Abstract
Background:
CTLA-4 impedes the immune system’s antitumor response. There are two Food and Drug Administration-approved anti-CTLA-4 agents – ipilimumab and tremelimumab – both used together with anti-PD-1/PD-L1 agents.
Objective:
To assess the prognostic implications and immunologic correlates of high CTLA-4 in tumors of patients on immunotherapy and those on non-immunotherapy treatments.
Design/methods:
We evaluated RNA expression levels in a clinical-grade laboratory and clinical correlates of CTLA-4 and other immune checkpoints in 514 tumors, including 489 patients with advanced/metastatic cancers and full outcome annotation. A reference population (735 tumors; 35 histologies) was used to normalize and rank transcript abundance (0–100 percentile) to internal housekeeping gene profiles.
Results:
The most common tumor types were colorectal (140/514, 27%), pancreatic (55/514, 11%), breast (49/514, 10%), and ovarian cancers (43/514, 8%). Overall, 87 of 514 tumors (16.9%) had high CTLA-4 transcript expression (⩾75th percentile rank). Cancers with the largest proportion of high CTLA-4 transcripts were cervical cancer (80% of patients), small intestine cancer (33.3%), and melanoma (33.3%). High CTLA-4 RNA independently/significantly correlated with high PD-1, PD- L2, and LAG3 RNA levels (and with high PD-L1 in univariate analysis). High CTLA-4 RNA expression was not correlated with survival from the time of metastatic disease [N = 272 patients who never received immune checkpoint inhibitors (ICIs)]. However, in 217 patients treated with ICIs (mostly anti-PD-1/anti-PD- L1), progression-free survival (PFS) and overall survival (OS) were significantly longer among patients with high versus non-high CTLA-4 expression [hazard ratio, 95% confidence interval: 0.6 (0.4–0.9) p = 0.008; and 0.5 (0.3–0.8) p = 0.002, respectively]; results were unchanged when 18 patients who received anti-CTLA-4 were omitted. Patients whose tumors had high CTLA-4 and high PD-L1 did best; those with high PD-L1 but non-high CTLA-4 and/or other expression patterns had poorer outcomes for PFS (p = 0.004) and OS (p = 0.009) after immunotherapy.
Conclusion:
High CTLA-4, especially when combined with high PD-L1 transcript expression, was a significant positive predictive biomarker for better outcomes (PFS and OS) in patients on immunotherapy.
Keywords: biomarker identification, biomarker validation, genomic signatures, immunotherapy biomarkers, prognostic biomarkers, response biomarkers
Plain language summary
High CTLA-4 expression and immunotherapy outcome
High CTLA-4 expression was not a prognostic factor for survival in patients not receiving ICIs but was a significant positive predictive biomarker for better outcome (PFS and OS) in patients on immunotherapy, perhaps because it correlated with expression of other checkpoints such as PD-1 and PD-L2.
Introduction
James Allison won the 2018 Nobel Prize in Physiology for his work on cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) and pioneered the dawn of immunotherapy for the treatment of many cancers.1,2 In March 2011, the Food and Drug Administration (FDA) approved the anti-CTLA-4 monoclonal antibody ipilimumab (Yervoy, Bristol-Myers Squibb) for advanced melanoma treatment, based on a large, randomized phase III clinical trial demonstrating that ipilimumab increased overall survival (OS) in melanoma patients who did not respond to standard therapy. 3 Subsequently, another anti-CTLA-4 antibody – tremelimumab – was approved (together with the anti-PD-L1 durvalumab) for non-small-cell lung cancer (NSCLC) and locally advanced or metastatic hepatocellular carcinoma.4,5
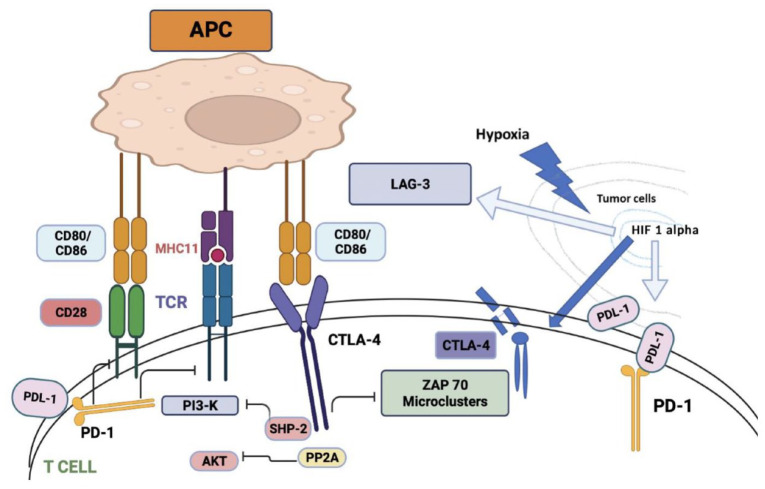
Cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) is a membrane glycoprotein expressed by activated effector T cells. CTLA-4 dampens immune function by inhibiting T-cell proliferation, cell cycle progression, and cytokine (IL-2, IFN-) production 6 (Figure 1). Both CD4+ and CD8+ T cells carry the homologous receptors cytotoxic T-lymphocyte antigen 4 (CTLA-4), (CD152), and CD28, which mediate opposing activities in T-cell activation.7,8
Figure 1.
CTLA-4 or cytotoxic T-lymphocyte-associated antigen 4 is a protein found on T cells that downregulate the immune response. CTLA-4 and PD-1 negatively regulate T-cell activation. CD28 mediates TCR and MHCII complex attachment. CTLA-4 acts as a competitive homolog to CD28 and binds to CD80/CD86, ligands for CD28, thereby preventing T-cell activation. PD-1 binding to PD-L1 also negatively regulates T-cell activation. Both pathways are activated by the activation of TCR. The phosphatase Src homology region-2 containing protein tyrosine phosphatase (SHP-2) is recruited and inhibits PI3K-A downstream signaling. CTLA-4 also reacts with serine/threonine phosphatase PP2A which dephosphorylates AKT and contributes to further inhibition of T-cell activation. CTLA-4 also blocks TCR induction of ZAP-70 micro-cluster formation. Tumor hypoxia-induced HIF-1-alpha stabilization upregulates the expression of CTLA-4, LAG-3, and PD-L1. Figure created in BioRender.
MHCII, major histocompatibility antigen; TCR, T-cell receptor.
