Abstract
Background
In the American population, the relationship between the triglyceride-glucose (TyG) index and TYG combined with indicators of obesity and cardiovascular disease (CVD) and its mortality has been less well studied.
Methods
This cross-sectional study included 11,937 adults from the National Health and Nutrition Examination Survey (NHANES) 2003–2018. Cox proportional hazards model, binary logistic regression analyses, restricted cubic spline (RCS), and receiver operating characteristic (ROC) were used to analyze the relationship between TyG and its combined obesity-related indicators and CVD and its mortality. Mediation analysis explored the mediating role of glycated hemoglobin and insulin in the above relationships.
Results
In this study, except for no significant association between TyG and CVD mortality, TyG, TyG-WC, TyG-WHtR, and TyG-BMI were significantly and positively associated with CVD and CVD mortality. TyG-WHtR is the strongest predictor of CVD mortality (HR 1.66, 95% CI 1.21–2.29). The TyG index correlated better with the risk of coronary heart disease (OR 2.52, 95% CI 1.66–3.83). TyG-WC correlated best with total CVD (OR 2.37, 95% CI 1.77–3.17), congestive heart failure (OR 2.14, 95% CI 1.31–3.51), and angina pectoris (OR 2.38, 95% CI 1.43–3.97). TyG-WHtR correlated best with myocardial infarction (OR 2.24, 95% CI 1.45–3.44). RCS analyses showed that most of the above relationships were linear (P-overall < 0.0001, P-nonlinear > 0.05). Otherwise, ROC curves showed that TyG-WHtR and TyG-WC had more robust diagnostic efficacy than TyG. In mediation analyses, glycated hemoglobin mediated in all the above relationships and insulin-mediated in partial relationships.
Conclusions
TyG-WC and TyG-WtHR enhance CVD mortality prediction, diagnostic efficacy of CVD and its mortality, and correlation with some CVD over and above the current hottest TyG. TyG-WC and TyG-WtHR are expected to become more effective metrics for identifying populations at early risk of cardiovascular disease and improve risk stratification.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-023-02115-9.
Keywords: Triglyceride glucose (TyG), Triglyceride glucose-waist circumference (TyG-WC), Triglyceride glucose-waist height ratio (TyG-WHtR), Triglyceride glucose-body mass index (TyG-BMI), Cardiovascular disease (CVD) mortality, Cardiovascular disease, National Health and Nutrition Examination Survey (NHANES)
Introduction
Cardiovascular disease (CVD) is the leading cause of death and years of healthy life lost in humans, the leading cause of the global disease burden, and a significant contributor to lost health and high healthcare cost [1, 2]. Identifying factors that predict CVD risk is essential to promote early disease prevention.
The Triglyceride-Glucose (TyG) index is a measure of insulin resistance that assesses the body's insulin sensitivity by combining two biomarkers, triglyceride and fasting blood glucose [3, 4]. Insulin resistance is a state in which the body’s sensitivity and response to insulin are reduced, resulting in the inability of insulin to efficiently transport glucose into cells, causing metabolic abnormalities such as hyperglycemia [5]. Insulin resistance is considered a significant risk factor for several metabolic diseases such as type 2 diabetes [6], obesity [7], cardiovascular disease [8–10], and so on. The TyG index is calculated using the following formula: TyG = Ln (triacylglycerol (mg/dL) × fasting blood glucose (mg/dL)/2). Recent studies have shown that the TyG index not only predicts the risk and severity of cardiovascular disease but is also associated with the prognosis of cardiovascular disease [11–14]. However, studies on TyG and cardiovascular disease have focused on European and Asian populations, and there are relatively few studies on TyG and cardiovascular disease and CVD mortality in the American population.
Obesity is prevalent worldwide and is closely associated with various health risks, such as poor glucose tolerance, insulin resistance, and metabolic disorders [15–17], which can lead to the onset progression and prognosis of cardiovascular disease [18–20]. TYG combined with obesity indices is closely associated with insulin resistance [21, 22], metabolic syndrome [23], uric acid [24], diabetes mellitus [25], and fatty liver [26]. Some studies have shown that TyG combined with adiposity indices is better than the TyG index for assessing [27]. However, these studies on TyG combined with obesity indices and cardiovascular disease were few and mainly focused on Asian and European populations [28]. The relationship between TyG combined with obesity indices and cardiovascular disease and CVD mortality in the American population is unclear. Our study used the National Health and Nutrition Examination Survey database to analyse the association of the TyG index and its combination of obesity indices with CVD mortality, total CVD, congestive heart failure, myocardial infarction, angina pectoris, and coronary heart disease.
Materials and methods
Data source and study population
This cross-sectional study included 11,937 adult participants from the National Health and Nutrition Examination Survey (NHANES) 2003–2018. The study procedure is illustrated in Fig. 1. Exclusion criteria comprised the following: (1) dietary energy intake below 800 or over 4200 kcal/day for male and below 500 or more than 3500 kcal/day for female [29]; (2) participants with missing triglyceride-glucose index and its combinations with indicators of obesity; (3) participants lacking the outcome or covariates. The NCHS Research Ethics Review Board approved the NHANES study protocol, and all participants provided written informed consent.
Fig. 1.
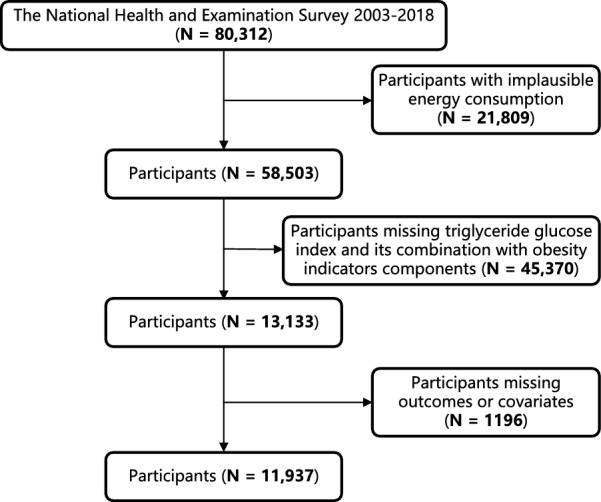
Flowchart depicting the participants’ selection
Definitions of TyG, TyG-WC, TyG-WHtR, and TyG-BMI
The TyG index quantifies insulin resistance by combining fasting glucose with triglyceride levels. Fasting blood glucose (FSG) and triglycerides were measured at baseline when the participants provided their blood samples. Body weight, height, and waist were obtained when people participated in the physical examinations at a mobile examination center. Furthermore, the body mass index along with the waist-to-height ratio were calculated. The participants were classified into four groups (Q1, Q2, Q3, Q4) by the quartiles of the TyG index, TyG-WC, TyG-WHtR, and TyG-BMI, respectively, and the Q1 group was used as the reference group.
TyG, TyG-WC, TyG-WHtR, and TyG-BMI were calculated according to the following formulas: (1) TyG = ln [triglycerides (mg/dl) × glucose (mg/dl)/2]; (2) BMI = body mass (kg)/height2(m2); (3) WHtR = waist circumference/height; (4) TyG-WC = TyG × waist circumference; TyG-WHtR = TyG × WHtR; TyG-BMI = TyG × BMI.
Total CVD, congestive heart failure, myocardium infarction, angina pectoris, and coronary heart disease ascertainment
The diagnosis of CVD was established by self-reported physician diagnoses obtained during an individual interview using a standardized medical condition questionnaire. The participants were asked, “Has a doctor or other health expert ever informed you that you have congestive heart failure/coronary heart disease/angina pectoris/myocardial infarction /stroke?” A person was regarded as having CVD if he or she replied “yes” to any of the above questions. Congestive heart failure, myocardial infarction, angina pectoris, and coronary heart disease are also defined according to the problems of the corresponding diseases mentioned above.
