, Lindenberg K, Harter PN, Braczynski AK, Plate KH, Dohmen H, Garvalov BK, Coras R, Hölsken A, Hewer E, Bewerunge-Hudler M, Schick M, Fischer R, Beschorner R, Schittenhelm J, Staszewski O, Wani K, Varlet P, Pages M, Temming P, Lohmann D, Selt F, Witt H, Milde T, Witt O, Aronica E, Giangaspero F, Rushing E, Scheurlen W, Geisenberger C, Rodriguez FJ, Becker A, Preusser M, Haberler C, Bjerkvig R, Cryan J, Farrell M, Deckert M, Hench J, Frank S, Serrano J, Kannan K, Tsirigos A, Brück W, Hofer S, Brehmer S, Seiz-Rosenhagen M, Hänggi D, Hans V, Rozsnoki S, Hansford JR, Kohlhof P, Kristensen BW, Lechner M, Lopes B, Mawrin C, Ketter R, Kulozik A, Khatib Z, Heppner F, Koch A, Jouvet A, Keohane C, Mühleisen H, Mueller W, Pohl U, Prinz M, Benner A, Zapatka M, Gottardo NG, Driever PH, Kramm CM, Müller HL, Rutkowski S, von Hoff K, Frühwald MC, Gnekow A, Fleischhack G, Tippelt S, Calaminus G, Monoranu C-M, Perry A, Jones C, Jacques TS, Radlwimmer B, Gessi M, Pietsch T, Schramm J, Schackert G, Westphal M, Reifenberger G, Wesseling P, Weller M, Collins VP, Blümcke I, Bendszus M, Debus J, Huang A, Jabado N, Northcott PA, Paulus W, Gajjar A, Robinson GW, Taylor MD, Jaunmuktane Z, Ryzhova M, Platten M, Unterberg A, Wick W, Karajannis MA, Mittelbronn M, Acker T, Hartmann C, Aldape K, Schüller U, Buslei R, Lichter P, Kool M, Herold-Mende C, Ellison DW, Hasselblatt M, Snuderl M, Brandner S, Korshunov A, von Deimling A, Pfister SM. DNA methylation-based classification of central nervous system tumours. Nature. 2018;555:469–474. doi: 10.1038/nature26000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Capper D, Zentgraf H, Balss J, Hartmann C, von Deimling A. Monoclonal antibody specific for IDH1 R132H mutation. Acta Neuropathol. 2009;118:599–601. doi: 10.1007/s00401-009-0595-z. [DOI] [PubMed] [Google Scholar]
- 11.Ceccarelli M, Barthel FP, Malta TM, Sabedot TS, Salama SR, Murray BA, Morozova O, Newton Y, Radenbaugh A, Pagnotta SM, Anjum S, Wang J, Manyam G, Zoppoli P, Ling S, Rao AA, Grifford M, Cherniack AD, Zhang H, Poisson L, Carlotti CG, Tirapelli DP da C, Rao A, Mikkelsen T, Lau CC, Yung WKA, Rabadan R, Huse J, Brat DJ, Lehman NL, Barnholtz-Sloan JS, Zheng S, Hess K, Rao G, Meyerson M, Beroukhim R, Cooper L, Akbani R, Wrensch M, Haussler D, Aldape KD, Laird PW, Gutmann DH, TCGA Research Network, Noushmehr H, Iavarone A, Verhaak RGW Molecular Profiling Reveals Biologically Discrete Subsets and Pathways of Progression in Diffuse Glioma. Cell. 2016;164:550–563. doi: 10.1016/j.cell.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Nunno V, Franceschi E, Tosoni A, Gatto L, Maggio I, Lodi R, Angelini D, Bartolini S, Brandes AA. Clinical and molecular features of patients with gliomas harboring IDH1 non-canonical mutations: a systematic review and meta-analysis. Adv Ther. 2022;39:165–177. doi: 10.1007/s12325-021-01977-3. [DOI] [PubMed] [Google Scholar]
