Abstract
Readily utilizable sugars down-regulate virulence gene expression in Listeria monocytogenes, which has led to the proposal that this regulation may be an aspect of global catabolite regulation (CR). We recently demonstrated that the metabolic enzyme α-glucosidase is under CR in L. monocytogenes. Here, we report the cloning and characterization from L. monocytogenes of an apparent ortholog of ccpA, which encodes an important mediator of CR in several low-G+C-content gram-positive bacteria. L. monocytogenes ccpA (ccpALm) is predicted to encode a 335-amino-acid protein with nearly 65% identity to the gene product of Bacillus subtilis ccpA (ccpABs). Southern blot analysis with a probe derived from ccpALm revealed a single strongly hybridizing band and also a second band of much lower intensity, suggesting that there may be other closely related sequences in the L. monocytogenes chromosome, as is the case in B. subtilis. Disruption of ccpALm resulted in the inability of the mutant to grow on glucose-containing minimal medium or increase its growth rate in the presence of preferred sugars, and it completely eliminated CR of α-glucosidase activity in liquid medium. However, α-glucosidase activity was only partially relieved from CR on solid medium. These results suggest that ccpA is an important element of carbon source regulation in L. monocytogenes. Nevertheless, utilizable sugars still down-regulate the expression of hly, which encodes the virulence factor hemolysin, in a ccpALm mutant, indicating that CcpA is not involved in carbon source regulation of virulence genes.
When the gram-positive, facultative intracellular human pathogen Listeria monocytogenes is grown in the presence of utilizable sugars, expression of its virulence genes is down-regulated (37, 44). However, sugars do not affect the level of the PrfA protein, the positive regulator of virulence determinants in L. monocytogenes. Based on these results, Milenbachs et al. proposed that regulation of virulence genes by sugars may represent an aspect of global catabolite control and could occur by modifying the activity of PrfA (37). This is consistent with the observation that a mutation in PrfA results in the deregulated expression of hly in the presence of utilizable sugars and other environmental factors (3, 5, 46). There is some evidence that PrfA requires an additional factor(s) (called PrfA-associated factor or Paf) for full activity in vivo (12, 46). Paf activity is present not only in pathogenic Listeria species, but also in the nonpathogenic soil organism Bacillus subtilis (4). Ripio et al. proposed a model in which environmental signals regulate PrfA-dependent virulence gene expression by altering the level or activity of Paf, thereby controlling the activation state of PrfA (46). The presence of Paf activity in both Bacillus and Listeria species implies that Paf has cellular roles other than regulation of virulence gene expression and could be subject to more general global regulatory controls, like catabolite regulation (CR), in response to environmental signals that modulate the activity of genes not necessarily involved in virulence.
In addition to its important role in the utilization of carbon sources, CR is known to influence diverse aspects of cell physiology in both gram-positive and gram-negative bacteria (15, 48, 49). Expression of virulence factors in several pathogenic bacteria (7, 35, 57) and important developmental processes like the initiation of sporulation in B. subtilis (15, 52) are under CR. However, the primary mechanisms that mediate CR in gram-negative and gram-positive bacteria are quite different. In gram-negative bacteria two global mechanisms of CR are known. The best-characterized mechanism involves the positive regulator cyclic AMP receptor protein (CRP) and its regulatory ligand cyclic AMP (10). The other mechanism is mediated by the catabolite repressor/activator (Cra) protein, which was initially characterized as the fructose repressor, FruR, in gram-negative enteric bacteria (43). In the low-G+C-content gram-positive bacteria, the predominant mechanism of CR involves transcriptional repression mediated by the catabolite control protein A (CcpA), a DNA-binding protein that belongs to the LacI-GalR family of transcription regulators (18, 22). A cis-acting element, called catabolite responsive element or CRE, has been identified in genes under CcpA regulation. CRE is a 14-bp region of dyad symmetry with the consensus sequence TG(A/T)NANCGNTN(A/T)CA, where N is A, G, C, or T (59). Specific binding of CcpA to CRE has been demonstrated by gel mobility shift assays and DNase footprinting analysis, strongly suggesting that CcpA exerts its effect on gene expression by its binding to CRE (16, 27–29). A key role in CR is also played by HPr, one of the components of the phosphoenolpyruvate:sugar phosphotransferase (PTS) sugar uptake system. HPr can be phosphorylated at the Ser-46 residue by an ATP-dependent HPr kinase. HPr(ser-P), but not free HPr, can bind to CcpA in vitro, and this interaction is dependent on high concentrations of fructose-1,6-bisphosphate (FBP), one of the intermediates of the glycolytic pathway (11, 27). Thus, the following model for CR in low-G+C-content gram-positive bacteria has been proposed: glucose or other rapidly metabolized sugars generate metabolic intermediates like FBP via the glycolytic pathway, metabolic intermediates activate the ATP-dependent HPr kinase that phosphorylates HPr at Ser-46, HPr(Ser-46) forms a complex with FBP and CcpA, and the complex binds to CRE in catabolite-repressed genes and blocks transcription initiation by RNA polymerase (22).
Since ccpA homologs have also been identified and characterized in Bacillus megaterium (24), Staphylococcus xylosus (13), Lactobacillus pentosus (34), and Lactobacillus casei (39), it appears that CcpA-mediated CR is a common theme in many low-G+C-content gram-positive bacteria. Disruption of the ccpA gene has pleiotropic effects on cell growth and enzyme regulation, as evidenced by the relief of sugar repression of several enzymes, slow growth rates on preferred carbon sources, and poor growth on minimal medium (MM) (18, 22). Nevertheless, considerable evidence points towards the existence of other mechanisms of CR in low-G+C-content gram-positive bacteria. In S. xylosus, CR of the activities of several enzymes mediated by glucose kinase has been demonstrated (58). In B. subtilis, a homolog of CcpA called CcpB can mediate CR when cells are grown on solid medium or in liquid medium without vigorous agitation (10). In B. subtilis, the regulation of the levanase operon is mediated both by CcpA-HPr and by a second independent pathway (36). In Streptococcus mutans, disruption of regM, a gene with homology to ccpA of other bacteria, paradoxically increases repression of several catabolite-controlled enzyme activities instead of relieving it (55). Thus, important differences in mechanisms of CR exist among low-G+C-content gram-positive bacteria, underscoring the importance of studying this phenomenon in bacteria other than B. subtilis.