According to the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes, cardiovascular disease (I00-I99), congestive heart failure (I50.0, I50.1, I50.9), coronary artery disease (I20-I25.9), myocardial infarction (I21-I23), and angina pectoris (I20.0-I20.9), CVD mortality (I00–I09, I11, I13, I20–I51, or I60–I69).
Assessment of covariates
Age and year were considered as a continuous variable, gender was divided into two groups of males and females, race/ethnicity was divided into five sections of Mexican Hispanics, non-Mexican Hispanics, non-Hispanic whites, non-Hispanic blacks, and other, educational attainment was grouped according to the Questionnaire on Educational Attainment for Adults 20 Years of Age and Older (QEAA), household income was grouped according to annual household income greater or less than $100,000, and smoking was defined as never smoking (participants who smoked less than 100 cigarettes in their lifetime), smoking (participants who smoked more than 100 cigarettes in their lifetime), physical activity (yes/no), drinking was defined as never drinking (less than 12 drinks per year), drinking (at least 12 drinks per year) and use of dietary supplements (yes/no), total energy and AHEI were considered as continuous variables. Family history of heart disease, self-reported cancer, and self-reported diabetes was established by self-reported physician diagnoses obtained during an individual interview using a standardized medical condition questionnaire. The participants were asked: “Close relative had a heart attack?” or “Ever been told you had cancer or malignancy?” or “Doctor told you you had diabetes?” and answered by yes or no. Weight, height, blood pressure, and waist circumference were obtained when people attended a physical examination at a mobile health center and were considered continuous type variables. In addition, fasting blood glucose (FSG), insulin, HbA1c, triglycerides, and total cholesterol were measured at baseline when participants provided blood samples and were considered continuous type variables. The AHEI was developed from the original Healthy Eating Index, which included 11 food components identified through a comprehensive review of studies. More details on the measurement of covariates can be found on the NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm).
Statistical analysis
All statistical analyses were carried out by the CDC guidelines (https://wwwn.cdc.gov/nchs/nhanes/tutorials/default.aspx). The statistical analyses for this study incorporated sample weights, clustering, and stratification due to the complex multi-stage stratified probability survey design employed in NHANES. Mean values (95% CI) were used to express participant characteristics for continuous variables, while percentages (95% CI) were used for categorical variables. Participants' baseline characteristics were described according to the quartiles of the TyG index, TyG-WC, TyG-WHtR, and TyG-BMI, respectively. They were subjected to homogeneity of variance tests and further Bonferroni tests. The Cox proportional hazards model was used to estimate hazard ratios (HRs) and 95% CIs for the association between TyG index and TyG combined with obesity metrics and CVD mortality. Binary weighted logistic regression analyses assessed the association between the TyG index and TyG combined with obesity metrics and CVD. Correlation results were expressed as odds ratios (OR) and 95% confidence intervals (CI) in the four predefined models. In addition, linear trends between TyG, TyG-WC, TyG-WHtR, and TyG-BMI quartiles were assessed by the median value within each quartile as a continuous variable. Model 1 was adjusted for age, sex, race, and year. Model 2 adjusted for age, sex, race, year, smoking, alcohol use, exercise, education level, income, and family history of cardiovascular disease. Model 3 adjusted for the same variables as Model 2 and for energy intake, alternative healthy eating index, and nutritional supplements. Model 4 adjusted for the same variables as Model 3: SBP, cholesterol, self-reported cancer, and self-reported diabetes.
To account for the dose–response relationship (linear or nonlinear) between TyG index, TyG-WC, TyG-WHtR, and TyG-BMI and the cardiovascular disease mortality and cardiovascular disease, restricted triple spline analyses adjusted for the same variables as in model 4 were performed at the 5, 50, and 95th percentiles of the distributions of TyG, TyG-WC, TyG-WHtR, and TyG-BMI Three nodes were set to exclude the most extreme 5% values to reduce the potential impact of outliers. Non-linearity tests were performed using the likelihood ratio test. Receiver operating characteristic (ROC) curves were used for diagnostic value analysis, and the area under the curve, as measured by the C-statistic, was computed to quantify the predictive power of TyG, obesity-related indices (WC et al.), and their combination for cardiovascular disease and its mortality.
In addition, mediation analyses were used to investigate whether the relevance of TyG and its obesity composite index to cardiovascular disease could be explained by glycated hemoglobin and insulin after adjusting for factors in the primary analysis model 4. Stratified analyses were conducted to assess potential moderating effects of age (> 50/ ≤ 50), sex (male/female), race (non-Hispanic white/other), smoking (yes/no), physical activity (yes/no), alcohol consumption (yes/no), and diabetes (yes/no). The above assessments were conducted using r4.1.1 software, and statistical significance was determined using a two-sided p-value threshold of less than 0.05.
Results
Basic characteristics of participants according to the quartile of TyG, TyG-WC, TyG-WHtR, and TyG-BMI indicators
The characteristics of the participants according to quartiles of TyG, TyG-WC, TyG-WHtR, and TyG-BMI are shown in Table 1 and Additional file 3: Tables S1, S2, and S3. Participants with higher TyG index, TyG-WC, TyG-WHtR, and TyG-BMI were more likely to be male, older, to have a higher physical activity level, and to be higher levels of BMI, waist circumference, triglycerides, fasting glucose, glycated hemoglobin, systolic blood pressure, insulin, cholesterol, self-reported cancer, self-reported diabetes, cardiovascular diseases mortality, cardiovascular disease, congestive heart failure, myocardial infarction, angina pectoris, and coronary heart disease, and lower levels of educational strata, income, and AHEI.
Table 1.