- 13.Felsberg J, Erkwoh A, Sabel MC, Kirsch L, Fimmers R, Blaschke B, Schlegel U, Schramm J, Wiestler OD, Reifenberger G. Oligodendroglial tumors: refinement of candidate regions on chromosome arm 1p and correlation of 1p/19q status with survival. Brain Pathol. 2004;14:121–130. doi: 10.1111/j.1750-3639.2004.tb00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felsberg J, Thon N, Eigenbrod S, Hentschel B, Sabel MC, Westphal M, Schackert G, Kreth FW, Pietsch T, Löffler M, Weller M, Reifenberger G, Tonn JC, Network GG. Promoter methylation and expression of MGMT and the DNA mismatch repair genes MLH1, MSH2, MSH6 and PMS2 in paired primary and recurrent glioblastomas. Int J Cancer. 2011;129:659–670. doi: 10.1002/ijc.26083. [DOI] [PubMed] [Google Scholar]
- 15.Felsberg J, Wolter M, Seul H, Friedensdorf B, Göppert M, Sabel MC, Reifenberger G. Rapid and sensitive assessment of the IDH1 and IDH2 mutation status in cerebral gliomas based on DNA pyrosequencing. Acta Neuropathol. 2010;119:501–507. doi: 10.1007/s00401-010-0647-4. [DOI] [PubMed] [Google Scholar]
- 16.Fortin Ensign SP, Jenkins RB, Giannini C, Sarkaria JN, Galanis E, Kizilbash SH. Translational significance of CDKN2A/B homozygous deletion in isocitrate dehydrogenase-mutant astrocytoma. Neuro Oncol. 2023;25:28–36. doi: 10.1093/neuonc/noac205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franceschi E, De Biase D, Di Nunno V, Pession A, Tosoni A, Gatto L, Tallini G, Visani M, Lodi R, Bartolini S, Brandes AA. IDH1 non-canonical mutations and survival in patients with glioma. Diagnostics (Basel) 2021;11:342. doi: 10.3390/diagnostics11020342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franceschi E, Tosoni A, Bartolini S, Minichillo S, Mura A, Asioli S, Bartolini D, Gardiman M, Gessi M, Ghimenton C, Giangaspero F, Lanza G, Marucci G, Novello M, Silini EM, Zunarelli E, Paccapelo A, Brandes AA. Histopathological grading affects survival in patients with IDH-mutant grade II and grade III diffuse gliomas. Eur J Cancer. 2020;137:10–17. doi: 10.1016/j.ejca.2020.06.018. [DOI] [PubMed] [Google Scholar]
- 19.Gramatzki D, Kickingereder P, Hentschel B, Felsberg J, Herrlinger U, Schackert G, Tonn J-C, Westphal M, Sabel M, Schlegel U, Wick W, Pietsch T, Reifenberger G, Loeffler M, Bendszus M, Weller M. Limited role for extended maintenance temozolomide for newly diagnosed glioblastoma. Neurology. 2017;88:1422–1430. doi: 10.1212/WNL.0000000000003809. [DOI] [PubMed] [Google Scholar]
- 20.Hartmann C, Hentschel B, Simon M, Westphal M, Schackert G, Tonn JC, Loeffler M, Reifenberger G, Pietsch T, von Deimling A, Weller M, Network GG. Long-term survival in primary glioblastoma with versus without isocitrate dehydrogenase mutations. Clin Cancer Res. 2013;19:5146–5157. doi: 10.1158/1078-0432.CCR-13-0017. [DOI] [PubMed] [Google Scholar]
- 21.Hartmann C, Hentschel B, Tatagiba M, Schramm J, Schnell O, Seidel C, Stein R, Reifenberger G, Pietsch T, von Deimling A, Loeffler M, Weller M, Network GG. Molecular markers in low-grade gliomas: predictive or prognostic? Clin Cancer Res. 2011;17:4588–4599. doi: 10.1158/1078-0432.CCR-10-3194. [DOI] [PubMed] [Google Scholar]
