Abstract
Two Escherichia coli genes, expressed from multicopy plasmids, are shown to cause partial induction of prophage λ in recA mutant lysogens. One is rcsA, which specifies a positive transcriptional regulator of the cps genes, which are involved in capsular polysaccharide synthesis. The other is dsrA, which specifies an 85-nucleotide RNA that relieves repression of the rcsA gene by histone-like protein H-NS. Genetic contexts known to increase Cps expression also cause RecA-independent λ induction: the rcsC137 mutation, which causes constitutive Cps expression, and the lon and rcsA3 mutations, which stabilize RcsA. Lambdoid phages 21, φ80, and 434 are also induced by RcsA and DsrA overexpression in recA lysogens. Excess λ cI repressor specifically blocks λ induction, suggesting that induction involves repressor inactivation rather than repressor bypass. RcsA-mediated induction requires RcsB, the known effector of the cps operon, whereas DsrA-mediated induction is RcsB independent in stationary phase, pointing to the existence of yet another RecA-independent pathway of prophage induction.
Temperate bacteriophages possess a dual mode of existence, able either to lyse sensitive cells or to form stable lysogens in which the phage genome is propagated as prophage by the bacterium, with phage lytic functions repressed by one or several phage repressors (28). For this reason, temperate phages have been attractive models for studying the mechanisms governing biological decisions: the choice between lysis and lysogeny after infection of a sensitive host and the choice of the established lysogen at each generation to continue propagating the prophage or to enter the lytic cycle.
The demonstration that, for certain lysogens, the vast majority of the population could be induced to enter the lytic cycle by UV irradiation was a major discovery, dispelling lingering doubts about the reality of the phenomenon of lysogeny (19, 20). Since that time, the combined efforts of many researchers over several decades have established the mechanism of lysogenic induction by UV irradiation and other DNA-damaging treatments. Under these conditions, the SOS response is induced; the RecA protein, activated by single-stranded DNA formed near DNA lesions, promotes autoproteolytic cleavage of certain prophage repressors as well as of the bacterial SOS repressor LexA (18, 28, 38, 39). The SOS response and SOS-inducible temperate phages have been described in a number of bacteria, including such distantly related species as Escherichia coli and Bacillus subtilis. In those cases tested, the SOS response is also the major pathway of spontaneous induction, which, under laboratory conditions at least, is severely reduced in recA mutant lysogens (28).
It has often been suggested that lysogenic induction via the SOS response is a selective mechanism permitting the phage to use its host’s detection system for DNA damage in order to leave a severely damaged cell which is likely to die, like a rat leaving a sinking ship. If so, there may well be other conditions in which the phage, using other host detection mechanisms to sense imminent cell death, would similarly choose to enter the lytic pathway. Furthermore, many temperate phages are not SOS inducible yet still exhibit spontaneous induction, even in recA hosts. Well-known examples in E. coli are Mu and P2; host functions involved in their induction have not been identified.
For E. coli, a large number of stress responses have been described, including those permitting the cell to sense changes in temperature, osmolarity, pH, or nutrient availability and adjust its pattern of gene expression appropriately. In theory at least, it is possible that for each stress response there is a set of phages tuned in to the cell’s detectors in such a way that, if cell survival seems threatened, the phage is induced.
In the present work we looked for E. coli genes, the amplification of which leads to RecA-independent induction of prophage λ. The selection used produced clones of the host genes rcsA and dsrA, which when overexpressed cause induction of λ and other lambdoid prophages in recA lysogens. Both genes code for positive regulators of capsular polysaccharide synthesis. Other genetic alterations leading to capsular polysaccharide overproduction also cause RecA-independent λ induction. From complementation studies, we conclude that DsrA participates in prophage induction via two different mechanisms. Results obtained for the induction of λcIind mutants suggest several possible mechanisms.
MATERIALS AND METHODS
Bacterial strains and bacteriophages.
The bacterial strains used in this study, all derivatives of E. coli K-12, are listed in Table 1, together with the bacteriophages. Standard techniques were used for growth of bacteria and bacteriophages, phage crosses, generalized transduction with P1vir, and lysogenization (23, 30). All recA strains were checked for UV sensitivity and lack of growth of λbio10 (or λimm434 bio10) phage, as described elsewhere (42). The rcsC137 and lon strains were mucoid on Luria-Bertani (LB) plates at 37°C and 30°C, respectively. The rcsA3 mutant formed mucoid colonies at 30°C on M9 plates but not on LB plates.
TABLE 1.
Bacterial strains and bacteriophages
| Strain or phage | Relevant genotype or phenotype | Source or reference |
|---|---|---|
| Strains | ||
| N99 | galK2 galT22 rpsL IN(rrnD-rrnE)1 | VKPMa |
| C600 | supE44 hsdR thi-1 thr-1 leuB6 lacY1 tonA21 | VKPM |
| LE392 | supE44 supF58 hsdR514 galK2 galT22 metB1 trpR55 lacY | VKPM |
| XL1-blue | supE44 hsdR17 recA1 endA1 gyrA46 thi relA1 lac F′[traD36 proAB+ lacIqlacZΔM15 Tn10] | VKPM |
| MW1184 | ara Δ(lac-proAB) rpsL thi (φ80 lacZΔM15) Δ(srlR-recA) 306::Tn10 | VKPM |
| K802 | supE44 hsdR2 galK2 galT22 metB1 lacY1 mcrA mcrB | VKPM |
| C600 Hfr | C600 + F′101(thr+ ara+ leu+) | VKPM |
| B3958 | K802 recA9::Tn5 | VKPM |
| B7218 | LE392 Apr | VKPM |
| B7237 | C600 hisG::Tn10 | VKPM |
| B7349 | B6905mal(λ) λ-resistant | VKPM |
| MT1 | ilv his rpsL λN7N53 cI+cro+::gal+ | 35 |
| MT2 | ilv his recA1 rpsL λN7N53 cI+cro+::gal+ | 35 |
| SG20322b | cps-11::lac-Mud1 lon-146::ΔTn10c | 3 |
| SG20645d | rcsA3 (RcsA*) zed-650::Tn10 | 3 |
| SG20797d | Δlon-510 rcsB11::ΔTn10c | 3 |
| SG20803d | rcsC137 ompC::Tn5 | 3 |
| SG20904d | Δlon-510 rcsA72::ΔTn10c | 3 |
| B6905 | C600 Δ(srlR-recA)306::Tn10 | C600 + P1(MW1184) |
| B7243 | C600 rcsA3 (RcsA*) Δ(srlR-recA)306::Tn10 | B7237 + P1(SG20645) + P1(MW1184)e |
| B7244 | N99 Δ(srl-recA)306::Tn10 | N99 + P1(MW1184) |
| B7246 | N99 dsrA::ΔTn903f | N99 + pDR301g |
| B7252 | C600 rcsA72::ΔTn10 recA9::Tn5c | C600 + P1(SG20904) + P1(B3958) |
| B7253 | C600 rcsB11::ΔTn10 recA9::Tn5c | C600 + P1(SG20797) + P1(B3958) |
| B7254 | C600 lon-146::ΔTn10 recA9::Tn5c | C600 + P1(SG20322) + P1(B3958) |
| B7307 | C600 dsrA::ΔTn903 Δ(srlR-recA)306::Tn10f | C600 + P1(B7246) + P1(MW1184) |
| B7315 | C600 rcsC137 ompC::Tn5 Δ(srlR-recA)306::Tn10 | C600 + P1(SG20803) + P1(MW1184) |
| Bacteriophages | ||
| λ+ | VKPM | |
| 21 | VKPM | |
| φ80 | VKPM | |
| 434 | VKPM | |
| Mucts62 | VKPM | |
| P1vir | VKPM | |
| λbio10 | VKPM | |
| λplac5 | VKPM | |
| λimm434bio10 | VKPM | |
| λimm434plac5 | VKPM | |
| λGE112 | b2 cI(Ind−) | 8 |
| λDY125 | b2 cI(Ind−) | 8 |
| λEK117 | b2 cI(Ind−) | 8 |
| λGR185 | b2 cI(Ind−) | 8 |
| λGE112 plac5 | cI(Ind−) | λGE112 + λimm434 plac5 |
| λDY125 plac5 | cI(Ind−) | λDY125 + λimm434 plac5 |
| λEK117 plac5 | cI(Ind−) | λEK117 + λimm434 plac5 |
| λGR185 plac5 | cI(Ind−) | λGR185 + λimm434 plac5 |
| λprecA | λcI857 recA+ | 22 |
| λ3B3 | rcsA+ dsrA+ dsrB+ | 17 |
VKPM, Russian National Collection of Industrial Microorganisms, GNIIGenetica, Moscow, Russia.