Baseline characteristics according to triglyceride-glucose (TyG) quartiles: NHANES, 2003–2018a
| Triglyceride-glucose (TyG) (N = 11,937) | P | Ptest | ||||
|---|---|---|---|---|---|---|
| ≤ 8.22 | 8.23–8.62 | 8.63–9.06 | > 9.07 | |||
| N = 2986 | N = 2986 | N = 2983 | N = 2982 | |||
| Age, years | 42.33 (41.36, 43.29) | 47.00 (46.13, 47.86) | 50.08 (49.32, 50.85) | 51.91 (51.15, 52.66) | < 0.001 | < 0.001 |
| Male, % | 37.60 (35.70, 39.60) | 47.40 (45.20, 49.70) | 51.20 (49.20, 53.20) | 58.50 (56.30, 60.70) | < 0.001 | < 0.001 |
| Non-Hispanic white, % | 68.00 (64.80, 71.10) | 71.00 (67.80, 73.90) | 72.80 (69.70, 75.70) | 73.10 (69.60, 76.30) | < 0.001 | < 0.001 |
| BMI, kg/m2 | 25.95 (25.71, 26.20) | 27.95 (27.68, 28.22) | 29.54 (29.23, 29.86) | 31.46 (31.10, 31.81) | < 0.001 | < 0.001 |
| Smoke, % | 19.80 (17.80, 22.00) | 24.20 (21.80, 26.70) | 25.90 (23.80, 28.20) | 27.20 (25.30, 29.30) | < 0.001 | < 0.001 |
| Drink, % | 74.00 (71.30, 76.50) | 73.90 (71.60, 76.10) | 74.60 (72.10, 76.90) | 73.20 (70.90, 75.40) | < 0.001 | < 0.001 |
| Regular exercise, % | 32.90 (30.30, 35.70) | 42.20 (39.90, 44.60) | 43.60 (41.20, 46.00) | 50.90 (48.10, 53.70) | < 0.001 | < 0.001 |
| College graduate or above, % | 36.90 (34.10, 39.80) | 30.80 (27.60, 34.10) | 27.10 (24.30, 30.10) | 21.20 (18.80, 23.80) | < 0.001 | < 0.001 |
| > 100,000 annual household income, % | 19.60 (16.80, 22.60) | 16.50 (13.80, 19.60) | 13.80 (11.90, 15.90) | 10.40 (8.10, 13.30) | 0.078 | 0.336 |
| Dietary supplements use, % | 51.00 (48.90, 53.10) | 51.30 (48.90, 53.60) | 50.20 (48.00, 52.50) | 52.70 (50.10, 55.20) | < 0.001 | < 0.001 |
| Total energy, kcal/day | 2045 (2015, 2027) | 2090 (2057, 2124) | 2060 (2018, 2102) | 2111 (2075, 2148) | < 0.001 | < 0.001 |
| AHEI sore | 50.86 (50.31, 51.41) | 50.03 (49.47, 50.59) | 50.21 (49.72, 50.71) | 49.58 (49.06, 50.09) | < 0.001 | < 0.001 |
| Family history of heart disease, % | 13.00 (11.60, 14.70) | 16.70 (14.70, 18.90) | 17.70 (15.90, 19.60) | 18.80 (16.70, 21.10) | 0.078 | 0.033 |
| Triglyceride, mmol/L | 0.68 (0.67, 0.69) | 1.07 (1.06, 1.07) | 1.53 (1.52, 1.54) | 2.91 (2.82, 3.00) | < 0.001 | < 0.001 |
| Fasting glucose, mmol/L | 5.15 (5.12, 5.18) | 5.46 (5.43, 5.49) | 5.76 (5.71, 5.80) | 6.93 (6.80, 7.05) | < 0.001 | < 0.001 |
| Waist circumference, cm | 89.93 (89.33, 90.52) | 96.67 (95.87, 97.47) | 101.12 (100.46, 101.78) | 107.34 (106.49, 108.19) | < 0.001 | < 0.001 |
| Glycohemoglobin, % | 5.35 (5.34, 5.37) | 5.52 (5.50, 5.54) | 5.65 (5.63, 5.68) | 6.34 (6.28, 6.40) | < 0.001 | < 0.001 |
| Standing height, cm | 167.49 (167.14, 167.83) | 167.86 (167.50, 168.22) | 167.41 (167.04, 167.79) | 168.01 (167.64, 168.38) | < 0.001 | < 0.001 |
| Insulin, pmol/L | 49.54 (48.07, 51.01) | 65.07 (63.15, 67.00) | 81.76 (79.41, 84.11) | 118.02 (112.65, 123.39) | < 0.001 | < 0.001 |
| Systolic blood pressure, mmHg | 115.63 (114.88, 116.38) | 120.18 (119.44, 120.91) | 122.24 (121.52, 122.96) | 127.11 (126.20, 128.02) | < 0.001 | < 0.001 |
| Cholesterol, mmol/L | 4.67 (4.63, 4.72) | 5.00 (4.95, 5.05) | 5.21 (5.17, 5.26) | 5.50 (5.44, 5.55) | < 0.001 | < 0.001 |
| Self-reported cancer, % | 7.10 (5.90, 8.60) | 9.50 (8.00, 11.20) | 10.70 (9.40, 12.30) | 11.20 (10.00, 12.60) | < 0.001 | < 0.001 |
| Self-reported diabetes, % | 2.20 (1.60, 2.80) | 4.20 (3.50, 5.10) | 7.70 (6.70, 8.90) | 21.40 (19.10, 23.80) | < 0.001 | < 0.001 |
| Death of cardiovascular diseases, % | 2.00 (1.50, 2.60) | 3.40 (2.90, 4.10) | 4.20 (3.50, 4.90) | 6.60 (5.70, 7.80) | < 0.001 | < 0.001 |
| Cardiovascular diseases, % | 4.80 (4.10, 5.60) | 7.30 (6.20, 8.60) | 10.00 (8.70, 11.50) | 13.70 (12.10, 15.50) | < 0.001 | < 0.001 |
| Congestive heart failure, % | 1.20 (0.90, 1.70) | 1.80 (1.30, 2.40) | 2.60 (2.00, 3.40) | 4.40 (3.60, 5.30) | < 0.001 | < 0.001 |
| Myocardial infarction, % | 1.70 (1.30, 2.40) | 3.20 (2.40, 4.20) | 4.00 (3.40, 4.80) | 5.50 (4.50, 6.60) | < 0.001 | < 0.001 |
| Angina pectoris, % | 1.10 (0.80, 1.60) | 1.60 (1.10, 2.20) | 3.10 (2.40, 0.40) | 3.80 (0.30, 4.80) | < 0.001 | < 0.001 |
| Coronary heart disease, % | 1.70 (1.30, 2.40) | 2.80 (2.10, 3.60) | 4.40 (3.50, 5.50) | 6.30 (5.30, 7.50) | < 0.001 | < 0.001 |
aContinuous variables were listed as weighted mean (95% CI). Categorical variables were listed as weighted percentage (95% CI). After adjusting for age, general linear models and chi-square tests were conducted to compare continuous and categorical baseline characteristics, respectively. And Ptest was the result of Bonfreni correction
Relationship between TyG, TyG-WC, TyG-WHtR, TyG-BMI, and cardiovascular disease mortality, total CVD, congestive heart failure, myocardial infarction, angina pectoris, and coronary heart disease
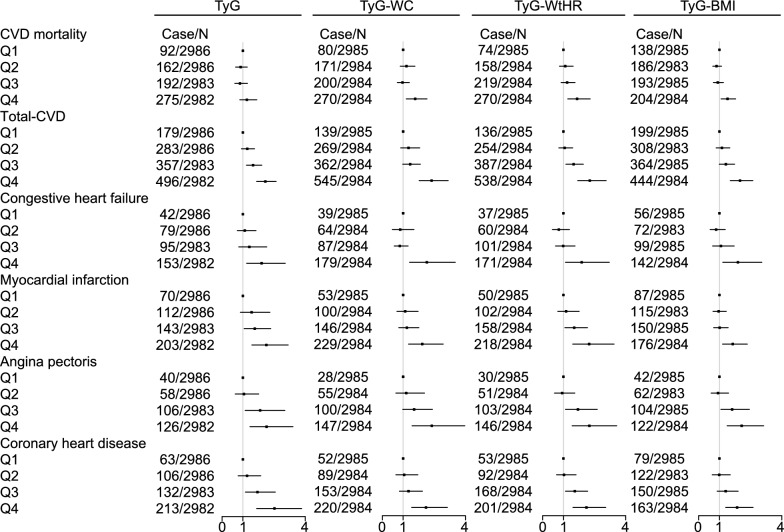
Figure 2 demonstrates the association of TyG and its combined obesity indicators with cardiovascular disease. Additional file 3: Tables S4–S9 provide detailed information on all associations. After adjustment for covariates, the results showed that except for no significant association between TyG and CVD mortality, TyG, TyG-WC, TyG-WHtR, and TyG-BMI were significantly and positively associated with CVD mortality, total cardiovascular disease, congestive heart failure, myocar-dial infarction, angina pectoris, and coronary heart disease (P trend < 0.05, Ptest < 0.05).
Fig. 2.
Forest plot of the TyG, TyG-WC, TyG-WHtR, and TyG-BMI association with CVD mortality, total CVD, congestive heart failure, myocardial infarction, angina pectoris, and coronary heart disease calculated using binomial logistic regression models/Cox proportional hazards model. The adjustments involved the covariables selected in the full binomial logistic regression model/Cox proportional hazards model. Case/N, the number of case subjects/total. Q quartile
For CVD mortality, TyG-WHtR had the highest predictive power (HR 1.66, 95% CI 1.21–2.29), followed by TyG-WC (HR 1.58, 95% CI 1.15–2.15). For total CVD, TyG-WC had the highest association (OR 2.37, 95% CI 1.77–3.17), followed by TyG-WHTR (OR 2.27, 95% CI 1.69–3.06). For congestive heart failure, TyG-WC had the highest association (OR 2.14, 95% CI 1.31–3.51), followed by TyG (OR 1.90, 95% CI 1.18–3.04). For myocardial infarction, TyG-WHTR had the highest association (OR 2.24, 95% CI 1.45–3.44), followed by TyG (OR 2.13, 95% CI 1.43–3.18). For angina pectoris, TyG-WC had the highest association (OR 2.38, 95% CI 1.43–3.97), followed by TyG-WHTR (OR 2.25, 95% CI 1.42–3.57). For coronary heart disease, TyG had the highest association (OR 2.52, 95% CI 1.66–3.83), followed by TyG-WHTR (OR 2.11, 95% CI 1.46–3.04).