- 22.Hartmann C, Hentschel B, Wick W, Capper D, Felsberg J, Simon M, Westphal M, Schackert G, Meyermann R, Pietsch T, Reifenberger G, Weller M, Loeffler M, von Deimling A. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol. 2010;120:707–718. doi: 10.1007/s00401-010-0781-z. [DOI] [PubMed] [Google Scholar]
- 23.Ichimura K, Schmidt EE, Goike HM, Collins VP. Human glioblastomas with no alterations of the CDKN2A (p16INK4A, MTS1) and CDK4 genes have frequent mutations of the retinoblastoma gene. Oncogene. 1996;13:1065–1072. [PubMed] [Google Scholar]
- 24.Intergroup Radiation Therapy Oncology Group Trial 9402, Cairncross G, Berkey B, Shaw E, Jenkins R, Scheithauer B, Brachman D, Buckner J, Fink K, Souhami L, Laperierre N, Mehta M, Curran W Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol. 2006;24:2707–2714. doi: 10.1200/JCO.2005.04.3414. [DOI] [PubMed] [Google Scholar]
- 25.Kinslow CJ, Mercurio A, Kumar P, Rae AI, Siegelin MD, Grinband J, Taparra K, Upadhyayula PS, McKhann GM, Sisti MB, Bruce JN, Canoll PD, Iwamoto FM, Kachnic LA, Yu JB, Cheng SK, Wang TJC. Association of MGMT promotor methylation with survival in low-grade and anaplastic gliomas after alkylating chemotherapy. JAMA Oncol. 2023 doi: 10.1001/jamaoncol.2023.0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korshunov A, Casalini B, Chavez L, Hielscher T, Sill M, Ryzhova M, Sharma T, Schrimpf D, Stichel D, Capper D, Reuss DE, Sturm D, Absalyamova O, Golanov A, Lambo S, Bewerunge-Hudler M, Lichter P, Herold-Mende C, Wick W, Pfister SM, Kool M, Jones DTW, von Deimling A, Sahm F. Integrated molecular characterization of IDH-mutant glioblastomas. Neuropathol Appl Neurobiol. 2019;45:108–118. doi: 10.1111/nan.12523. [DOI] [PubMed] [Google Scholar]
- 27.Kreth F-W, Thon N, Simon M, Westphal M, Schackert G, Nikkhah G, Hentschel B, Reifenberger G, Pietsch T, Weller M, Tonn J-C, Network GG. Gross total but not incomplete resection of glioblastoma prolongs survival in the era of radiochemotherapy. Ann Oncol. 2013;24:3117–3123. doi: 10.1093/annonc/mdt388. [DOI] [PubMed] [Google Scholar]
- 28.Kros JM, Rushing E, Uwimana AL, Hernández-Laín A, Michotte A, Al-Hussaini M, Bielle F, Mawrin C, Marucci G, Tesileanu CMS, Stupp R, Baumert B, van den Bent M, French PJ, Gorlia T (2022) Mitotic count is prognostic in IDH-mutant astrocytoma without homozygous deletion of CDKN2A/B. Results of consensus panel review of EORTC trials 26053 and EORTC 22033–26033. Neuro Oncol noac282. 10.1093/neuonc/noac282 [DOI] [PMC free article] [PubMed]
- 29.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 30.Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malta TM, de Souza CF, Sabedot TS, Silva TC, Mosella MS, Kalkanis SN, Snyder J, Castro AVB, Noushmehr H. Glioma CpG island methylator phenotype (G-CIMP): biological and clinical implications. Neuro Oncol. 2018;20:608–620. doi: 10.1093/neuonc/nox183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mellinghoff IK, Van Den Bent MJ, Blumenthal DT, Touat M, Peters KB, Clarke J, Mendez J, Yust-Katz S, Welsh L, Mason WP, Ducray F, Umemura Y, Nabors B, Holdhoff M, Hottinger AF, Arakawa Y, Sepulveda JM, Wick W, Soffietti R, Perry JR, Giglio P, De La Fuente M, Maher EA, Schoenfeld S, Zhao D, Pandya SS, Steelman L, Hassan I, Wen PY, Cloughesy TF (2023) Vorasidenib in IDH1- or IDH2-Mutant Low-Grade Glioma. N Engl J Med NEJMoa2304194. 10.1056/NEJMoa2304194 [DOI] [PMC free article] [PubMed]
