Abstract
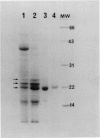
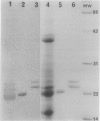
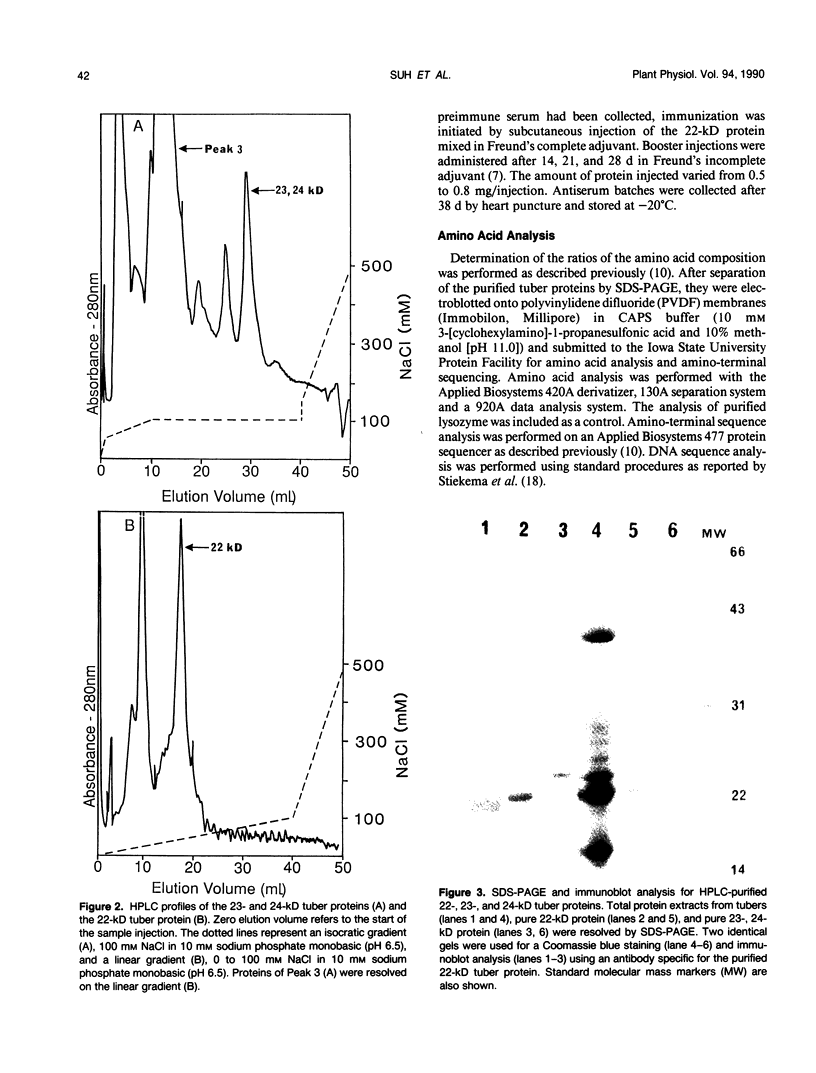
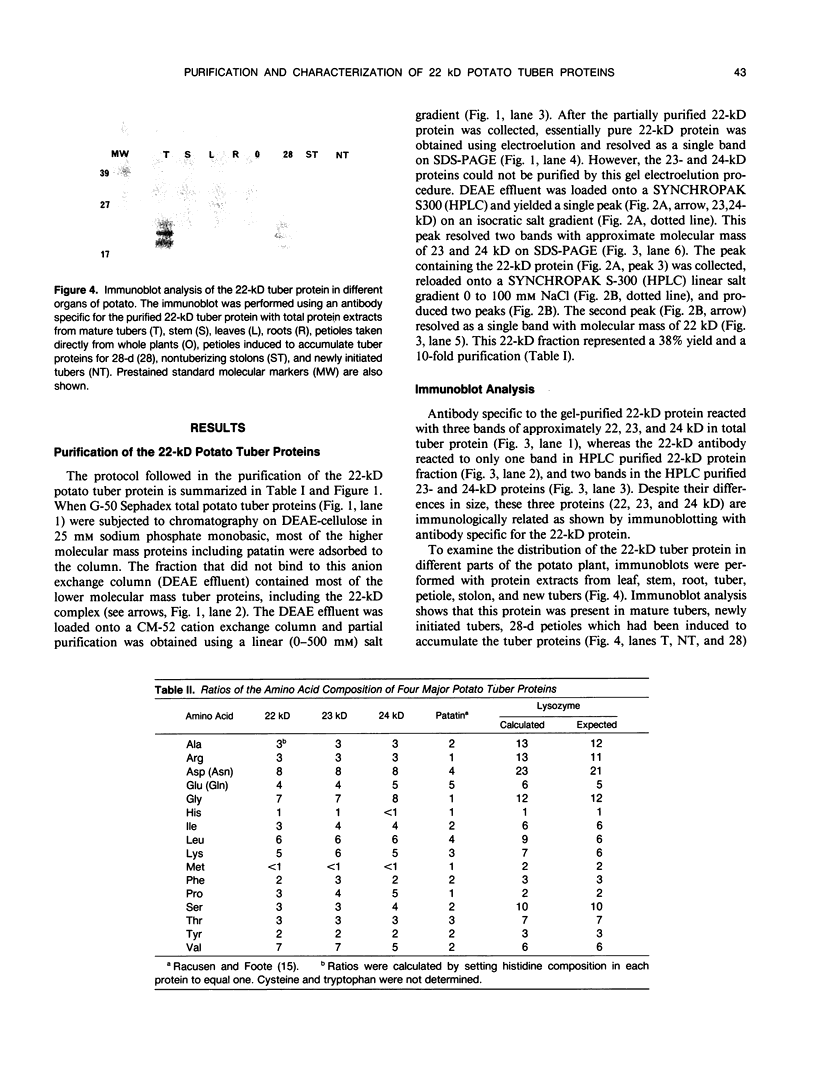
Three abundant proteins of approximate molecular masses of 22, 23, and 24 kilodaltons were purified from potato (Solanum tuberosum L.) tubers by DEAE cellulose and CM-52 cellulose ion exchange column chromatography, electroelution, and high-pressure liquid chromatography (HPLC). Antibodies specific to the gel-purified 22-kilodalton protein were prepared. Immunoblot analysis showed that the 22-, 23-, and 24-kilodalton proteins are immunologically related and that these proteins are present in tubers and as higher molecular mass forms in leaves, but not in stems, roots, and stolons. The ratios of amino acid composition were compared among the three purified proteins, and the aminoterminal amino acid sequences were determined for these three proteins. All three proteins have identical amino-terminal sequences that match the deduced amino acid sequence of an abundant tuber protein cDNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedbrook J. R., Coen D. M., Beaton A. R., Bogorad L., Rich A. Location of the single gene for the large subunit of ribulosebisphosphate carboxylase on the maize chloroplast chromosome. J Biol Chem. 1979 Feb 10;254(3):905–910. [PubMed] [Google Scholar]
- Bhown A. S., Bennett J. C. High-sensitivity sequence analysis of proteins recovered from sodium dodecyl sulfate gels. Methods Enzymol. 1983;91:450–455. doi: 10.1016/s0076-6879(83)91042-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bryant J., Green T. R., Gurusaddaiah T., Ryan C. A. Proteinase inhibitor II from potatoes: isolation and characterization of its protomer components. Biochemistry. 1976 Aug 10;15(16):3418–3424. doi: 10.1021/bi00661a004. [DOI] [PubMed] [Google Scholar]