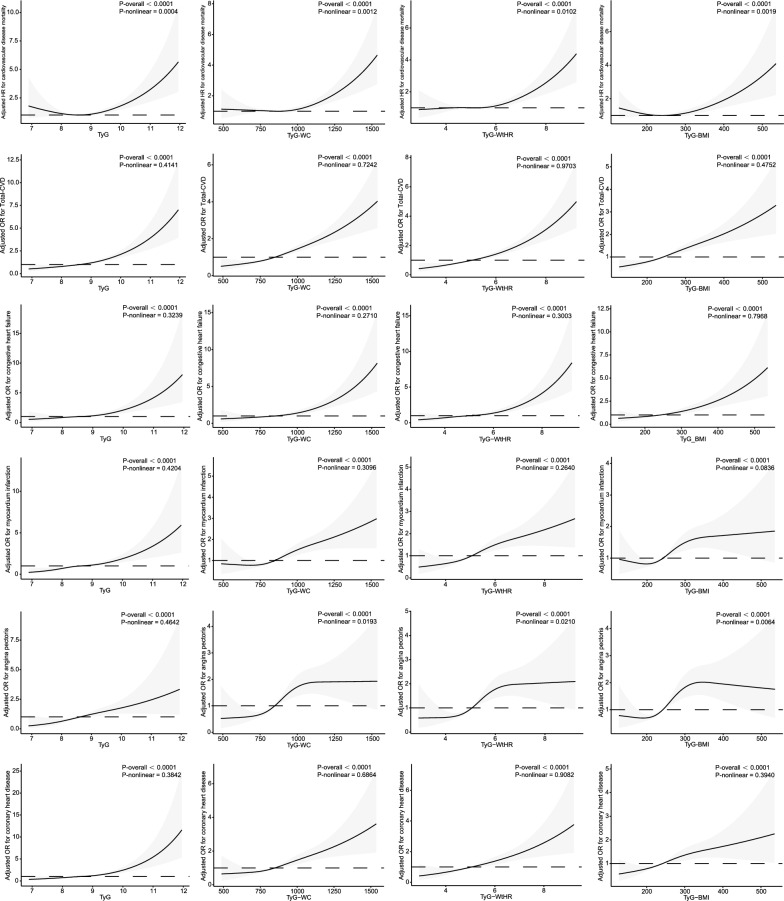
Restricted cubic splines (RCS) analysis investigating the relationship between TyG, TyG-WC, TyG-WHtR, and TyG-BMI and cardiovascular disease mortality, total CVD, congestive heart failure, myocardial infarction, angina pectoris, and coronary heart disease
In Fig. 3, We employed restricted cubic spline to flexibly model and visualize the associations between TyG, TyG-WC, TyG-WHtR, and TyG-BMI, and cardiovascular mortality, total cardiovascular disease, congestive heart failure, myocardial infarction, angina pectoris, and coronary heart disease. After adjusting for all covariates in the master analytical model 4 above, a linear correlation was observed between TyG and total CVD, congestive heart failure, myocardium infarction, angina pectoris, and coronary heart disease (P-overall < 0.0001, P-nonlinear > 0.05). Similarly, TyG-WC, TyG-WHtR, and TyG-BMI exhibited linear correlations with total CVD, myocardial infarction, and coronary heart disease (P-overall < 0.0001, P-nonlinear > 0.05). TyG, TyG-WC, TyG-WHtR, and TyG-BMI with CVD mortality exhibited nonlinear associations (P-overall < 0.0001 and P-nonlinear < 0.05), as well as TyG-WC, TyG-WHtR, and TyG-BMI with angina pectoris (P-overall < 0.0001 and P-nonlinear < 0.05).
Fig. 3.
Associations between TyG, TyG-WC, TyG-WHtR, and TyG-BMI with cardiovascular mortality, total CVD, congestive heart failure, myocardial infarction, angina pectoris, and coronary heart disease were evaluated by RCS after adjustment for the covariables. The solid black lines correspond to the central estimates, and the gray-shaded regions indicate the 95% confidence intervals
Mediation analysis of TyG, TyG-WC, TyG-WHtR, and TyG-BMI with CVD mortality, total CVD, congestive heart failure, myocardial infarction, angina pectoris, and coronary heart disease
Mediation analyses indicated that glycated hemoglobin partially mediated the association between TyG and its combined obesity indicators and CVD and CVD mortality (Additional file 1: Fig. S1). Glycated hemoglobin mediated a more significant proportion of indirect effects in the following relationships. For TyG, the proportion of indirect effects of glycated hemoglobin-mediated CVD mortality, total CVD, and congestive heart failure were 49.0, 41.6, and 42.2%. For the TyG-WHtR, the proportion of indirect effects of glycated hemoglobin-mediated coronary heart disease was 43.9%. For TyG-BMI, the ratios of indirect effects of glycated hemoglobin-mediated total CVD, myocardial infarction, and coronary heart disease were 42.3, 55.0, and 67.8%, respectively.
The proportions of indirect effects of insulin-mediated associations between TyG, TyG-WC, TyG-WHtR, and CVD mortality were − 11.2, − 25.1, and − 23.8%, respectively. For TyG, the proportions of indirect effects of insulin-mediated total CVD, congestive heart failure, and angina pectoris were 6.4, 10.2, and 10.6%, respectively. For TyG-BMI, the indirect effects of insulin-mediated total CVD and coronary heart disease were 11.4% and 29.5 (Additional file 1: Fig S1).
Receiver operating characteristic (ROC) curves of TyG, TyG-WC, TyG-WHtR, TyG-BMI, WC, WHtR, and BMI in relation to cardiovascular disease mortality, total CVD, congestive heart failure, myocardial infarction, angina pectoris, and coronary heart disease
The ROC curve showed that TyG-WHtR or TyG-WC had the highest diagnostic efficacy for CVD and its mortality, followed by WHtR or WC, TyG, TyG-BMI, and BMI (Additional file 2: Fig S2).
For CVD mortality, TyG-WHtR had the highest diagnostic efficacy (AUC: 0. 628, 95% CI 0.608–0.647), followed by TyG-WC (AUC: 0.614, 95% CI 0.595–0.634). For total CVD, TyG-WHtR had the highest diagnostic efficacy (AUC: 0. 655, 95% CI 0.640–0.670), followed by TyG-WC (AUC: 0.650, 95% CI 0.635–0.665). For congestive heart failure, TyG-WHtR had the highest diagnostic efficacy (AUC: 0.675, 95% CI 0.648–0.702), followed by TyG-WC (AUC: 0.669, 95% CI 0.641–0.696). For angina pectoris, TyG-WHtR had the highest diagnostic efficacy (AUC: 0.649, 95% CI 0.627–0.671), followed by TyG-WC (AUC: 0.648, 95% CI 0.625–0.670). For myocardial infarction, TyG-WHtR had the highest diagnostic efficacy (AUC: 0.665, 95% CI 0.639–0.692), followed by TyG-WC (AUC: 0.663, 95% CI 0.636–0.690). For coronary heart disease, TyG-WC had the highest diagnostic efficacy (AUC: 0.654, 95% CI 0.632–0.677), followed by TyG-WHtR (AUC: 0.642, 95% CI 0.620–0.664).