To address the hypothesis that central pathways of CR might mediate carbon source regulation of virulence genes in L. monocytogenes, we sought first to establish whether a homolog of ccpA exists and is responsible for regulating glucose repression of catabolic enzymes. We show here that a ccpA homolog is present in L. monocytogenes. Moreover, we demonstrate that a ccpALm mutant recapitulates the same range of phenotypes observed in a ccpABs mutant, including growth impairment on glucose-containing MM and abrogation of CR for the glucose-repressed enzyme α-glucosidase. Nevertheless, we observed no detectable effect on glucose repression of hly expression in the ccpALm mutant. Thus, if global pathways of CR influence the regulation of virulence genes in L. monocytogenes, they must do so through a mechanism that does not involve ccpA.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Escherichia coli DH5α mcr[φ80dlacZΔM15 recA1 endA1 gyrA96 thi-1 hsdR17 (rK− mK+) supE44 relA1 deoR Δ(lacZYA-argF)U169 mcr] (Gibco BRL) was used for all cloning. L. monocytogenes strains were grown on brain heart infusion (BHI) (Difco Laboratories, Detroit, Mich.) or Luria-Bertani (LB) medium. Cultures grown in LB medium were buffered with 100 mM 3-(N-morpholino)propane-sulfonic acid (MOPS) (pH 7.0 or 7.4). Ampicillin was used at a concentration of 100 μg/ml, and chloramphenicol and neomycin were each used at 5 μg/ml. The MM used was a modification of the Listeria minimal medium described previously (42) and contained 100 mM MOPS (pH 7.4), 4.82 mM KH2PO4, 11.55 mM Na2HPO4, 1.7 mM MgSO4, 1% (55 mM) glucose, 1 μg of thiamine/ml, 0.5 μg of riboflavin/ml, 0.5 μg of biotin/ml, 0.005 μg of thioctic acid/ml, and 0.1 mg each of leucine, isoleucine, valine, methionine, arginine, cysteine, and glutamine per ml. Sugar supplements were added to cultures or plates at a final concentration of 25 mM.
For calculation of doubling times, overnight cultures grown in LB medium buffered with 100 mM MOPS (pH 7.4) were subcultured at 1:50 in the same medium with or without each sugar (25 mM) and incubated at 37°C in a shaking water bath. Samples (1 ml) were collected at 30-min intervals from logarithmic-phase cultures, and the optical density at 600 nm was determined on a Shimadzu UV-1201 spectrophotometer.
Amplification of the ccpALm gene by PCR.
The degenerate primers CcpA-01R (5′ GARGCNAAYGTNWSIATGGC 3′; based on the amino acid sequence EANVSMA from the B. subtilis CcpA protein, GenBank accession no. 143024) and CcpA-04L (5′ CKCATNGCIACNGCNCC 3′; based on the amino acid sequence GAVAMR) were used to amplify the putative ccpALm homolog. Ambiguity codes are as follows: R represents A or G, Y represents T or C, N represents G, A, T, or C, W represents A or T, S represents C or G, and K represents G or T. PCR amplification was carried out as described previously (25).
Construction of pCON1-CCPA and disruption of the ccpALm gene.
A 615-bp internal ccpALm fragment was PCR amplified from chromosomal DNA with the primers CcpA-05R (5′ GTACGGATCCAAGATATTGCGA 3′) and CcpA-06L (5′ GCATGAATTCTGTATTGTTGCTTGT 3′). Incorporation of two mismatched bases in the primers (CC in GGATCC and the G and C in GAATTC) resulted in BamHI and EcoRI recognition sites (underlined) in CcpA-05R and CcpA-06L, respectively. The purified PCR product was digested with the two enzymes, ligated to similarly digested and purified pCON1 plasmid DNA (3), and transformed into E. coli with selection for ampicillin resistance. The resultant plasmid, pCON1-CCPA, was then transformed into the E. coli conjugation donor strain S17-1 and conjugated into L. monocytogenes 10403S as previously described (1). pCON1-CCPA, which has the temperature-sensitive origin of replication from plasmid pE194ts, was integrated into the L. monocytogenes chromosome by shifting an early-log-phase culture growing at the permissive temperature (30°C) to the nonpermissive temperature (41°C) for 3 h. The culture was diluted appropriately and plated onto BHI plates containing 5 μg of chloramphenicol/ml at 41°C. Integration of the vector at the ccpALm locus in this strain was confirmed by PCR amplification with primers flanking the cloned region followed by sequencing of the amplified product. The ccpALm mutant was named JB15 (10403S ccpA::pCON1).
To rescue sequences upstream of the insertion of pCON1-CCPA, chromosomal DNA was prepared from JB15 and digested to completion with XbaI, an enzyme that recognizes a sequence within the polylinker of pCON1-CCPA. After heat inactivation of the enzyme, the digested DNA was diluted, added to a ligation reaction mixture to favor self-ligation of DNA molecules, and transformed into E. coli. Plasmid DNA isolated from transformants was screened by restriction analysis, and the sequence of the rescued region was determined. Sequence from downstream of the pCON1-CCPA insertion was determined by sequencing of PCR products amplified by nested PCR with specific upstream primers based on the ccpALm sequence and random downstream primers. The sequence of the entire ccpALm coding region was then determined by directly sequencing the PCR products on both strands.
Transduction of hly-gus-neo cassette into JB15.
The hly-gus-neo cassette was introduced into JB15 by transduction from AML73 (3) by using the L. monocytogenes bacteriophage LMUP35 (20). One hundred microliters of phage dilution in TM buffer (10 mM Tris-HCl [pH 7.5], 10 mM MgSO4) was mixed with an equal volume of mid-logarithmic-phase culture of AML73 and incubated at room temperature for 40 min. Molten LB soft agar (3 ml) containing 10 mM MgSO4 and 10 mM CaCl2 was then added to each tube, and the mixture was poured over a plate of LB agar (containing 10 mM MgSO4 and 10 mM CaCl2) and incubated overnight at room temperature. Phages were recovered the next day by adding 5 ml of sterile TM buffer to the plates and leaving them overnight at room temperature. Phage stocks were sterilized by passing them through a 0.2-μm-pore-size filter, and their titers were determined. For transduction into JB15, 1 ml of culture (at a concentration of 108 CFU/ml) was centrifuged and the cell pellet was resuspended in 100 μl of BHI broth. The suspension was mixed with 107 PFU of phage and incubated at room temperature for 40 min. Molten BHI soft agar (3 ml) containing 10 mM sodium citrate, pH 7.5, was added to the cell-phage mix and poured onto BHI plates containing 10 mM sodium citrate, pH 7.5, and 5 μg each of neomycin and chloramphenicol per ml. Plates were incubated at 37°C for 2 days, and colonies were picked onto fresh BHI-citrate plates containing neomycin and chloramphenicol. Colonies that had acquired the hly-gus-neo cassette were nonhemolytic on blood-agar plates, blue on 5-bromo-4-chloro-3-indolyl-β-d-glucuronide (X-gluc) (United States Biological, Swampscott, Mass.) plates, neomycin resistant, and chloramphenicol resistant due to the cat gene carried on pCON1 inserted at the ccpALm locus.
DNA sequencing and analysis.
DNA sequencing was carried out by the method of Sanger et al. (51) by using the fmol thermal cycling sequencing system (Promega Corporation, Madison, Wis.). Custom-synthesized oligonucleotides were purchased either from DNAgency, Aston, Pa., or the University of Georgia Molecular Genetics Instrumentation Facility and end labeled with [γ-32P]ATP (Amersham, Arlington Heights, Ill.). Sequencing reactions were carried out on both strands of PCR products amplified from the L. monocytogenes chromosomal DNA. Protein and DNA sequences were analyzed with the MacVector software program (Kodak Scientific Imaging Systems, New Haven, Conn.) and the GCG software package (Genetics Computer Group, Madison, Wis.).
DNA preparation and Southern blot analysis.