Derived from MC4100, genotype F− Δ(argF-lac)U169 araD139 rpsL150 deoC1 relA1 ptsF25 flbB5301 rbsR.
ΔTn10 (Tn10Δ16Δ17) is defective Tn10 capable of transposition but deficient in transposase (40).
Derived from MC4100 and contains cpsB10::lacMu-immλ.
B7237 + P1(SG20645) + P1(MW1184) indicates that the strain was constructed by two successive P1 vir transductions in the order indicated.
ΔTn903 contains a drug resistance marker from transposon Tn903 which encodes for an aminoglycoside 3′-phosphotransferase gene conferring kanamycin resistance (37).
N99 + pDR301 indicates that the dsrA::ΔTn903 insertion of pDR301 was transferred to the chromosome by Hfr conjugation (see Materials and Methods).
Media and chemicals.
Permanent bacterial stocks were stored in 20% glycerol at −70°C; working stocks were maintained on LB agar at 4°C for up to 2 weeks. Except where otherwise stated, cells were grown in LB medium (23). M9 (23) and GT (24) plates were as described previously. Solid media were supplemented with 1.5% agar (Difco). Soft agar contained 0.2% MgSO4 with 0.8% agar. Eosin-methylene blue (EMB) galactose plates (23) were used to screen for Gal+ colonies. Plates containing 50 μg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) per ml and 50 μg of isopropyl-β-d-thiogalactopyranoside (IPTG) per ml were used to distinguish between Lac+ and Lac− colonies. Transducing phages were grown in NZCYM medium, as described by Sambrook et al. (29). Bacterial strains carrying the mini-Mu element pEG5005 and helper phage Mucts62 were induced in LB medium with CaCl2 (20 mg/ml) and MgSO4 (10 mg/ml), as described by Groisman and Casadaban (14). The following antibiotics were added, when needed, at the indicated concentration: ampicillin (Ap), 50 μg/ml (to maintain plasmids) or 20 μg/ml (to measure infective centers); chloramphenicol (Cm), 20 μg/ml; kanamycin (Km), 10 μg/ml; streptomycin (Sm), 10 μg/ml; and tetracycline (Tc), 10 μg/ml. Dilutions of cells and phages were in 0.8% saline.
Plasmid construction.
The plasmids used in this work are listed in Table 2. Isolation of plasmid and phage DNA and routine nucleic acid manipulations were as described previously (29). pUC19- and pGEM-7zf(−)-derived plasmids were constructed by restriction-enzyme digestion of the transducing phage and plasmid DNA followed by ligation with T4 DNA ligase. pDR406 and pDR416 were prepared in vivo after heat induction of strain C600, which carries both the mini-Mu element pEG5005 (Kmr) and a Mucts62 prophage, as described elsewhere (14). The resulting mixed phage lysate was used to transduce strain MT2 to Kmr on EMB galactose indicator plates containing Km to screen Gal+ colonies.
TABLE 2.
Plasmids
| Plasmid | Genotype or phenotype | Source or reference |
|---|---|---|
| pUC19 | bla+ | VKPMa |
| pGEM-7zf(−) | bla+ | Promega Corporation |
| pUC-4K | bla+, Kmr | 37 |
| pCM4 | bla+, Cmr | 4 |
| pEG5005 | pBCO::Mud5005; bla+, Kmr | 14 |
| pDR406 | rcsA+ dsrA+ dsrB+ derivative of pEG5005; Kmr | This work |
| pDR416 | rcsA+ dsrA+ dsrB+ derivative of pEG5005; Kmr | This work |
| pDR19 | bla+, Cmr | pUC19 + pCM4 |
| pDR500 | rcsA+ dsrA+ dsrB+bla+ | pUC19 + pDR416 |
| pDR501 | rcsA::ΔTn903 dsrA+ dsrB+bla+, Kmr | pDR500 + pUC4K |
| pDR100 | rcsA+ dsrA+ dsrB+bla+ | pUC19 + 3B3 |
| pDR200 | rcsA+bla+ | pUC19 + pDR100 |
| pDR300 | dsrA+ dsrB+bla+ | pUC19 + pDR100 |
| pDR201 | rcsA::ΔTn903 bla+, Kmr | pDR200 + pUC4K |
| pDR301 | dsrA::ΔTn903 dsrB+bla+, Kmr | pDR300 + pUC4K |
| pDR210 | rcsA+bla::cat, Cmr | pDR200 + pCM4 |
| pDR310 | dsrA+ dsrB+bla::cat, Cmr | pDR300 + pCM4 |
| pDR211 | rcsA::ΔTn903, bla::cat, Kmr Cmr | pDR201 + pCM4 |
| pDR311 | dsrA::ΔTn903 dsrB+bla::cat, Kmr Cmr | pDR301 + pCM4 |
| pDR12 | dsrA+ dsrB+bla+ | pGEM-7zf(−) + pDR100 |
| pDR1 | cI+, bla+ | pUC19 + λ+ |
VKPM, Russian National Collection of Industrial Microorganisms, GNIIGenetica, Moscow, Russia.
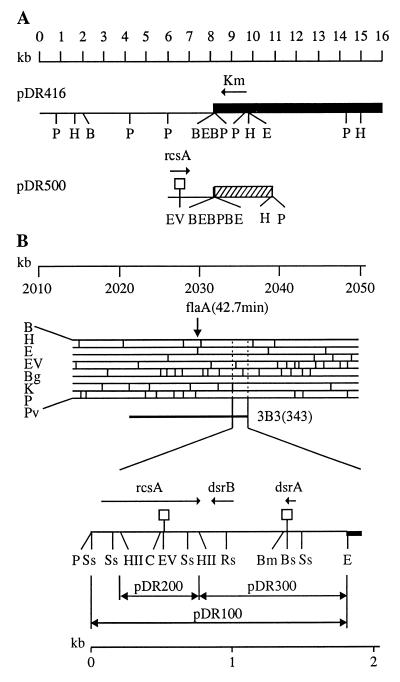
The plasmid bearing rcsA+ dsrA+ dsrB+ pDR500 was constructed by subcloning the isolated 2.0-kb PstI fragment of pDR416 in the unique PstI site of pUC19. A Lac− Apr mucoid transformant of XL1-blue was isolated, and plasmid DNA was extracted. pDR500 had the expected enzyme restriction pattern (Fig. 1A). pDR501 was constructed by insertion of ΔTn903 from pUC4K (37), cut with HincII, into the EcoRV site of pDR500.
FIG. 1.