Patients with cancer express increased numbers of T regulatory (Treg) cells; Tregs are CD4 T cells that constitutively express CTLA-4, preventing the body’s normal protective immune surveillance and impeding the immune system’s antitumor response.9,10 When Treg cells are depleted in mice, there is both a decrease in tumor growth rate and the development of increased endothelial venules, which indicate the destruction of tumor tissue. 11 Antigen-presenting cells that would normally play a role in anti-tumor immune surveillance are turned off by CTLA-4 once it has bound to B7-1 (CD80) or B7-2 (CD86). 12 CTLA-4 acts as a competitive homolog to CD28 and binds with greater avidity to CD80 and CD86, ligands for CD28, thereby preventing T-cell activation as CD28 mediates T-cell receptor (TCR) and major histocompatibility antigen (MHCII) complex attachment. 13 Thus, anti-CTLA-4 therapy can awaken the suppressed immune system by removing the competition for CD80 and CD86 and overcoming the hijacking of the immune system by cancer cells.
Monotherapy with anti-CTLA-4 agents may not be as efficacious as combination therapy with both anti-CTLA-4 and anti-PD-1/PD-L1 antibodies. 14 For instance, the anti-PD-1 nivolumab together with the anti-CTLA-4 ipilimumab are FDA approved for use in metastatic melanoma, advanced renal cell carcinoma, pleural mesothelioma, NSCLC, hepatocellular cancer, and colorectal cancer with high microsatellite instability (MSI-H).15 –19 Other approved anti-CTLA-4 agents include tremelimumab in combination with durvalumab (anti-PD-L1) and chemotherapy, which outperformed chemotherapy alone in patients with previously untreated metastatic NSCLC. 4 In 2022, the FDA also approved tremelimumab in combination with durvalumab for unresectable hepatocellular carcinoma. 5 Other novel agents targeting CTLA-4 include the humanized IgG1 anti-CTLA-4 monoclonal antibodies zalifrelimab (AGEN1884) and quavonlimab, which are in clinical trials for advanced cervical cancer and renal cell carcinoma, respectively20 –25 (Supplemental Table 1).
The role of PD-L1 as a tissue biomarker for response to anti-PD-1/PD-L1 therapies has been well studied, but there is a lack of research into the role of tissue CTLA-4 as a biomarker for outcomes. 26 There is however data to suggest that higher serum soluble CTLA-4 levels may predict ipilimumab response as well as immune-related adverse events. 27 The aim of this study was to assess the prognostic implications and immunologic correlates of high CTLA-4 in tumors of patients on immunotherapy as well as those on non-immunotherapy treatments.
Methods
Patients
Overall, 514 samples of solid tumors from the University of California, San Diego (UCSD) Moores Center for Personalized Cancer Therapy clinic were examined for CTLA-4 RNA expression levels at the clinical laboratory OmniSeq (https://www.OmniSeq.com/), which is licensed under the Clinical Laboratory Improvement Amendments (CLIA) and accredited by the College of American Pathologists (CAP). The primary cancers’ histological subtypes, patients’ ages, sexes, tumor mutational burdens (TMB), and levels of the anti-apoptotic protein PD-L1 (PD-L1) were all recorded. The earliest timestamped sample was used in this analysis if multiple distinct samples belonging to the same subject were examined on successive days. Study of Personalized Cancer Treatment to Determine Response and Toxicity, UCSD PREDICT, (NCT02478931) was carried out in conformity with the regulations of the UCSD Institutional Review Board, and any experimental interventions for which patients provided consent.
Tissue sampling and examination of cancer immunity markers
Following tumor collection, the samples were processed at the OmniSeq laboratory. Formalin-fixed, paraffin-embedded (FFPE) tissues were examined for RNA sequence. Using the truXTRAC FFPE extraction kit (Covaris, Inc., Woburn, MA), the RNA was extracted from FFPE mostly following the manufacturer’s protocol. The RNA was dissolved in 50 l of water after purification and, by the manufacturer’s instructions, the yield was determined using the Quant-iT RNA HS assay (Thermo Fisher Scientific, Waltham, MA). The pre-determined titer of 10 ng of RNA was approved for library creation. The immuneResponseRNA (v5.2.0.0, Thermo Fisher Scientific) 34 plugin for Torrent Suite was used for RNA expression absolute read count estimation. Custom scripts were used to conduct background subtraction, percentile ranking, and normalization.
Transcript abundance was standardized to a reference dataset consisting of 735 tumors encompassing 35 tumor histologies which were normalized to an internal housekeeping gene profile dataset. The low percentile was defined as 0–24 percentile, the moderate percentile as 25–74 percentile, and the high percentile as 75–100 percentile CTLA-4 RNA expression rank. The expression profiles were classified by transcript abundance rank values into ‘Low/Moderate’ (0–74) and ‘High’ (75–100) percentile.
For determination of tumor mutation burden (TMB) (determined at OmniSeq), genomic DNA was extracted from qualifying FFPE tumors (>30% tumor nuclei) using the truXTRAC FFPE extraction kit (Covaris), with 10 ng DNA input for library creation, to study TMB. Using the Comprehensive Cancer Panel, Ion AmpliSeq targeted sequencing chemistry was used to prepare DNA libraries, which were then prepared for enrichment and template preparation with the Ion Chef system, and then sequenced on the Ion S5XL 540 chip (Thermo Fisher Scientific). TMB was represented using eligible mutations per qualified panel size (mutations/megabase) after synonymous variants, indels, germline variants, and single nucleotide variations that had 5% variant allele fraction were deleted.
Outcome variables and statistical analysis
Patient characteristics and the expression pattern of immune markers were summarized by descriptive statistics. To investigate the correlation between CTLA-4 transcript levels and cancer diagnoses, as well as other immunomic markers, we performed univariable and subsequently multivariable analyses. Survival analyses were performed for patients with survival information using the Kaplan–Meier method. Overall survival (OS), when evaluated as a prognostic marker, was defined as the duration from the date of metastatic or locally advanced disease to the date of the last follow-up. The start date of immune checkpoint inhibitors (ICI) ranged from January 2015 to June 2021. The date of metastatic/advanced disease ranged from September 2004 to September 2020. The data cutoff date for the latest database was 14 June 2022. The reporting of this study conforms to STROBE guidelines (Supplemental Material 1).
For ascertaining the effect of ICIs on OS and on progression-free survival (PFS), Kaplan–Meier analysis was also performed. OS in the context of immunotherapy treatment was defined as the duration from the initial date of the immunotherapy to the date of the last follow-up; PFS, as the duration from the initial date of the immunotherapy to the date of the earliest of disease progression (clinical or radiological) or death from any cause. The data cutoff date for the current analysis was 24th June 2022. DN verified statistical analysis. IBM SPSS version 29 and R version 4.2.0 software were employed for statistical analysis. A p value of ⩽0.05 was deemed statistically significant.
Results
Patient characteristics
Overall, 514 patients were analyzed, including 489 patients with advanced/metastatic disease and relevant dates of diagnosis and outcomes available; 217 patients received ICIs sometime during the course of their disease and 272 never received immunotherapy. The median age of the cohort was 61 years (24–93 years), and 60% were women. There was a total of 16 tumor types with five or more samples per histology. The most common tumor types were colorectal cancer (27%), pancreatic cancer (11%), breast cancer (10%), and ovarian cancer (8%) (Supplemental Table 2).