Isolation of chromosomal DNA and Southern blot analysis were performed by standard techniques (50). A 615-bp internal ccpALm fragment to be used as the probe was amplified by PCR from chromosomal DNA, labeled with [32P]dCTP with a Prime-a-Gene labeling kit (Promega), and purified with a Sephadex G-50 quick spin column (Boehringer Mannheim, Indianapolis, Ind.). Hybridization was carried out overnight at 55°C, after which the membrane was washed three times with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and 0.1% sodium dodecyl sulfate (SDS) at room temperature and three times with 1× SSC and 0.1% SDS at 37°C and placed against a PhosphorImager screen.
α-Glucosidase and β-glucuronidase assays.
α-Glucosidase activity was assayed as described previously (8). β-Glucuronidase activity was assayed in samples collected from cultures that were 1 h into stationary phase as described previously (26). The specific activities of both enzymes are expressed as nanomoles of p-nitrophenol formed per minute per milligram of total protein. Protein concentration was measured by the modified method of Bradford (6), using the Bio-Rad protein assay reagent with bovine serum albumin as the standard.
Nucleotide sequence accession number.
The ccpA sequence from L. monocytogenes 10403S has been deposited in the GenBank database and assigned the accession no. AF076520.
RESULTS
Identification and cloning of ccpA from L. monocytogenes.
Based on sequences of CcpA homologs available in the GenBank database, degenerate PCR primers were designed to amplify possible homologs of ccpA from L. monocytogenes chromosomal DNA. Several primer pairs were tested. A band approximately 0.9 kb in size was obtained with the primer pair CcpA-01R and CcpA-04L (see Materials and Methods). The PCR product was purified and sequenced. An incomplete open reading frame (ORF) with base composition including 37.7% G+C content was identified. This value is consistent with the overall G+C content of 37 to 39% reported earlier for L. monocytogenes chromosomal DNA (47). A search of the GenBank database revealed over 60% identity of the translated sequence of the ORF with the CcpA proteins from B. subtilis and B. megaterium, suggesting that the product was derived from a true homolog of the ccpABs gene.
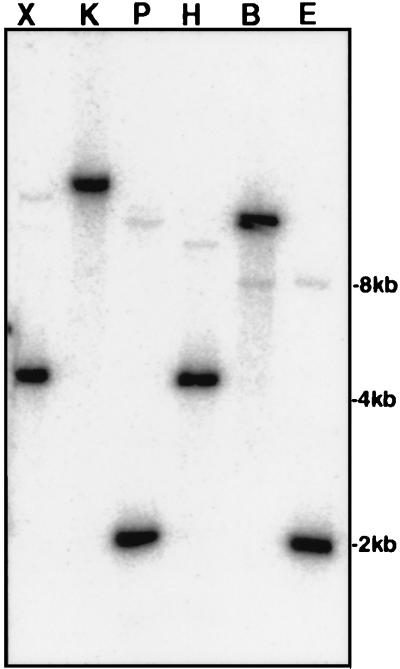
To confirm that the PCR-amplified fragment was from the chromosome of L. monocytogenes, we amplified a 615-bp fragment with the L. monocytogenes-specific primers CcpA-05R and CcpA-06L, labeled the fragment with [32P]dCTP, and used it as a probe in Southern blot analysis of chromosomal digests prepared with six different restriction enzymes. The probe consistently hybridized strongly with a single DNA band in each lane, indicating that a putative ccpALm homolog had been amplified and was not a contaminant. Interestingly, a low-stringency Southern blotting revealed a second band, of much lower intensity, in each lane, indicating the presence of another sequence closely related to the ccpALm sequence (Fig. 1). These data are consistent with results reported for B. subtilis, in which additional ccpA-like genes are known to exist (9).
FIG. 1.
Southern blot analysis of L. monocytogenes chromosomal DNA with a ccpALm probe. Chromosomal DNA (3 μg in each reaction) was digested with six different restriction enzymes (X, XbaI; K, KpnI; P, PstI; H, HindIII; B, BamHI; and E, EcoRI) and subjected to agarose gel electrophoresis. After transfer to membrane, the DNA was probed with a [32P]dCTP-labeled 615-bp ccpALm fragment.
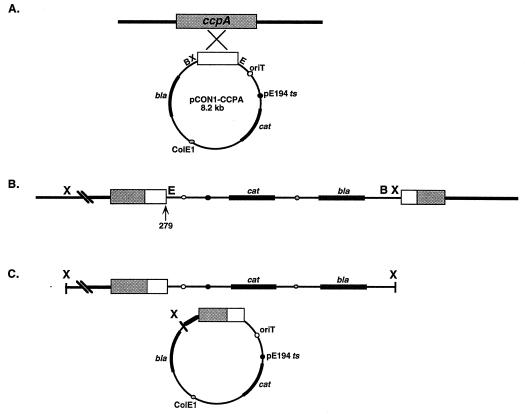
To disrupt the chromosomal ccpALm locus, the 615-bp internal ccpALm fragment was cloned into the integrational vector pCON1, conjugated into strain 10403S, and forced into the chromosome by Campbell integration by shifting to the nonpermissive temperature (Fig. 2A and B). Since pCON1 has a ColE1 origin of replication and the bla gene which confers ampicillin resistance, rescue of sequences adjacent to the site of integration was possible. A 1.1-kb fragment from the region upstream of ccpALm was isolated after digestion with XbaI and cloning in E. coli as described in Materials and Methods (Fig. 2C). We were unable to rescue sequences downstream from the site of vector insertion by this method. However, nested PCR with specific primers based on the ccpALm sequence and nonspecific downstream primers enabled us to amplify and determine the sequence of a region extending about 300 bp downstream of the putative stop codon of the ccpALm ORF.
FIG. 2.
Disruption of the gene and rescue of adjacent sequences. (A) A 615-bp internal ccpA fragment (open box) was PCR amplified from the L. monocytogenes chromosome with the primers CcpA-05R and CcpA-06L, cloned into the integrational vector pCON1, and conjugated into L. monocytogenes. (B) Shift of the pCON1-CCPA-containing strain to the non-permissive temperature forced integration of the vector into the chromosome at the ccpA locus, resulting in the truncation of the gene after codon 279 (arrow). (C) Sequences from upstream of the integrated vector were rescued by extracting chromosomal DNA from the mutant, digesting it with XbaI, and cloning it directly in E. coli. bla, β-lactamase gene; cat, chloramphenicol acetyltransferase gene; oriT, mobilization signal from plasmid RP4; pE194 ts, replication origin derived from plasmid pE194ts; ColE1, replication origin derived from pUC18.
Sequence analysis of the ccpALm gene.