Stratification of TyG, TyG-WC, TyG-WHtR, and TyG-BMI in relation to CVDmortality, total CVD, congestive heart failure, myocardial infarction, angina pectoris, and coronary heart disease
After controlling for variables, segmented analyses according to age, sex, race, exercise, smoking, alcohol consumption and diabetes (Additional file 3: Tables S10–S31) identified that significant correlation between TyG, TyG-WC, TyG-WHtR, and TyG-BMI with total CVD, congestive heart failure, myocardial infarction, angina pectoris, and coronary heart disease were more regularly observed within individuals who were ≤ 50 years of age, male, did not smoke, and no diabetes. Significant associations between TyG, TyG-WC, TyG-WHtR, and TyG-BMI and CVD mortality were more likely to occur among individuals aged ≤ 50 years, women, drinkers, and no diabetes.
Discussion
Our primary discoveries revealed that except for no significant association between TyG and CVD mortality, TyG, TyG-WC, TyG-WHtR, and TyG-BMI were significantly and positively associated with CVD mortality, total cardiovascular disease, congestive heart failure, myocardial infarction, angina pectoris, and coronary heart disease. Among the above indicators, TyG-WHtR had the best predictive ability for CVD mortality and the best correlation for myocardial infarction. TyG-WC had the highest correlation with total cardiovascular disease, congestive heart failure, and angina pectoris. TyG had the highest correlation with coronary heart disease. (2) TyG-WHtR and TyG-WC had more robust diagnostic efficacy than TyG for CVD and CVD mortality. (3) These associations were mainly mediated by hemoglobin. (4) Associations between TyG, TyG-WC, TyG-WHtR, and TyG-BMI and CVD were found to be more prevalent among individuals who were ≤ 50 years of age, male, did not smoke and no diabetes. Significant associations be-tween TyG, TyG-WC, TyG-WHtR, and TyG-BMI and CVD mortality were more likely to occur among individuals aged ≤ 50 years, women, drinkers, and no diabetes.
TyG, a biomarker derived from fasting glucose and triglyceride levels, has gained popularity as a substitute for insulin resistance owing to its user-friendly calculation and high degree of sensitivity and specificity [30–32]. Our research demonstrates a signifcant and positive connection between TyG and total CVD, congestive heart failure, myocardial infarction, angina pectoris, and coronary heart disease in the United States population, consistent with previous studies [33, 34]. However, current research on TyG and cardiovascular disease has centered on European and Asian populations, with a limited number of studies conducted in the United States [35]. Following the adjustment of nutritional data covariates such as total energy intake, AHEI, and dietary supplements, our investigation determined that TyG showed a significant and affirmative correlation with total CVD, congestive heart failure, myocardial infarction, angina pectoris, and coronary heart disease.
Our study is the inaugural investigation into the correlation between TyG combined with obesity indicators and CVD and CVD mortality in an American population. The findings of our study reveal that except for no significant association between TyG and CVD mortality, TyG, TyG-WC, TyG-WHtR, and TyG-BMI were significantly and positively associated with CVD mortality, total cardiovascular disease, congestive heart failure, myocar-dial infarction, angina pectoris, and coronary heart disease. RCS analyses showed that most of the above relationships were linear, but TyG-WC, TyG-WHtR, and TyG-BMI were non-linearly associated with cardiovascular mortality. This is consistent with previous reports [36]. In a prospective cohort study from the United States population, the TyG index was associated with mortality due to all-cause and cardiovascular disease non-linearly. In Model 3, the positive effect sizes become non-significant with cardiovascular death (HR, 1.37; 95% CI 0.78–2.42) [37]. It has been suggested that excessively high or low TyG, TyG-WC, and TyG-BMI indices may lead to an increased risk of cardiovascular death, which may be related to excessively high or low insulin resistance, inflammatory response, oxidative stress vascular endothelial function, which in turn may lead to an increased risk of cardiovascular death [38, 39]. TyG-WC, TyG-WHtR, and TyG-BMI were non-linearly associated with angina pectoris. The RCS curves do not represent the dispersion of the data. They may be related to the small number of angina patients, the disease's complexity, the angina data's multidimensionality, the setting of the parameters of the RCS curves, and the choice of their models.
Our research found that TyG-WHtR had the best predictive ability for CVD mortality and the best correlation for myocardial infarction. TyG-WC had the highest correlation with total cardiovascular disease, congestive heart failure, and angina pectoris. TyG had the highest correlation with coronary heart disease. The ROC curves showed that TyG-WHtR had the best diagnostic efficacy for cardiovascular disease mortality, total CVD, congestive heart failure, angina pectoris, and myocardial infarction. TyG-WC had the best diagnostic efficacy for coronary heart disease. The diagnostic efficacy of TyG-WC and TyG-WHtR for CVD and CVD mortality was higher than that of TyG, WC, and WHtR. TyG-BMI was more effective than BMI in diagnosing CVD and CVD mortality. The above results suggest that the correlation and diagnostic efficacy of TyG-WC and TyG-WHtR with cardiovascular diseases and their deaths are, to some extent, superior to the TyG index. A study involving 1145 participants from Korea [40], the results also showed that TyG-WC had better diagnostic efficacy for the progression of coronary artery calcification than TyG and TyG-BMI. Among the possible reasons, obesity can contribute directly to the development of cardiovascular risk factors, including dyslipidemia, type 2 diabetes, hypertension, and sleep disorders [41]. Obesity may also contribute to the development of cardiovascular disease and death from cardiovascular disease independently of other cardiovascular risk factors, especially the location of body fat distribution [41, 42]. Furthermore, integrating TyG with obesity indices presents a more accurate insulin resistance evaluation than the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) or TyG index alone [3, 21, 22, 27]. Our results provide significant proof for the literature on TyG, TyG-BMI, TyG-WC, and TyG-WHtR as predictors of cardiovascular disease risk.
Our research demonstrates that except for no significant association between TyG and CVD mortality, TyG, TyG-WC, TyG-WHtR, and TyG-BMI were significantly and positively associated with CVD and CVD mortality, especially in younger individuals. These findings are consistent with another study conducted among the American population, which revealed that the TyG index is significantly linked to a higher probability of heart failure in a younger age group (less than 60 years) [43]. Another study of an Iranian population showed that the TyG index was significantly associated with an increased risk of developing Cardiovascular disease/coronary heart disease and was more pronounced in younger people [44]. It is plausible that as one ages, there might be more risk factors for cardiovascular disease, making the predictive capabilities of TyG and its combination with obesity indices less potent in older populations. Our study also showed that the correlation of TyG, TyG-WC, TyG-WHtR, and TyG-BMI with CVD was higher in men than women. In contrast, the correlation of TyG-WC and TyG-WHtR with CVD mortality was higher in women than men. Current reports of sex differences in TyG, TyG-WC, TyG-WHtR, and TyG-BMI with CVD and CVD mortality are inconsistent. They may be related to the age and sex composition of the study population. A joint study of a prospective cohort and the Hong Kong Cohort Study showed that the association between the TyG index and the risk of heart failure events was stronger in women than in men [45]. In another study of the TyG index and the risk of a first major hard cardiovascular event within ten years, subgroup analyses showed that the above correlation was [β = 3.862 95% CI (3.274, 4.450), < 0.00001] in men and [β = 1.067, 95% CI (0.286, 1.849), = 0.00756] in women) [35]. Futhermore, our study suggests that associations between TyG, TyG-WC, TyG-WHtR, and TyG-BMI and CVD and CVD mortality was higher in no diabetes. Laura Sánchez-Íñigo et al. [46] and Liu Li et al. [47] were consistent with our findings. This may be related to glucose-lowering medication in diabetic patients, affecting the blood glucose level and directly influencing the TyG index. In addition, in the diabetic population, traditional cardiovascular risk factors have a more significant impact on cardiovascular events than IR [48].
