Abstract
Background:
Increasing evidence suggests that the endocannabinoid system (ECS) in the brain controls anxiety and may be a therapeutic target for the treatment of anxiety disorders. For example, both pharmacological and genetic disruption of cannabinoid receptor subtype-1 (CB1R) signaling in the central nervous system is associated with increased anxiety-like behaviors in rodents, while activating the system is anxiolytic. Sex is also a critical factor that controls the behavioral expression of anxiety; however, roles for the ECS in the gut in these processes and possible differences between sexes are largely unknown.
Objective:
In this study, we aimed to determine if CB1Rs in the intestinal epithelium exert control over anxiety-like behaviors in a sex-dependent manner.
Methods:
We subjected male and female mice with conditional deletion of CB1Rs in the intestinal epithelium (intCB1−/−) and controls (intCB1+/+) to the elevated plus maze (EPM), light/dark box, and open field test. Corticosterone (CORT) levels in plasma were measured at baseline and immediately after EPM exposure.
Results:
When compared with intCB1+/+ male mice, intCB1−/− male mice exhibited reduced levels of anxiety-like behaviors in the EPM and light/dark box. In contrast to male mice, no differences were found between female intCB1+/+ and intCB1−/− mice. Circulating CORT was higher in female versus male mice for both genotype groups at baseline and after EPM exposure; however, there was no effect of genotype on CORT levels.
Conclusions:
Collectively, these results indicate that genetic deletion of CB1Rs in the intestinal epithelium is associated with an anxiolytic phenotype in a sex-dependent manner.
Keywords: anxiety, cannabinoid receptors, sex differences, intestinal epithelium, animal behavior
Introduction
The endocannabinoid system (ECS) plays a critical role in the behavioral expression of anxiety.1–5 Indeed, mice treated with a low dose of the cannabinoid receptor subtype-1 (CB1R) agonist, WIN 55212-2, exhibited increased open-arm exploration in the elevated plus maze (EPM),6 which suggests that activating the ECS is associated with an anxiolytic phenotype. In contrast, mice lacking CB1Rs throughout the body spend less time exploring the open arms of the EPM when compared with wild-type mice,7 which suggests an anxiogenic effect for global CB1R deletion.
Similarly, mice lacking functional diacylglycerol lipase α, a key enzyme responsible for biosynthesis of the endocannabinoid, 2-arachidonoyl-sn-glycerol, in the brain, demonstrated reduced exploration of the central area of an open field test and increased anxiety-like behaviors in the light/dark box.4 Recent studies have also explored roles of the gut–brain axis in anxiety and related behavioral states. For example, the emergence of depression-like phenotypes was prevented in LPS-treated mice that had undergone subdiaphragmatic vagotomy.8
In addition, oral administration of specific gut microbiota led to altered neuronal activity in brainstem sites that receive sensory inputs from the intestine through the afferent vagus nerve, which led to stress-induced corticosterone (CORT) release, anxiety, and depressive behaviors.9–11 Notably, these effects were all prevented by vagotomy.
Together, these studies demonstrate the importance for the ECS in the central nervous system (CNS) in controlling anxiety-like behaviors in rodents, and a critical relationship between the gastrointestinal (GI) tract and emotional states. Specific roles of the ECS within the GI tract in anxiety-like behaviors, however, remain unclear.
The ECS is found throughout the GI tract, and controls food intake,12–15 gastric emptying and intestinal motility,16–18 and gut-barrier function.19–21 Moreover, recent studies suggest interactions between gut microbiota and local endocannabinoid formation, which may contribute to anxiety-like behaviors.22 In this study, mice colonized with Candida albicans in the gut had increased basal CORT production and alterations in the gut endocannabinoidome. These findings reveal a possible mechanism by which the gut–brain axis enables peripheral ECS control over CNS-mediated anxiety-like behaviors.
Vagal afferent fibers enable direct communication between the gut and the CNS.23–25 Vagal afferent neurons terminate in the brainstem at the nucleus of the solitary tract, which communicates with other brain structures that regulate fear and anxiety-like responses including the prefrontal cortex, hippocampus, and amygdala.24
Accordingly, it is possible that alterations in gut function may affect gut–brain signaling and ultimately the behavioral expression of anxiety. For example, Krieger et al. demonstrated that activation of vagal afferent neurons by both food intake and chemogenetic approaches increased anxiety-like behavior, while chemogenetic inhibition of vagal afferent neurons attenuated these responses.26
Importantly, the same study revealed sex differences in anxiety-like behaviors after chronic disruption of vagal afferent signaling from fibers originating in the gut. Notably, human females are more than twice as likely to be affected by mood disorders such as generalized anxiety disorder,27–29 so it is unsurprising that many rodent studies find similar sex-dependent outcomes when examining anxiety.30–33
Sex dictates many aspects of gut–brain signaling,34 ECS function,35,36 and physiology.37 Therefore, it is essential to understand how sex may differentially impact ECS function in the GI tract and the behavioral expression of anxiety. In this study, we tested the hypothesis that CB1Rs in the intestinal epithelium exert control over anxiety-like behaviors in male and female mice.
Materials and Methods
Animals
Male and female transgenic mice (described below in Transgenic Mouse Generation) 8–10 weeks of age were group-housed with ad libitum access to standard rodent laboratory diet (Teklad 2020x; Envigo, Huntingdon, United Kingdom; 16% kcal from fat, 24% kcal from protein, 60% kcal from carbohydrates) and water throughout all experiments unless otherwise stated. Mice were maintained on a 12-h dark/light cycle, with the dark cycle beginning at 18:00 h and light cycle beginning at 06:00 h. All procedures met the U.S. National Institute of Health guidelines for care and use of laboratory animals, and were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of California, Riverside.
Transgenic mouse generation
Conditional intestinal epithelium-specific CB1R-deficient mice (IntCB1−/−, Cnr1tm1.1 mrl/vil-cre ERT2) were generated by crossing Cnr1-floxed mice (IntCB1+/+, Cnr1tm1.1 mrl, Model #7599; Taconic, Oxnard, CA) with Vil-CRE ERT2 mice donated by Dr. Randy Seeley (University of Michigan, Ann Arbor, MI) with permission from Dr. Sylvie Robin (Curie Institute, Paris, France). Cre recombinase expression in the intestinal epithelium is driven by the villin promotor, which allows for conditional tamoxifen-dependent Cre recombinase action to remove the Cnr1 gene from these cells, as described by el Marjou et al.38 Cnr1tm1.1 mrl/vil-cre ERT2 mice used in these experiments are referred to as IntCB1−/−, and Cnr1tm1.1 mrl control mice (lacking Cre recombinase) are referred to as IntCB1+/+.
Tail snips were collected from pups at weaning, and DNA was extracted and analyzed by conventional polymerase chain reaction (PCR) using the following primers (5′-3′): GCAGGGATTATGTCCCTAGC (CNR1-ALT), CTGTTACCAGGAGTCTTAGC (1415-35), GGCTCAAGGAATACACTTATACC (1415-37), GAACCTGATGGACATGTTCAGG (vilcre, AA), AGTGCGTTCGAACGCTAGAGCCTGT (vilcre, SS), TTACGTCCATCGTGG-ACAGC (vilcre, MYO F), TGGGCTGGGTGTTAGCCTTA (vilcre, MYO R). Intestinal epithelial CB1R knockdown was verified by RT-qPCR immediately following experiments. Expression of the Cnr1 mRNA in the intestinal epithelium of intCB1−/− mice (0.1563±0.04848) is strongly reduced compared with intCB1+/+ controls (1.000±0.2223), t(19)=3.543, p=0.0022.
Gene expression
Total RNA from intestinal epithelium tissue was extracted using an RNeasy kit (Qiagen, Valencia, CA), and first-strand cDNA was generated using M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA). Areas used for tissue collection and processing were sanitized with 70% ethanol solution, then treated with RNAse inhibitor (RNAse Out; G-Biosciences, St. Louis, MO). Reverse transcription of total RNA was performed as previously described.13 Quantitative RT-PCR was performed using PrimePCR Assays (Biorad, Irvine, CA) with primers for CB1R (Cnr1) gene transcripts under preconfigured SYBR green assays (Biorad). Hprt was used as a housekeeping gene. Reactions were run in duplicates, and values expressed as relative mRNA expression.
