Abstract
Objective
To examine the role of patient‐perceived access to primary care in mediating and moderating racial and ethnic disparities in hypertension control and diabetes control among Veterans Health Administration (VA) users.
Data Source and Study Setting
We performed a secondary analysis of national VA user administrative data for fiscal years 2016–2019.
Study Design
Our primary exposure was race or ethnicity and primary outcomes were binary indicators of hypertension control (<140/90 mmHg) and diabetes control (HgbA1c < 9%) among patients with known disease. We used the inverse odds‐weighting method to test for mediation and logistic regression with race and ethnicity‐by‐perceived access interaction product terms to test moderation. All models were adjusted for age, sex, socioeconomic status, rurality, education, self‐rated physical and mental health, and comorbidities.
Data Collection/Extraction Methods
We included VA users with hypertension and diabetes control data from the External Peer Review Program who had contemporaneously completed the Survey of Healthcare Experience of Patients‐Patient‐Centered Medical Home. Hypertension (34,233 patients) and diabetes (23,039 patients) samples were analyzed separately.
Principal Findings
After adjustment, Black patients had significantly lower rates of hypertension control than White patients (75.5% vs. 78.8%, p < 0.01); both Black (81.8%) and Hispanic (80.4%) patients had significantly lower rates of diabetes control than White patients (85.9%, p < 0.01 for both differences). Perceived access was lower among Black, Multi‐Race and Native Hawaiian and Other Pacific Islanders compared to White patients in both samples. There was no evidence that perceived access mediated or moderated associations between Black race, Hispanic ethnicity, and hypertension or diabetes control.
Conclusions
We observed disparities in hypertension and diabetes control among minoritized patients. There was no evidence that patients' perception of access to primary care mediated or moderated these disparities. Reducing racial and ethnic disparities within VA in hypertension and diabetes control may require interventions beyond those focused on improving patient access.
Keywords: access to primary care; diabetes mellitus; health disparate, minority, and vulnerable populations; hypertension; mediation analysis; veterans health services
What is known on this topic
There are documented racial and ethnic disparities in hypertension and diabetes control among minoritized groups, including in the Veterans Health Administration (VA).
Racial and ethnic disparities in primary care access have also been documented, however, the role that perceived access plays in disparities in chronic disease control remains unknown.
What this study adds
This study identified persistent disparities in hypertension and diabetes control for Black and both Black and Hispanic (respectively) VA users who sought primary care at VA clinics, compared to non‐Hispanic White patients.
There was no evidence that perceived access mediated or moderated racial and ethnic disparities in hypertension and diabetes control.
1. INTRODUCTION
Disparities in chronic disease control between racially and ethnically minoritized (hereafter referred to as “minoritized”) and non‐Hispanic White (hereafter “White”) primary care patients in the United States are well‐documented. For example, the prevalence of hypertension and diabetes mellitus (hereafter “diabetes”) has consistently been found to be greater among non‐Hispanic Black (hereafter “Black”) and Hispanic populations versus White patients, while markers of quality of care and control of cardiovascular risk factors are consistently lower in these minoritized groups. 1 This likely contributes to reduced life expectancy among Black patients. 2 There are many overlapping factors that contribute to such disparities, with a recent dedicated focus on the social determinants of health. To improve the quality of primary care and outcomes for patients and narrow these disparities, healthcare systems must devise innovative, evidence‐based, and adequately resourced interventions that address the social determinants of health. 3 , 4
One element of the social determinants of health that could be targeted and improved to narrow racial and ethnic disparities in healthcare quality is access to primary care services. 5 , 6 Adequate access to primary care, with access defined broadly as the opportunity for patients and their communities to use appropriate healthcare services in proportion to their needs, 7 has been associated with improved healthcare quality and outcomes, including improved patient satisfaction via continuity of care, 8 reduced use of emergency services and high‐cost care, 9 improved self‐rated health, and lower mortality. 10 Though financial obstacles constitute major access barriers to healthcare use in many settings, even users of healthcare settings with minimal financial barriers to care, such as the Veterans Health Administration (VA), experience access barriers related to other social determinants of health. 11 , 12 Social determinants of health have been linked to adverse health outcomes among healthcare users in these settings. 13 , 14
While healthcare access is fundamental to health systems operations, it is an inherently challenging concept to define and has garnered various interpretations over time. 7 , 15 One recently developed, comprehensive, and increasingly used framework through which to conceptualize access is the conceptual model for access to health developed by Jean‐Frederic Levesque et al 7 , 16 (Figure 1). In brief, in this framework, access is determined as the opportunity to identify healthcare needs, to seek services, and to obtain services sought. Along this continuum, dimensions of accessibility are defined as approachability, acceptability, availability/accommodation, affordability, and appropriateness. Meanwhile, it accounts for the corresponding ability of populations to interact with these dimensions including abilities to perceive, seek, reach, pay for, and engage with care. It therefore can concurrently account for health systems and patients' perspectives in considering facilitators and barriers to healthcare access.