An essential finding of the study is that glycated hemoglobin has a partial mediating role in the association between TyG, TyG-WC, TyG-WHtR, and TyG-BMI and CVD mortality, total CVD, congestive heart failure, myocardial infarction, angina pectoris, and coronary heart disease. Glycated hemoglobin is the product of combining hemoglobin from red blood cells with sugars from serum. Glycated hemoglobin reflects the average blood glucose level over the last 2–3 months. Some studies have indicated a correlation between increased glycated hemoglobin levels and cardiovascular disease morbidity and mortality [49, 50]. We found a mediating role for insulin in the associations of TyG with total CVD, congestive heart failure, and angina pectoris and TyG-BMI with total CVD and coronary heart disease. Some studies have shown that insulin level increases the risk of cardiovascular disease [43, 51, 52]. Furthermore, the indirect effects of insulin-mediated associations between TyG, TyG-WC, TyG-WHtR, and CVD mortality were -11.2%, -25.1%, and -23.8%. In healthy people, insulin dilates and protects blood vessels. In diabetic populations, insulin is widely used to control blood glucose levels. The relevant literature shows that insulin has a protective effect on the heart and its functions [53–55]. However, further studies are needed to investigate the relationship between insulin and cardiovascular disease. Our findings suggest that effective interventions targeting glycated hemoglobin may be developed to prevent the risk of cardiovascular disease.
The mechanism behind TyG, TyG-WC, TyG-WHtR, and TyG-BMI and increased risk of CVD mortality, total CVD, congestive heart failure, myocardial infarction, angina pectoris, and coronary heart disease may be due to elevated levels of TyG, TyG-WC, TyG-WHtR, and TyG-BMI, which are associated with insulin resistance [56, 57]. Insulin resistance may induce glucose metabolism disorders and lipotoxicity. The release of inflammation factors by macrophages and adipocytes, inactivation of nitric oxide, activation of the sympathetic nervous system and renin–angiotensin–aldosterone system, hemorrhagic disorders, and platelet activation, can lead to cardiac dysfunction and myocardial injury, ultimately resulting in a range of cardiovascular diseases. Experimental research is needed to confirm the specific mechanisms.
Strengths and limitations
The study's strengths lie in its adjustment for covariates of nutritional data, including total energy intake, AHEI, and dietary supplements. Secondly, this study examines the impact of glycated hemoglobin and insulin on the relationship between TyG, TyG-WC, TyG-WHtR, and TyG-BMI and CVD mortality, total CVD, congestive heart failure, myocardial infarction, angina pectoris, and coronary heart disease. Lastly, the complex multi-stage probability sampling methodology guarantees that the participants in this study accurately represent the non-institutional population, facilitating the generalization of the results to the United States. There are also some limitations to this study. Firstly, data on CVD were collected by self-report, so patients with undiagnosed CVD were omitted. Secondly, the study relied on a single baseline blood sample to gather information on TyG, TyG-WC, TyG-WHtR, and TyG-BMI. Therefore, we could not assess their impact on CVD mortality, total CVD, congestive heart failure, myocardial infarction, angina pectoris, and coronary heart disease over time. Finally, our research is based on data from the United States and excluded people with abnormal energy intake. It remains to be seen whether these findings can be widely applied to other regions, suggesting that further research is warranted.
Conclusions
TyG-WC and TyG-WtHR enhance CVD mortality prediction, diagnostic efficacy of CVD and its mortality, and some CVD correlation over and above the current hottest TyG. Our study has important implications for identifying people at early risk of cardiovascular disease, improving risk stratification, and reducing the cost of screening, particularly in clinical practice and extensive epidemiological studies.
Supplementary Information
Additional file 1. Mediation analysis of TyG, TyG-WC, TyG-WHtR, and TyG-BMI with CVD mortality, total CVD, congestive heart failure, myocardial infarction, angina pectoris, and coronary heart disease.
Additional file 2. Receiver operating characteristic (ROC) curves of TyG, TyG-WC, TyG-WHtR, TyG-BMI, WC, WHtR, and BMI in relation to cardiovascular disease mortality, total CVD, congestive heart failure, myocardial infarction, angina pectoris, and coronary heart disease. A. CVD mortality; B. total CVD; C. congestive heart failure; D. myocardial infarction; E. angina pectoris; F. coronary heart disease.
Additional file 3. Supplementary Table 1-31.
Acknowledgements
All authors thank the NHANES 2003-2018 participants for their invaluable contributions. K.D., X.W., and Y.L. were responsible for the study design, provided statistical support, drafted the manuscript, and were primarily responsible for the final content. K.D., X.W., J.H., and Y.Z. were involved in preparing and analyzing the data and the visualization of the results. L.C., X.Q., L.L., Z.M., and X.T. thoroughly repeated and validated the statistical analysis. All authors actively participated in the research process made substantial contributions to manuscript revisions, and carefully reviewed and approved the final version.
Abbreviations
- TyG
Triglyceride-glucose
- CVD
Cardiovascular disease
- NHANES
National Health and Nutrition Examination Survey
- TyG-WC
Glucose triglyceride-waist circumference
- TyG-WHtR
Glucose triglyceride-waist height ratio
- TyG-BMI
Glucose triglyceride-body mass index
- RCS
Restricted cubic spline
- ROC
Receiver operating characteristic
- FSG
Fasting blood glucose
- OGTT 2 hPG
2-Hour glucose from an oral glucose tolerance test
- HDL-C
High-density lipoprotein-cholesterol
- LDL-C
Low-density lipoprotein-cholesterol
- SBP
Systolic Blood Pressure
- HOMA-IR
Homeostatic Model Assessment of Insulin Resistance
- HbA1c
Glycated hemoglobin
- AHEI
Alternative healthy eating index
- ORs
Odds ratios
- CIs
Confidence intervals
Author contributions
All authors thank the NHANES 2003-2018 participants for their invaluable contributions. K.D., X.W., and Y.L. were responsible for the study design, provided statistical support, drafted the manuscript, and were primarily responsible for the final content. K.D., X.W., J.H., and Y.Z. were involved in preparing and analyzing the data and the visualization of the results. L.C., X.Q., L.L., Z.M., and X.T. thoroughly repeated and validated the statistical analysis. All authors actively participated in the research process made substantial contributions to manuscript revisions, and carefully reviewed and approved the final version.
Funding
All authors received financial support from the National Natural Science Foundation (grant number 82030100), with funding specifically allocated to Ying Li.
Availability of data and materials
The data were obtained from publicly available sources, as previously stated.
Declarations
Competing interests
The authors declared that they had no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Keke Dang and Xuanyang Wang have contributed equally to this work and share first authorship.