- 33.Ohka F, Natsume A, Motomura K, Kishida Y, Kondo Y, Abe T, Nakasu Y, Namba H, Wakai K, Fukui T, Momota H, Iwami K, Kinjo S, Ito M, Fujii M, Wakabayashi T. The global DNA methylation surrogate LINE-1 methylation is correlated with MGMT promoter methylation and is a better prognostic factor for glioma. PLoS ONE. 2011;6:e23332. doi: 10.1371/journal.pone.0023332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olar A, Wani KM, Alfaro-Munoz KD, Heathcock LE, van Thuijl HF, Gilbert MR, Armstrong TS, Sulman EP, Cahill DP, Vera-Bolanos E, Yuan Y, Reijneveld JC, Ylstra B, Wesseling P, Aldape KD. IDH mutation status and role of WHO grade and mitotic index in overall survival in grade II-III diffuse gliomas. Acta Neuropathol. 2015;129:585–596. doi: 10.1007/s00401-015-1398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poetsch L, Bronnimann C, Loiseau H, Frénel JS, Siegfried A, Seizeur R, Gauchotte G, Cappellen D, Carpentier C, Figarella-Branger D, Eimer S, Meyronet D, Ducray F, POLA network Characteristics of IDH-mutant gliomas with non-canonical IDH mutation. J Neurooncol. 2021;151:279–286. doi: 10.1007/s11060-020-03662-x. [DOI] [PubMed] [Google Scholar]
- 36.Reifenberger G, Hentschel B, Felsberg J, Schackert G, Simon M, Schnell O, Westphal M, Wick W, Pietsch T, Loeffler M, Weller M, Network GG. Predictive impact of MGMT promoter methylation in glioblastoma of the elderly. Int J Cancer. 2012;131:1342–1350. doi: 10.1002/ijc.27385. [DOI] [PubMed] [Google Scholar]
- 37.Reifenberger G, Weber RG, Riehmer V, Kaulich K, Willscher E, Wirth H, Gietzelt J, Hentschel B, Westphal M, Simon M, Schackert G, Schramm J, Matschke J, Sabel MC, Gramatzki D, Felsberg J, Hartmann C, Steinbach JP, Schlegel U, Wick W, Radlwimmer B, Pietsch T, Tonn JC, von Deimling A, Binder H, Weller M, Loeffler M, Network GG. Molecular characterization of long-term survivors of glioblastoma using genome- and transcriptome-wide profiling. Int J Cancer. 2014;135:1822–1831. doi: 10.1002/ijc.28836. [DOI] [PubMed] [Google Scholar]
- 38.Reuss DE, Mamatjan Y, Schrimpf D, Capper D, Hovestadt V, Kratz A, Sahm F, Koelsche C, Korshunov A, Olar A, Hartmann C, Reijneveld JC, Wesseling P, Unterberg A, Platten M, Wick W, Herold-Mende C, Aldape K, von Deimling A. IDH mutant diffuse and anaplastic astrocytomas have similar age at presentation and little difference in survival: a grading problem for WHO. Acta Neuropathol. 2015;129:867–873. doi: 10.1007/s00401-015-1438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schlömer S, Felsberg J, Pertz M, Hentschel B, Löffler M, Schackert G, Krex D, Juratli T, Tonn JC, Schnell O, Vatter H, Simon M, Westphal M, Martens T, Sabel M, Bendszus M, Dörner N, Fliessbach K, Hoppe C, Reifenberger G, Weller M, Schlegel U, Network FTGG. Mid-term treatment-related cognitive sequelae in glioma patients. J Neurooncol. 