Structure of plasmids used. (A) Plasmid pDR416 is a derivative of the mini-Mu element pEG5005 and plasmid pDR500 is a derivative of pUC19. The cloned chromosomal DNA is represented by a thin line, and mini-Mu material is represented by a black box. The position and direction of transcription of the rcsA gene were inferred from earlier data (34) and from restriction analysis. The open square indicates the position of the ΔTn903 insertion which disrupts the rcsA gene in pDR500. The hatched box indicates the pUC19 DNA. (B) The physical structure of the chromosomal segment containing the rcsA, dsrA, and dsrB genes is shown together with the E. coli restriction map in the 42-min region (17). The position and direction of transcription of these genes were inferred from earlier data (31, 34) and from restriction analysis. The chromosomal insert in phage λ3B3 from the Kohara collection is indicated. The physical limits are indicated for the chromosomal DNA cloned in pDR100, pDR200, and pDR300, which are derivatives of pUC19. Open squares indicate the positions of the ΔTn903 insertions which disrupted the rcsA and dsrA genes in pDR201 and pDR301, respectively. Abbreviations for restrictions enzymes are as follows: B, BamHI; Bg, BglI; Bm, BsmI; Bs, Bsu36I; C, ClaI; E, EcoRI; EV, EcoRV; H, HindIII; HII, HincII; K, KpnI; P, PstI; Pv, PvuII; Rs, RsrII; Ss, SspII.
To construct the plasmid bearing rcsA+ dsrA+ dsrB+ pDR100, DNA preparations from phage λ3B3 from the Kohara collection (miniset 343) and from pUC19 were digested with EcoRI and PstI, mixed, and ligated. A Lac− Apr mucoid transformant of XL1-blue was isolated, and the plasmid was extracted. Restriction-enzyme and hybridization analysis confirmed the structure suggested by the genetics (Fig. 1B). Plasmids pDR200 (rcsA+) and pDR300 (dsrA+ dsrB+) were constructed by subcloning in pUC19 isolated HincII and HincII-EcoRI fragments, respectively, from pDR100. It is known that E. coli K-12 strains become mucoid when they carry several copies of the rcsA gene (36). pDR300 (dsrA+ dsrB+), like pDR200 (rcsA+), increased capsular polysaccharide synthesis, and the transformants formed slime colonies, but the pDR300 (dsrA+ dsrB+)-mediated effect was less pronounced and depended on the strain. We used C600 to select mucoid transformants. pDR200 (rcsA+) and pDR300 (dsrA+ dsrB+) had the expected 0.6-kb and 1.1-kb fragments, respectively. It should be noted that pDR300, in addition to the dsrA gene, also carries the adjacent dsrB gene. Both code for small RNAs, as described by Sledgjeski and Gottesman (31). The restriction map for the region containing rcsA, dsrA, and dsrB is shown in Fig. 1B; it is similar to that published earlier (17, 31, 34). Plasmid rcsA and dsrA genes were disrupted in vitro by inserting the HincII fragment from pUC4K, containing ΔTn903, into pDR200 (rcsA+) cut with EcoRV and pDR300 (dsrA+ dsrB+) cut with Bsu36I and blunted with Klenow polymerase. Nonmucoid Apr Kmr transformants of C600 were selected, and their plasmid DNA was extracted. The resulting plasmids, pDR201 (rcsA::ΔTn903) and pDR301 (dsrA::ΔTn903 dsrB+), had the expected restriction-enzyme cleavage patterns (Fig. 1B).
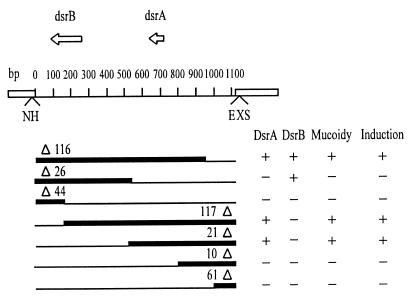
To define the physical limits of the dsrA+ and dsrB+ regions that confer the phenotype observed, we cloned the isolated 1.1-kb HincII-EcoRI fragment of pDR100 in pGEM-7zf(−) digested with SmaI and EcoRI. The resulting plasmid, pDR12, was digested to completion with SphI or NsiI as demonstrated by electrophoresis. Then pDR12 was digested again with XbaI or HindIII, and DNA deletion with exonuclease III was carried out as described in the Erase-a-Base system from Promega Biotec. The endpoints of the deletions were determined by sizing the linearized DNAs on agarose gels.
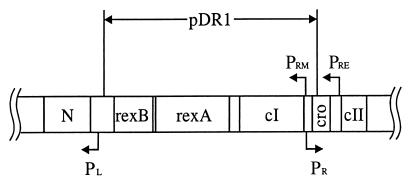
To clone the λ cI gene, λ+ DNA was digested with BglII and mixed with pUC19 digested with BamHI. Lac− Apr transformants of XL1-blue were checked for lambda immunity. Restriction analysis confirmed the genetic conclusion; plasmid pDR1 carries the fragment of λ DNA from 35,711 to 38,103 kb (5) (Fig. 2).
FIG. 2.
Structure of pDR1. pDR1 is a derivative of pUC19. The genetic and transcriptional map of the region surrounding the λ cI gene and a portion of the λ chromosome contained in pDR1 are indicated.
To measure infective centers it is necessary to prevent growth of lysogens. This is readily done by addition of ampicillin to the plates, provided the lysogens are Aps. We inactivated the plasmid bla gene in vitro as follows. First, we cloned the cat cassette from pCM4 (4) into the BamHI site of pUC19. From the resulting plasmid pDR19, the cassette was cut out by digestion with HincII and SmaI and inserted into the bla gene of pDR200 (rcsA+) and pDR201 (rcsA::ΔTn903) cut with ScaI and into the bla gene of pDR300 (dsrA+ dsrB+), and pDR301 (dsrA::ΔTn903 dsrB+) was partially digested with the same enzyme because of the presence of a second ScaI site outside of the bla gene. Restriction analysis of the selected plasmid confirmed insertion of the cat cassette in the bla gene.
Transfer of mutation from plasmid to chromosome.
The insertion mutation constructed in plasmid pDR301 was transferred to the chromosome as described by Parker and Marinus (26). Plasmid pDR301 (dsrA::ΔTn903 dsrB+) with a disrupted dsrA gene was introduced into C600 Hfr, and the resulting strain was used as a donor in conjugation experiments with N99 as the recipient. Smr Kmr colonies were checked for sensitivity to ampicillin. P1 transduction confirmed that the dsrA::ΔTn903 allele was linked to hisG::Tn10 (3% cotransduction) as expected for insertions in the rcsA dsrA region.
Halo test and quantitative measurement of prophage induction. (i) Halo test.
Lysogenic bacteria, grown overnight in LB medium at 30°C from single colonies on LB plates, were spotted on a lawn of the indicator strain LE392 in soft agar, and the plates were incubated overnight at 30°C. Herein, the term induction refers to appearance of a halo of lysis or of plaques within the spot.
(ii) Quantification of induction efficiency.
The efficiency of lysogenic induction was determined by calculating the ratio of infective centers to viable lysogens. Exponentially growing λ lysogens, washed to eliminate free phage, were mixed with indicator strain B7218 in soft agar and plated onto GT plates containing 20 μg of ampicillin/ml, as described by Moreau et al. (24). Plates were incubated overnight at 37°C. Ampicillin prevents uninduced bacteria from multiplying and releasing phage during overnight incubations but does not prevent induced lysogens from forming infective centers (25). This technique was used with Aps lysogens.
A second method of quantification of induction efficiency, measuring the concentration of free phage in the course of the induction experiment, was used. Overnight cultures from a single colony were prepared at 30°C in LB broth containing chloramphenicol when needed, 1 mM MgSO4, and 0.2% glucose (reducing readsorption by repressing lamB, the structural gene of the λ receptor). The cultures, washed to eliminate free phage, were diluted 50- to 100-fold in the same medium and incubated at 30°C with aeration. At various times, samples were assayed for total viable cells and the optical density at 590 nm was measured. Several drops of chloroform were then added, and the free phages were mixed with LE392 indicator bacteria in soft agar and plated on LB plates. Plates were incubated overnight at 37°C.