References
- 1.Vaduganathan M, Mensah GA, Turco JV, Fuster V, Roth GA. The global burden of cardiovascular diseases and risk. J Am Coll Cardiol. 2022;80(25):2361–2371. doi: 10.1016/j.jacc.2022.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019. J Am Coll Cardiol. 2020;76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee J, Kim B, Kim W, Ahn C, Choi HY, Kim JG, Kim J, Shin H, Kang JG, Moon S. Lipid indices as simple and clinically useful surrogate markers for insulin resistance in the U.S. population. Sci Rep. 2021 doi: 10.1038/s41598-021-82053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304. doi: 10.1089/met.2008.0034. [DOI] [PubMed] [Google Scholar]
- 5.Faerch K, Vaag A, Holst J, Hansen T, Jørgensen T, Borch-Johnsen K. Natural history of insulin sensitivity and insulin secretion in the progression from normal glucose tolerance to impaired fasting glycemia and impaired glucose tolerance: the Inter99 study. Diabetes Care. 2009;32(3):439–444. doi: 10.2337/dc08-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeFronzo R, Ferrannini E, Groop L, Henry R, Herman W, Holst J, Hu F, Kahn C, Raz I, Shulman G, et al. Type 2 diabetes mellitus. Nat Rev Dis Primers. 2015;1:15019. doi: 10.1038/nrdp.2015.19. [DOI] [PubMed] [Google Scholar]
- 7.Kahn B, Flier J. Obesity and insulin resistance. J Clin Investig. 2000;106(4):473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill M, Yang Y, Zhang L, Sun Z, Jia G, Parrish A, Sowers J. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metab Clin Exp. 2021;119:154766. doi: 10.1016/j.metabol.2021.154766. [DOI] [PubMed] [Google Scholar]
- 9.Bornfeldt K, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011;14(5):575–585. doi: 10.1016/j.cmet.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ingelsson E, Sundström J, Arnlöv J, Zethelius B, Lind L. Insulin resistance and risk of congestive heart failure. JAMA. 2005;294(3):334–341. doi: 10.1001/jama.294.3.334. [DOI] [PubMed] [Google Scholar]
- 11.Liang S, Wang C, Zhang J, Liu Z, Bai Y, Chen Z, Huang H, He Y. Triglyceride-glucose index and coronary artery disease: a systematic review and meta-analysis of risk, severity, and prognosis. Cardiovasc Diabetol. 2023;22(1):170. doi: 10.1186/s12933-023-01906-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Z, Liu L, Wang W, Cui H, Zhang Y, Xu J, Zhang W, Zheng T, Yang J. Triglyceride-glucose index in the prediction of adverse cardiovascular events in patients with premature coronary artery disease: a retrospective cohort study. Cardiovasc Diabetol. 2022;21(1):142. doi: 10.1186/s12933-022-01576-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tao L-C, Xu J-N, Wang T-T, Hua F, Li J-J. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022 doi: 10.1186/s12933-022-01511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding X, Wang X, Wu J, Zhang M, Cui M. Triglyceride–glucose index and the incidence of atherosclerotic cardiovascular diseases: a meta-analysis of cohort studies. Cardiovasc Diabetol. 2021 doi: 10.1186/s12933-021-01268-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang D, Mathurin P, Cortez-Pinto H, Loomba R. Global epidemiology of alcohol-associated cirrhosis and HCC: trends, projections and risk factors. Nat Rev Gastroenterol Hepatol. 2023;20(1):37–49. doi: 10.1038/s41575-022-00688-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lavie C, Laddu D, Arena R, Ortega F, Alpert M, Kushner R. Healthy weight and obesity prevention: JACC health promotion series. J Am Coll Cardiol. 2018;72(13):1506–1531. doi: 10.1016/j.jacc.2018.08.1037. [DOI] [PubMed] [Google Scholar]
- 17.Huang T, Qi Q, Zheng Y, Ley S, Manson J, Hu F, Qi L. Genetic predisposition to central obesity and risk of type 2 diabetes: two independent cohort studies. Diabetes Care. 2015;38(7):1306–1311. doi: 10.2337/dc14-3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan SS, Ning H, Wilkins JT, Allen N, Carnethon M, Berry JD, Sweis RN, Lloyd-Jones DM. Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiol. 2018 doi: 10.1001/jamacardio.2018.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iliodromiti S, Celis-Morales C, Lyall D, Anderson J, Gray S, Mackay D, Nelson S, Welsh P, Pell J, Gill J, et al. The impact of confounding on the associations of different adiposity measures with the incidence of cardiovascular disease: a cohort study of 296 535 adults of white European descent. Eur Heart J. 2018;39(17):1514–1520. doi: 10.1093/eurheartj/ehy057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wormser D, Kaptoge S, Di Angelantonio E, Wood A, Pennells L, Thompson A, Sarwar N, Kizer J, Lawlor D, Nordestgaard B, et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377(9771):1085–1095. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan S, Wang D, Jia Y. Comparison of insulin resistance-associated parameters in US adults: a cross-sectional study. Hormones. 2023;22(2):331–341. doi: 10.1007/s42000-023-00448-4. [DOI] [PubMed] [Google Scholar]
- 22.Lim J, Kim J, Koo S, Kwon G. Comparison of triglyceride glucose index, and related parameters to predict insulin resistance in Korean adults: an analysis of the 2007–2010 Korean national health and nutrition examination survey. PLoS ONE. 2019;14(3):e0212963. doi: 10.1371/journal.pone.0212963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Zhang T, He S, Jia S, Zhang Z, Ye R, Yang X, Chen X. Association of metabolic syndrome with TyG index and TyG-related parameters in an urban Chinese population: a 15-year prospective study. Diabetol Metab Syndr. 2022;14(1):84. doi: 10.1186/s13098-022-00855-4. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Zhou S, Yu Y, Zhang Z, Ma L, Wang C, Yang M, Cheng X, Liu T, Sun R, Chen Y. Association of obesity, triglyceride-glucose and its derivatives index with risk of hyperuricemia among college students in Qingdao, China. Front Endocrinol. 2022;13:1001844. doi: 10.3389/fendo.2022.1001844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xuan W, Liu D, Zhong J, Luo H, Zhang X. Impacts of triglyceride glucose-waist to height ratio on diabetes incidence: a secondary analysis of a population-based longitudinal data. Front Endocrinol. 2022;13:949831. doi: 10.3389/fendo.2022.949831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xue Y, Xu J, Li M, Gao Y. Potential screening indicators for early diagnosis of NAFLD/MAFLD and liver fibrosis: triglyceride glucose index-related parameters. Front Endocrinol. 2022;13:951689. doi: 10.3389/fendo.2022.951689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Er L, Wu S, Chou H, Hsu L, Teng M, Sun Y, Ko Y. Triglyceride glucose-body mass index is a simple and clinically useful surrogate marker for insulin resistance in nondiabetic individuals. PLoS ONE. 2016;11(3):e0149731. doi: 10.1371/journal.pone.0149731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng Y, Fang Z, Zhang X, Wen Y, Lu J, He S, Xu B. Association between triglyceride glucose-body mass index and cardiovascular outcomes in patients undergoing percutaneous coronary intervention: a retrospective study. Cardiovasc Diabetol. 2023;22(1):75. doi: 10.1186/s12933-023-01794-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hou W, Han T, Sun X, Chen Y, Xu J, Wang Y, Yang X, Jiang W, Sun C. Relationship between carbohydrate intake (quantity, quality, and time eaten) and mortality (total, cardiovascular, and diabetes): assessment of 2003–2014 national health and nutrition examination survey participants. Diabetes Care. 2022;45(12):3024–3031. doi: 10.2337/dc22-0462. [DOI] [PubMed] [Google Scholar]
- 30.Khan S, Sobia F, Niazi N, Manzoor S, Fazal N, Ahmad F. Metabolic clustering of risk factors: evaluation of Triglyceride-glucose index (TyG index) for evaluation of insulin resistance. Diabetol Metab Syndr. 2018;10:74. doi: 10.1186/s13098-018-0376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du T, Yuan G, Zhang M, Zhou X, Sun X, Yu X. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc Diabetol. 2014;13:146. doi: 10.1186/s12933-014-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guerrero-Romero F, Simental-Mendía L, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala M, Hernández-González S, Jacques-Camarena O, Rodríguez-Morán M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–3351. doi: 10.1210/jc.2010-0288. [DOI] [PubMed] [Google Scholar]
