Abstract
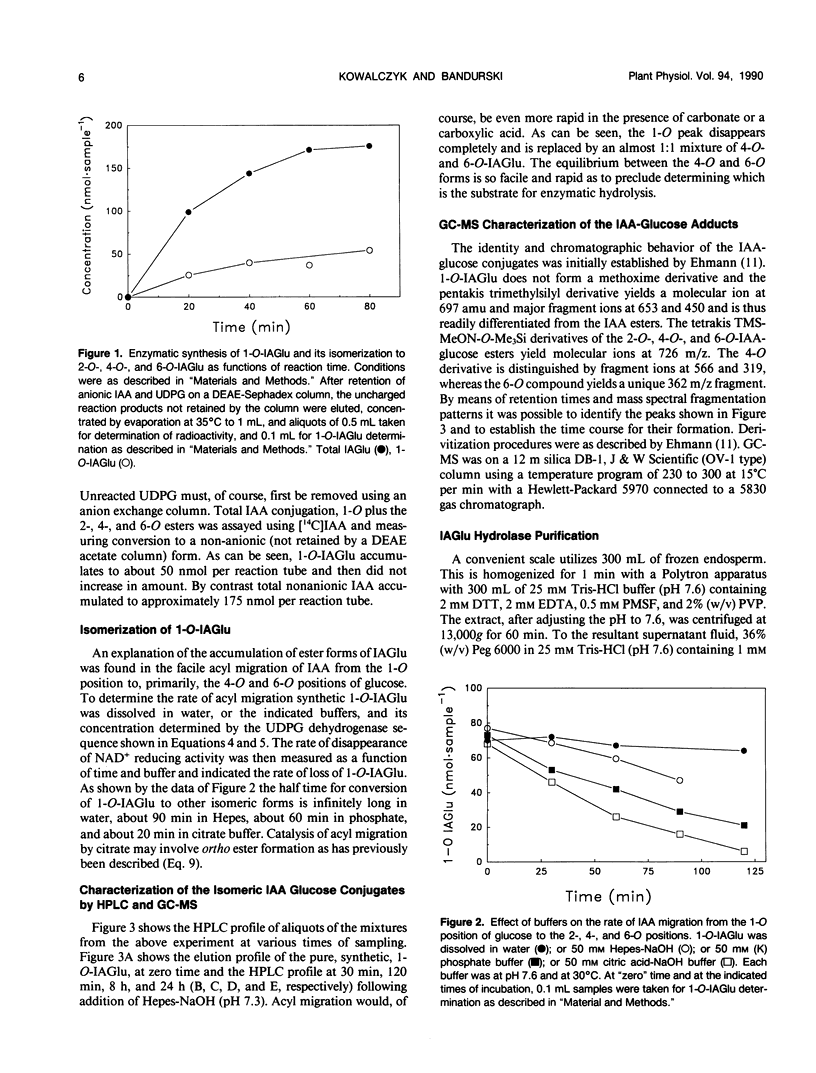
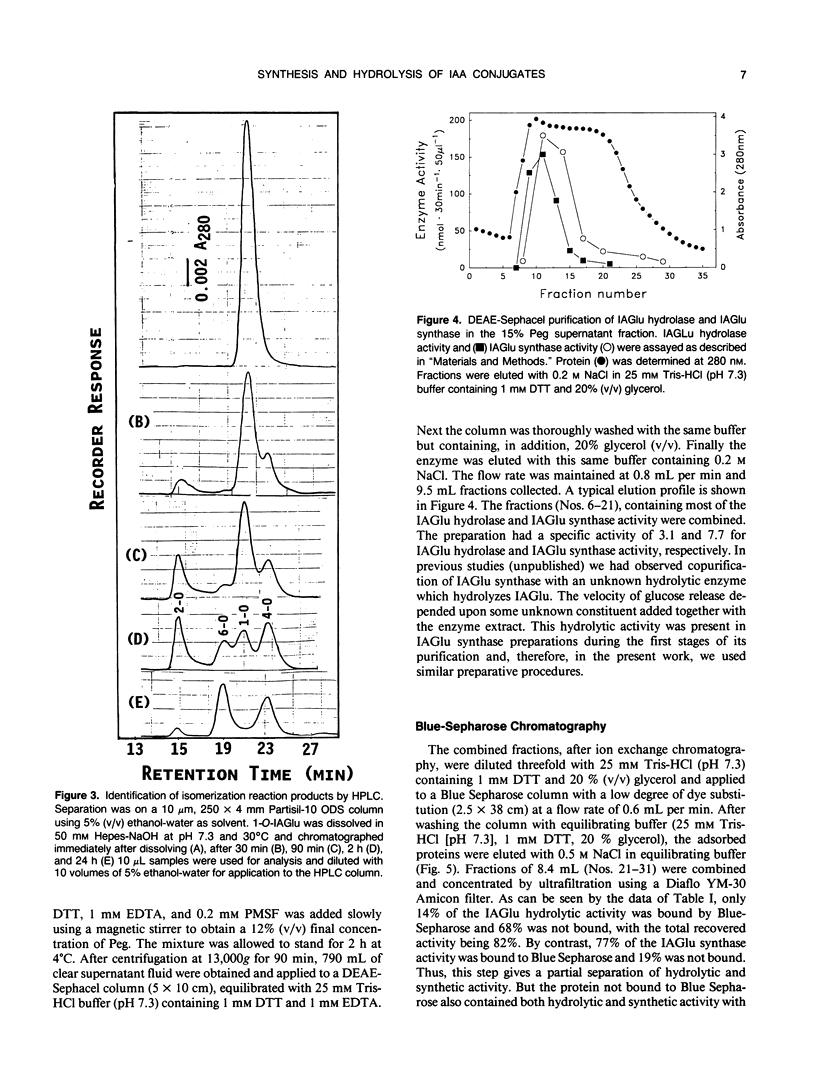
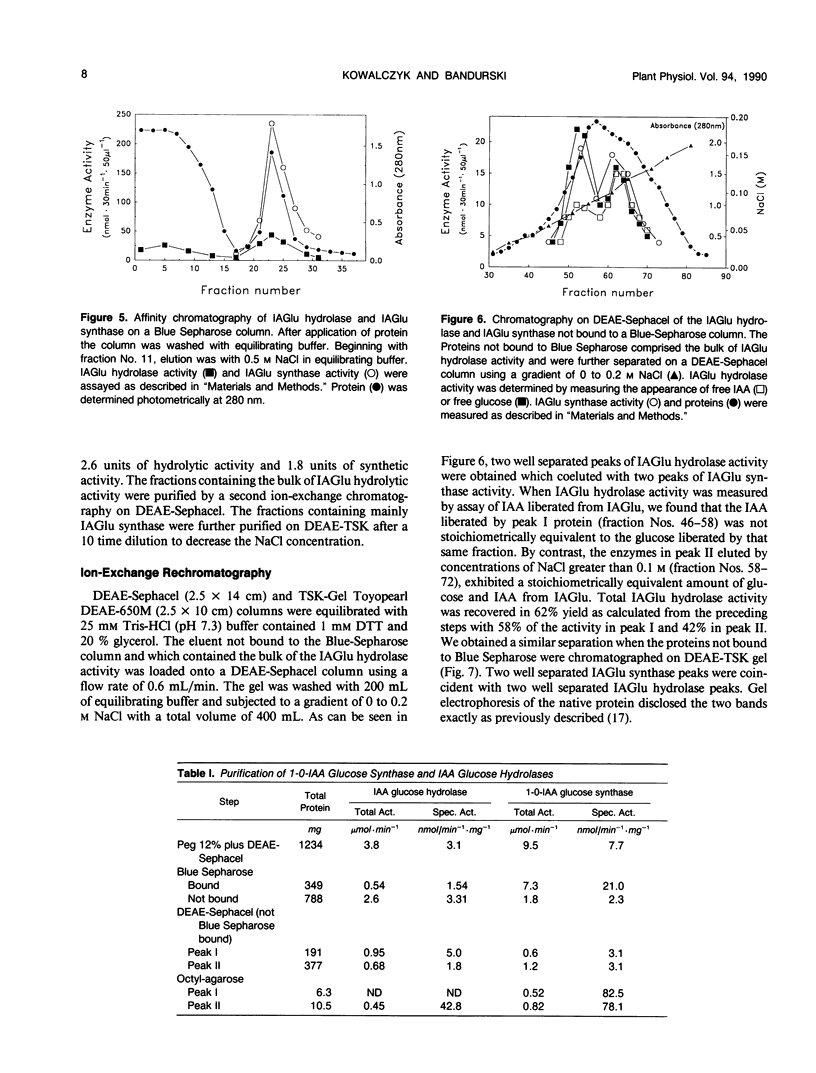
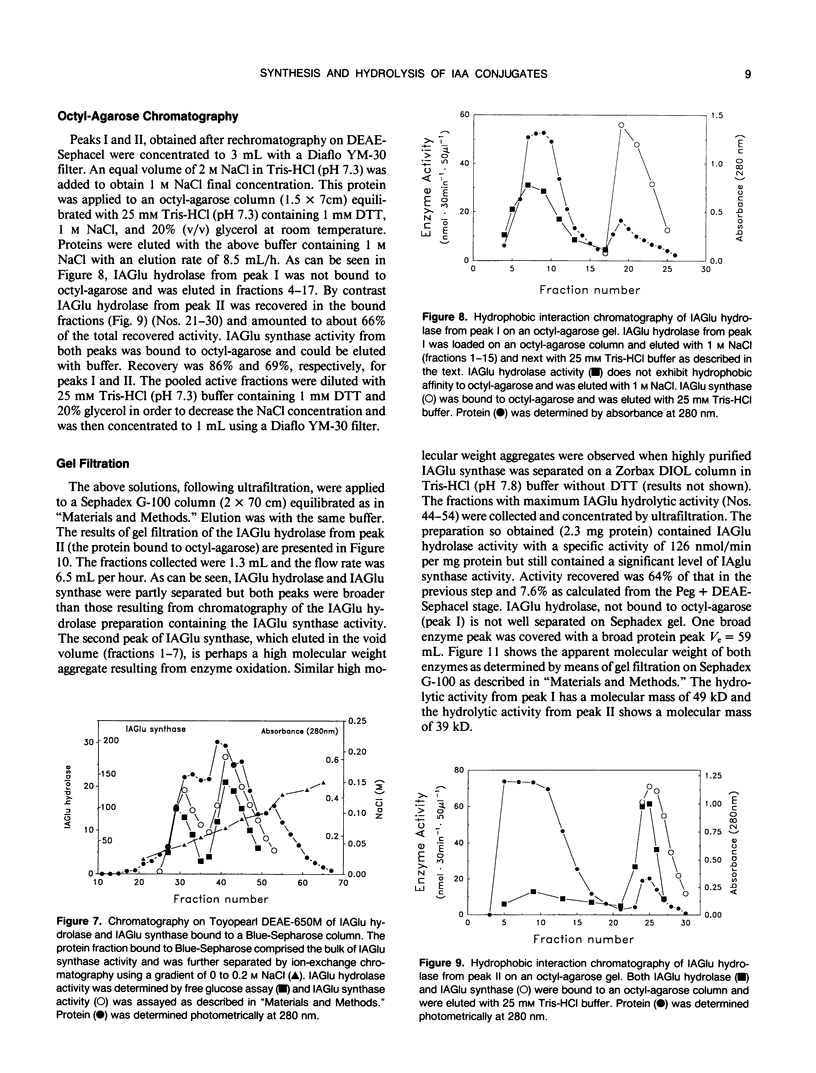
The first compound in the series of reactions leading to the ester conjugates of indole-3-acetic acid (IAA) in kernels of Zea mays sweet corn is the acyl alkyl acetal, 1-O-indol-3-ylacetyl-β-d-glucose (1-O-IAGlu). The enzyme catalyzing the synthesis of this compound is UDP-glucose:indol-3-ylacetate glucosyl-transferase (IAGlu synthase). The IAA moiety of the high energy compound 1-O-IAGlu may be enzymatically transferred to myo-inositol or to glycerol or the 1-O-IAGlu may be enzymatically hydrolyzed. Alternatively, nonenzymatic acyl migration may occur to yield the 2-O, 4-O, and 6-O esters of IAA and glucose. The 4-O and 6-O esters may then be enzymatically hydrolyzed to yield free IAA and glucose. This work reports new enzymatic activities, the transfer