RESULTS
Screening for clones of E. coli DNA that cause RecA-independent λ induction.
To screen for genes which, when overexpressed, can induce λ prophage, we took advantage of the characteristics of the MT strains constructed by Toman et al. (35). These strains carry a defective λ prophage with the N, cI, and cro genes. The gal operon is fused to the cro gene (and the normal gal promoter is deleted). When cI repressor is expressed, the strains are Gal−. If cI repressor is removed, the cro-gal operon is transcribed. The Cro protein then represses the cI gene and prevents turning off of cro-gal, so the strain becomes locked in a Gal+ configuration. The strains thus guard a “memory” of a past induction event. We used strain MT2, which carries a recA mutation and normally displays a much lower frequency of Gal+ colonies, <10−6, than the frequency, 10−3 to 10−4, in the recA+ strain MT1.
A gene library of E. coli wild type was prepared in vivo by using the mini-Mu element pEG5005 (14). Strain MT2 was then transformed with the library, and the transformants were plated on EMB galactose plates to identify Gal+ clones. Among 3,000 transformants, 6 Gal+ clones were isolated. Plasmid DNA was extracted and hybridized with the λprecA phage DNA. Four plasmids carried the recA gene. The other two plasmids, pDR406 and pDR416, were reintroduced into MT2 and also into a B6905(λ) (ΔrecA) lysogen. Both plasmids caused induction, as judged by the appearance of Gal+ clones in MT2 and by the formation of a halo of lysis when B6905(λ) (ΔrecA) transformants were spotted on a sensitive lawn.
Excess RcsA or DsrA causes RecA-independent induction of λ.
The two plasmids, pDR406 and pDR416, in addition to inducing λ in strains MT2 and B6905(λ) (ΔrecA), conferred a mucoid phenotype. Furthermore, in MT2 the mucoid phenotype was restricted to the Gal+ colonies, presumably reflecting the requirement for GalE enzyme to synthesize UDP-Gal, a precursor of colanic acid (21). Since this made scoring of MT2 difficult, we tested induction by monitoring the formation of a halo of lysis.
Mucoidy has been observed in cells overproducing RcsA (36). We therefore hybridized these plasmids with DNA from Kohara phage λ3B3, which carries the rcsA gene. Both plasmids carried the rcsA region. Furthermore, the restriction map of the cloned inserts accorded with that reported by Kohara et al. (17) for the region from 2,030 to 2,040 kb (Fig. 1). The 2.0-kb PstI fragment from pDR416 containing rcsA and dsrA was subcloned into pUC19. The resulting plasmid, pDR500, caused mucoidy and induced λ prophage in B6905(λ) (ΔrecA) cells. When the rcsA gene of pDR500 was interrupted by ΔTn903 (Fig. 1A; see Materials and Methods), the resulting plasmid, pDR501, caused mucoidy and induced λ. However, this plasmid carries the dsrA gene, overexpression of which can derepress chromosomal rcsA expression (reference 31; see below).
To determine conclusively the role of RcsA and DsrA in the induction observed, we recloned the rcsA and dsrA genes from Kohara phage λ3B3, making plasmids pDR200 (rcsA+) and pDR300 (dsrA+) (Fig. 1B). Both plasmids caused mucoidy and induced λ prophage in B6905(λ) (ΔrecA) cells. Inactivation of rcsA (plasmid pDR201; rcsA::ΔTn903) or of dsrA (plasmid pDR301; dsrA::ΔTn903) abolished both phenotypes. The dsrB gene, of unknown function, is present on the dsrA+ and dsrA::ΔTn903 plasmids but does not induce λ. Furthermore, it is not required for DsrA-mediated induction: reducing the size of the insert in plasmid pDR12 by the Erase-a-Base system showed that the dsrA gene alone was sufficient for RecA-independent λ induction (Fig. 3).
FIG. 3.
Detailed map of deletions generated in pDR12. Deletions were generated by the Erase-a-Base system (see Materials and Methods). The extent of each deletion is shown by a thin line. Mucoidy and induction were scored in strain B6905(λ) (ΔrecA) transformed with the various plasmids. Abbreviations for restrictions enzymes are as follows: E, EcoRI; H, HindIII; N, NsiI; S, SphI; X, XbaI.
These data confirm that overproduction of RcsA or DsrA (which derepresses the chromosomal rcsA gene) causes induction of λ in a recA lysogen.
We verified that the induced phage was indeed λ and not some hitherto unknown prophage. First, the induced phage was unable to grow on a λ lysogen. Second, transformants of the B6905 (ΔrecA) nonlysogen with plasmids pDR200 (rcsA+) and pDR300 (dsrA+) produced no lysis zone on a lawn of the indicator strain.
Effect of RcsA or DsrA overproduction on other lambdoid phages.
To determine whether the induction was strain or phage specific, we analyzed the effect of RcsA and DsrA overproduction on another recA mutant lysogenic for various lambdoid phages. Plasmids pDR200 (rcsA+) and pDR300 (dsrA+) were introduced into four lysogens of the ΔrecA strain B7244, and the ability of the resulting strains to produce halos on a sensitive lawn was observed. Lysogens having phages λ, 21, φ80, and 434 and both plasmids produced halos, but those without any plasmid did not. We conclude that overproduction of either RcsA or DsrA causes RecA-independent induction of all four of these phages and that induction is not strain specific.
Quantification of RecA-independent λ induction by RcsA or DsrA overproduction.
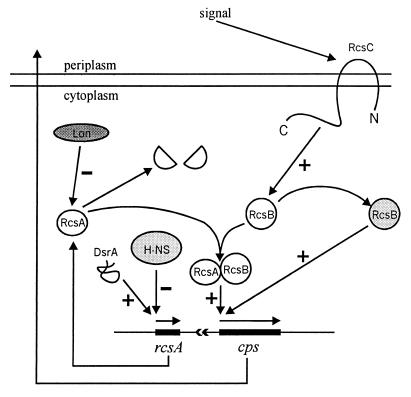
RcsA is known to be a transcriptional activator of the cps genes, which are involved in the synthesis of colanic acid, the capsular polysaccharide of E. coli. RcsA is normally limiting for the transcription of these genes, and its overexpression results in a mucoid phenotype (10, 12, 13, 34). The closely linked dsrA gene codes for a small, 85-nucleotide RNA molecule which, when overexpressed, derepresses rcsA transcription (overcoming repression by H-NS) (31). In addition to RcsA, the Cps regulon includes the RcsB and RcsC regulators, which form a sensor-effector pair. In response to an unknown environmental signal, the membrane sensor RcsC is thought to phosphorylate RcsB, thereby increasing expression of the Cps regulon (10, 12, 33) (Fig. 4).
FIG. 4.
Model of the regulation of cellular capsule synthesis in E. coli. Based in part on a figure presented by Stout and Gottesman (33).
RcsA is highly unstable and is degraded by Lon protease; lon mutants consequently have high levels of RcsA and high Cps expression (34, 36). The dominant rcsA3 mutation makes a stabler RcsA* protein and thus also induces high level of Cps expression (3). Finally, the rpsC137 allele results in constitutive activation of RcsB and high level of Cps expression, even in rcsA mutants (10, 12, 33). The rcsB gene product is absolutely necessary for Cps induction in all cases.
