Abstract
Background:
Gabapentin is increasingly used as an off-label, opioid-sparing pain medication in children. We investigated perioperative gabapentin administration and postoperative opioid use in children who underwent appendectomy for perforated appendicitis.
Methods:
A retrospective cohort study of healthy children ages 2–18 years undergoing appendectomy for perforated appendicitis from 2014 to 2019 was performed using the Pediatric Health Information System®. Propensity score matched (PSM) analysis was conducted with 1:1 matching based on patient and hospital characteristics. Multivariable linear regression analysis was used to evaluate an association between gabapentin, postoperative opioid use, and postoperative length of stay.
Results:
Of 29,467 children with perforated appendicitis who underwent appendectomy, 236 (0.8%) received gabapentin. In 2014, <10 children received gabapentin, but by 2019, 110 children received gabapentin. On univariate analysis of the PSM cohort, children receiving gabapentin had decreased total postoperative opiate use (2.3 SD ± 2.3 versus 3.0 SD ± 2.5 days, p < 0.001). On adjusted analysis, children receiving gabapentin had 0.65 fewer days of postoperative total opioid use (95% CI: −1.09, −0.21) and spent 0.69 fewer days in the hospital after surgery (95% CI: −1.30, −0.08).
Conclusion:
While overall use is infrequent, gabapentin is increasingly administered to children with perforated appendicitis who undergo an appendectomy and is associated with decreased postoperative opioid use and reduced postoperative length of stay. Multimodal pain management strategies incorporating gabapentin may reduce postoperative opioid consumption, but further studies of drug safety are needed for this off-label use in children undergoing surgery.
Level of evidence:
III.
Keywords: Appendicitis, Gabapentin, Opioid, Postoperative pain, Multimodal pain management
1. Introduction
Given the concerns around risks associated with excess opioid prescribing, healthcare institutions, physicians, and governmental agencies have pushed for expanded protocols to treat postoperative pain with fewer opioids in children [1]. Many children's hospitals across the country have reduced opioid administration by establishing Enhanced Recovery After Surgery (ERAS) protocols with multimodal analgesics to decrease opioid use during hospitalization as well as reduce length of stay after common pediatric surgeries [2-4]. Gabapentin is an example of a non-opioid analgesic that can be used to treat acute postoperative pain in children but not currently approved for this indication by the Federal Drug Administration (FDA). Gabapentin is thought to produce analgesia by binding to pre-synaptic calcium channels in the nervous system leading to inactivation by reducing the excitatory neurotransmitters involved in the pain cascade [5,6].
In adults, Hah et al.‘s randomized control trial cohort found that patients who were given preoperative gabapentin were more likely to discontinue opioids after surgery and no significance differences in adverse events were noted [7]. Single-institution retrospective studies of children undergoing appendectomy and tonsillectomy have shown that gabapentin decreases opioid consumption in children as well [8,9]. Furthermore, two randomized controlled trials of gabapentin use in children undergoing spinal fusion surgery for scoliosis found that it reduces opioid usage [10,11]. However, another randomized study of children undergoing spinal fusion for idiopathic scoliosis showed no difference in opioid consumption, pain scores, or side effects from gabapentin administration [12].
The primary objective of this study was to characterize perioperative gabapentin use in children who undergo appendectomy for perforated appendicitis by identifying a large cohort of healthy children undergoing this common pediatric surgery. Furthermore, we aimed to evaluate total postoperative opioid use in children who receive gabapentin compared to those children who do not. We hypothesize that children undergoing appendectomy for perforated appendicitis who receive gabapentin would demonstrate decreased postoperative opioid use and a reduced length of stay after surgery.
2. Methods
2.1. Study design and data source
This was a retrospective cohort studying using data from the Children Hospital Association's (CHA) Pediatric Health Information System (PHIS) database. PHIS is a multicenter administrative billing dataset that captures data from 52 children's hospitals across the United States. It captures data from inpatient, emergency department, and ambulatory surgery sites of CHA participating hospitals. Participating hospitals submit de-identified discharge and encounter data, which includes demographics, diagnoses, procedures, and charges. Data quality and consistency are assessed by a joint effort from the CHA and affiliated hospitals. Data are subjected to several validity checks before being included in the database. The Institutional Review Board reviewed this multicenter cohort study at Children's Hospital Los Angeles and granted exemption according to 45 Code of Federal Regulations 46.101(b) (4) for existing data, documents, records, and specimens.
