Abstract
Background:
The endogenous cannabinoid system (ECS), including the endocannabinoids (eCBs), anandamide (AEA), and 2-arachidonoylglycerol (2-AG), plays an integral role in psychophysiological functions. Although frequent cannabis use is associated with adaptations in the ECS, the impact of acute smoked cannabis administration on circulating eCBs, and the relationship between cannabis effects and circulating eCBs are poorly understood.
Methods:
This study measured the plasma levels of AEA, 2-AG, and Δ-9-tetrahydrocannabinol (THC), subjective drug-effects ratings, and cardiovascular measures at baseline and 15–180 min after cannabis users (n=26) smoked 70% of a cannabis cigarette (5.6% THC).
Results:
Cannabis administration increased the ratings of intoxication, heart rate, and plasma THC levels relative to baseline. Although cannabis administration did not affect eCB levels relative to baseline, there was a significant positive correlation between baseline AEA levels and peak ratings of “High” and “Good Drug Effect.” Further, baseline 2-AG levels negatively correlated with frequency of cannabis use (mean days/week) and with baseline THC metabolite levels.
Conclusions:
In a subset of heavy cannabis smokers: (1) more frequent cannabis use was associated with lower baseline 2-AG, and (2) those with lower AEA got less intoxicated after smoking cannabis. These findings contribute to a sparse literature on the interaction between endo- and phyto-cannabinoids. Future studies in participants with varied cannabis use patterns are needed to clarify the association between circulating eCBs and the abuse-related effects of cannabis, and to test whether baseline eCBs predict the intoxicating effects of cannabis and are a potential biomarker of cannabis tolerance.
Keywords: 2-arachidonoylglycerol, anandamide, endocannabinoid, cannabis use disorder, plasma concentration, subjective effects
Introduction
The endogenous cannabinoid system (ECS) comprises (1) endogenous lipid ligands, including the endocannabinoids (eCBs), anandamide (N-arachidonoylethanolamine or AEA), and 2-arachidonoylglycerol (2-AG); (2) cannabinoid receptor type 1 (CB1R) and type 2 (CB2R), to which the eCBs and other cannabinoid ligands bind; and (3) enzymes involved in the biosynthesis, transportation, and degradation of the eCBs.1–3
CB1 receptors (CB1Rs), the most abundant G protein-coupled receptors in the human brain, play an integral role in a diverse set of functions, including appetite and energy homeostasis, pain, immune response, stress response, mood, reward learning, and motivation.2,4 The CB1R also mediates the subjective and reinforcing effects of Δ-9-tetrahydrocannabinol (THC), the primary psychoactive constituent of cannabis.5 Given the rapid expansion of cannabis use worldwide,6,7 a better understanding of the impact of cannabis use on the ECS and the potential implications for cannabis effects is essential.
It is clear that repeated cannabis use is associated with a range of neuroadaptations in the ECS (see Jacobson et al.8 for review), including: (1) a ∼20% downregulation of brain CB1R concentrations in cannabis users relative to non-cannabis users, typically reversing after ∼2–14 days of abstinence9–12; (2) lower brain (14–20%)13 and serum14 levels of fatty acid amide hydroxylase (FAAH), the enzyme that metabolizes AEA and other fatty acids, relative to non-users, with significantly greater decreases in serum FAAH in longer-term (≥2 years) vs. shorter-term (<2 years) cannabis users14; and (3) lower cerebrospinal fluid levels of AEA but higher serum levels of 2-AG in frequent (≥10 times/month) compared with infrequent cannabis users (<10 times/month).15
Although these studies show that individual differences in ECS biomarkers vary as a function of cannabis use history, little is known about the direct effects of cannabis or its constituents on circulating eCBs in frequent cannabis users. In individuals who do not currently use cannabis, (1) a single dose of intravenous THC (0.1 mg/kg) has been shown to produce a small increase in plasma levels of 2-AG and AEA 30 min post-dose relative to baseline, followed by a marked reduction in both eCBs after 5 h,16 and (2) oral THC administration (20 mg) increased 2-AG and AEA levels 2 and 3 h post-dose relative to placebo.17
To our knowledge, no studies have investigated the acute effects of inhaled THC from smoked cannabis—the most widely used route and method of cannabis consumption—on circulating eCB levels in current cannabis smokers.
Thus, the purpose of the present study was to investigate: (1) whether smoked cannabis acutely alters concentrations of circulating eCBs in regular cannabis smokers relative to baseline, and (2) the relationship between circulating eCB levels and cannabis intoxication. Circulating levels of AEA, 2-AG, and THC, subjective drug-effects ratings, and cardiovascular measures were assessed at baseline (after ≥12 h abstinence from cannabis) and at eight timepoints from 15 to 180 min after cannabis users smoked 70% of a cannabis cigarette (5.6% THC).
Materials and Methods
Participants
Male and female volunteers, aged 21–50 years, were recruited from the New York City metropolitan area through newspaper advertisements and word-of-mouth. Those who met inclusion/exclusion criteria after an initial phone screen were invited to the New York State Psychiatric Institute (NYSPI) for further eligibility screening. Before enrolling, candidates provided a detailed substance use and medical history, received medical and psychiatric evaluations, and signed a consent form providing details of the study.