Drug preparation and administration
IntCB1−/− and intCB1+/+ mice were administered tamoxifen (IP, 40 mg/kg) every 24 h for five consecutive days. Tamoxifen (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in corn oil using bath sonication at a concentration of 10 mg/mL, then stored at 37°C protected from light until administration. Mice were group-housed in disposable cages throughout the injection period and for a 3-day postinjection period.
Elevated plus maze
On the day of the experiment, animals were allowed to acclimate to the testing room for 3–4 h before testing. Behavioral tests were conducted between 09:00 and 12:00 h. The EPM is a white plexiglass apparatus consisting of four equal-length arms (30×5 cm). The maze is elevated 39 cm off the ground. The “closed” arms of the EPM are enclosed by 15 cm tall walls on all sides, while the “open” arms have a 1 mm border around the edges of the arm to prevent animals from falling off. Light intensity on the open arms was ∼150 lux during testing.
At the time of the test, animals were placed at the center of the maze facing one of the open arms and were allowed to freely explore the maze for a 5-min period. The entire test was recorded by a stationary camera fixed on the ceiling above the maze, which allowed simultaneous tracking of the center-point and nose-point of the mouse by EthoVision 13 XT software (Noldus Information Technology, Wageningen, Netherlands). The mouse was only considered to be in a zone if both the nose-point and the center-point were in that zone at the same time for ∼0.1 sec.
Head dipping behavior was defined as the nose-point of the animal crossing the border of the open arm. Cumulative duration of head dips was calculated by the total amount of time an animal spent with its nose-point beyond the border of any open arm. Between tests, the maze was thoroughly cleaned with a 70% EtOH solution followed by deionized ultra-filtered water (DIUF) and allowed to completely dry before the next mouse entered the maze. The animals used for the EPM tests were not utilized for any additional behavioral testing.
Light/dark box test
On the day of the experiment, animals were allowed to acclimate to the testing room for 3–4 h before testing. Behavioral tests were conducted between 09:00 and 12:00 h. The light/dark box consists of two acrylic chambers. The “dark” box is an enclosed gray plexiglass chamber (8×20×30 cm) with a solid roof, approximately half the area of the “light” box. The “light” box is an open gray plexiglass chamber (18×20×30 cm) without a roof. The entire apparatus was placed on a table during recording. Light intensity in the light box was ∼150–200 lux during testing.
At the time of the test, animals were placed in the corner of the light box furthest from the entry to the dark box and were allowed to freely explore the apparatus for a 10-min period. The entire test was recorded by a stationary camera fixed on the ceiling above the box, which allowed simultaneous tracking of the center-point and nose-point of the mouse by EthoVision 13 XT software (Noldus Information Technology).
The mouse was only considered to be in a zone if both the nose-point and the center-point were in that zone at the same time for ∼0.1 sec. Between tests, the maze was thoroughly cleaned with a 70% EtOH solution followed by DIUF and allowed to completely dry before the next mouse entered the apparatus. The animals that were used for the light/dark box were allowed to rest for 7 days before exposure to the open field test (see the Open field test section below).
Open field test
On the day of testing, animals were allowed to acclimate to the testing room for 3–4 h before testing. Behavioral tests were conducted between 09:00 and 12:00 h. The open field is an open white plexiglass square (50×50×40 cm) without a roof. The open field apparatus was placed on a table during recording. Light intensity in the center of the open field was ∼150–200 lux during testing. The entire open field was divided into nine equal squares. The center square (1/9 of the area) was defined as the center zone.
At the time of the test, animals were placed in the bottom left corner of the open field apparatus and were allowed to freely explore the apparatus for a 10-min period. The entire test was recorded by a stationary camera fixed on the ceiling above the apparatus, which allowed simultaneous tracking of the center-point and nose-point of the mouse by EthoVision 13 XT software (Noldus Information Technology). The mouse was only considered to be in a zone if both the nose-point and the center-point were in that zone at the same time for ∼0.1 sec. Between tests, the open field was thoroughly cleaned with a 70% EtOH solution followed by DIUF and allowed to completely dry before the next mouse entered the apparatus.
CORT enzyme-linked immunosorbent assay
On the day of the experiment, mice were allowed to freely explore the EPM for 5 min. Thirty minutes after EPM exposure, mice were anesthetized by isoflurane, and blood was collected through retro-orbital bleed using nonheparinized capillary tubes and stored on ice. Blood samples were spun at 4900 RPM for 10 min at 4°C to isolate serum. Serum was collected and stored at −80°C until analysis. Serum CORT levels were quantified using the DetectX Corticosterone ELISA kit (Arbor Assays, Ann Arbor, MI).
Samples were diluted at 1:100 and plated in duplicate. The assay was completed as described by the kit insert. Average optical density (OD) values were calculated for each sample, and the mean OD for the NSB was subtracted from each average sample OD value. Sample concentrations interpolated on a 4PL %B/B0 standard curve and multiplied by the dilution factor of 100 to obtain neat sample concentrations. The animals used for CORT analysis were only subject to the EPM once; they were not subject to any additional behavioral testing.
Experimental design and statistical analysis
Details regarding the experimental design of individual experiments are provided in the figure legends. Data were analyzed by GraphPad Prism version 9.5.0 (GraphPad Software, La Jolla, CA) using unpaired Student's t-tests (two-tailed), two-way ANOVA, or three-way ANOVA with Holm-Sidak's multiple comparisons post hoc test when appropriate.
Results
Male, but not female, intCB1−/− mice exhibit anxiolytic behaviors in the EPM
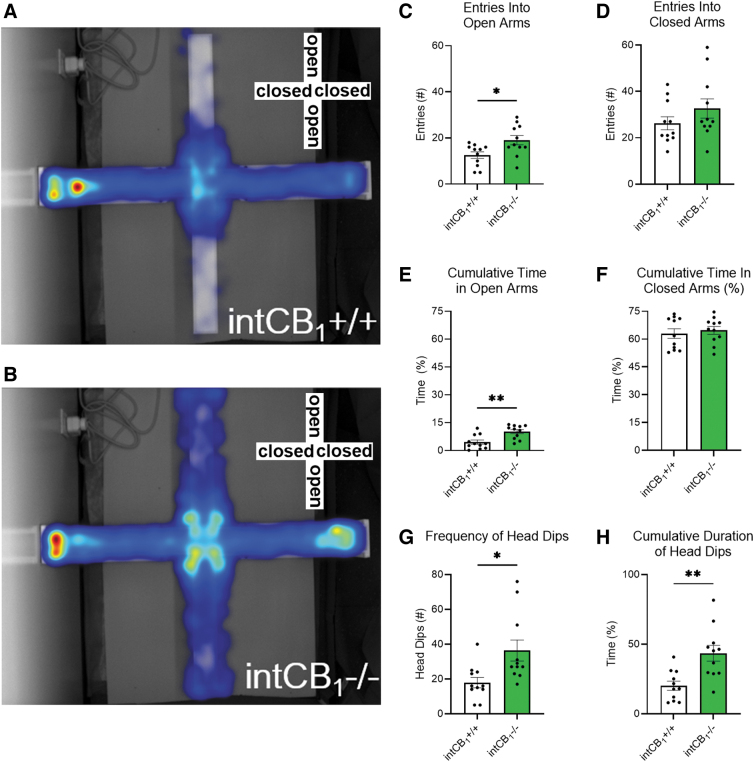
We tested the hypothesis that CB1Rs in the intestinal epithelium play a role in anxiety-like behaviors in the EPM. Male intCB1−/− mice entered the open arms of the EPM more often (Fig. 1B, C) and spent more time exploring the open arms (Fig. 1E) when compared with male intCB1+/+ control mice (Fig. 1A, E) during the 5-min test. There were no genotype differences in closed-arm entries or cumulative exploration time of the closed arm (Fig. 1D, F).
FIG. 1.
Male intCB1−/− mice exhibit anxiolytic behaviors in the EPM. Male intCB1−/− mice and intCB1+/+ controls were allowed to freely explore the EPM for 5 min. Merged heatmaps of all trials for (A) intCB1+/+ and (B) intCB1−/− mice show general exploration patterns of the open (vertical) and closed (horizontal) arms. Increasing time spent in area designated from blue to red, with red being most time. (C) IntCB1−/− male mice entered the open arms significantly more than controls [t(20)=2.602, p=0.0170]. (D) There were no differences in closed-arm entries between genotypes [t(20)=1.275, p=0.2170]. (E) IntCB1−/− male mice spent more time exploring the open arms when compared with controls [t(20)=3.570, p=0.0019], but there were no differences in (F) cumulative time of closed-arm exploration [t(20)=0.5128, p=0.6137]. (G) IntCB1−/− male mice exhibited an increased number of head dips compared with controls [t(20)=2.736, p=0.0127] and (H) spent more time performing the head dipping behavior than controls [t(20)=3.566, p=0.0019]. All analyses are unpaired Student's t-tests. Data presented as mean±SEM, n=11 mice per genotype. *p<0.05, **p<0.01. EPM, elevated plus maze; SEM, standard error of the mean.