2022;159:65–79. doi: 10.1007/s11060-022-04044-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seystahl K, Hentschel B, Loew S, Gramatzki D, Felsberg J, Herrlinger U, Westphal M, Schackert G, Thon N, Tatagiba M, Pietsch T, Reifenberger G, Löffler M, Wick W, Weller M, Network GG. Bevacizumab versus alkylating chemotherapy in recurrent glioblastoma. J Cancer Res Clin Oncol. 2020;146:659–670. doi: 10.1007/s00432-019-03086-9. [DOI] [PubMed] [Google Scholar]
- 41.Shirahata M, Ono T, Stichel D, Schrimpf D, Reuss DE, Sahm F, Koelsche C, Wefers A, Reinhardt A, Huang K, Sievers P, Shimizu H, Nanjo H, Kobayashi Y, Miyake Y, Suzuki T, Adachi J-I, Mishima K, Sasaki A, Nishikawa R, Bewerunge-Hudler M, Ryzhova M, Absalyamova O, Golanov A, Sinn P, Platten M, Jungk C, Winkler F, Wick A, Hänggi D, Unterberg A, Pfister SM, Jones DTW, van den Bent M, Hegi M, French P, Baumert BG, Stupp R, Gorlia T, Weller M, Capper D, Korshunov A, Herold-Mende C, Wick W, Louis DN, von Deimling A. Novel, improved grading system(s) for IDH-mutant astrocytic gliomas. Acta Neuropathol. 2018;136:153–166. doi: 10.1007/s00401-018-1849-4. [DOI] [PubMed] [Google Scholar]
- 42.Tesileanu CMS, van den Bent MJ, Sanson M, Wick W, Brandes AA, Clement PM, Erridge SC, Vogelbaum MA, Nowak AK, Baurain JF, Mason WP, Wheeler H, Chinot OL, Gill S, Griffin M, Rogers L, Taal W, Rudà R, Weller M, McBain C, van Linde ME, Sabedot TS, Hoogstrate Y, von Deimling A, de Heer I, van IJcken WFJ, Brouwer RWW, Aldape K, Jenkins RB, Dubbink HJ, Kros JM, Wesseling P, Cheung KJ, Golfinopoulos V, Baumert BG, Gorlia T, Noushmehr H, French PJ. Prognostic significance of genome-wide DNA methylation profiles within the randomized, phase 3, EORTC CATNON trial on non-1p/19q deleted anaplastic glioma. Neuro Oncol. 2021;23:1547–1559. doi: 10.1093/neuonc/noab088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tesileanu CMS, Vallentgoed WR, French PJ, van den Bent MJ. Molecular markers related to patient outcome in patients with IDH-mutant astrocytomas grade 2 to 4: A systematic review. Eur J Cancer. 2022;175:214–223. doi: 10.1016/j.ejca.2022.08.016. [DOI] [PubMed] [Google Scholar]
- 44.Tesileanu CMS, Vallentgoed WR, Sanson M, Taal W, Clement PM, Wick W, Brandes AA, Baurain JF, Chinot OL, Wheeler H, Gill S, Griffin M, Rogers L, Rudà R, Weller M, McBain C, Reijneveld J, Enting RH, Caparrotti F, Lesimple T, Clenton S, Gijtenbeek A, Lim E, de Vos F, Mulholland PJ, Taphoorn MJB, de Heer I, Hoogstrate Y, de Wit M, Boggiani L, Venneker S, Oosting J, Bovée JVMG, Erridge S, Vogelbaum MA, Nowak AK, Mason WP, Kros JM, Wesseling P, Aldape K, Jenkins RB, Dubbink HJ, Baumert B, Golfinopoulos V, Gorlia T, van den Bent M, French PJ. Non-IDH1-R132H IDH1/2 mutations are associated with increased DNA methylation and improved survival in astrocytomas, compared to IDH1-R132H mutations. Acta Neuropathol. 2021;141:945–957. doi: 10.1007/s00401-021-02291-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tran S, Thomas A, Aliouat I, Karachi C, Lozano F, Mokhtari K, Dehais C, Feuvret L, Carpentier C, Giry M, Doukani A, Lerond J, Marie Y, Sanson M, Idbaih A, Carpentier A, Hoang-Xuan K, Touat M, Capelle L, Bielle F. A threshold for mitotic activity and post-surgical residual volume defines distinct prognostic groups for astrocytoma IDH-mutant. Neuropathol Appl Neurobiol. 2023;49:e12928. doi: 10.1111/nan.12928. [DOI] [PubMed] [Google Scholar]