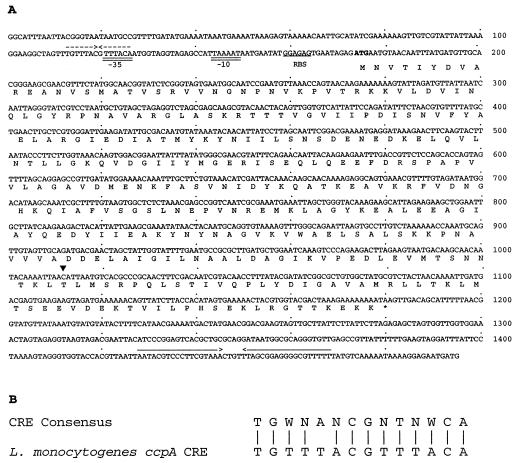
Nucleotide sequence analysis of the ccpALm locus revealed a 1,005-bp ORF, with a putative start codon (ATG) at position 178 (Fig. 3A) and stop codon at position 1179. A putative ribosome binding site, GGAGAG, is located 9 bp upstream of the apparent initiation codon. A putative −10 sequence, TAAAAT, with one difference from the canonical −10 sequence, is centered 27 nucleotides upstream of the putative start codon. The cognate −35 sequence, TTTACA, containing one mismatch with the consensus −35 sequence, is 17 bp upstream of the −10 sequence. Interestingly, a sequence resembling that of CRE is located 47 bp upstream of the putative start codon, partially overlapping the putative −35 sequence. The CRE-like sequence matches the consensus CRE sequence proposed by Weickert and Chambliss (59) at 13 of 14 positions (Fig. 3B). A potential rho-independent transcription terminator was identified 247 bp downstream from the ccpALm stop codon.
FIG. 3.
(A) Nucleotide sequence of the ccpALm gene. The deduced amino acid sequence is given in single-letter code below the DNA sequence. The putative start codon is shown in bold type, and the stop codon is indicated by an asterisk. A potential ribosome-binding site (RBS) is underlined. Putative −10 and −35 sequences are double underlined. A CRE-like sequence partially overlapping the putative −35 sequence is indicated by dashed inverted arrows. A possible factor-independent transcription terminator is marked with solid inverted arrows. The site of truncation of the ccpA coding sequence in JB15 is indicated with an arrowhead. (B) Comparison of the CRE-like sequence in the ccpALm promoter region with the CRE consensus sequence (59). Ambiguity codes are as follows: W denotes A or T and N denotes A, C, G, or T. Vertical bars indicate matching bases.
The ccpALm ORF is predicted to encode a protein of 335 amino acids with a calculated Mr of 36,900. The deduced amino acid sequence has 79.8% similarity to CcpA from B. subtilis (64.5% identity), with perfect conservation of the residues constituting the helix-turn-helix motif at the N terminus of the protein (Fig. 4). A high degree of identity was also observed with the B. megaterium (62%) and S. xylosus (55%) CcpA homologs. In every case, the degree of similarity was much higher at the N termini than at the C termini of the proteins. Significant but much weaker homology of the L. monocytogenes CcpA protein was also found with other members of the LacI-GalR family of proteins (data not shown) (19).
FIG. 4.
Alignment of L. monocytogenes CcpA with three other CcpA proteins. The B. subtilis (B_sub; GenBank accession no. 115950), B. megaterium (B_meg; accession no. 1168844), and S. xylosus (S_xyl; accession no. 3023459) CcpA proteins are compared with the deduced L. monocytogenes (L_mon) CcpA sequence. Positions identical in at least two of four sequences are shaded gray. Residues constituting the helix-turn-helix (HTH) domain at the N terminus are marked with a line. The alignment was performed with the Pileup program of the GCG software package.
Our sequence information indicated no ORF likely to be transcribed with this gene immediately downstream from ccpALm. In both B. subtilis (17) and B. megaterium (21), two ORFs (homologous to motA and motB from E. coli) are present immediately downstream from ccpA and appear to be cotranscribed with it. To investigate directly the possibility that the L. monocytogenes homolog might be part of a polycistronic operon, we performed Northern blot analysis with total RNA prepared from strain 10403S grown in LB medium with or without maltose or glucose. In every case, we detected a 1.4-kb transcript, indicating that the ccpALm gene is expressed as a monocistronic message (data not shown).
Growth characteristics of the ccpALm mutant.
The effect of ccpA disruption on growth characteristics was studied by comparing the growth of the ccpALm mutant JB15 with that of the parental wild-type strain 10403S on glucose-containing MM plates. The plates were incubated at 30°C, since even the wild-type strain was found to grow poorly at 37°C on solid medium and not at all in liquid MM (20). After 4 days of growth at 30°C, JB15 grew very poorly on MM plates compared to 10403S (Fig. 5A). On the other hand, JB15 grew well on the rich medium, BHI agar (Fig. 5B).
FIG. 5.
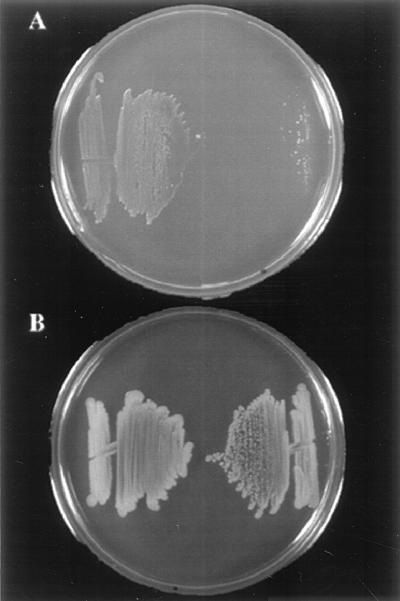
Growth of strains 10403S and JB15 (10403S ccpA::pCON1) on defined medium. The wild-type strain 10403S is on the left and JB15 is on the right in both panels. (A) Glucose-containing MM plate showing growth of the two strains after 4 days of incubation at 30°C. (B) Streaks made on BHI agar at the same time as on the plate shown in panel A, after 48-h incubation at 30°C.
We compared the doubling times of JB15 and 10403S in LB medium supplemented with various sugars. Although JB15 demonstrated a longer lag phase, its doubling time was similar to that of the wild-type strain in LB medium alone (data not shown). While doubling times of 10403S decreased from 44 min to approximately 36 min on addition of 25 mM (each) glucose, fructose, or cellobiose, the doubling times of the mutant in the presence of these sugars were nearly the same as in LB medium without added sugars. In all cases, the final cell densities reached by both strains were similar, indicating that JB15 was capable of utilizing these sugars (data not shown).
CR of α-glucosidase in the ccpALm mutant.
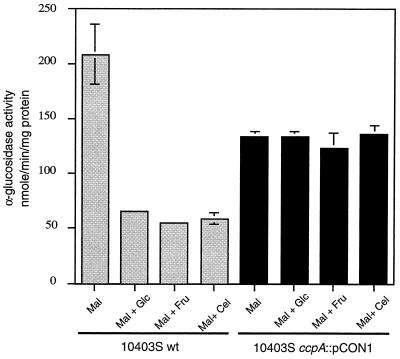
We showed recently that activity of the metabolic enzyme α-glucosidase in L. monocytogenes is inducible by maltose and subject to catabolite control in the presence of the repressing sugars glucose, fructose, and cellobiose (3). Egeter et al. have demonstrated that α-glucosidase activity is relieved from CR in an S. xylosus ccpA mutant (13). To test if the regulation of this enzyme in L. monocytogenes was also mediated by ccpA, we assayed α-glucosidase activity in JB15 and 10403S. For 10403S, the addition of either glucose, fructose, or cellobiose at a concentration of 25 mM to LB medium containing 25 mM maltose resulted in more than a fourfold decrease in α-glucosidase activity (Fig. 6). For JB15, while α-glucosidase activity was slightly lower than that of 10403S in LB medium containing maltose, almost complete derepression was observed in the presence of repressing sugars. Thus, these results strongly suggest that ccpALm mediates CR of α-glucosidase in liquid medium. We also constructed a strain, JB178, in which pCON1 was integrated into the L. monocytogenes 10403S chromosome immediately downstream of the ccpA coding region. Growth characteristics and α-glucosidase regulation of JB178 were found to be similar to those of the wild-type strain (data not shown). We conclude from these results that the phenotype of JB15 is manifested due to disruption of ccpA and not because of a polar effect on downstream genes.