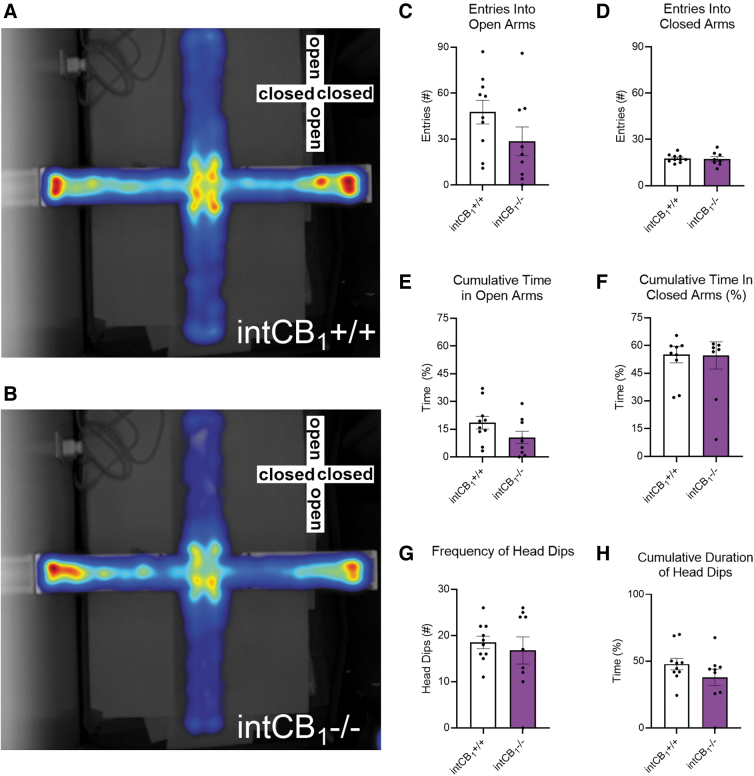
Male intCB1−/− mice had an increased number of head dips (Fig. 1G) and spent more time performing the head dipping behavior (Fig. 1H) when compared with male intCB1+/+ mice. In contrast to male mice, female intCB1−/− mice did not exhibit any differences in the number of open (Fig. 2B, C) or closed (Fig. 2B, D) arm entries when compared with female intCB1+/+ mice (Fig. 2A, C, D).
FIG. 2.
Female intCB1−/− mice do not perform differently from controls in the EPM. Female intCB1−/− mice and intCB1+/+ controls were allowed to freely explore the EPM for 5 min. Merged heatmaps of all trials for (A) intCB1+/+ and (B) intCB1−/− mice show general exploration patterns of the open (vertical) and closed (horizontal) arms. Increasing time spent in area designated from blue to red, with red being most time. (C) IntCB1−/− female mice did not exhibit any differences in open-arm entries compared with controls [t(17)=1.588, p=0.1307]. (D) There were no differences in closed-arm entries between genotypes [t(16)=0.1938, p=0.8488]. (E) IntCB1−/− female mice and controls spent a similar amount of time exploring the open arms [t(17)=1.665, p=0.1142] and (F) closed arms of the EPM [t(17)=0.05312, p=0.9853]. There were no genotype differences in the (G) total number of head dips [t(17)=0.5512, p=0.5886], or the (H) cumulative time spent performing head dip behavior [t(17)=1.334, p=0.1999] in female mice. All analyses are unpaired Student's t-tests. Data presented as mean±SEM, n=9–10 mice per genotype.
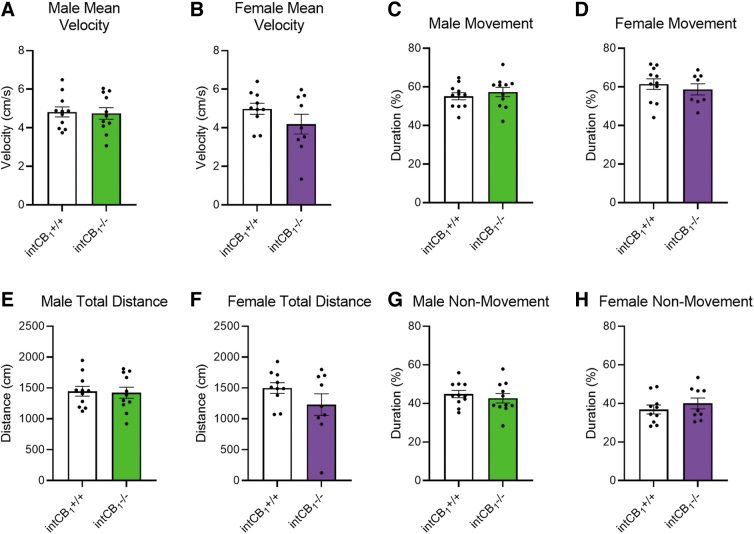
Furthermore, female intCB1−/− and female intCB1+/+ mice spent a similar amount of time exploring the open arms (Fig. 2E) and closed arms (Fig. 2F). There were no genotype differences in the frequency of head dips (Fig. 2G) or cumulative duration of head dipping behavior in female mice (Fig. 2H). We also evaluated general movement parameters in male and female intCB1−/− and intCB1+/+ mice. There were no significant differences in average velocity (Fig. 3A, B), total distance traveled (Fig. 3E, F), cumulative duration of movement (Fig. 3C, D), or cumulative duration of nonmovement (Fig. 3G, H) between genotypes of male or female mice.
FIG. 3.
Genotype differences in EPM exploration are not due to changes in movement. General movement parameters were quantified for both male and female mice on the EPM. (A) There were no differences in average velocity between male intCB1−/− mice and controls [t(20)=0.1997, p=0.8437] or (B) female intCB1−/− mice and controls [t(17)=1.394, p=0.1813]. (C) There were no differences in cumulative duration of movement between male intCB1−/− mice and controls [t(20)=0.7119, p=0.4847] or (D) female intCB1−/− mice and controls [t(17)=0.6774, p=0.5072]. (E) There were no differences in total distance traveled between male intCB1−/− mice and controls [t(20)=0.1986, p=0.8446] or (F) female intCB1−/− mice and controls [t(17)=1.427, p=0.1718]. (G) There were no differences in cumulative duration of nonmovement between male intCB1−/− mice and controls [t(20)=0.7119, p=0.4847] or (H) female intCB1−/− mice and controls [t(17)=0.8910, p=0.3854]. All analyses are unpaired Student's t-tests. Data presented as mean±SEM, n=9–11 mice per sex and genotype.
Male, but not female, intCB1−/− mice exhibit anxiolytic behaviors in the light/dark box
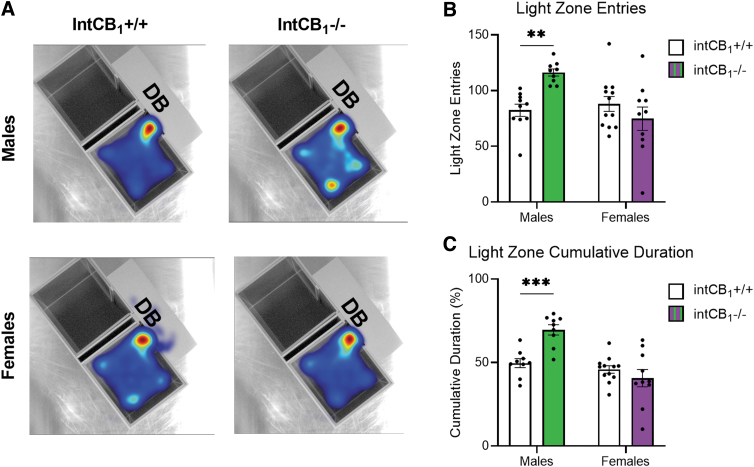
We next asked if CB1Rs in the intestinal epithelium play a role in anxiety-like behaviors in the light/dark box. Male intCB1−/− mice entered the light zone more frequently (Fig. 4A top right, B) and spent more time in the light box (Fig. 4A top right, C) than male intCB1+/+ control mice (Fig. 4A top left, B, C) during the 10-min test. In contrast to male mice; female mice did not exhibit any differences in light box exploration, irrespective of genotype (Fig. 4A bottom left and right, C, D).