2.2. Patient population and data elements
We identified all pediatric patients aged 2–18 years discharged between January 2014 and December 2019 who had an International Disease Classification Ninth or Tenth Revision, Clinical Modification (ICD-9/10-CM) for appendicitis and who underwent an appendectomy (Supplemental Table 1).
Individual demographics included age, sex, race, ethnicity (Hispanic or Latino, Not Hispanic or Latino, or Unknown), and insurance status (private, public, other). Hospital characteristics included the United States census region (Midwest, Northeast, South, and West) and hospital number. We excluded pediatric patients without available pharmacy data. To create a more uniform cohort of healthy children for this study, we excluded patients with a complex chronic condition defined by ICD-9 or ICD-10-CM Code for Pediatric complex chronic conditions classification system version 2 [13].
2.3. Definition of perforated appendicitis and open appendectomy
Cases of perforated appendicitis were classified by ICD-9-CM and ICD-10- CM codes (Supplemental Table 2). We focused on studying children with perforated appendicitis as they have a prolonged length of stay associated with increased opioid usage and have a high burden of pain [14]. Open and laparoscopic procedures were defined by ICD-9-CM and ICD-10-CM codes as well (Supplemental Table 3).
2.4. Treatment assignment & outcomes
Our primary treatment of interest was gabapentin use during hospitalization, defined as the receipt of gabapentin between admission and discharge date. Our primary outcome was total days of postoperative opioid analgesic use. Total opioid use was defined as any unique receipt of an opioid analgesic during the postoperative period. For example, a patient who received an opioid analgesic on postoperative day one, three, and four would have a total opioid use of three days. Opioid dosing and frequency are not available in the PHIS and so analysis was limited to total days of opioid use.
2.5. Statistical analysis and propensity score matching
Descriptive statistics (e.g., mean, standard deviation, frequencies, and percent) were calculated for overall and propensity score matched cohorts. Bivariate analyses were conducted to determine associations between gabapentin treatment and outcomes as well as covariates of interest. Chi-square (χ2) or Fisher's exact test were used for categorical variables and student's t or Mann–Whitney U test for continuous variables. To evaluate the impact of gabapentin use on postoperative use of opioids, we used propensity score matching (PSM) to adjust for potential confounding. A multivariable logistic regression model was used to generate propensity scores based on covariates (chosen a priori): age at surgery, sex, race, ethnicity, insurance status, open versus laparoscopic surgery, year of surgery, receipt of postoperative non-opioid medications (acetaminophen, ketorolac, and ibuprofen), postoperative antibiotic use, hospital, and hospital region. Given the large number of controls compared to cases, Greedy Matching without replacement was performed on the logit of the propensity score using a caliper 0.2 standard deviations of the logit of the PSM [15]. Following PSM patients were matched within each hospital receiving gabapentin and were compared to patients in the same hospital in the same year who did not receive gabapentin. The overall balance was assessed by bivariate differences as well as examining computed standard differences for each of the baseline characteristics included in the regression model. Assessment of the indicated covariates was comparable between the patients in the gabapentin treated group, and the patients who did not receive gabapentin. Standard differences between confounding variables had means with relatively small standardized differences between the treatment and control groups, which supports good balance among measured confounders [16]. In the PSM groups where a variable was not balanced, a double adjustment was performed in the subsequent linear regression by including the variable that was not balanced as a covariate in the final regression model. Our primary outcome of total postoperative opioid analgesic use was evaluated using the PS-matched cohort by linear regression. Secondary outcome of postoperative length of stay was also evaluated using linear regression. All analyses were conducted with two sided significance, α = 0.05. The analysis was performed using S.A.S. software 9.4 (S.A.S. Institute, Inc, Cary, North Carolina).