FIG. 6.
CR of α-glucosidase in strains 10403S and JB15 (10403S ccpA::pCON1) in liquid medium. Cells were grown at 37°C in LB medium buffered with 100 mM MOPS (pH 7.0), supplemented with the indicated sugars (each, 25 mM). Specific activity of α-glucosidase was measured in exponentially growing cells by using p-nitrophenyl-α-d-glucoside as the substrate. Mal, maltose; Glc, glucose; Fru, fructose; Cel, cellobiose; wt, wild type. Each sample was analyzed in triplicate, and the data represent the means and standard errors of the means of three independent experiments.
Recently Chauvaux et al. reported that CR of gnt and xyl operons is relieved only partially in ccpABs mutants grown on solid medium or in liquid cultures with little agitation. A mutation in a second gene, called ccpB, is required for full relief of CR under these conditions (9). To test if JB15 also exhibited only partial deregulation on solid medium, we used the chromogenic substrate X-α-d-glucoside (5-bromo-4-chloro-3-indolyl-α-d-glucopyranoside) to assay α-glucosidase activity on plates. Both the 10403S and the JB15 spots were blue on LB agar containing 25 mM maltose and 100 μg of X-α-d-glucoside/ml, indicating full expression of α-glucosidase (data not shown). When 25 mM glucose was incorporated in the medium, the 10403S spot was white; this result was expected due to the repression of α-glucosidase activity by glucose. Interestingly, JB15 was neither completely white (like the wild-type strain) nor as blue as in the absence of glucose, suggesting that α-glucosidase was only partially relieved from CR on solid medium in this strain (data not shown).
Disruption of ccpALm does not affect carbon source regulation of hly-gus.
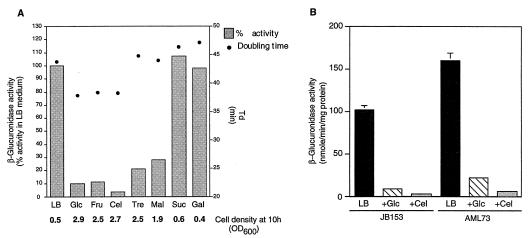
Several laboratories have shown that the presence of utilizable sugars in the medium results in downregulation of virulence genes in L. monocytogenes (37, 41, 44). We carefully examined the quantitative effects of various sugars on hly expression using AML73. This strain has the hly promoter fused to the gusA gene from E. coli, allowing colorimetric measurement of hly promoter activity (3). As shown in Fig. 7A, a correlation exists between how well a sugar stimulates growth and its extent of negative regulation of hly-gus expression. Sugars such as glucose, fructose, and cellobiose, which result in significant decreases in doubling times when added to LB medium at a concentration of 25 mM, also result in the most severe repression of hly-gus expression. Other sugars decrease doubling times only slightly (maltose) or not at all (trehalose). However, maltose and trehalose are clearly utilized by L. monocytogenes, as evidenced by the increase in final cell densities reached after 10 h of growth (Fig. 7A). Maltose and trehalose affect hly-gus expression significantly less (3- to 5-fold decrease in doubling times) than glucose, fructose, or cellobiose (10- to 25-fold decrease in doubling times). Sucrose and galactose are not utilized by L. monocytogenes and do not regulate hly-gus expression. Similar results were also obtained on plate assays with X-gluc (5-bromo-4-chloro-3-indolyl-α-d-glucuronide) used as a chromogenic substrate (data not shown).
FIG. 7.
(A) Growth rates of L. monocytogenes and levels of expression of hly-gus on different sugars. Strain AML73 was grown in LB medium buffered with 100 mM MOPS (pH 7.4), and doubling times and β-glucuronidase activity were measured in the presence of the indicated sugars (each, 25 mM). The data represent the means of either two (β-glucuronidase activity; each experiment was done in triplicate) or three (doubling times and final cell densities) separate experiments. Standard deviations were less than 10% in all cases. Glc, glucose; Fru, fructose; Cel, cellobiose; Tre, trehalose; Mal, maltose; Suc, sucrose; Gal, galactose. (B) hly-gus expression in JB153 (ccpA::pCON1) and AML73 (wild type). Sugars were added (each at a concentration of 25 mM) to LB medium buffered with 100 mM MOPS (pH 7.4), and β-glucuronidase activity was measured 2 h into stationary phase. Data represent means ± standard errors of the means of three independent experiments. LB, LB medium; Glc, glucose; Cel, cellobiose.
Since the disruption of ccpALm had effects on growth, doubling times in the presence of preferred carbon sources, and CR of α-glucosidase, we wondered whether the mutation would also relieve the carbon source regulation of hly-gus. We used the L. monocytogenes phage LMUP35 to transduce the hly-gus-neo cassette from AML73 to JB15 (resulting in the strain JB153). We then assayed β-glucuronidase activity in JB153. While there was a 35% decrease in the level of expression of hly-gus in strain JB153 compared to that in AML73, the relative extent of down-regulation by sugars was not affected in this strain (Fig. 7B). Therefore, we conclude from these data that CcpA does not mediate carbon source regulation of virulence genes in L. monocytogenes.
DISCUSSION
In this communication, we report the identification, cloning, and characterization of a ccpA homolog from L. monocytogenes. The deduced amino acid sequence encoded by the gene was found to have a high degree of similarity to that of B. subtilis CcpA, with perfect conservation of the residues constituting the helix-turn-helix domain. Disruption of ccpALm resulted in pleiotropic effects on cell growth and regulation, which is indicative of the important role CcpA plays in CR in this organism. However, ccpALm does not appear to mediate carbon source regulation of hly-gus, suggesting that other, as yet unidentified, factors are involved in down-regulating virulence genes in L. monocytogenes in the presence of utilizable sugars.
Sequence analysis of the region upstream of ccpALm revealed an ORF encoding a putative protein with approximately 68% identity with the B. subtilis aroA gene product (GenBank accession no. X65945) (2). The aroA gene encodes 3-deoxy-d-arabino-heptulosonate-7-phosphate synthase and is located upstream of ccpA in both B. subtilis and S. xylosus (accession no. X95439) (13). Immediately downstream from ccpA in B. subtilis and B. megaterium are two ORFs which show significant homology to the flagellar motor proteins from E. coli, motA and motB. Although the functions of these genes are not known, mutations disrupting them do not affect either motility or CR (18, 24). The genetic arrangement in S. xylosus, Streptococcus mutans, and L. casei appears to be different, since these organisms do not contain the two downstream ORFs (13, 39, 55). Our results indicate that L. monocytogenes also lacks these sequences, since we did not find any ORF in the sequenced region extending 300 bp downstream of ccpALm. Northern hybridization analysis did not reveal any ccpALm transcript longer than 1.4 kb. Furthermore, JB178, in which pCON1 is integrated into the chromosome immediately downstream of ccpA, had the wild-type phenotype. Therefore, ccpALm appears to be monocistronic, and the phenotype of JB15 is due to the disruption of ccpA and not a gene downstream of it.