Of 217 patients (42% of 514 patients) receiving ICIs, all but two patients received an anti-PD-1 or anti-PD-L1 agent; two patients received monotherapy with ipilimumab (anti-CTLA-4); altogether, 18 patients received an anti-CTLA-4 agent (including 2 patients as monotherapy, as mentioned, and 16 patients who received it in combination with an anti-PD-1/PD-L1 agent (Supplemental Table 2).
CTLA-4 RNA expression levels varied between and within tumor types
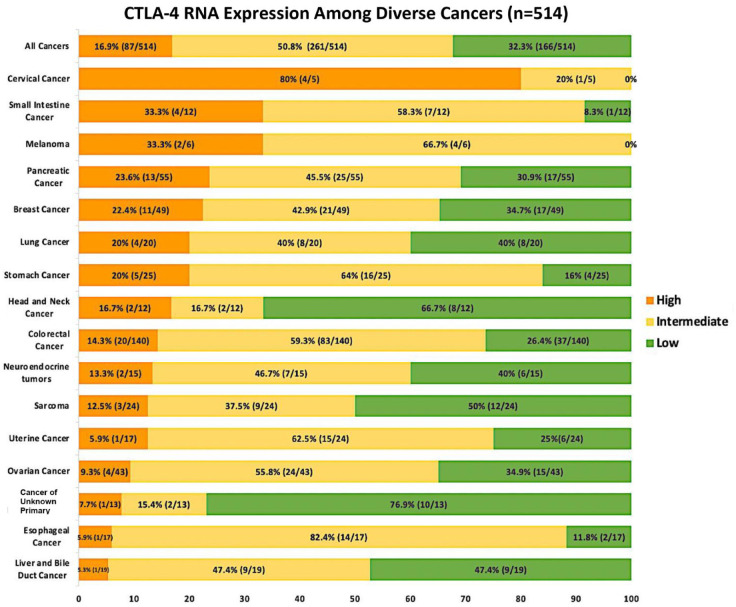
Overall, high CTLA-4 RNA expression (⩾75th percentile rank) was found in 17% of patients (87/514); moderate expression (25–74 percentile rank) in 51% (261/514); and low expression (<25th percentile rank) in 32% of patients (166/514). The tumor types with the greatest expression of CTLA-4 in our cohort included cervical cancer (4/5 or 80%), small intestine (4/12 or 33%), melanomas (2/6 or 33%), pancreatic cancers (13/55 or 24%), and breast cancers (11/49 or 22%; Figure 2).
Figure 2.
High is defined as 75–100 percentile CTLA-4 RNA expression rank; low–moderate is defined as 0–74. Percentages in the bar graph are of patients with that designated level of CTLA-4 RNA expression. Transcript abundance was normalized to an internal housekeeping gene profile dataset and ranked (0–100 percentile rank) in a standardized manner to a reference dataset of 735 tumors spanning 35 tumor histologies. Tumor types with ⩾ 10 samples were included. In addition, melanoma and cervical cancer were included because anti-CTLA-4 treatment is approved for melanoma and in the case of cervical cancer, CTLA-4 expression was high.
High CTLA-4 transcriptomic expression was associated with high PD-L1 RNA expression (univariate analysis) and with high PD-1, PD-L2, and LAG-3 RNA expression (multivariate analysis)
High CTLA-4 transcriptomic expression did not correlate with age (p = 0.7) or sex (p = 0.5) but did correlate significantly on multivariate analysis with high PD-1 (p < 0.0001), high PD-L2 (p = 0.02), high LAG-3 (p = 0.04) transcriptomic expression and with a diagnosis of cervical cancer (p = 0.005). Though high CTLA-4 correlated with high PD-L1 on univariate analysis (p < 0.0001), the correlation was not significant on multivariate analysis (p = 0.7). High CTLA-4 expression also did not correlate with high TMB (⩾10 mutations/mb) (p = 1.0) or other cancer diagnoses (Table 1).
Table 1.
Univariate and multivariate analysis of CTLA-4 clinical and immunomic features and transcriptomic expression (N = 514 patients).
| Feature* | Number of patients with ‘High’ CTLA-4$
(N (%)) |
Odds ratio (OR) for ‘High’ CTLA-4 (95% CI) | Univariate p-value | Multivariate OR for ‘High’ CTLA-4 (95% CI) ‡ | Multivariate p-value |
|---|---|---|---|---|---|
| Age ⩾ 61 years (n = 256) Age < 61 years (n = 258) |
45 (18%) 42 (16%) |
1.1 (0.7–1.7) 0.9 (0.9–1.4) |
p = 0.7 | ||
| Men (N = 204) Women (N = 310) |
32 (16%) 55 (18%) |
0.9 (0.5–1.4) 1.2 (0.7–1.9) |
p = 0.5 | ||
| ‘High’ PD-L1 (n = 67) ‘Low/Moderate’ PD-L1 (n = 447) |
29 (43%) 58 (13%) |
5.1 (2.9–8.9) 0.2 (0.1–0.3) | p < 0.0001 | 1.2 (0.5–2.7) 0.8 (0.4–0.8) |
p = 0.7 |
| ‘High’ PD-1 (n = 93) ‘Low/Moderate’ PD-1 (n = 421) |
54 (58%) 33 (8%) |
16.3 (9.4–28.0) 0.1 (0.03–0.1) |
p < 0.0001 | 9.7 (5.2–18.0) 0.2 (0.1–0.3) |
p < 0.0001 (‘High’ CTLA-4 found more frequently with ‘High’ PD-1) |
| ‘High’ PD-L2 (n = 100) ‘Low/Moderate’ PD-L2 (n = 414) |
40 (40%) 47 (11%) |
5.2 (3.2–8.6) 0.2 (0.1–0.3) | p < 0.0001 | 2.3 (1.2–4.5) 0.4 (0.2–0.9) |
p = 0.02 (‘High’ CTLA-4 found more frequently with ‘High’ PD-L2) |
| ‘High’ LAG-3 (n = 116) ‘Low/Moderate’ LAG-4 (n = 398) |
47 (41%) 40 (10%) |
6.1 (3.7–10.0) 0.2 (0.1–0.3) | p < 0.0001 | 2.0 (1.1–3.9) 0.5 (0.3–0.9) |