3. Results
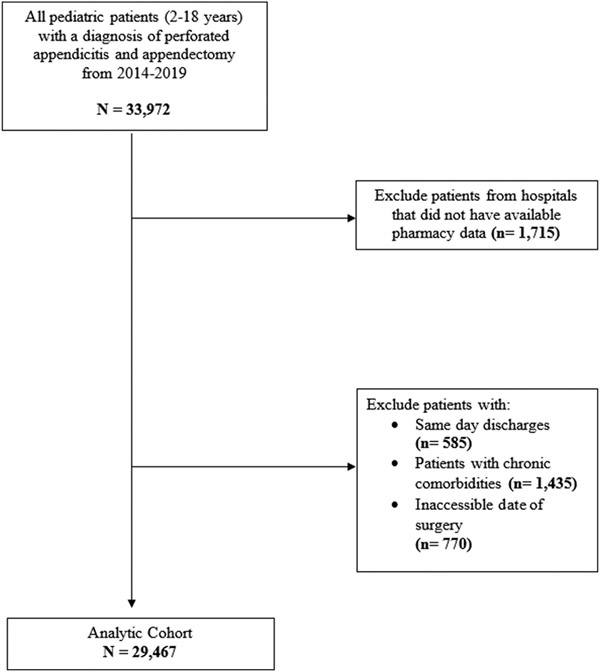
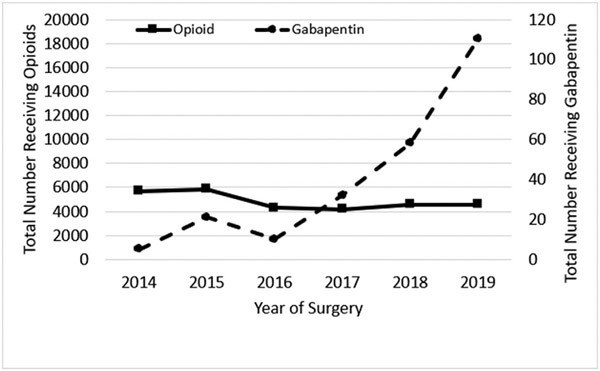
Overall, 29,467 children with perforated appendicitis were identified of which 236 (0.8%) received gabapentin during hospitalization (Fig. 1). In 2014, <10 children received gabapentin, but by 2019, 110 children received gabapentin, a 10-fold increase over five years (Fig. 2). Fifty hospitals were included in the analysis. Gabapentin was used in older children, 10.6 (± SD 4.0) versus 10.0 years of age (± SD 3.9) (p = 0.019). Most children on gabapentin were from the Southern region of the United States (n = 226, 95.8%). Children who were on gabapentin in comparison to those who were not on gabapentin did not significantly differ regarding sex or race (p > 0.05, Table 1).
Fig. 1.
Flow diagram of cohort selection.
Fig. 2.
Prevalence of Perioperative Gabapentin and Postoperative Opioid Use. The left vertical axis shows total patients receiving perioperative opioids, while the right vertical axis shows total patients receiving perioperative gabapentin. The horizontal axis denotes year of surgery.
Table 1.
Demographics and clinical variables of the overall cohort (N = 29,467) stratified by perioperative gabapentin administration.
| Gabapentin N = 236 |
No Gabapentin N = 29,231 |
P | |
|---|---|---|---|
| Age at surgery, years (mean, SDa) | 10.6 (4.0) | 10.0 (3.9) | 0.019 |
| Female Sex | 94 (39.8) | 11,944 (40.9) | 0.746 |
| Race | <0.001 | ||
| Unknown | 28 (11.9) | 2003 (6.9) | |
| White | 134 (56.8) | 18,293 (62.6) | |
| Black | 60 (25.4) | 2067 (7.1) | |
| Asian | 11 (4.7) | 770 (2.6) | |
| American Indian or Alaskan Native | <10 (<4.2) | 149 (0.5) | |
| Native Hawaiian or Pacific Islander | <10 (<4.2) | 70 (0.2) | |
| Other | <10 (<4.2) | 5879 (20.1) | |
| Ethnicity | <0.001 | ||
| Hispanic or Latino | 69 (29.2) | 11,906 (40.7) | |
| Not Hispanic or Latino | 167 (70.8) | 15,783 (54.1) | |
| Unknown | <10 (<4.2) | 1542 (5.3) | |
| Insurance | 0.675 | ||
| Private | 88 (37.3) | 11,112 (37.8) | |
| Public | 131 (55.5) | 16,543 (56.3) | |
| Other | 17 (7.2) | 1716 (5.8) | |
| Hospital Region | <0.001 | ||
| Midwest | <10 (<4.2) | 5599 (19.2) | |
| Northeast | <10 (<4.2) | 2389 (8.2) | |
| South | 226 (95.8) | 12,451 (42.6) | |
| West | <10 (<4.2) | 8792 (30.1) | |
| Open appendectomy | 16 (6.8) | 1290 (4.4) | 0.075 |
| Postoperative Analgesic Use | |||
| Opioid | 121 (51.3) | 20,201 (68.8) | <0.001 |
| Ibuprofen | 71 (30.1) | 8427 (28.7) | 0.646 |
| Ketorolac | 205 (86.9) | 20,788 (71.1) | <0.001 |
| Acetaminophen | 204 (86.4) | 20,520 (70.2) | <0.001 |
| IV acetaminophen | 11 (4.7) | 8152 (27.9) | <0.001 |
| Antibiotic Utilization | |||
| Any perioperative antibiotic use | 236 (100) | 29,055 (99.4) | – |
| Postoperative Antibiotic Use | 219 (92.8) | 27,570 (94.3) | 0.315 |
| Cumulative unique days of postop antibiotic use (Median, IQRb) | 4 (2.0–5.0) | 4 (3.0–5.0) | 0.565 |
| Postoperative outcomes | |||
| Postoperative LOS (in days) | 4.6 (3.3)a | 4.5 (3.3)a | 0.586 |
| Postoperative Opioid Use (in days) | 2.3 (2.2)a | 2.7 (2.1)a | <0.001 |
LOS, Length of stay.