Eligible participants were healthy (as determined by physical examination, psychiatric screening, electrocardiogram, blood pressure [BP] and heart rate [HR] measurements, and urine and blood chemistry analysis); were not regular users of drugs other than cannabis, tobacco, or caffeine; were not currently seeking treatment for their cannabis use; and reported smoking a minimum of 1 cannabis cigarette/day, at least 1 day/week for ≥ the past 4 weeks (confirmed by positive THC urine toxicology). Females were excluded if they were pregnant or nursing (confirmed by serum pregnancy testing).
All procedures were approved by the NYSPI Institutional Review Board and were in accordance with the Declaration of Helsinki.
Study design and procedures
Eligible participants completed a single 4-h outpatient visit (0900 to 1300) at the Cannabis Research Laboratory at NYSPI. They were instructed to not smoke either cannabis or tobacco cigarettes on the morning of their session (beginning at midnight) and underwent a baseline field sobriety assessment and a carbon monoxide (CO) test on arrival for verification. Participants were also given a breathalyzer test to ensure that they had not consumed alcohol before arrival. Urine samples were tested to confirm the absence of illicit drug use (other than cannabis) and pregnancy; anyone failing these initial screens did not participate that day.
TimeLine Follow-Back (TLFB) interviews were then conducted during which participants reported their substance use (e.g., frequencies, quantities) over the past week. As detailed in Table 1, baseline plasma eCB and THC levels, cardiovascular metrics, and subjective-effects ratings were collected at 30 min before cannabis administration. After baseline measurements, participants had a light breakfast (bagel or cereal, juice and/or coffee) and those who were tobacco cigarette smokers were allowed to have one tobacco cigarette before cannabis administration to avoid tobacco withdrawal symptoms during the session.
Table 1.
Timecourse of Study Sessions
| Time | Event | Time | Event |
|---|---|---|---|
| −60 | Begin session | 45 | Plasma |
| CO, breathalyzer, field sobriety test, urine toxicology, pregnancy test, TLFB, C-SSRS | 60 | Plasma, cardiovascular measures, VAS | |
| 90 | Plasma, cardiovascular measures, VAS | ||
| −30 | Plasma, cardiovascular measures, VAS | 120 | Plasma, cardiovascular measures, VAS |
| −20 | Breakfast, tobacco cigarette break (optional) | 150 | Plasma, cardiovascular measures, VAS |
| 0 | CAN administration | 180 | Plasma, cardiovascular measures, VAS |
| 15 | Plasma | 195 | Field sobriety test |
| 30 | Plasma, cardiovascular measures, VAS | End session |
Plasma=blood draw to extract plasma levels of THC, AEA, and 2-AG; cardiovascular measures=heart rate and systolic/diastolic blood pressure; VAS=VAS measuring mood and subjective effects of smoking CAN.
2-AG, 2-arachidonoylglycerol; AEA, anandamide; CAN, cannabis; CO, carbon monoxide; C-SSRS, Columbia-Suicide Severity Rating Scale; THC, Δ-9-tetrahydrocannabinol; TLFB, TimeLine Follow-Back; VAS, Visual Analog Scale.
Participants then smoked a controlled amount of cannabis (see details below). Outcome measures (i.e., plasma extractions, cardiovascular metrics, subjective-effects ratings) were collected at 15–30 min intervals for 3 h post-cannabis administration. On completion of data collection, participants were required to pass a field sobriety test before discharge from the Cannabis Laboratory, which was defined as a return to their baseline (non-intoxicated) field sobriety score.
Cannabis administration
During cannabis administration, participants received one cannabis cigarette (5.6% Δ9-THC; 0.35% cannabinol; nondetectable Δ8-THC and cannabidiol; terpenoid content unknown; w/w, ∼800 mg of cannabis plant material; provided by the National Institute of Drug Abuse [NIDA]). Cannabis cigarettes were stored frozen in an airtight container and humidified at room temperature for 24 h before use.
Investigators provided each participant with a cannabis cigarette, a lighter, and a smokeless ashtray, and the cannabis cigarette was smoked by using a cued paced-puffing smoking procedure: The investigator instructed the participant to “light the cigarette” (30 sec), “get ready” (5 sec), “inhale” (5 sec), “hold smoke in lungs” (10 sec), and “exhale,” with a 40-sec pause between each smoked puff.18 Participants smoked 70% of the cannabis cigarette (a line was drawn on the cigarette; participants stopped smoking once they reached the line).19
Assessments
Subjective drug effects and mood scales
Participants completed the Mood and Physical Symptoms Visual Analog Scale (VAS),20 a 44-item computerized subjective-effects questionnaire comprising a series of 100-mm lines labeled “Not at all” at one end (0 mm) and “Extremely” at the other end (100 mm). Participants rated the extent to which they were experiencing a range of mood and physical symptoms (i.e., “I Feel ‘Anxious’” or “I Feel ‘Energetic’”) as well as positive subjective effects of cannabis consumption (i.e., “I Feel ‘High’ and a ‘Good Drug Effect’”).