FIG. 4.
Male intCB1−/− mice, but not female, exhibit anxiolytic behaviors in the light/dark box. Male and female intCB1−/− mice and intCB1+/+ controls were allowed to freely explore the light/dark box for 10 min. (A) Merged heatmaps of all trials for male intCB1+/+, male intCB1−/− mice, female intCB1+/+, female intCB1−/− mice show general exploration patterns of the light box. Mice were unable to be recorded in the DB due to the opaque roof. Increasing time spent in area designated from blue to red, with red being most time. (B) Male intCB1−/− mice exhibited an increase in total light zone entries compared with controls, but there were no differences observed in light zone entries for female intCB1−/− mice and controls [sex×genotype interaction: F(1,37)=10.75; p=0.0023; sex main effect F(1,37)=6.236; p=0.0171; male intCB1−/− vs. male intCB1+/+ p=0.0053; two-way ANOVA followed by Holm-Sidak's multiple comparisons test]. (C) Male intCB1−/− mice exhibited an increase in light zone cumulative duration compared with controls, but there were no differences observed in light zone cumulative duration for female intCB1−/− mice and controls [sex×genotype interaction: F(1,36)=13.18; p=0.0009; sex main effect F(1,36)=22.21; p<0.0001; genotype main effect F(1,36)=4.521; p=0.0404; male intCB1−/− vs. male intCB1+/+ p=0.0008; two-way ANOVA followed by Holm-Sidak's multiple comparisons test]. Data presented as mean±SEM, n=9–12 mice per sex and genotype, **p<0.01, ***p<0.001. DB, dark box.
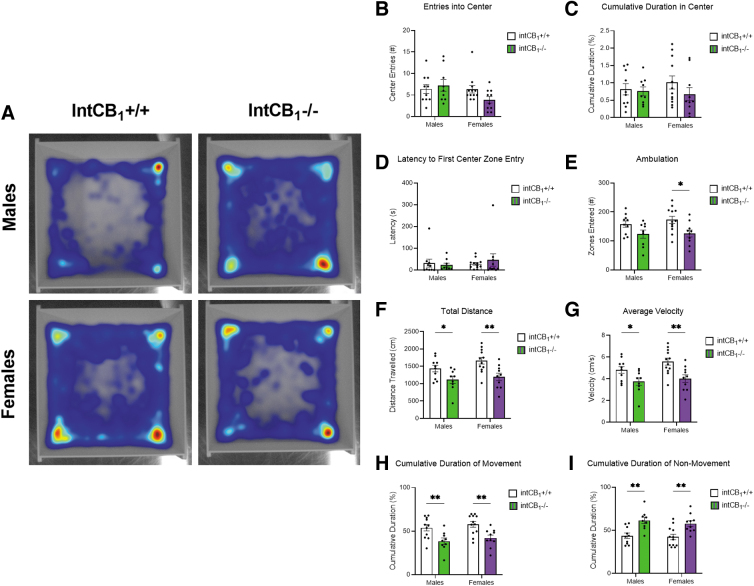
IntCB1−/− mice do not exhibit anxiolytic behaviors in the open field test
Exploratory behaviors of intCB1−/− mice were also evaluated in the open field test. Neither male nor female intCB1−/− mice exhibited a difference in center zone entries (Fig. 5B) or cumulative duration in center zone (Fig. 5C) when compared with intCB1+/+ control mice during the 10-min test. There were also no genotype differences observed in latency to first center zone entry (Fig. 5D) for male or female mice.
FIG. 5.
IntCB1−/− mice do not exhibit anxiolytic behaviors in the open field test. Male and female intCB1−/− mice and intCB1+/+ controls were allowed to freely explore the open field apparatus for 10 min. (A) Merged heatmaps of all trials for male intCB1+/+, male intCB1−/− mice, female intCB1+/+, female intCB1−/− mice show general exploration patterns of the open field. Increasing time spent in area designated from blue to red, with red being most time. There were no sex or genotype differences observed in (B) number of center zone entries, (C) cumulative duration in center zone, or (D) latency to first center zone entry. (E) IntCB1−/− female mice displayed a significant reduction in ambulation (total number of zones entered) compared with controls. There were no differences in ambulation between IntCB1−/− males and controls [genotype main effect F(1,37)=10.85; p=0.0022; female intCB1−/− vs. female intCB1+/+ p=0.0168]. (F) IntCB1−/− male and female mice demonstrated a reduction in total distance traveled compared with controls [genotype main effect F(1,37)=14.93; p=0.0004; male intCB1−/− vs. male intCB1+/+ p=0.0378; female intCB1−/− vs. female intCB1+/+ p=0.0037]. (G) IntCB1−/− male and female mice demonstrated a reduction in average velocity to controls [genotype main effect F(1,37)=14.96; p=0.0004; male intCB1−/− vs. male intCB1+/+ p=0.0379; female intCB1−/− vs. female intCB1+/+ p=0.0036]. (H) IntCB1−/− male and female mice demonstrated a reduction in the cumulative duration of movement to controls [genotype main effect F(1,38)=19.15; p<0.0001; male intCB1−/− vs. male intCB1+/+ p=0.0057; female intCB1−/− vs. female intCB1+/+ p=0.0057]. (I) IntCB1−/− male and female mice demonstrated an increase in the cumulative duration of movement compared with controls [genotype main effect F(1,37)=24.04; p<0.0001; male intCB1−/− vs. male intCB1+/+ p=0.0020; female intCB1−/− vs. female intCB1+/+ p=0.0020]. All analyses are two-way ANOVA followed by Holm-Sidak's multiple comparisons test. Data presented as mean±SEM, n=9–12 mice per sex and genotype. *p<0.05, **p<0.01.
Furthermore, female intCB1−/− mice exhibited a significant decrease in ambulation (Fig. 5E, total number of zones entered) when compared with female intCB1+/+ mice; however, no genotype differences in ambulation were found for male mice. Both male and female intCB1−/− mice exhibited a decrease in the total distance traveled (Fig. 5F) and average velocity (Fig. 5G) when compared with respective control mice. In addition, male and female intCB1−/− animals demonstrated a decrease in the cumulative duration of movement (Fig. 5H) and a corresponding increase in the cumulative duration of nonmovement (Fig. 5G) when compared with control mice.
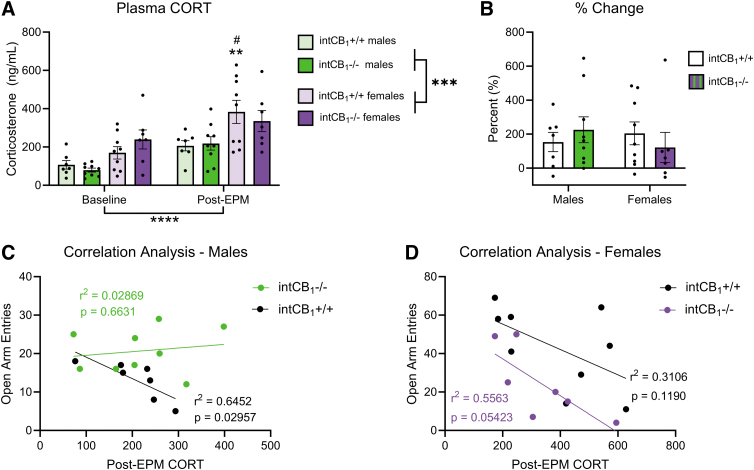
Circulating CORT levels are sex dependent
We next quantified circulating CORT levels in male and female intCB1−/− and intCB1+/+ control mice at baseline and 30 min after EPM exposure. There was a strong effect of sex and timepoint on plasma CORT levels (Fig. 6A). CORT was significantly higher in females than in males, regardless of genotype, both at baseline and after EPM exposure. CORT levels were also significantly elevated in all groups after EPM exposure when compared with their respective baseline levels. There was no effect of genotype on circulating CORT in either male or female mice at baseline or post-EPM.
FIG. 6.