p = 0.04 (‘High’ CTLA-4 found more frequently with ‘High’ LAG-3) |
| TMB ⩾ 10 mutations/mb (n = 33) TMB < 10 mutations/mb (n = 417) |
5 (15%) 63 (15%) |
1.0 (0.4–2.7) 1.0 (0.4–2.7) |
p = 1.0 | ||
| Colorectal cancer (n = 140) Other cancers (n = 374) |
20 (14%) 67 (18%) |
0.8 (0.4–1.3) 1.3 (0.8–2.3) |
p = 0.3 | ||
| Breast cancer (n = 49) Other cancers (n = 465) |
11 (22%) 76 (16%) |
1.5 (0.7–3.0) 0.7(0.3–1.4) |
p = 0.3 | ||
| Ovarian cancer (n = 43) Other cancers (n = 471) |
4 (9%) 83 (18%) |
0.5 (0.2–1.4) 2.1 (0.7–6.0) |
p = 0.1 | ||
| Cervical cancer (n = 5) Other cancers (n = 509) |
4 (80%) 83 (16%) |
20.5 (2.3–186.0) 0.04 (0.0–0.4) |
p = 0.002 | 32.7 (2.9–370.2) 0.03 (0.003–0.3) |
p = 0.005 (‘High’ CTLA-4 found more frequently with cervical cancer) |
| Pancreatic cancer (n = 55) Other cancers (n = 459) |
13 (24%) 74 (16%) |
1.6 (0.8–3.1) 0.6 (0.3–1.8) |
p = 0.2 | ||
| Uterine cancer (n = 24) Other cancers (n = 490) |
3 (13%) 84 (17%) |
0.7 (0.2–2.4) 1.4(0.4–5.0) |
p = 0.5 | ||
| Neuroendocrine cancer (n = 15) Other cancers (n = 499) |
2 (13%) 85 (17%) |
0.7 (0.2–3.4) 1.3 (0.3–6.0) |
p = 0.7 | ||
| Lung cancer (n = 20) Other cancers (n = 494) |
4 (20%) 83 (17%) |
1.2 (0.4–3.8) 0.8 (0.3–2.5) |
p = 0.7 | ||
| Sarcoma (n = 24) Other cancers (n = 490) |
3 (13%) 84 (17%) |
0.7 (0.2–2.4) 1.4 (0.4–5.0) |
p = 0.5 | ||
| Melanoma (n = 6) Other cancers (n = 508) |
2 (33%) 85 (17%) |
2.5 (0.4–13.8) 0.4 (0.1–2.2) |
p = 0.3 |
Data for some variables such as TMB was not available for all patients, so the total number of patients in some categories is lower. Only 15 patients had microsatellite instability and thus microsatellite status was not calculated.
High LAG-3 or PD-L1 or PD-1 or PD-L2 or CTLA-4 refers to ⩾75 transcript expression percentile rank; ‘Low/Moderate’ LAG-3 or PD-L1 or PD-1 or PD-L2 or CTLA-4 refers to <75 percentile rank transcript expression.
Variables with p value ⩽ 0.05 were used for multivariate analysis and p values are bolded to indicate significance.
High CTLA-4 transcriptomic expression is not a prognostic factor for overall survival in patients not exposed to immunotherapy
In our analysis, we found that high CTLA-4 expression was not associated with prognosis in the 272 patients who were not on ICIs. The median overall survival (OS) from the date of locally advanced or metastatic disease in the high CTLA-4 expressing group (n = 39) was 46.8 versus 42 months in the low to medium CTLA-4 expression group (n = 233), p = 0.5 [hazard ratio (HR) 0.8 (95% confidence interval 0.5–1.4)] (Figure 3).
Figure 3.
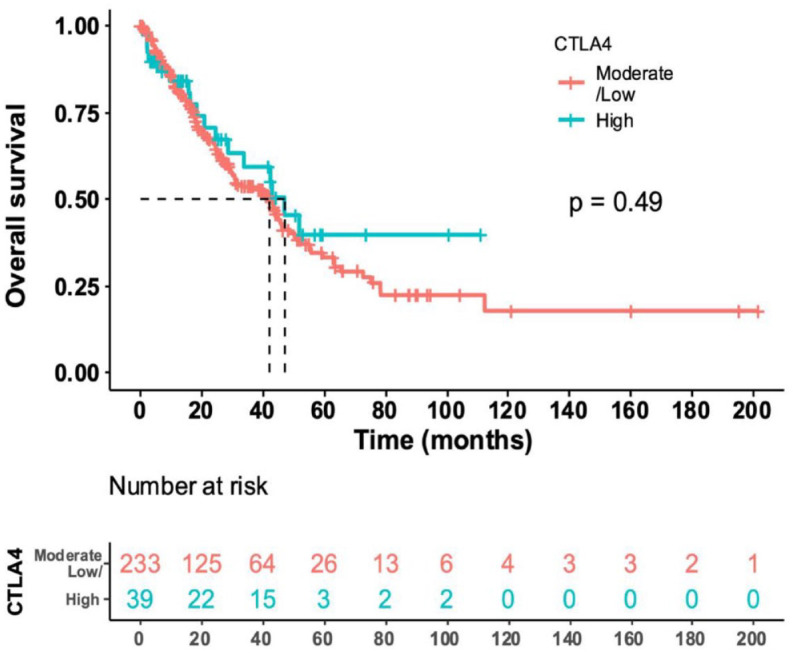
Median OS from date of locally advanced or metastatic disease in patients not treated with an ICI (N = 272 with available data). The median OS in the high CTLA-4 (N = 39) expressed cohort was 46.8 months (28.8 to NE, 95% CI) versus the median OS in the low/moderate CTLA-4 (N = 233) expressed cohort of 42 months [31.2–49.2, 95% CI; HR for high versus low/moderate expression cohort (95% CI) = 0.8 (0.5–1.4, 95% CI; p value = 0.49)]. These results indicate that high versus non-high CTLA-4 was not prognostic for OS.
*NE denotes not estimable.
ICI, immune checkpoint inhibitors; OS, overall survival.
High CTLA-4 transcriptomic expression is associated with better outcomes (longer PFS and OS) in ICI-treated patients
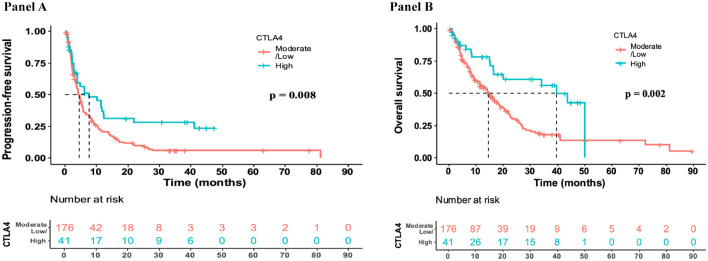
High CTLA-4 expression was found to significantly correlate with PFS in the 217 patients treated with ICI. The median PFS in patients from the date of start of ICI in the high CTLA-4 expression cohort (n = 41) was 7.7 versus 4.6 months in the low to moderate expression cohort (n = 176; Figure 4, Panel A; HR 0.6 (0.4–0.9), p = 0.008).
Figure 4.