Regulation of ccpA expression has been studied in several organisms. Its expression appears to be constitutive in B. subtilis (18), B. megaterium (24), and L. casei (39). However, in S. xylosus, there are two promoters upstream of ccpA (13). Transcription from one of these promoters is repressed when cultures are grown in the presence of glucose. This regulation is dependent on CcpA itself, since it is abolished in a ccpA mutant. Interestingly, there is a CRE-like sequence found just upstream of the ribosome-binding site of the S. xylosus ccpA gene, indicating that this autogenous regulatory loop may be affected by CcpA binding to its own promoter. We have identified a CRE-like sequence that has a single mismatch with the CRE consensus sequence proposed by Hueck et al. (23) located 47 bp upstream of the putative ccpALm start codon. Therefore, the interesting possibility exists that ccpALm expression may also be autoregulated.
Studies with B. subtilis have indicated that the intracellular signal linking carbon source availability and CcpA-mediated regulation may be HPr(Ser-P) (seryl-phosphorylated form of HPr) and that this interaction may be stimulated by one of the intermediates of the glycolytic pathway, fructose 1,6-bisphosphate (11). In L. monocytogenes, a fructose-specific PTS uptake system (of which HPr is one of the components) has been demonstrated biochemically (38). Recently, the sequence of L. monocytogenes ptsH (encoding HPr) and the partial sequence of ptsI (encoding enzyme I, another PTS component) have been deposited in the GenBank database (accession number AF030824). Therefore, our results demonstrating the role of CcpA in CR in L. monocytogenes raise the strong possibility that CcpA-HPr-dependent global CR may be operative in this organism.
While CcpA-HPr-mediated control is certainly an important global regulatory pathway, mounting evidence suggests that it is not the only mechanism responsible for CR in B. subtilis and other low-G+C-content gram-positive organisms (30, 31, 36, 53). Moreover, it was shown recently that disruption of regM, a ccpA homolog in S. mutans, not only had no effect on CR of several enzymes but also paradoxically increased their repression by sugars (55). It is also becoming apparent that other factors may interact with CcpA and/or HPr. Chauvaux et al. recently demonstrated that on solid medium and in cultures incubated with little agitation, a homolog of ccpABs, ccpB, interacts with HPr(Ser-P) and mediates CR of gnt and xyl operons along with CcpA (9). However, when cultures were incubated with vigorous agitation, CcpA alone seemed to be sufficient for CR. When we tested the regulation of the ccpALm mutant on plates, we too found that glucose repression of α-glucosidase was only partially relieved. This observation makes it very likely that additional factors are involved in α-glucosidase regulation in L. monocytogenes. The result is also intriguing since low-stringency Southern blotting demonstrated two bands that hybridized to a ccpALm probe, suggesting that there is at least one sequence closely related to ccpA in the L. monocytogenes chromosome. It is tempting to speculate that this sequence might be a ccpB homolog, since CcpA and CcpB have about 30% amino acid identity in B. subtilis (9).
In several bacterial pathogens, including L. monocytogenes, virulence genes are clustered on discrete regions of the chromosome referred to as pathogenicity islands (33). This finding suggests that bacterial pathogens evolved from related nonpathogenic species by acquiring contiguous blocks of DNA containing virulence genes (14). When the expression of genes acquired by horizontal transfer is regulated in response to environmental factors for which a broader regulatory mechanism already exists, it is to be expected that regulation of the acquired genes will employ or adapt to existing mechanisms. For example, in Salmonella species, regulation of the virulence operon spv is controlled not only by the plasmid-encoded virulence factor, SpvR, but also by the CRP-cyclic AMP complex, the global catabolite regulator (40). Similarly, in Vibrio cholerae, the expression of cholera toxin and toxin-coregulated pilus is also regulated by CRP-cyclic AMP (56). It is not to be expected that an independent mechanism of catabolite control would be reinvented for the specific purpose of modulating the expression of the PrfA-controlled regulon in L. monocytogenes. Therefore, it was surprising that disruption of ccpALm had a significant effect on growth and catabolite control but not on sugar regulation of virulence genes. Since expression of hly is not controlled by CcpA, one possibility is that its expression is under regulation of another catabolite control pathway in L. monocytogenes that remains to be defined. Another possibility is that the regulator of virulence genes, PrfA, is itself involved in sensing the presence of carbon sources. PrfA has been shown to possess structural and functional homology with the E. coli catabolite regulator, CRP (32, 54). PrfA is involved in the regulation of uptake of at least one sugar, glucose-1-phosphate (45). Furthermore, a single amino acid substitution (Gly145Ser) in the PrfA sequence makes hly-gus expression insensitive to negative control by sugars (3). Future experiments designed to test these hypotheses should yield interesting insights into this regulation.
ACKNOWLEDGMENTS
We thank David Hodgson for the generous gift of phage LMUP35 and for advice on transduction and preparation of minimal medium. We are grateful to David Brown, Janet Hatt, Andrea Milenbachs, and Paul Fawcett for helpful discussions and technical advice throughout this study. We thank Sidney Kushner, Caroline Ingle, and Bijoy Mohanty for advice on RNA preparation and Northern blot analysis. Additionally, we thank Kathy Spindler and Tad Seyler for helpful comments on the manuscript.
This work was supported by Public Health Service grant GM35495 from the National Institutes of Health.
REFERENCES
- 1.Barak I, Behari J, Olmedo G, Guzman P, Brown D P, Castro E, Walker D, Westpheling J, Youngman P. Structure and function of the Bacillus SpoIIE protein and its localization to sites of sporulation septum assembly. Mol Microbiol. 1996;19:1047–1060. doi: 10.1046/j.1365-2958.1996.433963.x. [DOI] [PubMed] [Google Scholar]
- 2.Behari, J., and P. Youngman. Unpublished data.