- 46.Tzaridis T, Schäfer N, Weller J, Steinbach J-P, Schlegel U, Seidel S, Sabel M, Hau P, Seidel C, Krex D, Goldbrunner R, Tonn J-C, Grauer O, Kebir S, Schneider M, Schaub C, Vatter H, Coch C, Glas M, Fimmers R, Pietsch T, Reifenberger G, Herrlinger U, Felsberg J. MGMT promoter methylation analysis for allocating combined CCNU/TMZ chemotherapy: lessons learned from the CeTeG/NOA-09 trial. Int J Cancer. 2021;148:1695–1707. doi: 10.1002/ijc.33363. [DOI] [PubMed] [Google Scholar]
- 47.Weller M, van den Bent M, Preusser M, Le Rhun E, Tonn JC, Minniti G, Bendszus M, Balana C, Chinot O, Dirven L, French P, Hegi ME, Jakola AS, Platten M, Roth P, Rudà R, Short S, Smits M, Taphoorn MJB, von Deimling A, Westphal M, Soffietti R, Reifenberger G, Wick W. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021;18:170–186. doi: 10.1038/s41571-020-00447-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weller M, Berger H, Hartmann C, Schramm J, Westphal M, Simon M, Goldbrunner R, Krex D, Steinbach JP, Ostertag CB, Loeffler M, Pietsch T, von Deimling A, Network GG. Combined 1p/19q loss in oligodendroglial tumors: predictive or prognostic biomarker? Clin Cancer Res. 2007;13:6933–6937. doi: 10.1158/1078-0432.CCR-07-0573. [DOI] [PubMed] [Google Scholar]
- 49.Weller M, Felsberg J, Hartmann C, Berger H, Steinbach JP, Schramm J, Westphal M, Schackert G, Simon M, Tonn JC, Heese O, Krex D, Nikkhah G, Pietsch T, Wiestler O, Reifenberger G, von Deimling A, Loeffler M. Molecular predictors of progression-free and overall survival in patients with newly diagnosed glioblastoma: a prospective translational study of the German Glioma Network. J Clin Oncol. 2009;27:5743–5750. doi: 10.1200/JCO.2009.23.0805. [DOI] [PubMed] [Google Scholar]
- 50.Weller M, Kaulich K, Hentschel B, Felsberg J, Gramatzki D, Pietsch T, Simon M, Westphal M, Schackert G, Tonn JC, von Deimling A, Davis T, Weiss WA, Loeffler M, Reifenberger G, Network GG. Assessment and prognostic significance of the epidermal growth factor receptor vIII mutation in glioblastoma patients treated with concurrent and adjuvant temozolomide radiochemotherapy. Int J Cancer. 2014;134:2437–2447. doi: 10.1002/ijc.28576. [DOI] [PubMed] [Google Scholar]
- 51.Weller M, Weber RG, Willscher E, Riehmer V, Hentschel B, Kreuz M, Felsberg J, Beyer U, Löffler-Wirth H, Kaulich K, Steinbach JP, Hartmann C, Gramatzki D, Schramm J, Westphal M, Schackert G, Simon M, Martens T, Boström J, Hagel C, Sabel M, Krex D, Tonn JC, Wick W, Noell S, Schlegel U, Radlwimmer B, Pietsch T, Loeffler M, von Deimling A, Binder H, Reifenberger G. Molecular classification of diffuse cerebral WHO grade II/III gliomas using genome- and transcriptome-wide profiling improves stratification of prognostically distinct patient groups. Acta Neuropathol. 2015;129:679–693. doi: 10.1007/s00401-015-1409-0. [DOI] [PubMed] [Google Scholar]