Circulating CORT levels are sex dependent. Circulating CORT levels were quantified in male and female intCB1−/− mice and intCB1+/+ controls at baseline and after a 5-min EPM exposure. Correlation analysis was performed between post-EPM CORT levels and number of open-arm entries on the EPM per mouse. (A) There was a significant effect of timepoint and sex on plasma CORT levels. IntCB1+/+ female mice exhibited significantly higher CORT after EPM exposure when compared with intCB1+/+ female mice at baseline. IntCB1+/+ females post-EPM also exhibited a significant increase in CORT when compared with intCB1+/+ males post-EPM [timepoint main effect F(1,28)=24.44; p<0.0001; sex main effect F(1,28)=19.76; p=0.0001; **baseline female intCB1+/+ vs. post-EPM female intCB1+/+ p=0.0036; #post-EPM female intCB1+/+ vs. post-EPM male intCB1+/+ p=0.0311; three-way ANOVA followed by Holm-Sidak's multiple comparisons test]. (B) There were no significant differences in % change of plasma CORT. % Change=((Post-EPM CORT − Baseline CORT)/Baseline CORT)×100. Data presented as mean±SEM, n=7–9 mice group. ***p<0.001, ****p<0.0001. (C) Male intCB1+/+ mice exhibited an inverse correlation between post-EPM CORT levels and number of open-arm entries (p=0.02957, r2=0.6452), while intCB1−/− males did not (p=0.6631, r2=0.02869). (D) Female intCB1+/+ mice (p=0.1190, r2=0.3106) and intCB1−/− mice (p=0.05423, r2=0.5563) exhibited a nonsignificant trend toward an inverse correlation between post-EPM CORT levels and number of open-arm entries. CORT, corticosterone.
Although there were strong effects of sex and timepoint on plasma CORT, no significant differences were observed in % change of CORT when comparing baseline and post-EPM levels (Fig. 6B). Analysis of correlations between post-EPM circulating CORT levels and the number of EPM open-arm entries in each mouse was next performed. A strong inverse correlation was observed in male intCB1+/+ animals, while there was a trending inverse correlation in intCB1+/+ females and intCB1−/− females (Fig. 6C, D). No relationship was observed between post-EPM CORT levels and number of open-arm entries in intCB1−/− males (Fig. 6C).
Discussion
We report that (i) male mice lacking CB1Rs in the intestinal epithelium exhibit anxiolytic behavior during the EPM and light/dark box tests, but not in the open field test; (ii) female mice lacking CB1Rs in the intestinal epithelium do not display an anxiolytic phenotype during any of the three tests; and (iii) sex differences in behaviors are associated with elevated levels of circulating CORT in female mice at baseline and immediately after behavioral testing. The findings reveal an important and sexually dimorphic role for CB1Rs in the intestinal epithelium in the behavioral expression of anxiety.
To better understand how peripheral components of the ECS contribute to the expression of anxiety-like behaviors, we utilized our transgenic mouse line that conditionally lacks CB1Rs selectively in the intestinal epithelium. Male intCB1−/− mice spent significantly more time exploring the open arms of the EPM when compared with the corresponding controls, as shown by total entries into open arms and cumulative time spent in open arms.
IntCB1−/− males also participated in head dipping behaviors more than corresponding controls. These anxiolytic behaviors observed in mice were not due to changes in mobility as evidenced by no differences detected versus control mice for mean velocity, total distance moved, and cumulative duration of movement or nonmovement. A similar anxiolytic phenotype was observed in the light/dark box: intCB1−/− male mice exhibited an increase in light box entries and cumulative duration in the light box when compared with control mice.
Since mice cannot be recorded in the dark box due to the opaque lid, measures of mobility in the dark compartment were not analyzed. Notably, intCB1−/− males did not display an anxiolytic phenotype on any measurable outcomes in the open field test when compared with corresponding controls. It is possible, however, that the 10-min testing period was not long enough for mice to display behavioral differences in the open field test.
Although many groups utilize a 5–10 min range for this test,39–41 others allow up to 30 min of exploration.42–44 Nonetheless, differential effects observed among genotypes in male mice on the three tests highlight the importance of utilizing a battery of behavioral tests to assess anxiety-like behaviors in rodents.45
In contrast to male mice, female intCB1−/− mice did not exhibit anxiolytic phenotypes on any of the three tests versus corresponding control mice. Female mice, however, displayed an increase in baseline EPM exploration when compared with male mice. This is particularly apparent when comparing the heat maps for intCB1+/+ control male and female mice (Figs. 1A and 2A, respectively). Specifically, female intCB1+/+ control mice exhibited a higher number of open-arm entries and cumulative duration in open arms when compared with those displayed by male control mice. This outcome was partially expected due to previous findings that female C57BL/6 mice exhibited higher activity levels than males on the EPM.46
Another study revealed sex differences in locomotion on the EPM in AKR and DBA/2 mice, but none of the other strains tested.47 Thus, it is possible that our findings are due to both strain and sex of the animals tested. In addition, Marcondes et al48 found that female rats in proestrus exhibited reduced anxiety-like behaviors compared with females in diestrus—a behavior mediated by estradiol levels. Further testing would be needed to determine if female mice in our study are similarly affected by estrous cycles. This sex difference in overall exploration may explain the lack of genotype differences observed in female anxiety-like behaviors.
Accordingly, it is possible that there is a “ceiling effect” for exploration of open arms in the EPM in female mice, thus preventing any further increases in open-arm exploration irrespective of genotype. To confirm this hypothesis, future experiments could be conducted in combination with administration of anxiety-reducing drugs (e.g., benzodiazepines) or stimuli in female intCB1−/− and control mice to evaluate if exploration can be increased above the baseline.
Both male and female intCB1−/− mice demonstrated a significant reduction in several locomotor parameters in the open field test when compared with corresponding control mice. The relationship between locomotor activity and rodent emotionality, however, is unclear.45,49–52
Indeed, inconsistencies have been widely noted in open field test protocols across laboratories, which suggest that measures of emotionality may confound analyses of locomotor activity, and vice versa.53 Therefore, genotype differences in ambulation, total distance traveled, average velocity, and cumulative duration of movement and nonmovement may reflect anxiety-related changes in behavior of mice, including intCB1−/− mice, or they may be a result of the other changes in behaviors. Nonetheless, the current results indicate that CB1Rs in the intestinal epithelium contribute to the expression of several behaviors that are widely used to analyze an “anxiety” phenotype in mice.
Although tamoxifen interacts with estrogen receptors, it was shown—at the same dosing regimen used in this study—to not affect anxiety-like behavior of male or female animals in a variety of behavioral tests, including the EPM and open field test.54 All animals in our study received tamoxifen at the same dose and dosing protocol, regardless of sex or genotype. Therefore, any behavioral effects caused by tamoxifen, if present, would be represented in all animals equally irrespective of sex, and thus does not likely contribute to sex differences found in our study.
Global and cell type-specific deletion of CB1R in the brain of rodents yields pronounced anxious-like phenotypes.3,55–57 To the best of our knowledge, we are the first group to test the effect of intestinal epithelium-specific CB1R deletion on anxiety-like behaviors. Unexpectedly, our findings indicate that male mice lacking CB1Rs in the intestinal epithelium exhibit an anxiolytic phenotype when compared with corresponding controls.
We also show that female mice lacking CB1Rs in the intestinal epithelium perform similarly to controls on the EPM, light/dark box, and open field test. It is important to note that deletion is specific to CB1 cannabinoid receptors. Some reports indicate a role for CB2Rs in the brain58–61 in the control of anxiety-like behaviors; however, roles of intestinal CB2Rs in anxiety are unknown, and their investigation in this context remains for future studies.
These should include use of mice with conditional deletion of CB2Rs in the intestinal epithelium in combination with pharmacological interventions to fully characterize the contribution of intestinal CB1- versus CB2–cannabinoid receptors in the behavioral expression of anxiety. Additional studies should utilize intCB1−/− mice in combination with CB1R-specific agonists to confirm the specific involvement of intestinal CB1Rs in this behavior.