- 3.Behari J, Youngman P. Regulation of hly expression in Listeria monocytogenes by carbon sources and pH occurs through separate mechanisms mediated by PrfA. Infect Immun. 1998;66:3635–3642. doi: 10.1128/iai.66.8.3635-3642.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bockmann R, Dickneite C, Middendorf B, Goebel W, Sokolovic Z. Specific binding of the Listeria monocytogenes transcriptional regulator PrfA to target sequences requires additional factor(s) and is influenced by iron. Mol Microbiol. 1996;22:643–653. doi: 10.1046/j.1365-2958.1996.d01-1722.x. [DOI] [PubMed] [Google Scholar]
- 5.Bohne J, Kestler H, Uebele C, Sokolovic Z, Goebel W. Differential regulation of the virulence genes of Listeria monocytogenes by the transcriptional activator PrfA. Mol Microbiol. 1996;20:1189–1198. doi: 10.1111/j.1365-2958.1996.tb02639.x. [DOI] [PubMed] [Google Scholar]
- 6.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 7.Busque P, Letellier A, Harel J, Dubreuil J D. Production of Escherichia coli STb enterotoxin is subject to catabolite repression. Microbiology. 1995;141:1621–1627. doi: 10.1099/13500872-141-7-1621. [DOI] [PubMed] [Google Scholar]
- 8.Chasin L A, Magasanik B. Induction and repression of the histidine-degrading enzymes of Bacillus subtilis. J Biol Chem. 1968;243:5165–5178. [PubMed] [Google Scholar]
- 9.Chauvaux S, Paulsen I T, Saier M., Jr CcpB, a novel transcription factor implicated in catabolite repression in Bacillus subtilis. J Bacteriol. 1998;180:491–497. doi: 10.1128/jb.180.3.491-497.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crasnier M. Cyclic AMP and catabolite repression. Res Microbiol. 1996;147:479–482. doi: 10.1016/0923-2508(96)84002-2. [DOI] [PubMed] [Google Scholar]
- 11.Deutscher J, Kuster E, Bergstedt U, Charrier V, Hillen W. Protein kinase-dependent HPr/CcpA interaction links glycolytic activity to carbon catabolite repression in Gram-positive bacteria. Mol Microbiol. 1995;15:1049–1053. doi: 10.1111/j.1365-2958.1995.tb02280.x. [DOI] [PubMed] [Google Scholar]
- 12.Dickneite C, Bockmann R, Spory A, Goebel W, Sokolovic Z. Differential interaction of the transcription factor PrfA and the PrfA-activating factor (Paf) of Listeria monocytogenes with target sequences. Mol Microbiol. 1998;27:915–928. doi: 10.1046/j.1365-2958.1998.00736.x. [DOI] [PubMed] [Google Scholar]
- 13.Egeter O, Bruckner R. Catabolite repression mediated by the catabolite control protein CcpA in Staphylococcus xylosus. Mol Microbiol. 1996;21:739–749. doi: 10.1046/j.1365-2958.1996.301398.x. [DOI] [PubMed] [Google Scholar]
- 14.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher S H, Sonenshein A. Control of carbon and nitrogen metabolism in Bacillus subtilis. Annu Rev Microbiol. 1991;45:107–135. doi: 10.1146/annurev.mi.45.100191.000543. [DOI] [PubMed] [Google Scholar]
- 16.Fujita Y, Miwa Y, Galinier A, Deutscher J. Specific recognition of the Bacillus subtilis gnt cis-acting catabolite-responsive element by a protein complex formed between CcpA and seryl-phosphorylated HPr. Mol Microbiol. 1995;17:953–960. doi: 10.1111/j.1365-2958.1995.mmi_17050953.x. [DOI] [PubMed] [Google Scholar]
- 17.Grundy F J, Waters D A, Takova T Y, Henkin T M. Identification of genes involved in utilization of acetate and acetoin in Bacillus subtilis. Mol Microbiol. 1993;10:259–271. doi: 10.1111/j.1365-2958.1993.tb01952.x. [DOI] [PubMed] [Google Scholar]
- 18.Henkin T M. The role of CcpA transcriptional regulator in carbon metabolism in Bacillus subtilis. FEMS Microbiol Lett. 1996;135:9–15. doi: 10.1111/j.1574-6968.1996.tb07959.x. [DOI] [PubMed] [Google Scholar]
- 19.Henkin T M, Grundy F J, Nicholson W L, Champliss G H. Catabolite repression of alpha-amylase gene expression in Bacillus subtilis involves a trans-acting gene product homologous to the Escherichia coli Lacl and GalR repressors. Mol Microbiol. 1991;5:575–584. doi: 10.1111/j.1365-2958.1991.tb00728.x. [DOI] [PubMed] [Google Scholar]
- 20.Hodgson, D. A. Personal communication.
- 21.Hueck C, Kraus A, Hillen W. Sequences of ccpA and two downstream Bacillus megaterium genes with homology to the motAB operon from Bacillus subtilis. Gene. 1994;143:147–148. doi: 10.1016/0378-1119(94)90621-1. [DOI] [PubMed] [Google Scholar]
- 22.Hueck C J, Hillen W. Catabolite repression in Bacillus subtilis: a global regulatory mechanism for the Gram-positive bacteria? Mol Microbiol. 1995;15:395–401. doi: 10.1111/j.1365-2958.1995.tb02252.x. [DOI] [PubMed] [Google Scholar]
- 23.Hueck C J, Hillen W, Saier M., Jr Analysis of a cis-acting sequence mediating catabolite repression in Gram-positive bacteria. Res Microbiol. 1994;145:503–518. doi: 10.1016/0923-2508(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 24.Hueck C J, Kraus A, Schmiedel D, Hillen W. Cloning, expression and functional analyses of the catabolite control protein CcpA from Bacillus megaterium. Mol Microbiol. 1995;16:855–864. doi: 10.1111/j.1365-2958.1995.tb02313.x. [DOI] [PubMed] [Google Scholar]
- 25.Innis M A, Gelfand D H, Sninsky J J, White T J. PCR protocols. A guide to methods and applications. San Diego, Calif: Academic Press, Inc.; 1990. [Google Scholar]
- 26.Jefferson R A, Burgess S M, Hirsh D. β-Glucuronidase from Escherichia coli as a gene-fusion marker. Proc Natl Acad Sci USA. 1986;83:8447–8451. doi: 10.1073/pnas.83.22.8447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones B E, Dossonnet V, Kuster E, Hillen W, Deutscher J, Klevit R E. Binding of the catabolite repressor protein CcpA to its DNA target is regulated by phosphorylation of its corepressor HPr. J Biol Chem. 1997;272:26530–26535. doi: 10.1074/jbc.272.42.26530. [DOI] [PubMed] [Google Scholar]
- 28.Kim J H, Chambliss G H. Contacts between Bacillus subtilis catabolite regulatory protein CcpA and amyO target site. Nucleic Acids Res. 1997;25:3490–3496. doi: 10.1093/nar/25.17.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim J H, Guvener Z T, Cho J Y, Chung K C, Chambliss G H. Specificity of DNA binding activity of the Bacillus subtilis catabolite control protein CcpA. J Bacteriol. 1995;177:5129–5134. doi: 10.1128/jb.177.17.5129-5134.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kraus A, Hueck C, Gartner D, Hillen W. Catabolite repression of the Bacillus subtilis xyl operon involves a cis element functional in the context of an unrelated sequence, and glucose exerts additional xylR-dependent repression. J Bacteriol. 1994;176:1738–1745. doi: 10.1128/jb.176.6.1738-1745.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kruger S, Gertz S, Hecker M. Transcriptional analysis of bglPH expression in Bacillus subtilis: evidence for two distinct pathways mediating carbon catabolite repression. J Bacteriol. 1996;178:2637–2644. doi: 10.1128/jb.178.9.2637-2644.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lampidis R, Gross R, Sokolovic Z, Goebel W, Kreft J. The virulence regulator protein of Listeria ivanovii is highly homologous to PrfA from Listeria monocytogenes and both belong to the Crp-Fnr family of transcription regulators. Mol Microbiol. 1994;13:141–151. doi: 10.1111/j.1365-2958.1994.tb00409.x. [DOI] [PubMed] [Google Scholar]