- Hannapel D. J., Miller J. C., Park W. D. Regulation of potato tuber protein accumulation by gibberellic Acid. Plant Physiol. 1985 Aug;78(4):700–703. doi: 10.1104/pp.78.4.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Hurn B. A., Chantler S. M. Production of reagent antibodies. Methods Enzymol. 1980;70(A):104–142. doi: 10.1016/s0076-6879(80)70044-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Paiva E., Lister R. M., Park W. D. Induction and accumulation of major tuber proteins of potato in stems and petioles. Plant Physiol. 1983 Jan;71(1):161–168. doi: 10.1104/pp.71.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park W. D., Blackwood C., Mignery G. A., Hermodson M. A., Lister R. M. Analysis of the Heterogeneity of the 40,000 Molecular Weight Tuber Glycoprotein of Potatoes by Immunological Methods and by NH(2)-Terminal Sequence Analysis. Plant Physiol. 1983 Jan;71(1):156–160. doi: 10.1104/pp.71.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikaard C. S., Brusca J. S., Hannapel D. J., Park W. D. The two classes of genes for the major potato tuber protein, patatin, are differentially expressed in tubers and roots. Nucleic Acids Res. 1987 Mar 11;15(5):1979–1994. doi: 10.1093/nar/15.5.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pēna-Cortés H., Sánchez-Serrano J. J., Mertens R., Willmitzer L., Prat S. Abscisic acid is involved in the wound-induced expression of the proteinase inhibitor II gene in potato and tomato. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9851–9855. doi: 10.1073/pnas.86.24.9851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker-Simmons M., Ryan C. A. Immunological Identification of Proteinase Inhibitors I and II in Isolated Tomato Leaf Vacuoles. Plant Physiol. 1977 Jul;60(1):61–63. doi: 10.1104/pp.60.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]