Mean days (Standard Deviation).
Median days (Interquartile Range).
Overall, more than 99% (29,344/29,467) of children received opioids during hospital admission for appendectomy, including administration on the day of surgery. Approximately, 31% were administered opiates for one day or less while 69% received opiates for two days or longer. Children who received gabapentin during their hospitalization for appendectomy were less likely to receive a postoperative opioid (51.3% versus 68.8%, p < 0.001) and more likely to receive an alternative, multimodal pain medication such as ketorolac (86.9% versus 71.1%, p < 0.001), IV acetaminophen (86.4% versus 70.2%, p < 0.001), or PO acetaminophen (4.7% versus 27.9%, p < 0.001). The total mean postoperative days an opioid was prescribed was shorter for children receiving gabapentin in comparison to those children not on gabapentin (2.3 ± 2.2 days versus 2.7 ± 2.1 days, p < 0.001). There was no significant difference in the postoperative length of stay between groups on unadjusted univariate analysis (p = 0.586) (Table 1).
Propensity score (1:1) matching (PSM) produced two groups of 229 children each (Table 2). Baseline characteristics were similar between both groups, including sex, race, ethnicity, insurance status, and perioperative characteristics. The groups were also well balanced by postoperative pain regimens with no differences in use of ibuprofen, and ketorolac after surgery (Table 2). However, postoperative oral acetaminophen was more commonly used in the gabapentin naïve group (86% vs. 93%, p = 0.015). In the PSM cohort, children who received gabapentin had a significantly reduced amount of mean postoperative days on an opioid of 2.4 (± SD 2.3) days in comparison to children not on gabapentin of 3.2 (± SD 2.3) days (p < 0.001, Table 2). Children who received gabapentin had a decreased postoperative mean length of stay of 4.6 (± SD 3.3) days in comparison to those children not on gabapentin of 5.4 (± SD 2.9) days (p < 0.001) (Table 2). In the PSM cohort, gabapentin was given to 9% (21/233) of children pre-operatively, 65% (153/233) on the day of surgery, and 94% (220/233) after the day of surgery. In the PSM cohort, gabapentin was given for a median of 3 days (IQR = 2.0–5.0) postoperatively.
Table 2.
Demographics and clinical variables of the propensity score matched cohort (N = 458) stratified by perioperative gabapentin administration.