FIGURE 1.
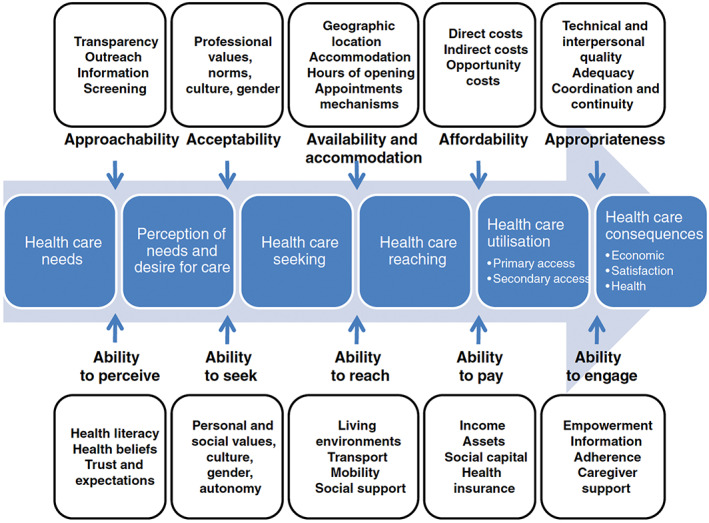
The conceptual framework for patient‐centered access to health care, as developed by Levesque et al.7 (Reprinted with permission from Biomed Central; Levesque J‐F, Harris MF, Russell G. Patient‐centered access to health care: conceptualizing access at the interface of health systems and populations. International Journal for Equity in Health 2013;12:18). [Color figure can be viewed at wileyonlinelibrary.com]
The Levesque framework also provides a helpful model for conceptualizing racial and ethnic disparities in primary care access. 17 Prior research has demonstrated that racially and ethnically minoritized individuals, including Black, Hispanic, and American Indian or Alaska Native (AIAN) groups, are less able to access necessary medical care. 18 , 19 In reference to the Levesque framework, this is due to gaps in insurance coverage (affordability/ability to pay), cost‐related barriers (affordability/ability to pay) and not having usual source of care (availability and accommodation/ability to reach). 18 , 19 These systemic issues are rooted in structurally racist factors that influence neighborhood investment and resultant available healthcare infrastructure, such that neighborhoods with predominantly minority populations have fewer and lower‐quality healthcare services available (availability and accommodation). 20 , 21
In addition to inequities by race and ethnicity in primary care access, as indicated by measures of insurance coverage, there are also documented gaps in perceptions of primary care access as a component of patient care experience. 22 , 23 , 24 , 25 Using the Levesque framework, perception of access correlates with the upstream domain of perception of needs and desire for care, the dimensions of accessibility of approachability, acceptability and availability/accommodation, and the population interaction dimensions of ability to perceive, ability to seek, and ability to reach. 7 Perceived access to primary care has been associated with positive health behaviors including improved self‐care, access to necessary mental health services, and shared decision‐making with providers. 26 , 27 , 28 Literature suggests that Black Medicare and Medicaid beneficiaries have lower ratings of perceived primary care access compared to White patients. 23 , 24 , 25 , 29 , 30 Although the VA is the nation's largest integrated, single‐payer healthcare system and has low barriers to access for eligible beneficiaries, 31 in an analysis of 2010 VA Survey of Healthcare Experiences of Patients (SHEP) data, Black and Hispanic patients had significantly lower patient experience ratings of perceived access to primary care, namely “getting needed care” and “getting care quickly”. 22 Thus, lower satisfaction in primary access among minoritized patients may serve as an impediment to engagement in care and have negative downstream consequences. Theoretically, interventions to improve primary care access for minoritized patients could in turn lead to improved outcomes for these populations and reduce disparities in chronic disease control. The Levesque framework could be helpful in facilitating researchers' ability to isolate and test the impacts of specific dimensions of access in understanding this relationship.