To quantify the level of induction due to RcsA and DsrA overproduction, we assayed the number of lysogens able to form an infective center on a sensitive lawn. To limit the growth of uninduced lysogens, ampicillin was added to the plates and an ampicillin-resistant indicator was used. Ampicillin does not affect phage growth but kills uninduced cells after about one generation (25). This prevents the formation of tiny plaques due to late induction in microcolonies of lysogens. Since these assays require the use of Aps lysogens, we inactivated the bla gene in the plasmids carrying rcsA+, dsrA+ and their inactivated derivatives. The resulting plasmids—pDR210 (rcsA+ bla::cat), pDR310 (dsrA+ bla::cat), pDR211 (rcsA::ΔTn903 bla::cat) and pDR311 (dsrA::ΔTn903 bla::cat)—were introduced into the tester strains and analyzed for inducibility. All conditions leading to high level of Cps expression also caused RecA-independent induction of λ (Table 3). Our results do not contradict those of Gottesman et al. (11), who observed no influence of lon mutation on the frequency of λ prophage induction, because the level of RcsABC-mediated induction is several orders of magnitude lower than that of induction via the RecA pathway.
TABLE 3.
Efficiency of induction of prophage λa
| Plasmid and gene(s) | Concn (cells/ml) at t1 | Frequency of infective centers (λc+) at t1 | Concn of phage (λc+/ml) at:
|
||
|---|---|---|---|---|---|
| t1 | t2 | t3 | |||
| C600recA(λ) | |||||
| No plasmid | 1.4 × 108 | 1.1 × 10−7 | 1.8 × 102 | 60 | 20 |
| pDR211 (rcsA::ΔTn903) | 1.4 × 108 | 2.0 × 10−7 | 1.0 × 102 | 50 | 30 |
| pDR311 (dsrA::ΔTn903) | 1.0 × 108 | 2.7 × 10−7 | 40 | 40 | 1.0 × 102 |
| pDR210 (rcsA+) | 9.0 × 107 | 1.8 × 10−6 | 2.0 × 103 | 9.0 × 102 | 4.0 × 102 |
| pDK311 (dsrA+) | 9.0 × 107 | 7.8 × 10−6 | 8.0 × 102 | 9.0 × 102 | 3.6 × 103 |
| lon | 1.0 × 108 | 1.3 × 10−6 | 5.0 × 102 | 2.0 × 102 | 60 |
| rcsA3 | 1.0 × 108 | 1.9 × 10−6 | 1.5 × 103 | 6.0 × 102 | 1.0 × 102 |
| rcsC137 | 1.1 × 108 | 1.4 × 10−5 | 1.5 × 103 | 9.5 × 103 | 7.0 × 103 |
| C600(λ) | 1.0 × 108 | 9.0 × 10−5 | 2.0 × 104 | 8.0 × 104 | 2.0 × 104 |
An overnight culture, washed to eliminate free phage, was diluted 50- to 100-fold in fresh LB broth and incubated at 30°C. The concentrations of cells at the start of the experiment ranged from 2 × 107 to 4 × 107. The frequency of infective centers in exponential phase and the free-phage concentration were measured at various times, as described in Materials and Methods. t1 (2 to 3 h), t2 (6 to 8 h), and t3 (24 h) correspond to exponential-, postexponential-, and stationary-phase time points. All experiments were carried out at least three times; averages are given in the table. Deviations from the averages were insignificant.
The concentration of free phage was determined at several time points during the induction experiment, to see whether the level depended on the phase of cell growth.
The event we are interested in is λ+ prophage induction caused by RcsA or DsrA overproduction, but lytic phage development can also result when λcI mutants arise, contributing to the number of infective centers. To distinguish between induction and mutation, we periodically monitored the levels of free phage in growing cultures, scoring λc+ and λc separately. λc+ and λc phages can be recognized because they form turbid and clear plaques, respectively. Both rcsA+ and dsrA+ that were cloned on plasmids, like the rcsC137, rcsA3, or lon mutation, stimulated λ prophage induction (Table 3). The results confirm that the genes regulating the synthesis of capsular polysaccharide affect prophage stability. It is interesting that RcsA overproduction causes an increase in free phage in exponential phase whereas DsrA overproduction produces the maximum free-phage titer on entry to the stationary phase of growth.
The mucoid phenotype of strains carrying rcsA+ and dsrA+ plasmids could reduce phage readsorption, thereby apparently increasing the free-phage titer. Indeed, adsorption to these cells was slower (data not shown). However, similar free-phage curves were obtained with λ-resistant recA(λ) lysogens, in which readsorption was prevented (data not shown). Furthermore, the number of infective centers, which should not be significantly influenced by phage readsorption, showed a similar 10- to 100-fold increase in recA lysogens bearing rcsA+ or dsrA+ plasmids or carrying an rcsC137, rcsA3, or lon allele (Table 3). The growth rates of the various strains used differed little, although mucoid strains reached stationary phase at a lower concentration (data not shown). We thus conclude that rcsA+ or dsrA+ plasmids increase the free-phage titer not by changing cell vigor, phage burst size, or adsorption efficiency but rather from genuine prophage induction.
Neither pDR210 (rcsA+) nor pDR310 (dsrA+) significantly increased the concentration of λc in the culture medium, showing that the burst of λc mutants is independent of RcsA and DsrA. This once again implies that the observed increase in the concentration of λc+ free phage reflects true induction of λ+ prophage, since other factors would be expected to affect λc+ and λc titers similarly.
Genetics of RcsA- or DsrA-mediated induction.
To clarify the role of the rcsA, dsrA, and rcsB genes in RecA-independent λ induction, we carried out complementation tests in which lysogenic strains B7252(λ) (rcsA recA), B7307(λ) (dsrA recA), and B7253(λ) (rcsB recA) were tested for their ability to induce the prophage in the presence of rcsA- and dsrA-bearing plasmids (Table 4).
TABLE 4.
Role of the rcsA, dsrA, and rcsB genes in λ inductiona
| Plasmid and gene(s) | Concn (cells/ml) at t1 | Frequency of infective centers (λc+) at t1 | Concn of phage (λc+/ml) at:
|
||
|---|---|---|---|---|---|
| t1 | t2 | t3 | |||
| B7252(λ) (rcsA recA) | |||||
| No plasmid | 2.5 × 108 | 2.9 × 10−7 | 70 | 40 | 40 |
| pDR211 (rcsA::ΔTn903) | 1.7 × 108 | 1.4 × 10−7 | 70 | 60 | 20 |
| pDR311 (dsrA::ΔTn903) | 1.3 × 108 | 8.0 × 10−8 | 70 | 60 | 30 |
| pDR210 (rcsA+) | 1.3 × 108 | 1.5 × 10−6 | 5.0 × 102 | 4.0 × 102 | 2.0 × 102 |
| pDR311 (dsrA+) | 9.0 × 107 | 1.2 × 10−5 | 2.0 × 102 | 1.5 × 103 | 4.0 × 103 |
| B7307(λ) (dsrA recA) | |||||
| No plasmid | 1.0 × 108 | 1.1 × 10−7 | 10 | 20 | 1.0 × 102 |
| pDR211 (rcsA::ΔTn903) | 9.0 × 107 | 3.0 × 10−7 | 30 | 30 | 60 |
| pDR311 (dsrA::ΔTn903) | 9.0 × 107 | 4.7 × 10−7 | 50 | 60 | 1.5 × 102 |
| pDR210 (rcsA+) | 1.2 × 108 | 9.1 × 10−7 | 8.0 × 102 | 9.0 × 102 | 3.0 × 102 |
| pDR311 (dsrA+) | 1.1 × 108 | 8.9 × 10−6 | 8.0 × 102 | 1.1 × 103 | 3.7 × 103 |
| B7253(λ) (rcsB recA) | |||||
| No plasmid | 2.5 × 108 | 2.3 × 10−7 | 20 | 50 | 30 |
| pDR211 (rcsA::ΔTn903) | 1.0 × 108 | 1.0 × 10−7 | 10 | 30 | 20 |
| pDR311 (dsrA::ΔTn903) | 9.0 × 107 | 1.6 × 10−7 | 10 | 40 | 20 |
| pDR210 (rcsA+) | 1.2 × 108 | 1.9 × 10−7 | 20 | 40 | 10 |
| pDR311 (dsrA+) | 1.4 × 108 | 5.9 × 10−6 | 4.0 × 102 | 1.0 × 103 | 4.0 × 103 |
Induction mediated by RcsA, as seen from the frequency of infective centers and the free-phage concentration, does not require intact chromosomal rcsA and dsrA genes but is strictly dependent on an intact rcsB gene (Table 4). It is clear that the activation of cps transcription and prophage induction by RcsA have common regulatory steps.