- 33.Lee C A. Pathogenicity islands and the evolution of bacterial pathogens. Infect Agents Dis. 1996;5:1–7. [PubMed] [Google Scholar]
- 34.Likman B C, Heerikhuisen M, Leer R J, van den Broek A, Borsboom Y, Chaillou S, Postma P W, Pauwels P H. Regulation of expression of the Lactobacillus pentosus xylAB operon. J Bacteriol. 1997;179:5391–5397. doi: 10.1128/jb.179.17.5391-5397.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez-Cadena M G, Guzman-Verduzco L M, Stieglitz H, Kupersztoch-Portnoy Y M. Catabolite repression of Escherichia coli heat-stable enterotoxin activity. J Bacteriol. 1981;145:722–728. doi: 10.1128/jb.145.2.722-728.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin-Verstraete I, Stulke J, Klier A, Rapoport G. Two different mechanisms mediate catabolite repression of the Bacillus subtilis levanase operon. J Bacteriol. 1995;177:6919–6927. doi: 10.1128/jb.177.23.6919-6927.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milenbachs A A, Brown D P, Moors M, Youngman P. Carbon-source regulation of virulence gene expression in Listeria monocytogenes. Mol Microbiol. 1997;23:1075–1085. doi: 10.1046/j.1365-2958.1997.2711634.x. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell W J, Reizer J, Herring C, Hoischen C, Saier M., Jr Identification of a phosphoenolpyruvate:fructose phosphotransferase system (fructose-1-phosphate forming) in Listeria monocytogenes. J Bacteriol. 1993;175:2758–2761. doi: 10.1128/jb.175.9.2758-2761.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monedero V, Gosalbes M J, Perez-Martinez G. Catabolite repression in Lactobacillus casei ATCC 393 is mediated by CcpA. J Bacteriol. 1997;179:6657–6664. doi: 10.1128/jb.179.21.6657-6664.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Byrne C P, Dorman C J. The spv virulence operon of Salmonella typhimurium LT2 is regulated negatively by the cyclic AMP (cAMP)-cAMP receptor protein system. J Bacteriol. 1994;176:905–912. doi: 10.1128/jb.176.3.905-912.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park S F, Kroll R G. Expression of listeriolysin and phosphatidylinositol-specific phospholipase C is repressed by the plant-derived molecule cellobiose in Listeria monocytogenes. Mol Microbiol. 1993;8:653–661. doi: 10.1111/j.1365-2958.1993.tb01609.x. [DOI] [PubMed] [Google Scholar]
- 42.Premaratne R J, Lin W J, Johnson E A. Development of an improved chemically defined minimal medium for Listeria monocytogenes. Appl Environ Microbiol. 1991;57:3046–3048. doi: 10.1128/aem.57.10.3046-3048.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramseier T M. Cra and the control of carbon flux via metabolic pathways. Res Microbiol. 1996;147:489–493. doi: 10.1016/0923-2508(96)84003-4. [DOI] [PubMed] [Google Scholar]
- 44.Renzoni A, Klarsfeld A, Dramsi S, Cossart P. Evidence that PrfA, the pleiotropic activator of virulence genes in Listeria monocytogenes, can be present but inactive. Infect Immun. 1997;65:1515–1518. doi: 10.1128/iai.65.4.1515-1518.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ripio M T, Brehm K, Lara M, Suarez M, Vazquez-Boland J A. Glucose-1-phosphate utilization by Listeria monocytogenes is PrfA dependent and coordinately expressed with virulence factors. J Bacteriol. 1997;179:7174–7180. doi: 10.1128/jb.179.22.7174-7180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ripio M T, Dominguez-Bernal G, Lara M, Suarez M, Vazquez-Boland J A. A Gly145Ser substitution in the transcriptional activator PrfA causes constitutive overexpression of virulence factors in Listeria monocytogenes. J Bacteriol. 1997;179:1533–1540. doi: 10.1128/jb.179.5.1533-1540.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rocourt J, Grimont P A D. Listeria welshimeri sp. nov. and Listeria seeligeri sp. nov. Int J Sys Bacteriol. 1983;33:866–869. [Google Scholar]
- 48.Saier M., Jr Cyclic AMP-independent catabolite repression in bacteria. FEMS Microbiol Lett. 1996;138:97–103. doi: 10.1111/j.1574-6968.1996.tb08141.x. [DOI] [PubMed] [Google Scholar]
- 49.Saier M, Jr, Chauvaus S, Cook G M, Deutscher J, Paulsen I T, Reizer J, Ye J J. Catabolite repression and inducer control in Gram-positive bacteria. Microbiology. 1996;142:217–230. doi: 10.1099/13500872-142-2-217. [DOI] [PubMed] [Google Scholar]
- 50.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 51.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schaeffer P, Millet J, Aubert J-P. Catabolite repression of bacterial sporulation. Proc Natl Acad Sci USA. 1965;54:704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schonert S, Buder T, Dahl M K. Identification and enzymatic characterization of the maltose-inducible α-glucosidase MalL (sucrase-isomaltase-maltase) of Bacillus subtilis. J Bacteriol. 1998;180:2574–2578. doi: 10.1128/jb.180.9.2574-2578.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sheehan B, Klarsfeld A, Ebright R, Cossart P. A single substitution in the putative helix-turn-helix motif of the pleiotropic activator PrfA attenuates Listeria monocytogenes virulence. Mol Microbiol. 1996;20:785–797. doi: 10.1111/j.1365-2958.1996.tb02517.x. [DOI] [PubMed] [Google Scholar]
- 55.Simpson C L, Russell R R. Identification of a homolog of CcpA catabolic repressor protein in Streptococcus mutans. Infect Immun. 1998;66:2085–2092. doi: 10.1128/iai.66.5.2085-2092.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skorupski K, Taylor R K. Cyclic AMP and its receptor protein negatively regulate the coordinate expression of cholera toxin and toxin-coregulated pilus in Vibrio cholerae. Proc Natl Acad Sci USA. 1997;94:265–270. doi: 10.1073/pnas.94.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith J L, Bencivengo M M, Kunsch C A. Enterotoxin A synthesis in Staphylococcus aureus: inhibition by glycerol and maltose. J Gen Microbiol. 1986;132:3375–3380. doi: 10.1099/00221287-132-12-3375. [DOI] [PubMed] [Google Scholar]
- 58.Wagner E, Marcandier S, Egeter O, Deutscher J, Gotz F, Bruckner R. Glucose kinase-dependent catabolite repression in Staphylococcus xylosus. J Bacteriol. 1995;177:6144–6152. doi: 10.1128/jb.177.21.6144-6152.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weickert M J, Chambliss G H. Site-directed mutagenesis of a catabolite repression operator sequence in Bacillus subtilis. Proc Natl Acad Sci USA. 1990;87:6238–6242. doi: 10.1073/pnas.87.16.6238. [DOI] [PMC free article] [PubMed] [Google Scholar]