Cardiovascular effects
The HR and systolic and diastolic BP were measured by using a Sentry II combination HR/BP vital signs monitor (Model 6100; NBS Medical Services, Costa Mesa, CA).
Plasma THC and eCB levels
A nurse or phlebotomist inserted a 20-gauge venous catheter (Quik-Cath®; Treavenol Laboratories, Deerfield, IL) into a peripheral vein in the arm for blood withdrawal (6 mL for each of the nine timepoints; total of 54 mL). The EDTA-coated tubes (Becton, Dickinson and Company, Franklin Lakes, NY) were used for plasma preparation. Syringes and EDTA tubes were ice-chilled before blood collection, and storage cups were prepared with 1% PMSF solution (10 mg PMSF in 1 mL methanol) and 5% 1 N HCL at final concentration. Samples were snap frozen in liquid nitrogen immediately and kept at −80°C until analysis.
Samples were analyzed by using a previously validated method that involves liquid/liquid extraction, derivatization, and gas chromatography-tandem mass spectrometry.21,22 After homogenization of plasma and extraction with chloroform/methanol/Tris-HCl 50 mM pH 7.5 (2:1:1, v/v) containing internal deuterated standards (THC-d3, AEA-d4 and 2-AG-d5), the dried lipid extract was purified by using solid-phase extraction (SPE C18; Agilent). THC, AEA, and 2-AG fractions were obtained by eluting the column with 1:1 (by vol.) cyclohexane/ethylacetate.
Samples were then subjected to isotope-dilution liquid chromatography-chemical ionization-tandem mass spectrometric analysis. Mass spectral analyses were performed on a TSQ Quantum Access triple quadrupole instrument (Thermo-Finnigan) equipped with an APCI (atmospheric pressure chemical ionization) source and operating in positive ion mode. The TSQ Quantum Access triple quadrupole instrument was used in conjunction with a Surveyor LC Pump Plus (Supelco C18 Discovery Analytical column) and cooled autosampler.
To evaluate between-run and within-run accuracy and precision, quality control samples were prepared by directly supplementing a plasma pool control with our compounds of interest and run for each batch of samples analyzed. Intra- and inter-assay coefficients of variation met acceptance criteria (<20%) for THC, AEA, and 2-AG. Using calibration curves (linearity R2>0.99), the amounts of THC, AEA, and 2-AG were determined by isotopic dilution and expressed as ng/mL.
Data analysis
Analyses were conducted in GraphPad Prism Version 8.3.0 (GraphPad Software, San Diego, CA). To determine whether there was a significant effect of smoked cannabis on subjective ratings of cannabis intoxication (i.e., “High,” “Good Drug Effect”), cardiovascular measures (i.e., BP, HR), and plasma THC/THC metabolite and eCB (AEA, 2-AG) concentrations as a function of time, a one-way repeated-measures analysis of variance was conducted for each measured outcome variable, with time since cannabis administration as the within-subject factor.
Post hoc multiple comparison-adjusted tests for differences were conducted to identify post-dose timepoints significantly differing from baseline. Results were considered statistically significant at multiple comparison-adjusted p≤0.05.
After assessing data normality, relationships between baseline and peak plasma eCB and THC levels, acute cannabis intoxication (peak positive subjective effects after smoked cannabis administration), and self-reported cannabis use history, respectively, were evaluated by using Pearson's or Spearman's (if data non-normal) correlations. Correlation coefficients with p≤0.05 were considered statistically significant.
Results
Participant characteristics
Table 2 presents demographic and substance use characteristics assessed via self-report questionnaires and in-person interviews. Participants reported smoking, on average, roughly 5 g of cannabis on each of 6 days per week, with an average length of consistent cannabis use of 16 years, representing a sample of heavy, near-daily, long-term cannabis smokers.
Table 2.
Demographic and Drug Use Characteristics
| N =26, total sample | |
|---|---|
| Demographics | |
| Sex | 20 M, 6 F |
| Age (years) | 34.2 (±6.7) [22–49] |
| Race (Black/White/Other) | 14/8/4 |
| Ethnicity (Hispanic/non-Hispanic) | 8/14 |
| Education (years) | 13.4 (±1.8) [11–17] |
| CAN use | |
| CAN days/week | 5.7 (±1.6) [2.5–7] |
| CAN g/day | 5.1 (±5.5) [0.75–21] |
| Age of first CAN use (years) | 15.4 (±4.2) [7–28] |
| Total duration of CAN use (years) | 15.8 (±8.1) [2–32] |
| Other substance use | |
| Tobacco cigarette smokers | 17 (69%) |
| Tobacco cigarettes/day | 8.7 (±9.6) [1.5–40] |
| Alcohol drinkers | 9 (35%) |
| Alcohol drinking days/week | 1.8 (±1.1) [1–3.5] |
| Alcohol drinks/occasion | 2.2 (±1.3) [1–4.5] |
Values with parentheses listed in either mean (± standard deviation) [range] or number (percentage). Tobacco cigarette smokers were defined as individuals smoking at least 1 cigarette per day. Alcohol drinkers were defined as individuals having regular alcohol exposure at one or more alcoholic drinks per week. Race: Other=Asian, Native American, Mixed Race.