Panel A: Median PFS from date of start of ICI (N = 217). High CTLA-4 indicates ⩾ 75th percentile RNA expression; low/moderate CTLA-4 indicates < 75th percentile RNA expression. The median PFS in the high CTLA-4 (N = 41) expressed cohort was 7.7 months (4–21.8 months, 95% CI) versus 4.6 months (3.7–5.3, 95% CI) in the low/moderate CTLA-4 cohort (N = 176) (HR, 95% CI for high versus non-high CTLA-4 PFS was 0.6 [0.4–0.9, 95% CI; p = 0.008]). These results indicate that high versus non-high CTLA-4 was predictive for a longer PFS after immunotherapy. Panel B: Median OS from the date of start of ICI in patients treated with an ICI (N = 217). High CTLA-4 indicates ⩾ 75th percentile RNA expression; low/moderate CTLA-4 indicates < 75th percentile RNA expression. The median OS in the high CTLA-4 (N = 41) expressed cohort was 39.6 months (16.8–NE, 95% CI) versus the median OS in the low/moderate CTLA-4 (N = 176) expressed cohort at 14.5 months (11.4–18.5 months, 95% CI). HR (95% CI) = 0.5 (0.3–0.8; p = 0.002) for high versus non-high CTLA-4 OS. These results indicate high versus non-high CTLA-4 RNA expression was predictive for longer OS after ICI.
ICI, immune checkpoint inhibitors; PFS, progression-free survival.
High CTLA-4 expression was also significantly correlated with better OS in the 217 patients treated with ICI. The median OS in patients from the date of start of ICI in the high CTLA-4 expression cohort (n = 41) was 39.6 versus 14.5 months in the low to moderate expression cohort (n = 176; HR 0.5 (0.3–0.8), p = 0.002; Figure 4, Panel B).
The results above, that is, longer PFS and OS in the patients with high versus non-high CTLA-4 treated with ICI remained significant (p = 0.03 and 0.002, respectively) when 18 of 217 patients who had an anti-CTLA-4 agent included in their regimen were excluded from analysis (data not shown).
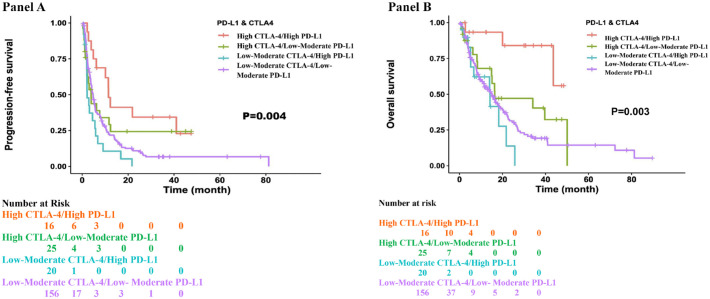
High CTLA-4/high PD-L1 expression was associated with longer PFS and OS as compared to other expression patterns of CTLA-4 and PD-L1 in ICI-treated patients
Median PFS in the high CTLA-4/high PD-L1 (N = 16) expressed cohort was 11.4 months (6.14 to NE) and median OS was not reached (assessed from the time of start of ICI), both longer than the other groups as seen in Figure 5, Panels A and B). Notably, patients with high CTLA-4 and low–moderate PD-L1 did better than patients with low–moderate CTLA-4 but with high PD-L1.
Figure 5.
Panel A: Patients with high CTLA-4 and high PD-L1 expression had the longest PFS. Patients with high CTLA-4 and low–moderate PD-L1 did better than patients with low–moderate CTLA-4 but with high PD-L1. PFS was assessed from the time of the start of ICI. Panel B: Patients with high CTLA-4 and high PD-L1 expression had the longest OS. Patients with high CTLA-4 and low–moderate PD-L1 did better than patients with low–moderate CTLA-4 but with high PD-L1. OS was assessed from the time of the start of ICI.
ICI, immune checkpoint inhibitors; PD-L1, PFS, progression-free survival; OS, overall survival.
Discussion
With the rapidly advancing field of immune checkpoint blockade for the treatment of a multitude of cancers, it is important to examine the role of expression of various checkpoints as prognostic factors for outcome as well as predictive factors for PFS and OS after immunotherapy. One of the most frequently used biomarkers for PD-1/PD-L1 inhibitors in clinical practice is tumor PD-L1 expression level.26,28 –30 Negative PD-L1 expression, however, cannot rule out a response to anti-PD-1/PD-L1 inhibitors. 31 Recently, a large meta-analysis of a hundred papers concluded that PD-L1 immunohistochemistry and tumor mutational burden (TMB) classified responders and non-responders to ICI therapy about 50% of the time, with considerable variability among tumor types. 32 Other biomarkers may also predict response to immunotherapy, including but not limited to the presence of aberrant chromatin remodeling genes, T-cell repertoire, and expression of PD-1 on tumor-infiltrating lymphocytes.33 –35
While CTLA-4 is established as an immune checkpoint, like PD-L1 and PD-1, with a primary immune dampening effect, there has been no comprehensive research into tumoral expression or its impact on response to ICI. There is however evidence that patients with melanoma treated with an anti-CTLA-4 antibody who had higher levels of circulating Tregs cells at baseline had better survival. 36 Tregs constitutively express CTLA-4 and thus higher numbers of circulatory Tregs suggest higher levels of CTLA-4. 37 Similarly, other studies have shown that patients with melanoma on ipilimumab (anti-CTLA-4 antibody) with a soluble CTLA-4 (sCTLA-4) >200 pg/ml had a significantly lower death rate, suggesting better response to ICI. 27 Likewise, another small study of nine patients demonstrated that serum CTLA-4 levels were significantly elevated in responders to ipilimumab (n = 9) compared to non-responders (n = 5). In 11 patients not on ipilimumab, the serum CTLA-4 did not play a prognostic role. 38
Our study demonstrated that CTLA-4 expression level was high in ~17% of cancers overall, but the percent of tumors with high CTLA-4 varied between and within tumor types, perhaps reflecting the heterogeneity of the immune landscape across tumors.
High CTLA-4 (⩾75th percentile rank RNA expression) was associated with improved PFS in patients receiving anti-PD-1/PD-L1 therapies, including in patients who never received an anti-CTLA-4 antibody. Of interest, patients with both high CTLA-4 and high PD-L1 expression had the longest PFS and OS after ICI therapy; patients whose tumors had high PD-L1 expression but non-high CTL-4 expression or other expression patterns did worse. Our study provides new data, as prior studies have not taken an in-depth look at the role of CTLA-4 expression in determining outcomes after anti-PD-1/PD-L1 therapies, since most prior work has concentrated on the role of CTLA-4 expression in patients treated with anti-CTLA-4 agents.