Since DsrA overproduction stimulates RcsA synthesis, we expected DsrA induction to reflect the same genetic requirements. However, we found a 10- to 100-fold increase in the frequency of infective centers in both rcsA recA mutants and rcsB recA mutants compared to control cells (Table 4). This observation suggests that induction by DsrA is independent of the RcsABC system.
For DsrA-mediated induction, there was a difference between the exponential and stationary phases of growth, as seen from the free-phage concentration. In exponential phase, rcsA and rcsB mutations reduced the effect of pDR310 (dsrA+) about 10-fold, compatible with the positive action of DsrA RNA on rcsA transcription. However, in stationary phase there was a marked increase in the titer of λc+ free phage in rcsA and rcsB mutants carrying a dsrA+ plasmid. This observation is difficult to reconcile with the hypothesis that DsrA RNA causes prophage induction only through its positive regulation of rcsA transcription (antagonizing repression by H-NS). It suggests that in the late phase of cell growth, an increased level of DsrA RNA induces λ prophage via an RcsA-independent pathway.
The levels of λc in all experiments were similar (data not shown).
Effect of λ repressor overproduction on RcsA-mediated induction.
If the RcsABC system provides a repressor bypass for the various phages, overproduction of repressor would not be expected to affect induction. If, on the other hand, some component of the RcsABC system (or a gene product induced by these activators) interacts directly with the various phage repressors, then repressor overproduction should reduce induction frequency. Using a multicopy plasmid pDR1 (cI+), we overproduced λ repressor in recA rcsC137(λ) and recA lon(λ) lysogens. Induction was completely abolished (data not shown). This suggests that RcsA-mediated induction involves repressor inactivation. The same plasmid in recA lon strains that are lysogenic for the heteroimmune phages 21, φ80, and 434 had no effect on induction. Similar specificity has been shown for SOS induction, where excess λ cI, although preventing λ induction, does not saturate the system since 434 repressor can still be inactivated (1).
Influence of cIind mutations on λ induction by RcsA and DsrA.
To clarify the mechanism of prophage induction by RcsA and DsrA, we tested the inducibility of mutant λcIind prophages, described by Gimble and Sauer (8). The repressors of all λcIind mutants used—λGR185, λEK117, λDY125, and λGE112—have a defect in RecA-stimulated proteolysis, but all except λGE112 (mutated in the cleavage site) can carry out self cleavage, at various efficiencies, under alkaline condition in vitro (9). Since the λcIind mutants all carried the b2 deletion, covering the att site, we crossed them with λimm434 plac5 to obtain λcIind plac5 att+ recombinants, which we tested for inducibility (Table 5).
TABLE 5.
Efficiency of induction of λcIind prophagesa
| Plasmid and gene(s) | Concn (cells/ml) at t1 | Frequency of infective centers (λc+)b at t1 | Concn of phage (λc+/ml)b at:
|
||
|---|---|---|---|---|---|
| t1 | t2 | t3 | |||
| C600ΔrecA(λ plac5) | |||||
| No plasmid | 2.3 × 108 | 9.0 × 10−9 | 1.5 × 102 | 1.3 × 102 | 1.6 × 102 |
| pDR211 (rcsA::ΔTn903) | 2.4 × 108 | 3.3 × 10−8 | 2.8 × 102 | 1.8 × 102 | 3.7 × 102 |
| pDR311 (dsrA::ΔTn903) | 1.4 × 108 | 1.5 × 10−8 | 1.0 × 102 | 1.0 × 102 | 1.4 × 102 |
| pDR210 (rcsA+) | 1.8 × 108 | 2.0 × 10−7 | 1.7 × 103 | 9.2 × 102 | 4.3 × 102 |
| pDR311 (dsrA+) | 1.4 × 108 | 1.6 × 10−6 | 8.0 × 102 | 3.1 × 103 | 8.5 × 103 |
| C600 (λDY125 plac5) | |||||
| No plasmid | 3.6 × 108 | 4.0 × 10−8 | 3.0 × 102 | 1.7 × 102 | 1.6 × 102 |
| pDR211 (rcsA::ΔTn903) | 2.8 × 108 | 3.6 × 10−8 | 1.7 × 102 | 2.0 × 102 | 2.1 × 102 |
| pDR311 (dsrA::ΔTn903) | 3.3 × 108 | 1.8 × 10−8 | 2.9 × 102 | 2.4 × 102 | 2.1 × 102 |
| pDR210 (rcsA+) | 3.6 × 108 | 1.1 × 10−7 | 4.3 × 103 | 2.2 × 103 | 6.2 × 102 |
| pDR311 (dsrA+) | 2.7 × 108 | 2.5 × 10−7 | 1.4 × 103 | 4.0 × 103 | 2.9 × 103 |
| C600(λGE112 plac5) | |||||
| No plasmid | 1.8 × 108 | 2.0 × 10−8 | 90 | 50 | 50 |
| pDR211 (rcsA::ΔTn903) | 2.9 × 108 | 2.0 × 10−8 | 50 | 30 | 40 |
| pDR311 (dsrA::ΔTn903) | 2.2 × 108 | 2.0 × 10−8 | 50 | 30 | 30 |
| pDR210 (rcsA+) | 2.0 × 108 | 4.0 × 10−8 | 3.6 × 102 | 6.0 × 102 | 2.0 × 102 |
| pDR311 (dsrA+) | 2.3 × 108 | 3.8 × 10−7 | 1.2 × 103 | 9.4 × 102 | 7.9 × 103 |
As can be seen in Table 5, spontaneous induction of λDY125 plac5 in exponential phase increases two- to tenfold when the cells carry pDR210 (rcsA+) or pDR311 (dsrA+), as judged by the frequency of infective centers and the level of free phage. This observation shows that the action of RcsA and DsrA on the prophage is not inhibited by the cIind mutation. Similar results were obtained with λGR185 plac5 and λEK117 plac5 (data not shown). However, the action of RcsA was lowered in the case of prophage λGE112 plac5 (Table 5). As mentioned above, the repressor of this phage is mutated in the cleavage site and differs from the other cIind proteins in that it does not undergo self cleavage under alkaline condition in vitro (9). This result suggests that prophage induction in exponential phase is by repressor inactivation, possibly reflecting the action of an alternative RecA coprotease.
As shown in Table 5, on entry to stationary phase pDR311 (dsrA+) causes a similar, severalfold increase in the concentration of free phage with λplac5 and λcIind. The effects of the plasmid were the same on all λcIind phages. DsrA RNA thus acts on the prophage independently of cIind mutations in the repressor gene.
The amounts of λc mutants differed little among the strains tested (data not shown).
DISCUSSION
Prophage stability is coupled to cell physiology. Under conditions in which cell viability is jeopardized, the prophage may manage to survive by entering the lytic pathway. The paradigm for lysogenic induction—and in fact the only induction mechanism known at present to exist—is the SOS response and its positive regulator, the RecA protein. In response to DNA damage, RecA stimulates the autoproteolytic cleavage of certain prophage repressors (28).