F, females; M, males.
Acute effects of smoked cannabis
Subjective drug effects and mood ratings
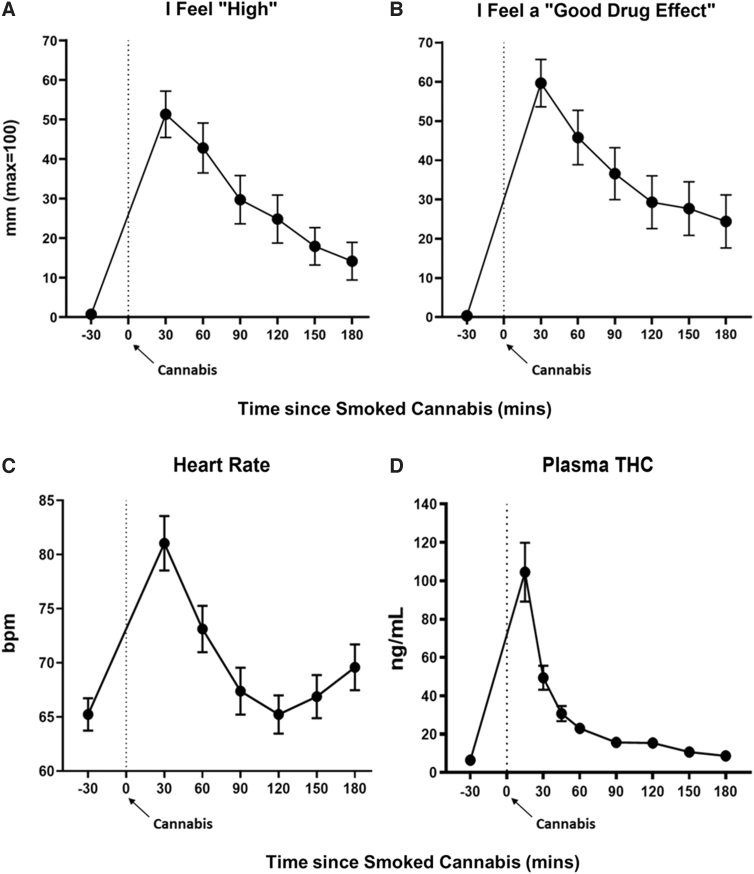
Figure 1A and B portrays ratings of subjective drug effects as a function of time since smoking cannabis, beginning at baseline (30 min before smoking cannabis) and ending 3 h after cannabis administration. Smoked cannabis significantly increased ratings of feeling “High” and “Good Drug Effect” at each timepoint relative to baseline (multiple comparison-adjusted p<0.05 for all timepoints), with peak ratings of “High” (mean=51 mm) and “Good Drug Effect” (mean=60 mm) both occurring, on average, at the first post-dose timepoint measured (30 min after smoking).
FIG. 1.
Acute subjective and physiological effects of smoked (5.6% THC) cannabis administration. Effect of smoked cannabis on (A, B) subjective drug effects and (C) heart rate, as a function of time, beginning at baseline (30 min before cannabis administration) and ending 3 h after cannabis administration. Time “0” refers to the time (in min) in which cannabis (70% of a NIDA cannabis cigarette) was smoked. Error bars represent ±SEM. (D) Mean (± SEM) plasma THC levels after smoked cannabis (from Time=0 min) through 3 h post-dose. bpm, beats per min; NIDA, National Institute of Drug Abuse; SEM, standard error of the mean; THC, Δ-9-tetrahydrocannabinol.
Cardiovascular effects
Figure 1C, portraying HR as a function of time, shows that smoked cannabis significantly increased HR (multiple comparison-adjusted p<0.0001 at timepoint T=30 min, and p<0.05 at T=60 and 180 min) relative to baseline (mean baseline HR=65 beats per min [bpm]), with peak HR (mean peak HR=81 bpm) occurring at the first timepoint measured after smoking (T=30 min). Cannabis did not significantly alter systolic or diastolic BP (p>0.05; data not shown).
Plasma THC levels
Figure 1D, which portrays mean plasma THC levels as a function of time, shows that THC levels were low at baseline (mean baseline plasma THC [± standard error of the mean, SEM]=6.46 [± 1.87] ng/mL; range: 0.02–49.64 ng/mL), suggesting that the participants did not appear to have smoked cannabis the morning of the session, as requested. Plasma THC levels peaked (Tmax) at the first timepoint measured after smoking (T=15 min), with peak plasma THC concentrations (Cmax) ranging from 15.4 to 410.8 ng/mL (mean peak plasma THC [± SEM]=104.8 [± 15.09] ng/mL). Relative to baseline, there was a statistically significant increase in plasma THC (at multiple comparison-adjusted p≤0.01) at timepoints T=15 (peak), 30, 45, 60, 90, and 120 min.