- 52.Wick W, Hartmann C, Engel C, Stoffels M, Felsberg J, Stockhammer F, Sabel MC, Koeppen S, Ketter R, Meyermann R, Rapp M, Meisner C, Kortmann RD, Pietsch T, Wiestler OD, Ernemann U, Bamberg M, Reifenberger G, von Deimling A, Weller M. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27:5874–5880. doi: 10.1200/JCO.2009.23.6497. [DOI] [PubMed] [Google Scholar]
- 53.Wick W, Meisner C, Hentschel B, Platten M, Schilling A, Wiestler B, Sabel MC, Koeppen S, Ketter R, Weiler M, Tabatabai G, von Deimling A, Gramatzki D, Westphal M, Schackert G, Loeffler M, Simon M, Reifenberger G, Weller M. Prognostic or predictive value of MGMT promoter methylation in gliomas depends on IDH1 mutation. Neurology. 2013;81:1515–1522. doi: 10.1212/WNL.0b013e3182a95680. [DOI] [PubMed] [Google Scholar]
- 54.Wick W, Weller M, van den Bent M, Sanson M, Weiler M, von Deimling A, Plass C, Hegi M, Platten M, Reifenberger G. MGMT testing–the challenges for biomarker-based glioma treatment. Nat Rev Neurol. 2014;10:372–385. doi: 10.1038/nrneurol.2014.100. [DOI] [PubMed] [Google Scholar]
- 55.Wolter M, Felsberg J, Malzkorn B, Kaulich K, Reifenberger G. Droplet digital PCR-based analyses for robust, rapid, and sensitive molecular diagnostics of gliomas. Acta Neuropathol Commun. 2022;10:42. doi: 10.1186/s40478-022-01335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong QH-W, Li KK-W, Wang W-W, Malta TM, Noushmehr H, Grabovska Y, Jones C, Chan AK-Y, Kwan JS-H, Huang QJ-Q, Wong GC-H, Li W-C, Liu X-Z, Chen H, Chan DT-M, Mao Y, Zhang Z-Y, Shi Z-F, Ng H-K. Molecular landscape of IDH-mutant primary astrocytoma Grade IV/glioblastomas. Mod Pathol. 2021;34:1245–1260. doi: 10.1038/s41379-021-00778-x. [DOI] [PubMed] [Google Scholar]
- 57.Yang AS, Estécio MRH, Doshi K, Kondo Y, Tajara EH, Issa J-PJ. A simple method for estimating global DNA methylation using bisulfite PCR of repetitive DNA elements. Nucleic Acids Res. 2004;32:e38. doi: 10.1093/nar/gnh032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zacher A, Kaulich K, Stepanow S, Wolter M, Köhrer K, Felsberg J, Malzkorn B, Reifenberger G. Molecular diagnostics of gliomas using next generation sequencing of a glioma-tailored gene panel. Brain Pathol. 2017;27:146–159. doi: 10.1111/bpa.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zheng S, Houseman EA, Morrison Z, Wrensch MR, Patoka JS, Ramos C, Haas-Kogan DA, McBride S, Marsit CJ, Christensen BC, Nelson HH, Stokoe D, Wiemels JL, Chang SM, Prados MD, Tihan T, Vandenberg SR, Kelsey KT, Berger MS, Wiencke JK. DNA hypermethylation profiles associated with glioma subtypes and EZH2 and IGFBP2 mRNA expression. Neuro Oncol. 2011;13:280–289. doi: 10.1093/neuonc/noq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data may be shared upon appropriate request of qualified investigators for purposes of replicating procedures and results.