of IAA from 1-O-IAGlu to glycerol, and the enzymecatalyzed hydrolysis of 4-O- and 6-O-IAGlu. Data is also presented on the rate of non-enzymatic acyl migration of IAA from the 1-O to the 4-O and 6-O positions of glucose. We also report that enzymes catalyzing the synthesis of 1-O-IAGlu and the hydrolysis of 1-O, 4-O, and 6-O-IAGlu fractionate as a hormone metabolizing complex. The association of synthetic and hydrolytic capabilities in enzymes which cofractionate may have physiological significance.
Full text
PDF

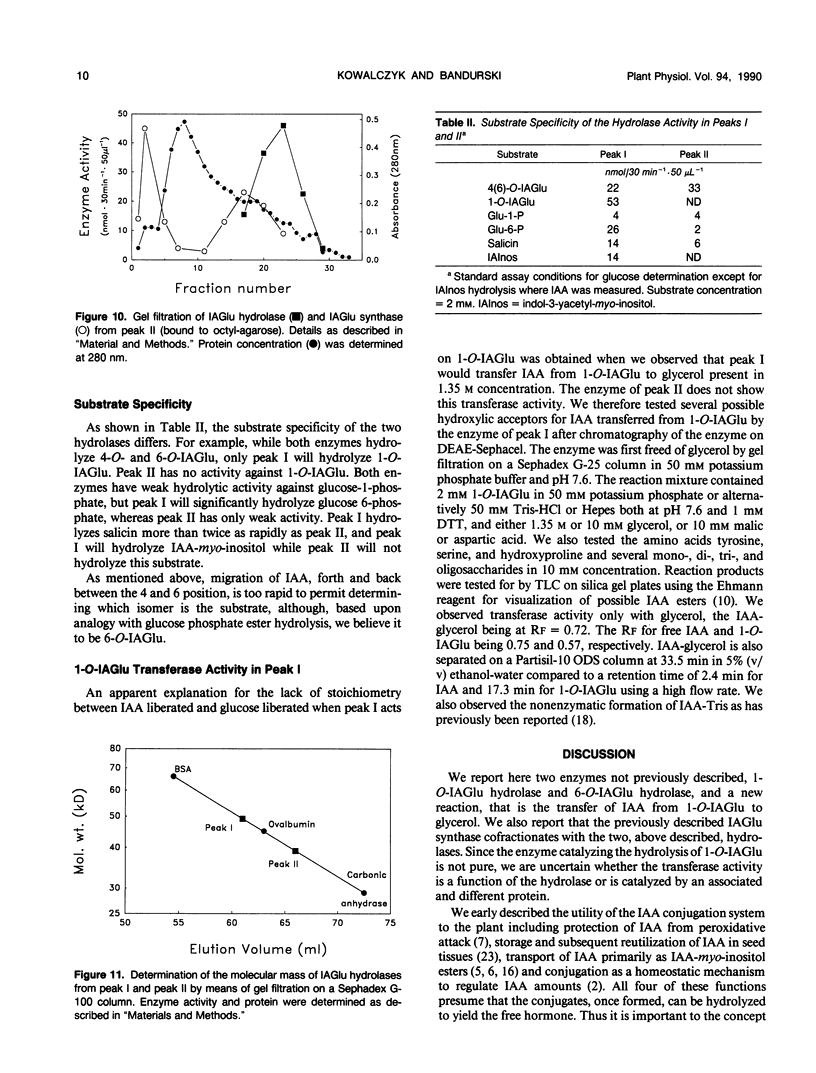
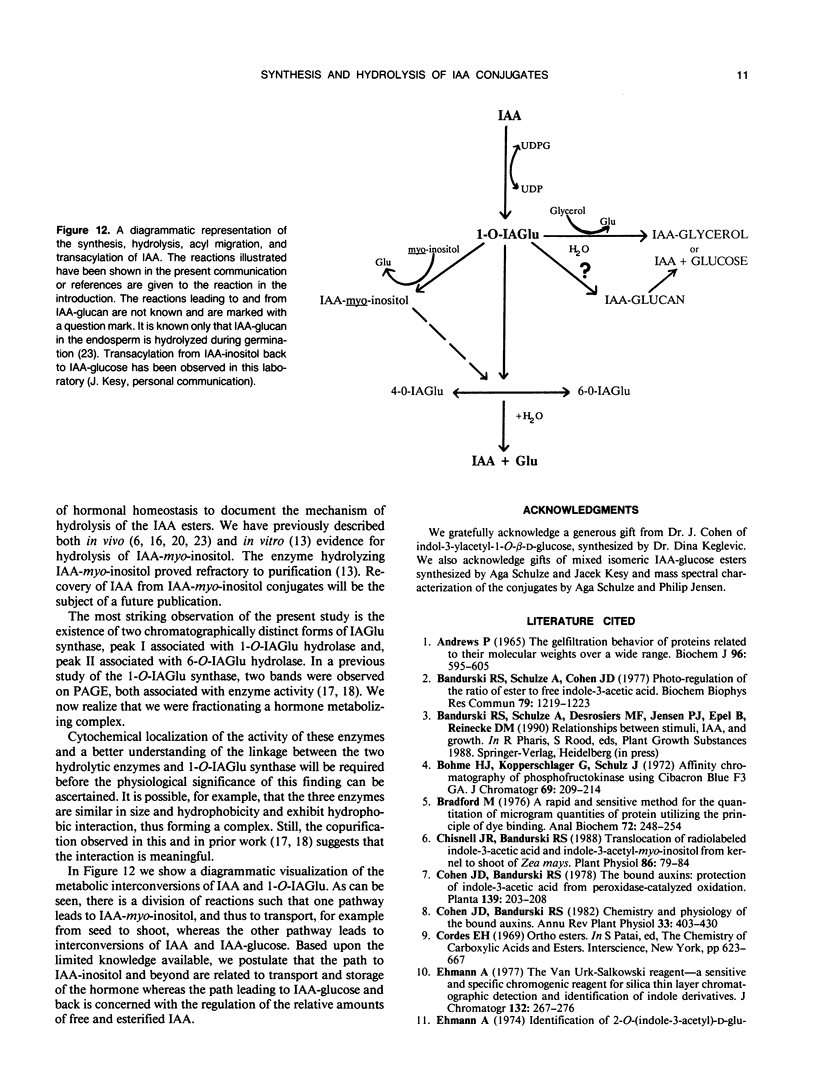

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandurski R. S., Schulze A., Cohen J. D. Photo-regulation of the ratio of ester to free indole-3-acetic acid. Biochem Biophys Res Commun. 1977 Dec 21;79(4):1219–1223. doi: 10.1016/0006-291x(77)91136-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Böhme H. J., Kopperschläger G., Schulz J., Hofmann E. Affinity chromatography of phosphofructokinase using Cibacron blue F3G-A. J Chromatogr. 