Plasma eCB levels
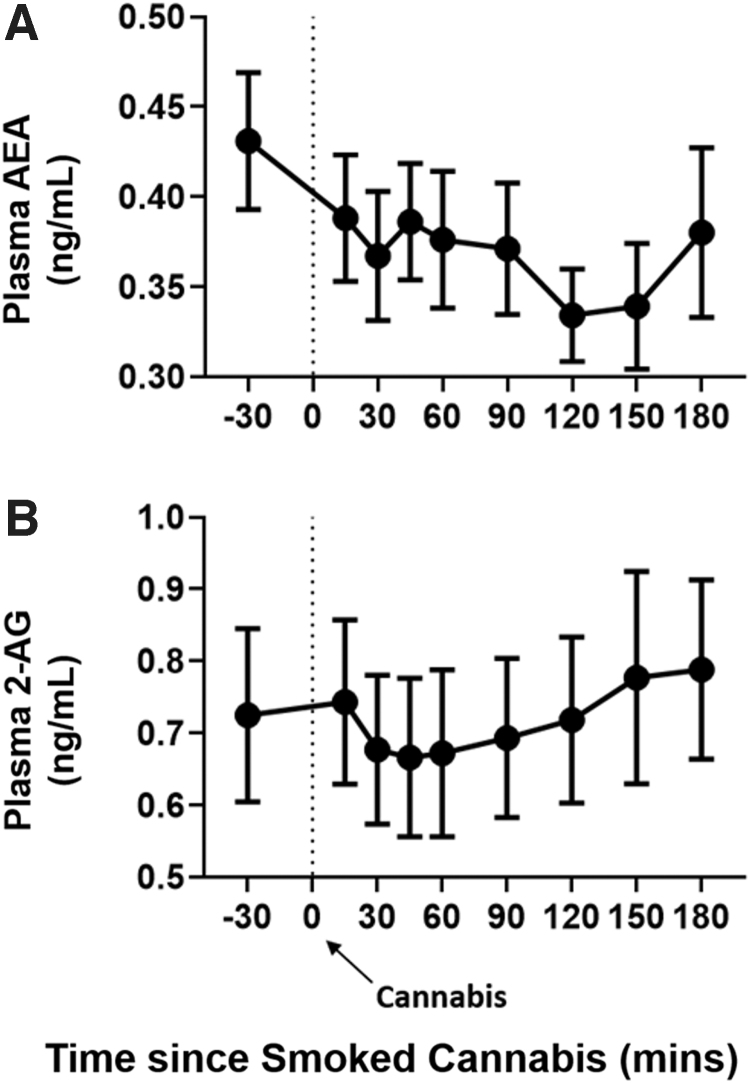
Plasma AEA and 2-AG levels did not significantly change from baseline (mean [± SEM]: AEA=0.43 [± 0.04] ng/mL and 2-AG=0.73 [± 0.12] ng/mL) at any timepoint after smoked cannabis (p>0.05; Fig. 2A, B).
FIG. 2.
Acute effects of smoked (5.6% THC) cannabis administration on circulating endocannabinoid levels. Effect of smoked cannabis on plasma levels of (A) AEA and (B) 2-AG as a function of time, beginning at baseline (30 min before cannabis administration) and ending 3 h after cannabis administration. Time “0” refers to the time (in min) in which cannabis (70% of an NIDA cannabis cigarette) was smoked. Error bars represent ±SEM. 2-AG, 2-arachidonoylglycerol; AEA, anandamide.
Relationships between variables
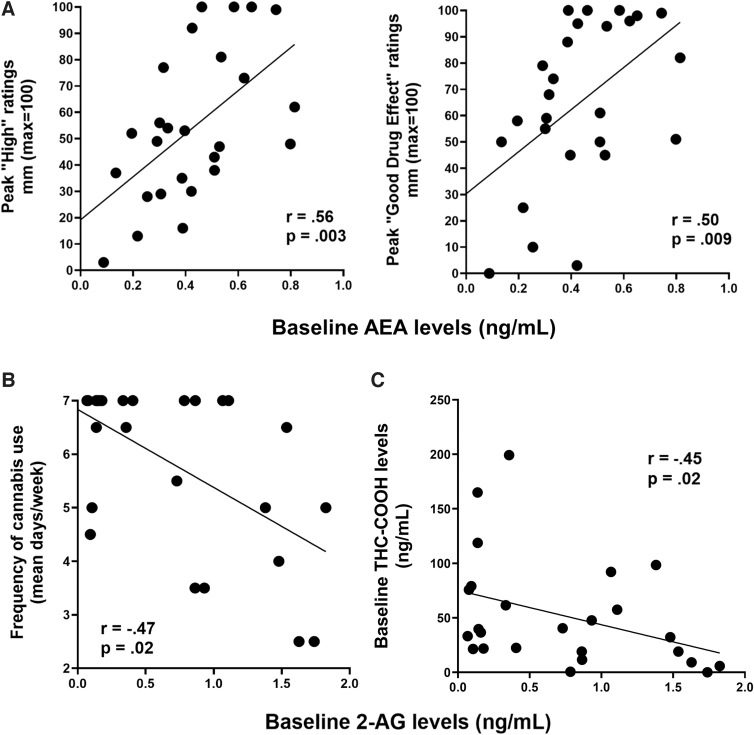
Relationship between basal plasma eCB levels and abuse-related subjective cannabis effects
Figure 3A illustrates that plasma AEA levels at baseline (before cannabis administration) were positively correlated with peak ratings of “High” (r=0.56, p<0.01) and “Good Drug Effect” (r=0.50, p<0.01) after cannabis administration, with higher baseline AEA levels corresponding to greater positive cannabis effects ratings. There was no significant correlation between plasma 2-AG levels and peak abuse-related effects of cannabis (p>0.05; data not shown).