| Gabapentin N = 229 |
No Gabapentin N = 229 |
P | |
|---|---|---|---|
| Age at surgery, years (mean, SDa) | 10.6 (4.0) | 10.4 (3.9) | 0.750 |
| Female sex | 90 (39.3) | 92 (40.2) | 0.849 |
| Race | 0.449 | ||
| Unknown | 28 (12.2) | 18 (7.9) | |
| White | 134 (58.5) | 150 (65.5) | |
| Black | 53 (23.1) | 48 (21.0) | |
| Asian | 11 (4.8) | 12 (5.2) | |
| American Indian or Alaskan Native | <10 (<4.2) | <10 (<4.2) | |
| Native Hawaiian or Pacific Islander | <10 (<4.2) | <10 (<4.2) | |
| Other | <10 (<4.2) | <10 (<4.2) | |
| Ethnicity | 0.114 | ||
| Hispanic or Latino | 69 (30.1) | 54 (23.6) | |
| Not Hispanic or Latino | 160 (69.9) | 175 (76.4) | |
| Unknown | <10 (<4.2) | <10 (<4.2) | |
| Insurance | 0.139 | ||
| Private | 87 (38.0) | 108 (47.2) | |
| Public | 125 (54.6) | 107 (46.7) | |
| Other | 17 (7.4) | 14 (6.1) | |
| Hospital Region | 0.354 | ||
| Midwest | <10 (<4.2) | <10 (<4.2) | |
| Northeast | <10 (<4.2) | 11 (4.7) | |
| South | 219 (95.6) | 217 (94.8) | |
| West | <10 (<4.2) | <10 (<4.2) | |
| Open appendectomy | 16 (7.0) | 17 (7.4) | 0.857 |
| Postoperative Outcomes | |||
| Analgesic Use | |||
| Opioidb | 118 (51.5) | 157 (68.6) | <0.001 |
| Ibuprofen | 64 (28.0) | 70 (30.6) | 0.534 |
| Ketorolac | 200 (87.3) | 202 (88.2) | 0.775 |
| Acetaminophen | 197 (86.0) | 213 (93.0) | 0.015 |
| IV acetaminophen | 11 (4.8) | 14 (6.1) | 0.537 |
| Antibiotic Utilization | |||
| Any perioperative antibiotic useb | 229 (100) | 229 (100) | – |
| Postoperative Antibiotic Use | 213 (93.0) | 217 (94.8) | 0.435 |
| Cumulative unique days of postop antibiotic use (Median, IQRc) | 4 (2.0–5.0) | 5 (3.0–6.0) | 0.923 |
| Postoperative LOS (in days)b | 4.6 (3.3)a | 5.3 (3.3)a | <0.001 |
| Postoperative Opioid Use (in days)b | 2.3 (2.3)a | 3.0 (2.5)a | <0.001 |
LOS, Length of stay.
Mean days (Standard Deviation).
Outcome variables not included in propensity score matching.
Median days (Interquartile Range).
A linear regression analysis of the PSM cohort demonstrated that children receiving gabapentin during their hospitalization had on average 0.65 fewer days of opioid use compared to children who did not receive gabapentin (−95% CI: − 1.09, − 0.21). A separate linear regression for the total postoperative length of stay demonstrated that children who received gabapentin had 0.69 fewer hospital days compared to children who did not receive gabapentin (95% CI: − 1.30, − 0.29). When double adjustment was performed using postoperative acetaminophen as a covariate, children on gabapentin had a total 0f 0.57 days less of postoperative opioid use in comparison to children who did not receive gabapentin (95% CI: −1.01, −0.13) (Supplemental Table 4). Postoperative length of stay was less in the gabapentin group in comparison to the group not receiving gabapentin, but this was not significant (−0.54, 95% CI: −1.15, 0.07).
4. Discussion
Although infrequent, gabapentin use has increased in recent years among otherwise healthy children undergoing appendectomy for perforated appendicitis. Children with perforated appendicitis who underwent an appendectomy and received gabapentin demonstrated a significant decrease in opioid use and a shorter length of stay. These findings suggest that incorporating gabapentin as part of a multimodal pain management regimen for children with appendicitis may decrease opioid requirements as well as reduce hospitalization.
A number of studies have used gabapentin for acute postoperative pain in children, indicating a trend towards increasing usage in children of off-label medications. Currently, up to 39–44% of adult medications prescribed in children are administered without a clear FDA indication for children [8,11,12,17]. The side effect profile in children who take gabapentin is not widely reported or studied given no randomized trials or other prospective studies in children have been submitted to the FDA for approval or post-marketing surveillance of these medications. From the existing literature, some authors note severe reactions from gabapentin like suicidality and depression and other less severe reactions such as ataxia, somnolence, dizziness, fatigue, nausea, and vomiting, among other complications in adults [18,19]. These studies primarily focus on the side effects in adults given the lack of longitudinal post-marketing surveillance studies in children, which would reveal these side effect profiles as seen with FDA approved drugs. Furthermore, although in some studies, the use of opiates was significantly reduced with gabapentin, there was a higher risk of sedation when adults took this medication and largely unknown whether this side effect would be observed in children [20].