1972 Jun 28;69(1):209–214. doi: 10.1016/s0021-9673(00)83103-9. [DOI] [PubMed] [Google Scholar]
- Chisnell J. R., Bandurski R. S. Translocation of radiolabeled indole-3-acetic acid and indole-3-acetyl-myo-inositol from kernel to shoot of Zea mays L. Plant Physiol. 1988;86:79–84. doi: 10.1104/pp.86.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcuera L. J., Michalczuk L., Bandurski R. S. Enzymic synthesis of indol-3-ylacetyl-myo-inositol galactoside. Biochem J. 1982 Nov 1;207(2):283–290. doi: 10.1042/bj2070283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehmann A. Identification of 2-O (indole-3-acetyl)-D-glucopyranose, 4-O-(indole-3-acetyl)-D-glucopyranose and 6-O-(indole-3-acetyl)-D-glucopyranose from kernels of Zea mays by gas-liquid chromatography-mass spectrometry. Carbohydr Res. 1974 May;34(1):99–114. doi: 10.1016/s0008-6215(00)80374-2. [DOI] [PubMed] [Google Scholar]
- Ehmann A. The van urk-Salkowski reagent--a sensitive and specific chromogenic reagent for silica gel thin-layer chromatographic detection and identification of indole derivatives. J Chromatogr. 1977 Feb 11;132(2):267–276. doi: 10.1016/s0021-9673(00)89300-0. [DOI] [PubMed] [Google Scholar]
- Epstein E., Cohen J. D., Bandurski R. S. Concentration and Metabolic Turnover of Indoles in Germinating Kernels of Zea mays L. Plant Physiol. 1980 Mar;65(3):415–421. doi: 10.1104/pp.65.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall P. J., Bandurski R. S. [3H]Indole-3-acetyl-myo-inositol hydrolysis by extracts of Zea mays L. vegetative tissue. Plant Physiol. 1986;80:374–377. doi: 10.1104/pp.80.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keglević D., Pokorny M. The chemical synthesis of 1-O-(indol-3'-ylacetyl)-beta-D-glucopyranose. The higher