FIG. 3.
Correlating circulating endocannabinoid levels with cannabis use measures and plasma THC/THC metabolites. (A) Baseline AEA levels (measured at 30 min before smoked cannabis administration) positively correlated with peak positive subjective drug effects (after smoked cannabis). (B) Baseline 2-AG levels (measured at 30 min before smoked cannabis administration) negatively correlated with frequency of cannabis use (mean use in days/week) as self-reported in drug use history. (C) Baseline 2-AG levels (measured at 30 min before smoked cannabis administration) negatively correlated with baseline plasma levels of the THC metabolite, THC-COOH. THC-COOH, 11-nor-9-carboxy-Δ9-tetrahydrocannabinol.
Relationship between basal plasma eCB levels and cannabis use patterns
Figure 3B illustrates that baseline plasma 2-AG levels (before cannabis administration) were negatively correlated with frequency of cannabis use (mean days cannabis use/week; Spearman's rho=−0.47, p<0.05), with heavier cannabis use associated with lower baseline 2-AG levels. Baseline plasma AEA levels did not correlate with patterns of cannabis use (p>0.05; data not shown).
Relationship between basal plasma eCB levels and plasma THC/THC metabolites
Baseline plasma THC levels did not correlate with either basal AEA or 2-AG levels (p>0.05; data not shown). However, baseline plasma levels of the THC metabolite THC-COOH (11-nor-9-carboxy-Δ9-tetrahydrocannabinol), which has a longer timecourse of detection in plasma than THC or the THC metabolite 11-OH-THC (11-Hydroxy-Δ9-tetrahydrocannabinol),23 ranged from 0.00 to 199.20 ng/mL at baseline (mean [± SEM]=51.57 [± 9.74] ng/mL) and negatively correlated with baseline plasma 2-AG (Spearman's rho=−0.45, p<0.05) (Fig. 3C), but not AEA (p>0.05; data not shown).
Baseline plasma levels of the THC metabolite 11-OH-THC ranged from 0.00 to 11.98 ng/mL (baseline mean [± SEM]=2.44 [± 0.51] ng/mL), and there was a trending negative relationship between basal 11-OH-THC levels and baseline plasma 2-AG (Spearman's rho=−0.37, p=0.07; data not shown), but not AEA (p>0.05; data not shown).
Relationship between cannabis use patterns and abuse-related subjective cannabis effects
Baseline cannabis use patterns (i.e., years of cannabis use, cannabis use frequency, cannabis use quantity) did not significantly correlate with peak subjective effects ratings (p>0.05; data not shown).
Discussion
This study shows that relative to baseline, smoking a single cannabis cigarette (5.6% THC) produced time-dependent increases in ratings of intoxication (i.e., “High” and “Good Drug Effects”), HR, and plasma THC levels in cannabis smokers without significantly altering plasma concentrations of eCBs AEA and 2-AG over a 3-h period post-smoking.
There was, however, an association between circulating eCB levels and clinically relevant cannabis use end-points: Baseline AEA significantly predicted the positive subjective effects of smoked cannabis, with higher baseline AEA levels corresponding to greater ratings of intoxication. In addition, heavier cannabis use (number of days cannabis smoked per week; THC metabolite levels in plasma at baseline) was associated with lower baseline 2-AG levels.
There are several possible explanations for the current findings. First, the association between lower circulating 2-AG could suggest that (1) cannabis use results in compensatory reductions in circulating eCB levels. Preclinical data showing that eCB concentrations are reduced with exogenous cannabinoid agonist administration support this explanation,2 along with other cannabis-induced adaptations in the ECS noted in the Introduction section (i.e., altered brain FAAH and CB1R levels).
Or, (2) preexisting eCB deficiencies predict a vulnerability for heavy cannabis use, perhaps in an attempt to compensate for endogenous deficits.2 In addition, individuals with higher baseline levels of the long-lasting metabolite, THC-COOH, had lower basal 2-AG, consistent with the notion that heavier cannabis use predicts lower levels of 2-AG.
Second, the association between lower circulating AEA and less intoxication could suggest that plasma eCBs are a peripheral biomarker of cannabis tolerance, that is, heavier cannabis smokers have lower circulating eCBs and having lower circulating eCBs is associated with being less sensitive to the intoxicating effects of cannabis.