Other stress responses of the bacterial cell, alongside the SOS response, may also induce certain prophages, allowing them to abandon a host in physiological difficulty. Under laboratory conditions, these putative systems of lysogenic induction are likely to be inefficient unless the right stress is applied. Here we present evidence that this is indeed the case for E. coli. In particular, we have demonstrated RecA-independent induction of λ and other lambdoid prophages by overproduction of RcsA, a transcriptional regulator of the cps operons involved in capsular polysaccharide synthesis (10, 12, 13, 34), and by overproduction of DsrA, an 85-nucleotide RNA able to antagonize H-NS repression (31). In both cases, the numbers of free phage and of infective centers in overproducing strains were increased 10- to 100-fold compared to those in control strains.
The synthesis of capsular polysaccharide, or colanic acid, is regulated by the two component system RcsC-RcsB, in which RcsC, a membrane protein, is similar to histidine kinase sensor proteins and RcsB, which is absolutely required for cps gene expression, is similar to DNA-binding effectors (33). RcsA, and probably RcsF as well (7), are additional transcriptional activators of the Cps regulon. The RcsA level is regulated by H-NS repression of rcsA, which is antagonized by DsrA RNA (31), and by degradation by protease Lon (34, 36).
We show here that the RcsABC-dependent system induces λ, 21, φ80, and 434, and its action may well extend to other lambdoid phages and possibly also to nonlambdoid, SOS-inducible phages. It is interesting that spontaneous induction of lambdoid phages is principally via the SOS response, as evinced by its sharp reduction in recA lysogens (cf. Table 3). The second mechanism seems to be via RcsABC, since induction is essentially abolished in recA rcsB(λ) lysogens (cf. Table 4).
At present we have no information as to the nature of the environmental signal transduced by the RcsABC system that stimulates capsular polysaccharide synthesis. Nevertheless, all strains with genetic backgrounds that resulted in increased cps gene expression also displayed RecA-independent prophage induction. These include strains overproducing RcsA or DsrA from multicopy plasmids, lon and rcsA3 mutants in which RcsA is stabilized (and therefore present at high levels), and the cps constitutive mutant rcsC137, thought to phosphorylate RcsB spontaneously. It is a curious fact that, although lon mutants have higher cps expression than the rcsA3 mutant (3), the latter strain has a higher level of λ induction (Table 3). This suggests that prophage induction does not result from transcriptional activation of a cps operon.
Prophage induction by the RcsABC system, which takes place in exponential phase, depends on the concentration of repressor in the cells. One can speculate that this induction results either from a lowering of cI repressor synthesis from the promoter PRM or from decreased repressor activity via a direct interaction between cI and some cell protein(s) whose synthesis induces the RcsABC system. The second hypothesis seems more likely since the mutation in phage λGE112, which blocks self cleavage of the repressor, lowers prophage induction via RcsA. The RcsABC system may regulate the expression of an alternative RecA coprotease. The known homology between RcsA and the LuxR family of transcriptional activators (34) suggests the activation of the gene of an alternative RecA coprotease. In addition, the complete dependence of RcsA action on the presence of an intact rcsB gene and the effect of the rcsC137 mutation also argue in favor of this proposition.
Prophage induction in the presence of excess DsrA RNA in exponential phase depends on the presence of intact rcsA and rcsB genes. This indicates that the action of DsrA RNA on the prophage in exponentially growing cells is via rcsA transcription. However, on entry to stationary phase, as we have seen, induction by DsrA RNA no longer depends on the RcsABC system. This leads us to postulate the existence of a second induction pathway of λ prophage by DsrA RNA, independent of both RecA and the RcsABC system. Furthermore, this pathway is not affected by the presence of a cIind mutation in the prophage repressor gene, even if the normal cleavage site is mutated. DsrA action on the prophage in this phase of growth is probably at the transcriptional level, possibly creating a repressor bypass.
Retallack and Friedman (27) have reported that 10Sa RNA, the ssrA gene product, binds to λ cI repressor, competing with binding to the operator OR2 and reducing repression. It is not known whether an excess of this small RNA, like DsrA, can cause RecA-independent prophage induction. However, the recently established role of 10Sa RNA in tagging proteins produced from truncated mRNA molecules (16) suggests that gene regulation is not its primary function.
DsrA RNA was originally discovered because of its ability, when overproduced, to derepress rcsA expression (31). It was shown to relieve turning off by H-NS of several operons, including rcsA and rpoS, the structural gene of sigma S (31, 32). Derepression of rcsA is clearly involved in DsrA-mediated prophage induction in exponential-phase cells, since chromosomal rcsA and rcsB mutations lead to a significant drop in the induction level (Table 4). In stationary phase, however, excess DsrA causes efficient prophage induction even in the absence of RcsA and RcsB (Table 4). Stationary-phase cells normally have high levels of both H-NS (6) and RpoS (15). DsrA increases the amount of RpoS (32) and antagonizes H-NS action (31), which itself normally decreases the amount of RpoS (2, 41). Since both H-NS and RpoS regulate a number of operons, DsrA overexpression, with a larger-than-normal increase in RpoS and less-efficient action of H-NS, could radically alter the pattern of gene expression in stationary phase. How this configuration results in prophage induction remains to be shown.
Induction of λ prophage via RecA-assisted repressor cleavage was an important step forward in elucidating the molecular regulation of the SOS response. We have presented here two new, RecA-independent systems of prophage induction. One is observed in stationary phase in the presence of excess DsrA RNA and probably involves sigma S and the histone-like protein H-NS; its characterization will add to our knowledge of gene expression in stationary phase. The other induces prophages via RcsABC regulatory network; knowledge of this circuit should help reveal the environmental signal inducing capsular polysaccharide synthesis. It is likely that additional mechanisms of lysogenic induction exist. Our search, for example, would not have identified systems inoperative on λ or systems that require RecA in a non-SOS role. A complete catalogue of lysogenic induction mechanisms would reveal what aspects of host physiology viruses are sensitive to and would provide new tools for elucidating the molecular signals indicating cellular disorders.
ACKNOWLEDGMENTS
We thank S. Gottesman, R. Sauer, and Y. Kohara for generously providing bacterial strains, plasmids, and phages.