activity of the glucoside in comparison with exogenous indol-3-ylacetic acid in plant-section elongation tests. Biochem J. 1969 Oct;114(4):827–832. doi: 10.1042/bj1140827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komoszynski M., Bandurski R. S. Transport and metabolism of indole-3-acetyl-myo-inositol-galactoside in seedlings of Zea mays. Plant Physiol. 1986;80:961–964. doi: 10.1104/pp.80.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leznicki A. J., Bandurski R. S. Enzymic synthesis of indole-3-acetyl-1-O-beta-d-glucose. I. Partial purification and characterization of the enzyme from Zea mays. Plant Physiol. 1988;88:1474–1480. doi: 10.1104/pp.88.4.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leznicki A. J., Bandurski R. S. Enzymic synthesis of indole-3-acetyl-1-O-beta-d-glucose. II. Metabolic characteristics of the enzyme. Plant Physiol. 1988;88:1481–1485. doi: 10.1104/pp.88.4.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowacki J., Bandurski R. S. Myo-Inositol Esters of Indole-3-acetic Acid as Seed Auxin Precursors of Zea mays L. Plant Physiol. 1980 Mar;65(3):422–427. doi: 10.1104/pp.65.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogita Z. I., Markert C. L. A miniaturized system for electrophoresis on polyacrylamide gels. Anal Biochem. 1979 Nov 1;99(2):233–241. doi: 10.1016/s0003-2697(79)80001-9. [DOI] [PubMed] [Google Scholar]
- Piskornik Z., Bandurski R. S. Purification and Partial Characterization of a Glucan Containing Indole-3-acetic Acid. Plant Physiol. 1972 Jul;50(1):176–182. doi: 10.1104/pp.50.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda M., Bandurski R. S. A Quantitative Estimation of Alkali-labile Indole-3-Acetic Acid Compounds in Dormant and Germinating Maize Kernels. Plant Physiol. 1969 Aug;44(8):1175–1181. doi: 10.1104/pp.44.8.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]