The present findings are consistent with our prior study of non-opioid analgesic use in children with appendicitis which found that persistent opioid use after surgery was associated with prolonged hospitalization [14]. Furthermore, our findings align with previous reports of reduced opioid use among children receiving perioperative gabapentin [8,9]. Baxter et al.‘s 1-year retrospective study matched 29 children receiving gabapentin postoperatively with 58 children who did not receive gabapentin for simple and complicated cases of appendicitis requiring surgery [9]. Children on gabapentin took fewer morphine equivalents in comparison to gabapentin naïve children. However, Baxter et al. did not observe differences patients' pain scores or length of stay with gabapentin usage. The authors also noted that several children on gabapentin were also on ketorolac postoperatively, which we did control for in our analysis. They also reported all appendicitis cases as opposed to just perforated appendicitis, which may have been underpowered to look at the impact of gabapentin on length of stay as most cases of uncomplicated appendicitis, which constituted 40% of their cohort, went home soon after surgery. In considering our study results, gabapentin could be most efficacious for children with perforated appendicitis who have a prolonged length of stay associated with opioid usage [14].
Usage of gabapentin as an opioid-sparing agent is also increasing for other pediatric surgical populations. Amani et al. conducted a randomized clinical trial giving a single preoperative dose of gabapentin to children who undergo tonsillectomy with their standard of care pain medications [8]. Children who received one dose of preoperative gabapentin had decreased pain scores compared to the local anesthetic group. Other studies, however, showed no effect of gabapentin on postoperative pain [12]. Mayell et al. randomized children undergoing spinal fusion for idiopathic scoliosis to receive postoperative gabapentin. They demonstrated no difference in opioid consumption, pain scores, or side effects from post-intervention gabapentin administration [12]. In contrast, Rusy et al. in their randomized trial of children with idiopathic scoliosis who underwent surgery and received preoperative and postoperative gabapentin, showed a reduction in their overall morphine usage associated with gabapentin administration [11]. Moreover, Anderson et al. also show that children who received preoperative and postoperative gabapentin in addition to a standard pain regimen had decreased total postoperative opioid use and visual analog pain scales [10]. The use of non-opioid medication such as gabapentin in conjunction with opioids may allude to a synergistic effect in patients that may augment the effect of opioids, and which is why many recommend giving gabapentin preoperatively to derive the most benefit from gabapentin in postoperative pain control. A possible mechanism in animal models of the synergy is that gabapentin may enhance inhibitory signals and decrease excitatory signals along the spinal cord while altering opioid levels in the amygdala, both affecting central and peripheral pathways of pain [5,6,21]. Hence, it may augment opioids especially in cases of neuropathic pain with animal neuropathic pain models indicating increased sensitivity to opioids after gabapentin administration [6].
Our data showed a decreased postoperative length of stay for children who received gabapentin compared to those who did not receive gabapentin. Similar results are reported in adults receiving ERAS protocols that include gabapentin [22]. Other literature has not shown any difference in gabapentin administration alone or as part of an ERAS protocol on length of stay after surgery in adults or children [9,23]. This lack of a difference may imply that gabapentin usage needs to be tailored to the procedures and populations with most pain exposure and duration that may impact global outcomes like the length of stay such as children admitted with perforated appendicitis. Furthermore, more research and uniformity on how it is administered either preoperatively, postoperatively, or both are needed.
Notably, this study also found a higher proportion of children taking gabapentin in conjunction with other multimodal pain therapies. This likely is a result of the generational shift away from opioids and the increasing popularity of enhanced recovery after surgery protocols. We attempted to minimize this effect by matching the PSM cohorts by postoperative non-opioid analgesic use, year of surgery, region in the U.S. and institution. Notably, the present analysis could not account for subjective patient-reported pain thresholds which may have changed among children receiving less pain medication overall due to prescribers’ reluctance to give opioids. Such patient-reported metrics should be part of future studies aiming to optimize postoperative pain management regiments for children recovering from surgery.
Limitations of our study include the usage of an administrative database susceptible to errors in billing codes and missing data. However, PHIS is well validated with multiple studies in children with appendicitis and subjected to extensive and rigorous quality assurance audits [24-26]. This database's administrative nature also allowed us to identify trends in gabapentin usage for children undergoing surgery as PHIS represents 20% of pediatric hospitalizations in the United States [27,28]. Additionally, as gabapentin is infrequently prescribed for children with perforated appendicitis, using administrative data enabled a sufficiently powered analysis of a rare pediatric medication exposure. Another limitation of this study is that we could not measure morphine equivalents nor pain scores to quantify medication use and determine patient pain levels for each child after surgery. Instead, we used the surrogate measure of cumulative days of opioid use. Although this metric does require subsequent validation with clinical records, it represents a previously published measurement of opioid using the PHIS dataset [14]. This study also did not capture side effects for children who received gabapentin which may prove significant. In addition, for those that received gabapentin on the day of surgery, we cannot determine if it was given prior to surgery or after surgery, which may be particularly relevant for the surgeon caring for a patient with possible perforated appendicitis. Ultimately, larger prospective studies are needed to further elucidate optimal multimodal analgesic regimens for children requiring surgery. Finally, while the association of gabapentin use with decreased postoperative opioid use remained significant in our sensitivity analysis double adjusting for postoperative acetaminophen use, postoperative length of stay no longer significantly differed between groups. Notably, the point estimates were similar to the PSM analysis without double adjustment, indicating that while the direction of the association remains the same, it is likely a relatively weak association dependent on regression model selection.