In contrast to the impact of CTLA-4 levels on outcomes after ICIs, for patients in our study not on ICI therapy, the level of CTLA-4 expression was not correlated with outcome. The prior literature has shown mixed results. Contrary to our findings that CTLA-4 tumoral expression is not prognostic for patients not on ICI-based therapy, some studies have shown CTLA-4 to be a poor prognostic marker in cancers such as triple-negative breast cancer, renal cell carcinomas, and nasopharyngeal cancers.39 –41 However, in a review of 21 manuscripts looking at the prognostic correlation of increased CTLA-4 in the cytoplasm or cell surface, 11/21 studies showed a poor outcome with high tumor CTLA-4 expression, 7/21 studies demonstrated that CTLA-4 was associated with a better prognosis, and 3 were inconclusive. 42 In addition, a study of patients with non-small-cell lung carcinomas demonstrated a positive prognostic effect of high CTLA-4 expression on overall survival. 43
The PD-1/PD-L1 axis and CTLA-4 levels have been highly correlated to T cell and other immunocyte marker expression as well as the extent of tumor infiltration by lymphocytes. 44 In addition, though not fully categorized, the PD-1/PD-L1 axis plays a role in the differentiation of Tregs, converting T helper lymphocytes to Tregs that constitutively express CTLA-4. The PD-1/PD-L1 axis can regulate the differentiation and function of Tregs, and Tregs will influence the therapeutic efficacy of PD-1/PD-L1 blockade; in turn, CTLA-4 expressed by tumor cells can regulate PD-L1 expression as seen in non-small-cell lung cancers treated with anti-CTLA-4 antibody.45,46 Although not well studied, PD-L1 expression levels also reflect IFN-γ-inducible biology and thus theoretically could predict response to other ICT classes. One study did observe that melanomas with PD-L1 IHC TPS > 1% had a significantly improved response to all ICI monotherapies, including anti-CLTA-4 monotherapy. 47 However, other studies do not support this, including a study in melanoma showing that CD274 (PD-L1) was a significant predictor of anti-PD-1, but not anti-CTLA-4, response. 48 High LAG-3 expression may also be important in some cases. 49 Of interest in this regard, in our study, high CTLA-4 RNA expression independently correlated with high RNA expression of PD-1, PD-L2, and LAG-3 (and correlated with PD-L1 expression in univariate but not in multivariate analysis).
By recent estimates, 46.3% of advanced cancer patients are eligible for immune checkpoint inhibitor therapy and around 12.5% of patients will respond to ICI. 50 It is important to identify potential responders to personalize therapy as well as avoid side effects for non-responders. Identifying biomarkers for responsiveness is therefore important. In our study, high CTLA-4 tumor expression correlated with longer PFS and OS on ICI therapy and merits further exploration as a predictive biomarker to guide therapy choice.
There are several important limitations to the study. First, multiple different tumor types were included, and so the impact of CTLA-4 in individual histologies was not assessed, as many histologies had only small numbers of patients; still, our data may speak to the generalizability of the observations across tumor types. Future study of larger numbers of patients with individual histologies is warranted. The correlation between high CTLA-4 and cervical cancer especially merits further exploration in larger groups of patients, since only five cervical tumors were included in our cohort. Importantly, protein levels of CTLA-4 were not assessed, nor were individual cell types examined, as we used a bulk RNA transcriptomic methodology. Finally, this was a real-world dataset, based on physicians using clinical-grade immunomic testing in patients with advanced/metastatic disease. As such, there was no specific inclusion or exclusion criteria. The disadvantage of real-world datasets is that they may have specific unknown biases in the collection; on the other hand, they do not suffer from restrictive inclusion and exclusion criteria typical of prospective clinical trials.
Conclusion
To our knowledge, this is one of the first studies to examine the clinical implications of CTLA-4 transcriptomic levels across tumor types. CTLA-4 expression patterns were heterogeneous, varying between and within tumor types. High CTLA-4 RNA independently correlated with high PD-1, PD-L2, and LAG3 RNA levels. Key findings were that CTLA-4 did not correlate with survival from the time of metastatic disease in patients who never received immunotherapy. However, for patients treated with immunotherapy, high CTLA-4 transcript levels correlated with significant improvements in PFS and OS. CTLA-4 and PD-L1 co-high expression were associated with the best outcomes; patients whose tumors had high PD-L1, but non-high CTLA-4 did worse on anti-PD-1/PD-L1 therapies. Taken together, these data suggest that further evaluation of the complex interplay between PD-L1 and CTLA-4 tumoral expression and immunotherapy response is warranted.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359231220510 for High CTLA-4 transcriptomic expression correlates with high expression of other checkpoints and with immunotherapy outcome by Nithya Krishnamurthy, Daisuke Nishizaki, Scott M. Lippman, Hirotaka Miyashita, Mary K. Nesline, Sarabjot Pabla, Jeffrey M. Conroy, Paul DePietro, Shumei Kato and Razelle Kurzrock in Therapeutic Advances in Medical Oncology
Supplemental material, sj-pdf-2-tam-10.1177_17588359231220510 for High CTLA-4 transcriptomic expression correlates with high expression of other checkpoints and with immunotherapy outcome by Nithya Krishnamurthy, Daisuke Nishizaki, Scott M. Lippman, Hirotaka Miyashita, Mary K. Nesline, Sarabjot Pabla, Jeffrey M. Conroy, Paul DePietro, Shumei Kato and Razelle Kurzrock in Therapeutic Advances in Medical Oncology
Acknowledgments
None.
Footnotes
ORCID iD: Nithya Krishnamurthy  https://orcid.org/0009-0009-9091-1927
https://orcid.org/0009-0009-9091-1927
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Nithya Krishnamurthy, Icahn School of Medicine at Mount Sinai, 1 Gustave L. Levy Place, New York, NY 10029-6574, USA.
Daisuke Nishizaki, Center for Personalized Cancer Therapy and Division of Hematology and Oncology, Department of Medicine, University of California San Diego, Moores Cancer Center, La Jolla, CA, USA.
Scott M. Lippman, Center for Personalized Cancer Therapy and Division of Hematology and Oncology, Department of Medicine, University of California San Diego, Moores Cancer Center, La Jolla, CA, USA
Hirotaka Miyashita, Dartmouth Cancer Center, Hematology and Medical Oncology, Lebanon, NH, USA.
Mary K. Nesline, OmniSeq Inc. (Labcorp), Buffalo, NY, USA
Sarabjot Pabla, OmniSeq Inc. (Labcorp), Buffalo, NY, USA.
Jeffrey M. Conroy, OmniSeq Inc. (Labcorp), Buffalo, NY, USA
Paul DePietro, OmniSeq Inc. (Labcorp), Buffalo, NY, USA.
Shumei Kato, Center for Personalized Cancer Therapy and Division of Hematology and Oncology, Department of Medicine, University of California San Diego, Moores Cancer Center, La Jolla, CA, USA.