Sex differences related to ECS control of behavior have been described by other groups as well. For example, female rats displayed both anxiolytic and anxiogenic effects in response to treatment with the fatty acid amide inhibitor, URB587, and the monoacylglycerol lipase inhibitor, MJN110, which were dependent on estrous cycles, while male rats responded to the same treatments with only anxiolytic or anxiogenic behaviors, respectively.36
In a different study, inhibition of anandamide or 2-AG hydrolysis had no effect in males, but did alter fear–memory extinction in females.35 These differences may be attributed, in part, to sexual dimorphism and function of the amygdala, hippocampus, and medial prefrontal cortices,62,63 all of which densely express ECS components64,65 and estrogen receptors.66–68 Moreover, significant elevations in circulating levels of CORT were observed in female mice in this study, regardless of genotype, both at baseline and immediately after EPM exposure. These female-specific CORT elevations may seem contradictory to the fact that they explored the EPM more than males. However, the fact that females exhibited higher CORT levels at baseline may not be directly related to stress or anxiety, but rather reflect ethological differences in male versus female mouse behavior and physiology.
As reviewed by Kokras et al., depressive and anxiety-like behaviors in female rodents are significantly less dependent on CORT levels than in males.69 This is consistent with our findings that male control mice exhibited a robust inverse correlation between the number of open-arm entries and post-EPM CORT levels (Fig. 6C), while female mice did not (Fig. 6D). It is possible that elevated CORT levels in female mice may prevent the deletion of CB1Rs in the intestinal epithelium from having anxiolytic effects.
Differential levels of circulating CORT, however, are insufficient to explain the genotype differences observed in male mice. The finding that intCB1−/− males do not exhibit the inverse correlation between CORT levels and open-arm entries (Fig. 6C) that was observed in the controls may be indicative of HPA-axis dysregulation and should be further explored as a possible explanation for the genotype differences observed only in male mice.
Sex differences in the gut microbiome may also account for the variability observed between males and females in this study. Bacteria are directly involved in estrogen metabolism,70 while estrogen, in turn, can modulate bacterial metabolism through the estrogen receptor β.71 Furthermore, treatment with 17β-estradiol reduced the presence of proteobacteria and LPS in male mice, while it increased inflammation and metabolic endotoxemia in ovariectomized female mice.72 Other studies indicate that levels of estrogen and testosterone influence diversity of the microbiota.73,74
In light of findings that indicate a causative relationship between the gut microbiome and expression of anxiety-like behaviors through the gut–brain axis,9–11,75 it is possible that differences in the microbiota of males and females are a source of the sex differences in anxiety behaviors measured in this study. To fully understand the contribution of the microbiome to this process, future studies should profile microbial species diversity in intCB1+/+ and intCB1−/− male and female mice.
CB1Rs are expressed on a variety of cells expressed in the intestinal epithelium, including enteroendocrine I cells.13,76 Nutrient-induced CCK release by I cells enables gut–brain satiation communication through CCKA receptors located on vagal afferent fibers.77 Indeed, vagal afferent fibers may play a critical role in the transmission of affective signals from the gut to the brain.23,78 Accordingly, it is possible that the absence of CB1Rs in I cells in intCB1−/− mice leads to alterations in gut–brain signaling that impacts anxiety-like behaviors.
Moreover, several studies report that vagal afferent signaling has a direct impact on anxiety-like behaviors. For example, subdiaphragmatic vagal deafferentation in rats caused a reduction in anxiety-like behaviors on the EPM, open field test, and food neophobia test.79 Another group demonstrated that both feeding and chemogenetic activation of gut-innervating vagal afferents increased anxiety-like behaviors, while fasting and chemogenetic inhibition of the same fibers blocked increases in anxiety-like behaviors.26
Similarly, Maniscalco et al. found that an overnight fast attenuated anxious behavior in rats tested on the EPM and acoustic startle test.80 It is unclear whether the anxiolysis observed in intCB1−/− males in this study is the direct result of changes in vagal afferent neuronal signaling, but future studies should evaluate roles of gut–brain signaling in these processes.
Conclusions
Collectively these results suggest that genetic deletion of CB1Rs in the intestinal epithelium is associated with an anxiolytic phenotype in a sex-dependent manner, with a robust phenotype found for male mice. Future studies will investigate the mechanism(s) by which intestinal CB1Rs control anxiety-like behaviors.
Acknowledgments
The authors thank Jose Luis Martin for his contribution to chemical analyses.
Abbreviations Used
- CB1R
cannabinoid receptor subtype-1
- CNS
central nervous system
- CORT
corticosterone
- DB
dark box
- DIUF
deionized ultra-filterd water
- ECS
endocannabinoid system
- ELISA
enzyme-linked immunosorbent assay
- EPM
elevated plus maze
- GI
gastrointestinal
- OD
optical density
- RT-qPCR
reverse transcription quantitative polymerase chain reaction
- SEM
standard error of the mean
Authors' Contributions
C.P.W. contributed to experimental design, data collection, analysis and interpretation of results, article preparation; B.A. and C.A. performed data collection; N.V.D. assisted with experimental design, analysis and interpretation of results, article preparation and editing, funding.
Data Availability Statement
All data that support the findings of this study are available in the figures of this article.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
Research is funded by the National Institutes of Health (R01 DK119498 and L30 DK1149978) and the Tobacco-Related Disease Research Program (T29KT0232) to Nicholas. V. DiPatrizio.
Cite this article as: Wood CP, Avalos B, Alvarez C, DiPatrizio NV (2023) A sexually dimorphic role for intestinal cannabinoid receptor subtype-1 in the behavioral expression of anxiety, Cannabis and Cannabinoid Research 8:6, 1045–1059, DOI: 10.1089/can.2023.0150.
References
- 1. Ruehle S, Rey AA, Remmers F, et al. The endocannabinoid system in anxiety, fear memory and habituation. J Psychopharmacol 2012;26(1):23–39; doi: 10.1177/0269881111408958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Petrie GN, Nastase AS, Aukema RJ, et al. Endocannabinoids, cannabinoids and the regulation of anxiety. Neuropharmacology 2021;195:108626; doi: 10.1016/j.neuropharm.2021.108626 [DOI] [PubMed] [Google Scholar]
- 3. Lutz B, Marsicano G, Maldonado R, et al. The endocannabinoid system in guarding against fear, anxiety and stress. Nat Rev Neurosci 2015;16(12):705–718; doi: 10.1038/nrn4036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jenniches I, Ternes S, Albayram O, et al. Anxiety, stress, and fear response in mice with reduced endocannabinoid levels. Biol Psychiatry 2016;79(10):858–868; doi: 10.1016/j.biopsych.2015.03.033 [DOI] [PubMed] [Google Scholar]
- 5. Patel S, Hill MN, Cheer JF, et al. The endocannabinoid system as a target for novel anxiolytic drugs. Neurosci Biobehav Rev 2017;76(Pt A):56–66; doi: 10.1016/j.neubiorev.2016.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patel S, Hillard CJ. Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: Further evidence for an anxiolytic role for endogenous cannabinoid signaling. J Pharmacol Exp Ther 2006;318(1):304–311; doi: 10.1124/jpet.106.101287 [DOI] [PubMed] [Google Scholar]
- 7. Haller J, Bakos N, Szirmay M, et al. The effects of genetic and pharmacological blockade of the CB1 cannabinoid receptor on anxiety. Eur J Neurosci 2002;16(7):1395–1398; doi: 10.1046/j.1460-9568.2002.02192.x [DOI] [PubMed] [Google Scholar]