Although we did not find that cannabis use patterns negatively correlated with cannabis intoxication herein, which appears to contradict this idea, the present sample comprised heavy cannabis users (averaging ≥5 g cannabis/day). It may be that clearer interrelations between circulating eCBs, acute intoxication, and cannabis use patterns would emerge when participants with a wider variation of cannabis smoking patterns are tested.
Future research could test these hypothesized explanations by measuring circulating eCB levels over time in recently abstinent daily cannabis smokers to determine whether levels increase over the length of abstinence (as occurs with brain CB1Rs, e.g.; see the Introduction section). An increase in circulating eCBs with abstinence would support the idea that cannabis directly reduces circulating eCB levels. If, by contrast, low circulating plasma eCBs do not recover as a function of abstinence, prospective studies could be conducted to determine whether preexisting low circulating eCB levels predict a vulnerability to developing a pattern of regular cannabis use.
The present study also shows that although smoked cannabis produced expected increases in plasma THC and ratings of intoxication, it did not alter eCB levels relative to baseline in this sample of heavy cannabis users, contrasting with studies showing that a single dose of oral or intravenous THC acutely increased circulating eCBs within 3 h of administration among individuals with no recent cannabis use.16,17 As noted earlier, more studies in humans are needed—testing a diverse sample of participants (from infrequent to long-term daily users) and a range of cannabis or cannabinoid doses and routes of administration—to further clarify the acute effects of these frequently used compounds on the ECS.
There are limitations to consider with the design of the present study. First, the study sample was 77% male, so the potential impact of sex on circulating eCB levels and their relationship to acute cannabis effects was not assessed. Second, the study compared changes in outcome measures relative to baseline and tested one strength of cannabis, which was low potency relative to what is currently available for recreational use.24
We note that participants experienced robust prototypical cannabis effects, that is, time-dependent increases in ratings of “High” and “Good Drug Effect” and increased HR after smoking 70% of a cannabis cigarette (5.6% THC) using our standardized paced-puffing approach,18 supporting the validity of the current findings. However, it is possible that differences in outcomes would have emerged over the 3-h measurement period if compared with a placebo condition and/or a more potent and varied cannabis chemovar.
In conclusion, this is the first study to our knowledge to assess the direct impact of smoking cannabis—the most common25 and thus relevant way that cannabis is used—on circulating eCB levels in current cannabis smokers, and to evaluate their relationship to acute subjective cannabis effects and self-reported patterns of cannabis use.
Although smoked cannabis did not alter plasma eCB levels in the short term, baseline eCB levels were positively related to the positive subjective effects of cannabis, and they were negatively related to measures of chronic cannabis use (frequency and quantity). Further research is needed to determine whether low basal eCB levels predict increased cannabis use, or whether heavier cannabis use causes lower eCB levels and reflects cannabis tolerance.
Acknowledgments
T.K.-R. gratefully acknowledges salary support from the NYSPI's Hadar Foundation Fellowship.
Abbreviations Used
- 11-OH-THC
11-hydroxy-Δ9-tetrahydrocannabinol
- 2-AG
2-arachidonoylglycerol
- AEA
anandamide
- BP
blood pressure
- bpm
beats per min
- CAN
cannabis
- CB1R
cannabinoid receptor type 1
- CO
carbon monoxide
- C-SSRS
Columbia-Suicide Severity Rating Scale
- eCB
endocannabinoid
- ECS
endogenous cannabinoid system
- F
females
- FAAH
fatty acid amide hydroxylase
- HR
heart rate
- M
males
- NIDA
National Institute of Drug Abuse
- NYSPI
New York State Psychiatric Institute
- SEM
standard error of the mean
- THC
Δ-9-tetrahydrocannabinol
- THC-COOH
11-nor-9-carboxy-Δ9-tetrahydrocannabinol
- TLFB
TimeLine Follow-Back
- VAS
Visual Analog Scale
Authors' Contributions
M.H. and P.V.P. were responsible for the concept and design of the overall research study. M.H. and E.S.H. screened participants and oversaw cannabis sessions. I.B., I.M., M.V., and S.M. conducted the extraction, derivatization, and gas chromatography-tandem mass spectrometry of the plasma samples. T.K.-R. conducted data analyses and drafted the article. M.H. provided critical revision of the article for intellectual content. All authors reviewed and approved the final version for publication.
Author Disclosure Statement
During the past three years, M.H. has been serving on the scientific advisory board of Pleo Pharma and has received cannabis capsules from Tilray, Inc., to conduct a research study on cannabis and neuropathy (NCT03782402). All other authors declare no conflicts of interest.
Funding Information
The study was funded by a gift account from the Institut National de la Santé et de la Recherche Médical.
Cite this article as: Kearney-Ramos T, Herrmann ES, Belluomo I, Matias I, Vallee M, Monlezun S, Piazza PV, Haney M (2023) The relationship between circulating endogenous cannabinoids and the effects of smoked cannabis, Cannabis and Cannabinoid Research 8:6, 1069–1078, DOI: 10.1089/can.2021.0185.