Razelle Kurzrock, MCW Cancer Center and Genomic Sciences and Precision Medicine Center, Medical College of Wisconsin, Milwaukee, WI, USA; WIN Consortium, Paris, France.
Declarations
Ethics approval and consent to participate: Study of Personalized Cancer Treatment to Determine Response and Toxicity, UCSD PREDICT, (NCT02478931) was carried out in conformity with the regulations of the UCSD Institutional Review Board, and any experimental interventions for which patients provided consent.
Consent for publication: Included in IRB approved protocol above NCT02478931.
Author contributions: Nithya Krishnamurthy: Data curation; Formal analysis; Investigation; Writing – original draft; Writing – review & editing.
Daisuke Nishizaki: Data curation; Formal analysis; Writing – original draft.
Scott M. Lippman: Formal analysis; Writing – review & editing.
Hirotaka Miyashita: Formal analysis; Writing – review & editing.
Mary K. Nesline: Data curation; Methodology; Writing – review & editing.
Sarabjot Pabla: Data curation; Methodology; Writing – review & editing.
Jeffrey M. Conroy: Data curation; Investigation; Writing – review & editing.
Paul DePietro: Data curation; Methodology; Writing – review & editing.
Shumei Kato: Conceptualization; Data curation; Writing – review & editing.
Razelle Kurzrock: Conceptualization; Project administration; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: RK is funded in part by 5U01CA180888-08 and 5UG1CA233198-05.
RK has received research funding from Boehringer Ingelheim, Debiopharm, Foundation Medicine, Genentech, Grifols, Guardant, Incyte, Konica Minolta, Medimmune, Merck Serono, OmniSeq, Pfizer, Sequenom, Takeda, and TopAlliance and from the NCI; as well as consultant and/or speaker fees and/or advisory board/consultant for Actuate Therapeutics, AstraZeneca, Bicara Therapeutics, Inc., Biological Dynamics, Caris, Datar Cancer Genetics, Daiichi, EISAI, EOM Pharmaceuticals, Iylon, LabCorp, Merck, NeoGenomics, Neomed, Pfizer, Prosperdtx, Regeneron, Roche, TD2/Volastra, Turning Point Therapeutics, X-Biotech; has an equity interest in CureMatch Inc. and IDbyDNA; serves on the Board of CureMatch and CureMetrix, and is a co-founder of CureMatch.
SK serves as a consultant for Medpace, Foundation Medicine, NeoGenomics, and CureMatch. He receives speaker’s fee from Chugai, Roche/Genentech and Bayer, and advisory board for Pfizer. He has research funding from ACT Genomics, Sysmex, Konica Minolta, OmniSeq, Personalis, and Function Oncology. MN, SP, JC, and PD are employees of OmniSeq. NK, SL, and DN have no disclosures.
Availability of data and material: Data and material available upon reasonable request.
References
- 1. Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996; 271: 1734–1736. [DOI] [PubMed] [Google Scholar]
- 2. Huang PW, Chang JW. Immune checkpoint inhibitors win the 2018 Nobel Prize. Biomed J 2019; 42: 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Camacho LH. CTLA-4 blockade with ipilimumab: biology, safety, efficacy, and future considerations. Cancer Med 2015; 4: 661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rizvi NA, Cho BC, Reinmuth N, et al. Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic Non-Small cell lung cancer: the MYSTIC phase 3 randomized clinical trial. JAMA Oncol 2020; 6: 661–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abou-Alfa G, Lau G, Kudo M, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evidence 2022; 1: EVIDoa2100070. [DOI] [PubMed] [Google Scholar]
- 6. Rowshanravan B, Halliday N, Sansom DM. CTLA-4: a moving target in immunotherapy. Blood 2018; 131: 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Linsley P, Brady W, Urnes M, et al. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med 1991; 174: 561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schwartz JC, Zhang X, Fedorov AA, et al. Structural basis for co-stimulation by the human CTLA-4/B7-2 complex. Nature 2001; 410: 604–608. [DOI] [PubMed] [Google Scholar]
- 9. Shitara K, Nishikawa H. Regulatory T cells: a potential target in cancer immunotherapy. Ann N Y Acad Sci 2018; 1417: 104–115. [DOI] [PubMed] [Google Scholar]
- 10. Zeng G, Jin L, Ying Q, et al. Regulatory T cells in cancer immunotherapy: basic research outcomes and clinical directions. Cancer Manag Res 2020; 12: 10411–10421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li C, Jiang P, Wei S, et al. Regulatory T cells in tumor microenvironment: new mechanisms, potential therapeutic strategies and future prospects. Mol Cancer 2020; 19: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sobhani N, Tardiel-Cyril DR, Davtyan A, et al. CTLA-4 in regulatory T cells for cancer immunotherapy. Cancers 2021; 13: 1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Noel P, Boise L, Thompson C. Regulation of T cell activation by CD28 and CTLA4. Adv Exp Med Biol 1996; 406: 209-17. [DOI] [PubMed] [Google Scholar]
- 14. Li J, Gu J. Efficacy and safety of ipilimumab for treating advanced melanoma: a systematic review and meta-analysis. J Clin Pharm Ther 2019; 44: 420–429. [DOI] [PubMed] [Google Scholar]
- 15. Rotte A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J Exp Clin Cancer Res 2019; 38: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nikoo M, Rabiee F, Mohebbi H, et al. Nivolumab plus ipilimumab combination therapy in cancer: current evidence to date. Int Immunopharmacol 2023; 117: Article No. 109881. [DOI] [PubMed] [Google Scholar]
- 17. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2019; 381: 1535–1546. [DOI] [PubMed] [Google Scholar]
- 18. Paz-Ares LG, Ramalingam SS, Ciuleanu TE, et al. First-line nivolumab plus ipilimumab in advanced NSCLC: 4-year outcomes from the randomized, open-label, phase 3 checkmate 227 part 1 trial. J Thorac Oncol 2022; 17: 289–308. [DOI] [PubMed] [Google Scholar]
- 19. Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018; 378: 1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O’Malley DM, Randall LM, Jackson CG, et al. RaPiDS (GOG-3028): randomized Phase II study of balstilimab alone or in combination with zalifrelimab in cervical cancer. Future Oncol 2021; 17: 3433–3443. [DOI] [PubMed] [Google Scholar]
- 21. Perez C, Wesolowski R, Wilky B, et al. Single-agent zalifrelimab (anti-CTLA-4) shows clinical benefit in rare tumors-case reports from a phase 2 study (NCT02694822). J Immunother Cancer 2020; 8: A279. [Google Scholar]
- 22. Bullock A, Grossman J, Fakih M, et al. LBA O-9 botensilimab, a novel innate/adaptive immune activator, plus balstilimab (anti-PD-1) for metastatic heavily pretreated microsatellite stable colorectal cancer. Ann Oncol 2022; 33: S376. [Google Scholar]