5. Conclusion
While overall utilization is infrequent, perioperative gabapentin use has recently increased in children with perforated appendicitis undergoing appendectomy. Children who received gabapentin had fewer days of postoperative opioid use and shorter hospitalization. Multimodal pain management strategies incorporating gabapentin may reduce postoperative opioid consumption and decrease the length of stay in children with perforated appendicitis undergoing appendectomy. Future prospective studies of non-opioid analgesic protocols are needed to better understand the safety, side effects and optimal timing of gabapentin administration to optimize pain control in children undergoing surgery.
Supplementary Material
Funding
Dr. Kelley-Quon is supported by grant KL2TR001854 from the National Center for Advancing Translational Science (NCATS) of the U.S. National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funder did not take part in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Abbreviations:
- FDA
Food and Drug Administration
- PSM
Propensity Score Matched
- CHA
Children's Hospital Association
- PHIS
Pediatric Health Information System®
- ERAS
Enhanced Recovery After Surgery
- ICD-9/10-CM
International Classification of Diseases, Ninth/Tenth Revision, Clinical Modification.
Footnotes
Declaration of competing interest
None.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpedsurg.2023.03.009.
Presented as an oral presentation at the American College of Surgeons Clinical Congress 2020, October 2020, Virtual Event.
References
- [1].Schuler MS, Heins SE, Smart R, Griffin BA, Powell D, Stuart EA, et al. The state of the science in opioid policy research. Drug Alcohol Depend 2020;214:108137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Leeds IL, Boss EF, George JA, Strockbine V, Wick EC, Jelin EB. Preparing enhanced recovery after surgery for implementation in pediatric populations. J Pediatr Surg 2016;51(12):2126–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rove KO, Brockel MA, Saltzman AF, Donmez MI, Brodie KE, Chalmers DJ, et al. Prospective study of enhanced recovery after surgery protocol in children undergoing reconstructive operations. J Pediatr Urol 2018;14(3):252 e1–e9. [DOI] [PubMed] [Google Scholar]
- [4].Saltzman AF, Warncke JC, Colvin AN, Carrasco A Jr, Roach JP, Bruny JL, et al. Development of a postoperative care pathway for children with renal tumors. J Pediatr Urol 2018;14(4):326 e1–e6. [DOI] [PubMed] [Google Scholar]
- [5].Bennett MI, Simpson KH. Gabapentin in the treatment of neuropathic pain. Palliat Med 2004;18(1):5–11. [DOI] [PubMed] [Google Scholar]
- [6].Matthews EA, Dickenson AH. A combination of gabapentin and morphine mediates enhanced inhibitory effects on dorsal horn neuronal responses in a rat model of neuropathy. Anesthesiology 2002;96(3):633–40. [DOI] [PubMed] [Google Scholar]
- [7].Hah J, Mackey SC, Schmidt P, McCue R, Humphreys K, Trafton J, et al. Effect of perioperative gabapentin on postoperative pain resolution and opioid cessation in a mixed surgical cohort: a randomized clinical trial. JAMA Surg 2018;153(4):303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Amani S, Abedinzadeh MR. Effects of oral gabapentin, local bupivacaine and intravenous pethidine on post tonsillectomy pain. Iran J Otorhinolaryngol 2015;27(82):343–8. [PMC free article] [PubMed] [Google Scholar]
- [9].Baxter KJ, Hafling J, Sterner J, Patel AU, Giannopoulos H, Heiss KF, et al. Effectiveness of gabapentin as a postoperative analgesic in children undergoing appendectomy. Pediatr Surg Int 2018;34(7):769–74. [DOI] [PubMed] [Google Scholar]
- [10].Anderson DE, Duletzke NT, Pedigo EB, Halsey MF. Multimodal pain control in adolescent posterior spinal fusion patients: a double-blind, randomized controlled trial to validate the effect of gabapentin on postoperative pain control, opioid use, and patient satisfaction. Spine Deform 2020;8(2):177–85. [DOI] [PubMed] [Google Scholar]