References
- 1. Atkinson DL, Abbott JK. Chapter 3-cannabinoids and the brain: the effects of endogenous and exogenous cannabinoids on brain systems and function. In: The complex connection between cannabis and schizophrenia. Compton MT, Manseau MW, eds. Academic Press: San Diego, CA, 2018, pp. 37–74. [Google Scholar]
- 2. Hillard CJ. Circulating endocannabinoids: from whence do they come and where are they going?. Neuropsychopharmacology. 2018;43:155–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pacher P, Bátkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Covey DP, Wenzel JM, Cheer JF. Cannabinoid modulation of drug reward and the implications of marijuana legalization. Brain Res. 2015;1628:233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cooper ZD, Haney M. Cannabis reinforcement and dependence: role of the cannabinoid CB1 receptor. Addict Biol. 2008;13:188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Compton WM, Han B, Jones CM, et al. Marijuana use and use disorders in adults in the USA, 2002–2014: analysis of annual cross-sectional surveys. Lancet Psychiatry. 2016;3:954–964. [DOI] [PubMed] [Google Scholar]
- 7. Hasin DS. US epidemiology of cannabis use and associated problems. Neuropsychopharmacology. 2018;43:195–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jacobson MR, Watts JJ, Boileau I, et al. A systematic review of phytocannabinoid exposure on the endocannabinoid system: implications for psychosis. Eur Neuropsychopharmacol. 2019;29:330–348. [DOI] [PubMed] [Google Scholar]
- 9. Ceccarini J, Kuepper R, Kemels D, et al. [18F]MK-9470 PET measurement of cannabinoid CB1 receptor availability in chronic cannabis users. Addict Biol. 2015;20:357–367. [DOI] [PubMed] [Google Scholar]
- 10. D'Souza DC, Cortes-Briones JA, Ranganathan M, et al. Rapid changes in CB1 receptor availability in cannabis dependent males after abstinence from cannabis. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hirvonen J, Goodwin RS, Li CT, et al. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry. 2012;17:642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spindle TR, Kuwabara H, Eversole A, et al. Brain imaging of cannabinoid type I (CB1) receptors in women with cannabis use disorder and male and female healthy controls. Addict Biol. 2021;26:e13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boileau I, Mansouri E, Williams B, et al. Fatty acid amide hydrolase binding in brain of cannabis users: imaging with the novel radiotracer [11C]CURB. Biol Psychiatry. 2016;80:691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. El-Gohary M, Eid MA. Effect of cannabinoid ingestion (in the form of bhang) on the immune system of high school and university students. Hum Exp Toxicol. 2004;23:149–156. [DOI] [PubMed] [Google Scholar]
- 15. Morgan CJ, Page E, Schaefer C, et al. Cerebrospinal fluid anandamide levels, cannabis use and psychotic-like symptoms. Br J Psychiatry. 2013;202:381–382. [DOI] [PubMed] [Google Scholar]
- 16. Thieme U, Schelling G, Hauer D, et al. Quantification of anandamide and 2-arachidonoylglycerol plasma levels to examine potential influences of tetrahydrocannabinol application on the endocannabinoid system in humans. Drug Test Anal. 2014;6:17–23. [DOI] [PubMed] [Google Scholar]
- 17. Walter C, Ferreirós N, Bishay P, et al. Exogenous delta9-tetrahydrocannabinol influences circulating endogenous cannabinoids in humans. J Clin Psychopharmacol. 2013;33:699–705. [DOI] [PubMed] [Google Scholar]
- 18. Foltin RW, Fischman MW, Pedroso JJ, et al. Marijuana and cocaine interactions in humans: cardiovascular consequences. Pharmacol Biochem Behav. 1987;28:459–464. [DOI] [PubMed] [Google Scholar]
- 19. Haney M, Malcolm RJ, Babalonis S, et al. Oral cannabidiol does not alter the subjective, reinforcing or cardiovascular effects of smoked cannabis. Neuropsychopharmacology. 2016;41:1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haney M, Hart CL, Vosburg SK, et al. Marijuana withdrawal in humans: effects of oral THC or divalproex. Neuropsychopharmacology. 2004;29:158–170. [DOI] [PubMed] [Google Scholar]
- 21. Drummen M, Tischmann L, Gatta-Cherifi B, et al. Role of endocannabinoids in energy-balance regulation in participants in the postobese state—a PREVIEW study. J Clin Endocrinol Metab. 2020;105:e2511–e2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gatta-Cherifi B, Matias I, Vallée M, et al. Simultaneous postprandial deregulation of the orexigenic endocannabinoid anandamide and the anorexigenic peptide YY in obesity. Int J Obes (Lond). 2012;36:880–885. [DOI] [PubMed] [Google Scholar]
- 23. Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids. I. Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. J Anal Toxicol. 1992;16:276–282. [DOI] [PubMed] [Google Scholar]
- 24. Schwabe AL, Hansen CJ, Hyslop RM, et al. Comparative genetic structure of Cannabis sativa including federally produced, wild collected, and cultivated samples. Front Plant Sci. 2021;12:675770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wenzel JM, Cheer JF. Endocannabinoid regulation of reward and reinforcement through interaction with dopamine and endogenous opioid signaling. Neuropsychopharmacology. 2018;43:103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]