- 8. Zhang J, Ma L, Chang L, et al. A key role of the subdiaphragmatic vagus nerve in the depression-like phenotype and abnormal composition of gut microbiota in mice after lipopolysaccharide administration. Transl Psychiatry 2020;10(1):186; doi: 10.1038/s41398-020-00878-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bravo JA, Forsythe P, Chew MV, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A 2011;108(38):16050–16055; doi: 10.1073/pnas.1102999108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goehler LE, Gaykema RP, Opitz N, et al. Activation in vagal afferents and central autonomic pathways: Early responses to intestinal infection with Campylobacter jejuni. Brain Behav Immun 2005;19(4):334–344; doi: 10.1016/j.bbi.2004.09.002 [DOI] [PubMed] [Google Scholar]
- 11. Gaykema RP, Goehler LE, Lyte M. Brain response to cecal infection with Campylobacter jejuni: Analysis with Fos immunohistochemistry. Brain Behav Immun 2004;18(3):238–245; doi: 10.1016/j.bbi.2003.08.002 [DOI] [PubMed] [Google Scholar]
- 12. Argueta D, DiPatrizio N. Peripheral endocannabinoid signaling controls hyperphagia in Western diet-induced obesity. Physiol Behav 2017;171:32–39; doi: 10.1016/j.physbeh.2016.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Argueta D, Perez P, Makriyannis A, et al. Cannabinoid CB1 receptors inhibit gut-brain satiation signaling in diet-induced obesity. Front Physiol 2019;10:704; doi: 10.3389/fphys.2019.00704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Avalos B, Argueta D, Perez P, et al. Cannabinoid CB1 receptors in the intestinal epithelium are required for acute Western-diet preferences in mice. Nutrients 2020;12(9):2874; doi: 10.3390/nu12092874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Quarta C, Mazza R, Obici S, et al. Energy balance regulation by endocannabinoids at central and peripheral levels. Trends Mol Med 2011;17(9):518–526; doi: 10.1016/j.molmed.2011.05.002 [DOI] [PubMed] [Google Scholar]
- 16. Di Marzo V, Capasso R, Matias I, et al. The role of endocannabinoids in the regulation of gastric emptying: Alterations in mice fed a high-fat diet. Br J Pharmacol 2008;153:1272–7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Camilleri M, Carlson P, McKinzie S, et al. Genetic variation in endocannabinoid metabolism, gastrointestinal motility, and sensation. Am J Physiol Gastrointest Liver Physiol 2008;294(1):G13–G19; doi: 10.1152/ajpgi.00371.2007 [DOI] [PubMed] [Google Scholar]
- 18. Aviello G, Romano B, Izzo AA. Cannabinoids and gastrointestinal motility: animal and human studies. Eur Rev Med Pharmacol Sci 2008;12(Suppl 1):81–93. [PubMed] [Google Scholar]
- 19. Wiley MB, DiPatrizio NV. Diet-induced gut barrier dysfunction is exacerbated in mice lacking cannabinoid 1 receptors in the intestinal epithelium. Int J Mol Sci 2022;23(18):10549; doi: 10.3390/ijms231810549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kimball ES, Schneider CR, Wallace NH, et al. Agonists of cannabinoid receptor 1 and 2 inhibit experimental colitis induced by oil of mustard and by dextran sulfate sodium. Am J Physiol Gastrointest Liver Physiol 2006;291(2):G364–G371; doi: 10.1152/ajpgi.00407.2005 [DOI] [PubMed] [Google Scholar]
- 21. Storr MA, Keenan CM, Zhang H, et al. Activation of the cannabinoid 2 receptor (CB2) protects against experimental colitis. Inflamm Bowel Dis 2009;15(11):1678–1685; doi: 10.1002/ibd.20960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Markey L, Hooper A, Melon LC, et al. Colonization with the commensal fungus Candida albicans perturbs the gut-brain axis through dysregulation of endocannabinoid signaling. Psychoneuroendocrinology 2020;121:104808; doi: 10.1016/j.psyneuen.2020.104808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mayer EA. Gut feelings: The emerging biology of gut-brain communication. Nat Rev Neurosci 2011;12(8):453–466; doi: 10.1038/nrn3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci 2000;85(1–3):1–17; doi: 10.1016/S1566-0702(00)00215-0 [DOI] [PubMed] [Google Scholar]
- 25. Critchley HD, Harrison NA. Visceral influences on brain and behavior. Neuron 2013;77(4):624–638; doi: 10.1016/j.neuron.2013.02.008 [DOI] [PubMed] [Google Scholar]
- 26. Krieger JP, Asker M, van der Velden P, et al. Neural pathway for gut feelings: Vagal interoceptive feedback from the gastrointestinal tract is a critical modulator of anxiety-like behavior. Biol Psychiatry 2022;92(9):709–721; doi: 10.1016/j.biopsych.2022.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Seedat S, Scott KM, Angermeyer MC, et al. Cross-national associations between gender and mental disorders in the World Health Organization World Mental Health Surveys. Arch Gen Psychiatry 2009;66(7):785–795; doi: 10.1001/archgenpsychiatry.2009.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005;62(6):593–602; doi: 10.1001/archpsyc.62.6.593 [DOI] [PubMed] [Google Scholar]
- 29. Bekker MH, van Mens-Verhulst J. Anxiety disorders: Sex differences in prevalence, degree, and background, but gender-neutral treatment. Gend Med 2007;4(Suppl B):S178–S193; doi: 10.1016/s1550-8579(07)80057-x [DOI] [PubMed] [Google Scholar]
- 30. An XL, Zou JX, Wu RY, et al. Strain and sex differences in anxiety-like and social behaviors in C57BL/6J and BALB/cJ mice. Exp Anim 2011;60(2):111–123; doi: 10.1538/expanim.60.111 [DOI] [PubMed] [Google Scholar]
- 31. Caldarone BJ, King SL, Picciotto MR. Sex differences in anxiety-like behavior and locomotor activity following chronic nicotine exposure in mice. Neurosci Lett 2008;439(2):187–191; doi: 10.1016/j.neulet.2008.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nyuyki KD, Cluny NL, Swain MG, et al. Altered brain excitability and increased anxiety in mice with experimental colitis: Consideration of hyperalgesia and sex differences. Front Behav Neurosci 2018;12:58; doi: 10.3389/fnbeh.2018.00058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leussis MP, Thanos JM, Powers A, et al. Sex differences in long-term behavioral alterations, especially anxiety, following prenatal fluoxetine exposure in C57BL/6 mice. Pharmacol Biochem Behav 2021;211:173293; doi: 10.1016/j.pbb.2021.173293 [DOI] [PubMed] [Google Scholar]
- 34. Holingue C, Budavari AC, Rodriguez KM, et al. Sex differences in the gut-brain axis: Implications for mental health. Curr Psychiatry Rep 2020;22:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Morena M, Nastase AS, Santori A, et al. Sex-dependent effects of endocannabinoid modulation of conditioned fear extinction in rats. Br J Pharmacol 2021;178(4):983–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Salemme BW, Raymundi AM, Sohn JMB, et al. The estrous cycle influences the effects of fatty acid amide hydrolase and monoacylglycerol lipase inhibition in the anxiety-like behavior in rats. Cannabis Cannabinoid Res 2023. [Epub ahead of print]; doi: 10.1089/can.2022.0329 [DOI] [PubMed] [Google Scholar]
- 37. Rubino T, Parolaro D. Sexually dimorphic effects of cannabinoid compounds on emotion and cognition. Front Behav Neurosci 2011;5:64; doi: 10.3389/fnbeh.2011.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. el Marjou F, Janssen KP, Chang BH, et al. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 2004;39(3):186–193; doi: 10.1002/gene.20042 [DOI] [PubMed] [Google Scholar]
- 39. Kraeuter AK, Guest PC, Sarnyai Z. The open field test for measuring locomotor activity and anxiety-like behavior. Methods Mol Biol 2019;1916:99–103; doi: 10.1007/978-1-4939-8994-2_9 [DOI] [PubMed] [Google Scholar]
- 40. Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur J Pharmacol 2003;463(1–3):3–33; doi: 10.1016/s0014-2999(03)01272-x [DOI] [PubMed] [Google Scholar]
- 41. Gould TD, Dao DT, Kovacsics CE. The Open Field Test. In: Mood and Anxiety Related Phenotypes in Mice: Characterization Using Behavioral Tests. (Gould TD. ed.) Humana Press: New York, New York; 2009; pp. 1–332. [Google Scholar]
- 42. Choleris E, Thomas AW, Kavaliers M, et al. A detailed ethological analysis of the mouse open field test: Effects of diazepam, chlordiazepoxide, and an extremely low frequency pulsed magnetic field. Neurosci Biobehav Rev 2001;25(3):235–260. [DOI] [PubMed] [Google Scholar]
- 43. Dulawa SC, Holick KA, Gundersen B, et al. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology 2004;29(7):1321–1330; doi: 10.1038/sj.npp.1300433 [DOI] [PubMed] [Google Scholar]