REFERENCES
- 1.Bailone A, Levine A, Devoret R. Inactivation of prophage λ repressor in vivo. J Mol Biol. 1979;131:553–572. doi: 10.1016/0022-2836(79)90007-x. [DOI] [PubMed] [Google Scholar]
- 2.Barth M, Marschall C, Muffler A, Fisher D, Hengge-Aronis R. Role for the histone-like protein H-NS in growth phase-dependent and osmotic regulation of ςS and many ςS-dependent genes in Escherichia coli. J Bacteriol. 1995;177:3455–3464. doi: 10.1128/jb.177.12.3455-3464.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brill J A, Quinlan-Walshe C, Gottesman S. Fine-structure mapping and identification of two regulators of capsule synthesis in Escherichia coli K-12. J Bacteriol. 1988;170:2599–2611. doi: 10.1128/jb.170.6.2599-2611.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Close T J, Rodriguez R L. Construction and characterization of the chloramphenicol-resistance gene cartridge: a new approach to the transcriptional mapping of extrachromosomal elements. Gene. 1982;20:305–316. doi: 10.1016/0378-1119(82)90048-8. [DOI] [PubMed] [Google Scholar]
- 5.Daniels D L, Sanger F, Coulson A R. Features of bacteriophage λ: analysis of the complete nucleotide sequence. Cold Spring Harbor Symp Quant Biol. 1983;48:1009–1024. doi: 10.1101/sqb.1983.047.01.115. [DOI] [PubMed] [Google Scholar]
- 6.Dersch P, Schmidt K, Bremer E. Synthesis of the Escherichia coli K-12 nucleoid-associated DNA-binding protein H-NS is subject to growth-phase control and autoregulation. Mol Microbiol. 1993;8:875–889. doi: 10.1111/j.1365-2958.1993.tb01634.x. [DOI] [PubMed] [Google Scholar]
- 7.Gervais F G, Drapeau G R. Identification, cloning, and characterization of rcsF, a new regulator gene for exopolysaccharide synthesis that suppresses the division mutation ftsZ84 in Escherichia coli K-12. J Bacteriol. 1992;174:8016–8022. doi: 10.1128/jb.174.24.8016-8022.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gimble F S, Sauer R T. Mutations in bacteriophage λ repressor that prevent RecA-mediated cleavage. J Bacteriol. 1985;162:147–154. doi: 10.1128/jb.162.1.147-154.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gimble F S, Sauer R T. λ repressor inactivation: properties of purified ind− proteins in the autodigestion and RecA-mediated cleavage reactions. J Mol Biol. 1986;192:39–47. doi: 10.1016/0022-2836(86)90462-6. [DOI] [PubMed] [Google Scholar]
- 10.Gottesman S. Regulation of capsule synthesis: modification of the two-component paradigm by an accessory unstable regulator. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C: American Society for Microbiology; 1995. pp. 253–262. [Google Scholar]
- 11.Gottesman S, Gottesman M, Shaw J E, Pearson M L. Protein degradation in E. coli: the lon mutation and bacteriophage lambda N and cII protein stability. Cell. 1981;24:225–233. doi: 10.1016/0092-8674(81)90518-3. [DOI] [PubMed] [Google Scholar]
- 12.Gottesman S, Stout V. Regulation of capsular polysaccharide synthesis in Escherichia coli K12. Mol Microbiol. 1991;5:1599–1606. doi: 10.1111/j.1365-2958.1991.tb01906.x. [DOI] [PubMed] [Google Scholar]
- 13.Gottesman S, Trisler P, Torres-Cabassa A. Regulation of capsular polysaccharide synthesis in Escherichia coli K-12: characterization of three regulatory genes. J Bacteriol. 1985;162:1111–1119. doi: 10.1128/jb.162.3.1111-1119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groisman E A, Casadaban M J. Mini-Mu bacteriophage with plasmid replicon for in vivo cloning and lac gene fusing. J Bacteriol. 1986;168:357–364. doi: 10.1128/jb.168.1.357-364.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hengge-Aronis R. Regulation of gene expression during entry into stationary phase. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C, Low K B, Magasanic B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1996. pp. 1497–1512. [Google Scholar]
- 16.Keiler K C, Waller P R, Sauer R T. A peptide tagging system in degradation of proteins synthesized from damaged messenger RNA. Science. 1996;271:990–993. doi: 10.1126/science.271.5251.990. [DOI] [PubMed] [Google Scholar]
- 17.Kohara Y, Akiyama K, Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987;50:495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- 18.Little J W. Mechanism of specific LexA cleavage: autodigestion and the role of RecA coprotease. Biochimie. 1991;73:411–422. doi: 10.1016/0300-9084(91)90108-d. [DOI] [PubMed] [Google Scholar]
- 19.Lwoff A. Lysogeny. Bacteriol Rev. 1953;17:269–237. doi: 10.1128/br.17.4.269-337.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lwoff A, Siminovitch L, Kjeldgaard N. Induction de la production de bactériophages chez une bactérie lysogène. Ann Inst Pasteur (Paris) 1950;79:815–859. [PubMed] [Google Scholar]
- 21.Markovitz A. Genetics and regulation of bacterial capsular polysaccharide synthesis and radiation sensitivity. In: Sutherland I, editor. Surface carbohydrates of the prokaryotic cell. London, United Kingdom: Academic Press, Ltd.; 1977. pp. 415–462. [Google Scholar]
- 22.McEntee K, Epstein W. Isolation and characterization of specialized transducing bacteriophages for the recA gene of Escherichia coli. Virology. 1977;77:306–318. doi: 10.1016/0042-6822(77)90427-5. [DOI] [PubMed] [Google Scholar]
- 23.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 24.Moreau P, Bailone A, Devoret R. Prophage λ induction in Escherichia coli K12 envA uvrB: a highly sensitive test for potential carcinogens. Proc Natl Acad Sci USA. 1976;73:3700–3704. doi: 10.1073/pnas.73.10.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogava T, Tomizava J I. Abortive lysogenization of bacteriophage lambda b2 and residual immunity of non-lysogenic segregants. J Mol Biol. 1967;23:225–245. doi: 10.1016/s0022-2836(67)80030-5. [DOI] [PubMed] [Google Scholar]
- 26.Parker B, Marinus M G. A simple and rapid method to obtain substitution mutations in Escherichia coli: isolation of a dam deletion/insertion mutation. Gene. 1988;73:531–535. doi: 10.1016/0378-1119(88)90517-3. [DOI] [PubMed] [Google Scholar]
- 27.Retallack D M, Friedman D I. A role for a small stable RNA in modulating the activity of DNA-binding proteins. Cell. 1995;83:227–235. doi: 10.1016/0092-8674(95)90164-7. [DOI] [PubMed] [Google Scholar]
- 28.Roberts J, Devoret R. Lysogenic induction. In: Hendrix R W, Roberts J W, Stahl F W, Weisberg R A, editors. Lambda II. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1983. pp. 123–144. [Google Scholar]
- 29.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 30.Silhavy T J, Berman M L, Enquest L W. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1984. [Google Scholar]
- 31.Sledgjeski D, Gottesman S. A small RNA acts as antisilencer of the H-NS-silenced rcsA gene of Escherichia coli. Proc Natl Acad Sci USA. 1995;92:2003–2007. doi: 10.1073/pnas.92.6.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sledgjeski D, Gupta A, Gottesman S. The small RNA, DsrA, is essential for the low temperature expression of RpoS during exponential growth in Escherichia coli. EMBO J. 1996;15:3993–4000. [PMC free article] [PubMed] [Google Scholar]
- 33.Stout V, Gottesman S. RcsB and RcsC: a two-component regulator of capsule synthesis in Escherichia coli. J Bacteriol. 1990;172:659–669. doi: 10.1128/jb.172.2.659-669.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stout V, Torres-Cabassa A, Maurizi M R, Gutnick D, Gottesman S. RcsA, an unstable positive regulator of capsular polysaccharide synthesis. J Bacteriol. 1991;173:1738–1747. doi: 10.1128/jb.173.5.1738-1747.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toman Z, Dambly-Chaudière C, Tenenbaum L, Radman V. A system for detection of genetic and epigenetic alterations in Escherichia coli induced by DNA-damaging agents. J Mol Biol. 1985;186:97–105. doi: 10.1016/0022-2836(85)90260-8. [DOI] [PubMed] [Google Scholar]
- 36.Torres-Cabassa A S, Gottesman S. Capsule synthesis in Escherichia coli K-12 is regulated by proteolysis. J Bacteriol. 1987;169:981–989. doi: 10.1128/jb.169.3.981-989.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vieira J, Messing J. The pUC plasmids, an M13 mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982;19:259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 38.Walker G C. Mutagenesis and inducible responses to DNA damage in Escherichia coli. Microbiol Rev. 1984;48:60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walker G C. The SOS response of Escherichia coli. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C, Low K B, Magasanic B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1996. pp. 1400–1416. [Google Scholar]
- 40.Way J C, Davis M S, Morisato D, Roberts D E, Kleckner N. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene. 1984;32:369–379. doi: 10.1016/0378-1119(84)90012-x. [DOI] [PubMed] [Google Scholar]
- 41.Yamashino T, Ueguchi C, Mizuno T. Quantitative control of the stationary phase specific sigma factor, ςS, in Escherichia coli: involvement of the nucleoid protein H-NS. EMBO J. 1995;14:594–602. doi: 10.1002/j.1460-2075.1995.tb07035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zissler J, Signer E, Schaffer F. The role of recombination in growth of bacteriophage lambda. I. The gamma gene. In: Hershey A D, editor. The bacteriophage lambda. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1971. pp. 455–468. [Google Scholar]