- [11].Rusy LM, Hainsworth KR, Nelson TJ, Czarnecki ML, Tassone JC, Thometz JG, et al. Gabapentin use in pediatric spinal fusion patients: a randomized, double-blind, controlled trial. Anesth Analg 2010;110(5):1393–8. [DOI] [PubMed] [Google Scholar]
- [12].Mayell A, Srinivasan I, Campbell F, Peliowski A. Analgesic effects of gabapentin after scoliosis surgery in children: a randomized controlled trial. Paediatr Anaesth 2014;24(12):1239–44. [DOI] [PubMed] [Google Scholar]
- [13].Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr 2014;14: 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mahdi EM, Ourshalimian S, Russell CJ, Zamora AK, Kelley-Quon LI. Fewer postoperative opioids are associated with decreased duration of stay for children with perforated appendicitis. Surgery 2020. Nov;168(5):942–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bergstralh EJ, Kosanke JL. Computerized matching of cases to controls. Technical Report Series; 1995. p. 1–35. number 56. [Google Scholar]
- [16].Analysis of observational Health care data using SAS ®. North Carolina, USA: SAS Institute Inc.; 2010. [Google Scholar]
- [17].Hoon D, Taylor MT, Kapadia P, Gerhard T, Strom BL, Horton DB. Trends in off-label drug use in ambulatory settings: 2006-2015. Pediatrics 2019;144(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Larsen Burns M, Kinge E, Stokke Opdal M, Johannessen SI, Johannessen Landmark C. Therapeutic drug monitoring of gabapentin in various indications. Acta Neurol Scand 2019;139(5):446–54. [DOI] [PubMed] [Google Scholar]
- [19].Verret M, Lauzier F, Zarychanski R, Perron C, Savard X, Pinard A-M, et al. Perioperative use of gabapentinoids for the management of postoperative acute pain. Anesthesiology 2020;133(2):265–79. [DOI] [PubMed] [Google Scholar]
- [20].Ho KY, Gan TJ, Habib AS. Gabapentin and postoperative pain–a systematic review of randomized controlled trials. Pain 2006;126(1–3):91–101. [DOI] [PubMed] [Google Scholar]
- [21].Rocha L, Ondarza-Rovira R, Maidment NT. Gabapentin modifies extracellular opioid peptide content in amygdala: a microdialysis study. Epilepsy Res 1999;35(1):13–20. [DOI] [PubMed] [Google Scholar]
- [22].Chapman JS, Roddy E, Ueda S, Brooks R, Chen LL, Chen LM. Enhanced recovery pathways for improving outcomes after minimally invasive gynecologic oncology surgery. Obstet Gynecol 2016;128(1):138–44. [DOI] [PubMed] [Google Scholar]
- [23].Fan KL, Luvisa K, Black CK, Wirth P, Nigam M, Camden R, et al. Gabapentin decreases narcotic usage: enhanced recovery after surgery pathway in free autologous breast reconstruction. Plast Reconstr Surg Glob Open 2019;7(8):e2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Anandalwar SP, Cameron DB, Graham DA, Melvin P, Dunlap JL, Kashtan M, et al. Association of intraoperative findings with outcomes and resource use in children with complicated appendicitis. JAMA Surg 2018;153(11):1021–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chisholm AG, Little BD, Johnson RF. Validating peritonsillar abscess drainage rates using the Pediatric hospital information system data. Laryngoscope 2020;130(1):238–41. [DOI] [PubMed] [Google Scholar]
- [26].Rice-Townsend S, Hall M, Barnes JN, Lipsitz S, Rangel SJ. Variation in risk-adjusted hospital readmission after treatment of appendicitis at 38 children's hospitals: an opportunity for collaborative quality improvement. Ann Surg 2013;257(4):758–65. [DOI] [PubMed] [Google Scholar]
- [27].Colvin JD, Hall M, Gottlieb L, Bettenhausen JL, Shah SS, Berry JG, et al. Hospitalizations of low-income children and children with severe Health conditions: implications of the patient protection and affordable care act. JAMA Pediatr 2016;170(2):176–8. [DOI] [PubMed] [Google Scholar]
- [28].Kane JM, Colvin JD, Bartlett AH, Hall M. Opioid-related critical care resource use in US children's hospitals. Pediatrics 2018;141(4). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.