- 23. Choueiri T, Plimack E, Powles T, et al. Phase 3 study of first-line treatment with pembrolizumab+ belzutifan+ lenvatinib or pembrolizumab/quavonlimab+ lenvatinib versus pembrolizumab+ lenvatinib for advanced renal cell carcinoma (RCC). J Clin Oncol 2022; 40. [Google Scholar]
- 24. Perets R, Bar J, Rasco DW, et al. Safety and efficacy of quavonlimab, a novel anti-CTLA-4 antibody (MK-1308), in combination with pembrolizumab in first-line advanced non-small-cell lung cancer. Ann Oncol 2021; 32: 395–403. [DOI] [PubMed] [Google Scholar]
- 25. Deva S, Mackiewicz J, Dalle S, et al. Abstract CT557: phase 1/2 study of quavonlimab (Qmab)+ pembrolizumab (pembro) in patients (pts) with advanced melanoma that progressed on a PD-1/PD-L1 inhibitor. Cancer Res 2022; 82: CT557. [Google Scholar]
- 26. Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther 2015; 14: 847–856. [DOI] [PubMed] [Google Scholar]
- 27. Pistillo MP, Fontana V, Morabito A, et al. Soluble CTLA-4 as a favorable predictive biomarker in metastatic melanoma patients treated with ipilimumab: an Italian melanoma intergroup study. Cancer Immunol Immunother 2019; 68: 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016; 375: 1823–1833. [DOI] [PubMed] [Google Scholar]
- 29. Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 2014; 20: 5064–5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fountzilas E, Vo HH, Mueller P, et al. Correlation between biomarkers and treatment outcomes in diverse cancers: A systematic review and meta-analysis of Phase I and II immunotherapy clinical trials. Eur J Cancer 2023; 189: 112927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang F, Wang JF, Wang Y, et al. Comparative analysis of predictive biomarkers for PD-1/PD-L1 inhibitors in cancers: developments and challenges. Cancers 2021; 14: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mariam A, Kamath S, Schveder K, et al. Biomarkers for response to Anti–PD-1/Anti–PD-L1 immune checkpoint inhibitors: A large meta-analysis. Oncology 2023; 37: 210–219. [DOI] [PubMed] [Google Scholar]
- 33. Krishnamurthy N, Kato S, Lippman S, et al. Chromatin remodeling (SWI/SNF) complexes, cancer, and response to immunotherapy. J Immunother Cancer 2022; 10: e004669. [Google Scholar]
- 34. Aran A, Garrigós L, Curigliano G, et al. Evaluation of the TCR repertoire as a predictive and prognostic biomarker in cancer: diversity or clonality? Cancers 2022; 14: 1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bevins NJ, Okamura R, Montesion M, et al. Tumor infiltrating lymphocyte expression of PD-1 predicts response to anti-PD-1/PD-L1 immunotherapy. J Immunother Precis Oncol 2022; 5: 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Simeone E, Gentilcore G, Giannarelli D, et al. Immunological and biological changes during ipilimumab treatment and their potential correlation with clinical response and survival in patients with advanced melanoma. Cancer Immunol Immunother 2014; 63: 675–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Retseck J, Nasr A, Lin Y, et al. Long term impact of CTLA4 blockade immunotherapy on regulatory and effector immune responses in patients with melanoma. J Transl Med 2018; 16: 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Leung AM, Lee AF, Ozao-Choy J, et al. Clinical benefit from ipilimumab therapy in melanoma patients may be associated with serum CTLA4 levels. Front Oncol 2014; 4: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Peng Z, Su P, Yang Y, et al. Identification of CTLA-4 associated with tumor microenvironment and competing interactions in triple negative breast cancer by co-expression network analysis. J Cancer 2020; 11: 6365–6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang PY, Guo SS, Zhang Y, et al. Tumor CTLA-4 overexpression predicts poor survival in patients with nasopharyngeal carcinoma. Oncotarget 2016; 7: 13060–13068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kahlmeyer A, Stöhr CG, Hartmann A, et al. Expression of PD-1 and CTLA-4 are negative prognostic markers in renal cell carcinoma. J Clin Med 2019; 8: 743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Abdulkhaleq F, Larossi N, Ogbonda O, et al. CTLA-4 expression by human tumor cells and its impact on immunotherapeutic strategies:a systematic review. Oncol Insights 2021; 2: 151–169. [Google Scholar]
- 43. Salvi S, Fontana V, Boccardo S, et al. Evaluation of CTLA-4 expression and relevance as a novel prognostic factor in patients with non-small cell lung cancer. Cancer Immunol Immunother 2012; 61: 1463–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu JN, Kong XS, Huang T, et al. Clinical implications of aberrant PD-1 and CTLA4 expression for cancer immunity and prognosis: a pan-cancer study. Front Immunol 2020; 11: 2048. 2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cai J, Wang D, Zhang G, et al. The role of PD-1/PD-L1 axis in treg development and function: implications for cancer immunotherapy. Onco Targets Ther 2019; 12: 8437–8445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang H, Dutta P, Liu J, et al. Tumour cell-intrinsic CTLA4 regulates PD-L1 expression in non-small cell lung cancer. J Cell Mol Med 2019; 23: 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Conroy J, Pabla S, Nesline M, et al. Next generation sequencing of PD-L1 for predicting response to immune checkpoint inhibitors. J Immunother Cancer 2019; 7: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brüggemann C, Kirchberger MC, Goldinger SM, et al. Predictive value of PD-L1 based on mRNA level in the treatment of stage IV melanoma with ipilimumab. J Cancer Res Clin Oncol 2017; 143: 1977–1984. [DOI] [PubMed] [Google Scholar]
- 49. Adashek JJ, Kato S, Nishizaki D, et al. LAG-3 transcriptomic expression patterns across malignancies: Implications for precision immunotherapeutics. Cancer Med 2023; 12: 13155–13166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open 2019; 2: e192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359231220510 for High CTLA-4 transcriptomic expression correlates with high expression of other checkpoints and with immunotherapy outcome by Nithya Krishnamurthy, Daisuke Nishizaki, Scott M. Lippman, Hirotaka Miyashita, Mary K. Nesline, Sarabjot Pabla, Jeffrey M. Conroy, Paul DePietro, Shumei Kato and Razelle Kurzrock in Therapeutic Advances in Medical Oncology
Supplemental material, sj-pdf-2-tam-10.1177_17588359231220510 for High CTLA-4 transcriptomic expression correlates with high expression of other checkpoints and with immunotherapy outcome by Nithya Krishnamurthy, Daisuke Nishizaki, Scott M. Lippman, Hirotaka Miyashita, Mary K. Nesline, Sarabjot Pabla, Jeffrey M. Conroy, Paul DePietro, Shumei Kato and Razelle Kurzrock in Therapeutic Advances in Medical Oncology