- 33.Che B, Zhong C, Zhang R, Pu L, Zhao T, Zhang Y, Han L. Triglyceride-glucose index and triglyceride to high-density lipoprotein cholesterol ratio as potential cardiovascular disease risk factors: an analysis of UK biobank data. Cardiovasc Diabetol. 2023 doi: 10.1186/s12933-023-01762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park K, Ahn C, Lee S, Kang S, Nam J, Lee B, Kim J, Park J. Elevated TyG index predicts progression of coronary artery calcification. Diabetes Care. 2019;42(8):1569–1573. doi: 10.2337/dc18-1920. [DOI] [PubMed] [Google Scholar]
- 35.Qu H, Long L, Chen L, Wu H, Fu C, Zhang S. Triglyceride-glucose index and estimated 10-year risk of a first hard cardiovascular event. Front Cardiovasc Med. 2022;9:994329. doi: 10.3389/fcvm.2022.994329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Q, Xiao S, Jiao X, Shen Y. The triglyceride-glucose index is a predictor for cardiovascular and all-cause mortality in CVD patients with diabetes or pre-diabetes: evidence from NHANES 2001–2018. Cardiovasc Diabetol. 2023;22(1):279. doi: 10.1186/s12933-023-02030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu X, He G, Lo K, Huang Y, Feng Y. The triglyceride-glucose index, an insulin resistance marker, was non-linear associated with all-cause and cardiovascular mortality in the general population. Front Cardiovasc Med. 2020;7:628109. doi: 10.3389/fcvm.2020.628109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kizer J, Arnold A, Jenny N, Cushman M, Strotmeyer E, Ives D, Ding J, Kritchevsky S, Chaves P, Hirsch C, et al. Longitudinal changes in adiponectin and inflammatory markers and relation to survival in the oldest old: the cardiovascular health study all stars study. J Gerontol A Biol Sci Med Sci. 2011;66(10):1100–1107. doi: 10.1093/gerona/glr098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ludin A, Gur-Cohen S, Golan K, Kaufmann K, Itkin T, Medaglia C, Lu X, Ledergor G, Kollet O, Lapidot T. Reactive oxygen species regulate hematopoietic stem cell self-renewal, migration and development, as well as their bone marrow microenvironment. Antioxid Redox Signal. 2014;21(11):1605–1619. doi: 10.1089/ars.2014.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cho Y, Lee J, Kim H, Kim E, Lee M, Yang D, Kang J, Jung C, Park J, Kim H, et al. Triglyceride glucose-waist circumference better predicts coronary calcium progression compared with other indices of insulin resistance: a longitudinal observational study. J Clin Med. 2020 doi: 10.3390/jcm10010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lavie C, Milani R, Ventura H. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53(21):1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 42.Rexrode K, Carey V, Hennekens C, Walters E, Colditz G, Stampfer M, Willett W, Manson J. Abdominal adiposity and coronary heart disease in women. JAMA. 1998;280(21):1843–1848. doi: 10.1001/jama.280.21.1843. [DOI] [PubMed] [Google Scholar]
- 43.Wang H, He S, Wang J, An Y, Wang X, Li G, Gong Q. Hyperinsulinemia and plasma glucose level independently associated with all-cause and cardiovascular mortality in Chinese people without diabetes-A post-hoc analysis of the 30-year follow-up of Da Qing diabetes and IGT study. Diabetes Res Clin Pract. 2023;195:110199. doi: 10.1016/j.diabres.2022.110199. [DOI] [PubMed] [Google Scholar]
- 44.Barzegar N, Tohidi M, Hasheminia M, Azizi F, Hadaegh F. The impact of triglyceride-glucose index on incident cardiovascular events during 16 years of follow-up: Tehran Lipid and Glucose Study. Cardiovasc Diabetol. 2020;19(1):155. doi: 10.1186/s12933-020-01121-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X, Chan J, Guan B, Peng S, Wu X, Lu X, Zhou J, Hui J, Lee Y, Satti D, et al. Triglyceride-glucose index and the risk of heart failure: evidence from two large cohorts and a Mendelian randomization analysis. Cardiovasc Diabetol. 2022;21(1):229. doi: 10.1186/s12933-022-01658-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sánchez-Íñigo L, Navarro-González D, Fernández-Montero A, Pastrana-Delgado J, Martínez J. The TyG index may predict the development of cardiovascular events. Eur J Clin Invest. 2016;46(2):189–197. doi: 10.1111/eci.12583. [DOI] [PubMed] [Google Scholar]
- 47.Liu L, Wu Z, Zhuang Y, Zhang Y, Cui H, Lu F, Peng J, Yang J. Association of triglyceride-glucose index and traditional risk factors with cardiovascular disease among non-diabetic population: a 10-year prospective cohort study. Cardiovasc Diabetol. 2022;21(1):256. doi: 10.1186/s12933-022-01694-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014;10(5):293–302. doi: 10.1038/nrendo.2014.29. [DOI] [PubMed] [Google Scholar]
- 49.Sinning C, Makarova N, Völzke H, Schnabel R, Ojeda F, Dörr M, Felix S, Koenig W, Peters A, Rathmann W, et al. Association of glycated hemoglobin A levels with cardiovascular outcomes in the general population: results from the BiomarCaRE (biomarker for cardiovascular risk assessment in Europe) consortium. Cardiovasc Diabetol. 2021;20(1):223. doi: 10.1186/s12933-021-01413-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Selvin E, Steffes M, Zhu H, Matsushita K, Wagenknecht L, Pankow J, Coresh J, Brancati F. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362(9):800–811. doi: 10.1056/NEJMoa0908359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pyöräla K. Relationship of glucose tolerance and plasma insulin to the incidence of coronary heart disease: results from two population studies in Finland. Diabetes Care. 1979;2(2):131–141. doi: 10.2337/diacare.2.2.131. [DOI] [PubMed] [Google Scholar]
- 52.Lamarche B, Tchernof A, Mauriège P, Cantin B, Dagenais G, Lupien P, Després J. Fasting insulin and apolipoprotein B levels and low-density lipoprotein particle size as risk factors for ischemic heart disease. JAMA. 1998;279(24):1955–1961. doi: 10.1001/jama.279.24.1955. [DOI] [PubMed] [Google Scholar]
- 53.Sinha A, Bhattacharya S, Acharya K, Mazumder S. Stimulation of nitric oxide synthesis and protective role of insulin in acute thrombosis in vivo. Life Sci. 1999;65(25):2687–2696. doi: 10.1016/S0024-3205(99)00537-8. [DOI] [PubMed] [Google Scholar]
- 54.Xi L, Kukreja R. Pivotal role of nitric oxide in delayed pharmacological preconditioning against myocardial infarction. Toxicology. 2000;155:37–44. doi: 10.1016/S0300-483X(00)00275-4. [DOI] [PubMed] [Google Scholar]
- 55.Yazar A, Polat G, Un I, Levent A, Kaygusuz A, Büyükafşar K, Çamdeviren H. Effects of glibenclamide, metformin and insulin on the incidence and latency of death by oubain-induced arrhythmias in mice. Pharmacol Res. 2002;45(3):183–187. doi: 10.1006/phrs.2001.0944. [DOI] [PubMed] [Google Scholar]
- 56.Ren J, Wu N, Wang S, Sowers J, Zhang Y. Obesity cardiomyopathy: evidence, mechanisms, and therapeutic implications. Physiol Rev. 2021;101(4):1745–1807. doi: 10.1152/physrev.00030.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga F. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17(1):122. doi: 10.1186/s12933-018-0762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Mediation analysis of TyG, TyG-WC, TyG-WHtR, and TyG-BMI with CVD mortality, total CVD, congestive heart failure, myocardial infarction, angina pectoris, and coronary heart disease.
Additional file 2. Receiver operating characteristic (ROC) curves of TyG, TyG-WC, TyG-WHtR, TyG-BMI, WC, WHtR, and BMI in relation to cardiovascular disease mortality, total CVD, congestive heart failure, myocardial infarction, angina pectoris, and coronary heart disease. A. CVD mortality; B. total CVD; C. congestive heart failure; D. myocardial infarction; E. angina pectoris; F. coronary heart disease.
Additional file 3. Supplementary Table 1-31.
Data Availability Statement
The data were obtained from publicly available sources, as previously stated.