- 44. McIlwain KL, Merriweather MY, Yuva-Paylor LA, et al. The use of behavioral test batteries: effects of training history. Physiol Behav 2001;73(5):705–717; doi: 10.1016/s0031-9384(01)00528-5 [DOI] [PubMed] [Google Scholar]
- 45. Ramos A. Animal models of anxiety: Do I need multiple tests? Trends Pharmacol Sci 2008;29(10):493–498; doi: 10.1016/j.tips.2008.07.005 [DOI] [PubMed] [Google Scholar]
- 46. Tucker LB, McCabe JT. Behavior of male and female C57BL/6J mice is more consistent with repeated trials in the elevated zero maze than in the elevated plus maze. Front Behav Neurosci 2017;11:13; doi: 10.3389/fnbeh.2017.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. O'Leary TP, Gunn RK, Brown RE. What are we measuring when we test strain differences in anxiety in mice? Behav Genet 2013;43(1):34–50; doi: 10.1007/s10519-012-9572-8 [DOI] [PubMed] [Google Scholar]
- 48. Marcondes FK, Miguel KJ, Melo LL, et al. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiol Behav 2001;74(4–5):435–440; doi: 10.1016/s0031-9384(01)00593-5 [DOI] [PubMed] [Google Scholar]
- 49. Gray JA. Emotionality in male and female rodents: A reply to Archer. Br J Psychol 1979;70(3):425–440; doi: 10.1111/j.2044-8295.1979.tb01713.x [DOI] [PubMed] [Google Scholar]
- 50. Archer J. Tests for emotionality in rats and mice: A review. Anim Behav 1973;21(2):205–235; doi: 10.1016/s0003-3472(73)80065-x [DOI] [PubMed] [Google Scholar]
- 51. Seibenhener ML, Wooten MC. Use of the open field maze to measure locomotor and anxiety-like behavior in mice. J Vis Exp 2015(96):e52434; doi: 10.3791/52434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Walsh RN, Cummins RA. The open-field test: A critical review. Psychol Bull 1976;83(3):482–504. [PubMed] [Google Scholar]
- 53. Stanford SC. The open field test: Reinventing the wheel. J Psychopharmacol 2007;21(2):134–135; doi: 10.1177/0269881107073199 [DOI] [PubMed] [Google Scholar]
- 54. Rotheneichner P, Romanelli P, Bieler L, et al. Tamoxifen activation of cre-recombinase has no persisting effects on adult neurogenesis or learning and anxiety. Front Neurosci 2017;11:27; doi: 10.3389/fnins.2017.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Soriano D, Brusco A, Caltana L. Further evidence of anxiety- and depression-like behavior for total genetic ablation of cannabinoid receptor type 1. Behav Brain Res 2021;400:113007; doi: 10.1016/j.bbr.2020.113007 [DOI] [PubMed] [Google Scholar]
- 56. Lutz B, Häring M, Enk V, et al. Cannabinoid type-1 receptor signaling in central serotonergic neurons regulates anxiety-like behavior and sociability. Front Behav Neurosci 2015;9:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Urigüen L, Pérez-Rial S, Ledent C, et al. Impaired action of anxiolytic drugs in mice deficient in cannabinoid CB1 receptors. Neuropharmacology 2004;46(7):966–973; doi: 10.1016/j.neuropharm.2004.01.003 [DOI] [PubMed] [Google Scholar]
- 58. Li J, Wang H, Liu D, et al. CB2R activation ameliorates late adolescent chronic alcohol exposure-induced anxiety-like behaviors during withdrawal by preventing morphological changes and suppressing NLRP3 inflammasome activation in prefrontal cortex microglia in mice. Brain Behav Immun 2023;110:60–79; doi: 10.1016/j.bbi.2023.02.001 [DOI] [PubMed] [Google Scholar]
- 59. García-Gutiérrez MS, Manzanares J. Overexpression of CB2 cannabinoid receptors decreased vulnerability to anxiety and impaired anxiolytic action of alprazolam in mice. J Psychopharmacol 2011;25(1):111–120; doi: 10.1177/0269881110379507 [DOI] [PubMed] [Google Scholar]
- 60. Almeida-Santos AF, Gobira PH, Rosa LC, et al. Modulation of anxiety-like behavior by the endocannabinoid 2-arachidonoylglycerol (2-AG) in the dorsolateral periaqueductal gray. Behav Brain Res 2013;252:10–17; doi: 10.1016/j.bbr.2013.05.027 [DOI] [PubMed] [Google Scholar]
- 61. Ishiguro H, Horiuchi Y, Tabata K, et al. Cannabinoid CB2 receptor gene and environmental interaction in the development of psychiatric disorders. Molecules 2018;23(8):724; doi: 10.3390/molecules23081836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Goldstein JM, Kennedy DN, Caviness VS. Images in neuroscience. Brain development, XI: sexual dimorphism. Am J Psychiatry 1999;156(3):352; doi: 10.1176/ajp.156.3.352 [DOI] [PubMed] [Google Scholar]
- 63. Lebron-Milad K, Milad MR. Sex differences, gonadal hormones and the fear extinction network: Implications for anxiety disorders. Biol Mood Anxiety Disord 2012;2:3; doi: 10.1186/2045-5380-2-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Marsicano G, Kuner R.. Anatomical Distribution of Receptors, Ligands and Enzymes in the Brain and in the Spinal Cord: Circuitries and Neurochemistry. In: Cannabinoids and the Brain (Kofalvi A. ed.) Springer Science+Business Media: New York, New York; 2008; pp. 161–743. [Google Scholar]
- 65. Katona I, Freund TF. Multiple functions of endocannabinoid signaling in the brain. Annu Rev Neurosci 2012;35:529–558; doi: 10.1146/annurev-neuro-062111-150420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Montague D, Weickert CS, Tomaskovic-Crook E, et al. Oestrogen receptor alpha localisation in the prefrontal cortex of three mammalian species. J Neuroendocrinol 2008;20(7):893–903; doi: 10.1111/j.1365-2826.2008.01743.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Spencer JL, Waters EM, Romeo RD, et al. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol 2008;29(2):219–237; doi: 10.1016/j.yfrne.2007.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology 2006;31(6):1097–1111; doi: 10.1038/sj.npp.1301067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kokras N, Krokida S, Varoudaki TZ, et al. Do corticosterone levels predict female depressive-like behavior in rodents? J Neurosci Res 2021;99(1):324–331; doi: 10.1002/jnr.24686 [DOI] [PubMed] [Google Scholar]
- 70. Kwa M, Plottel CS, Blaser MJ, et al. The intestinal microbiome and estrogen receptor-positive female breast cancer. J Natl Cancer Inst 2016;108(8):djw029; doi: 10.1093/jnci/djw029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Menon R, Watson SE, Thomas LN, et al. Diet complexity and estrogen receptor? Status affect the composition of the murine intestinal microbiota. Appl Environ Microbiol 2013;79(18):5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kaliannan K, Robertson RC, Murphy K, et al. Estrogen-mediated gut microbiome alterations influence sexual dimorphism in metabolic syndrome in mice. Microbiome 2018;6:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Shin JH, Park YH, Sim M, et al. Serum level of sex steroid hormone is associated with diversity and profiles of human gut microbiome. Res Microbiol 2019;170(4–5):192–201; doi: 10.1016/j.resmic.2019.03.003 [DOI] [PubMed] [Google Scholar]
- 74. Yurkovetskiy L, Burrows M, Khan AA, et al. Gender bias in autoimmunity is influenced by microbiota. Immunity 2013;39(2):400–412; doi: 10.1016/j.immuni.2013.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sampson TR, Mazmanian SK. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe 2015;17(5):565–576; doi: 10.1016/j.chom.2015.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sykaras AG, Demenis C, Case RM, et al. Duodenal enteroendocrine I-cells contain mRNA transcripts encoding key endocannabinoid and fatty acid receptors. PLoS One 2012;7(8):e42373; doi: 10.1371/journal.pone.0042373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Clemmensen C, Muller TD, Woods SC, et al. Gut-brain cross-talk in metabolic control. Cell 2017;168(5):758–774; doi: 10.1016/j.cell.2017.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Forsythe P, Sudo N, Dinan T, et al. Mood and gut feelings. Brain Behav Immun 2010;24(1):9–16; doi: 10.1016/j.bbi.2009.05.058 [DOI] [PubMed] [Google Scholar]
- 79. Klarer M, Arnold M, Günther L, et al. Gut vagal afferents differentially modulate innate anxiety and learned fear. J Neurosci 2014;34(21):7067–7076; doi: 10.1523/JNEUROSCI.0252-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Maniscalco JW, Zheng H, Gordon PJ, et al. Negative energy balance blocks neural and behavioral responses to acute stress by “Silencing” central glucagon-like peptide 1 signaling in rats. J Neurosci 2015;35(30):10701–10714; doi: 10.1523/JNEUROSCI.3464-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data that support the findings of this study are available in the figures of this article.