In this study, our objective was to determine whether access, as measured by patients' perceptions of access to primary care services, mediated racial and ethnic disparities in hypertension and diabetes control and whether access to primary care modified the magnitudes of disparities in hypertension and diabetes control among different racial and ethnic groups. Hypertension and diabetes control in patients with known disease could be especially dependent on access (i.e., patients would need regular access to primary care to titrate their antihypertensive and antihyperglycemic agents to achieve disease control), and therefore we focused on these quality measures in this analysis. The VA is an ideal environment in which to address this question as it provides lower cost‐sharing for eligible beneficiaries, 32 which effectively controls for the “affordability” and “ability to pay” dimensions of the Levesque framework. 7 To conduct this study, we linked data files containing measures of VA beneficiaries' assessment of access from the SHEP‐patient‐centered medical home (SHEP‐PCMH) survey and clinical care quality data at the beneficiary level. We first tested racial and ethnic differences in diabetes and hypertension control among VA beneficiaries. We then tested whether access, as measured by patient perception of access, mediated or moderated the association between race and ethnicity and hypertension and diabetes control. We hypothesized that patient‐perceived access mediates the association between race and ethnicity and hypertension and diabetes control especially for larger minoritized racial and ethnic groups in the VA (i.e., Black and Hispanic) groups, such that being Black or Hispanic is associated with having lower perceived access than Whites, which is, in turn, associated with having poorer hypertension/diabetes control than Whites. In addition, we hypothesized that the magnitude of the association between race and ethnicity and hypertension and diabetes control changes as a function of access, such that higher levels of access are associated with reduced associations between race/ethnicity and hypertension/diabetes control (i.e., access moderates this relationship).
2. METHODS
2.1. Design and data sources
We linked data from two sources: the VA SHEP‐PCMH survey and the VA External Peer Review Program (EPRP) from fiscal year 2016 through fiscal year 2019. The SHEP is an annual survey of a healthcare system‐stratified random sample of 600,000 VA patients who are 18 years or older, who have seen a Patient Aligned Care Team (PACT, the VA model of the PCMH 33 ) provider at least once in the previous 10 months. 34 It is administered by the VA Office of Quality and Patient Safety. Data were anonymized prior to our accessing of records. The overall response rate for the SHEP surveys was 38%. Analytic weights were derived to account for both the sampling design characteristics and non‐response. The EPRP is a validated tool for tracking VA quality performance metrics derived from VA electronic medical record data. 35 It incorporates data from a sample of Veterans who had been enrolled in the VA for at least 2 years and who had at least one primary care visit in the previous 13–24 months.
We limited our linked analytic sample to participants for whom we had SHEP survey data contemporaneous to or prior to their EPRP data. This was to avoid including participants for whom we had obtained measures of chronic disease control prior to obtaining their perceived access scores. We then linked PCMH‐SHEP and EPRP data to VA Corporate Data Warehouse (CDW) electronic health record data to obtain measures of participant demographic and clinical variables, including socioeconomic status and comorbidity. We derived the remaining covariates (described below) from SHEP variables.
This project received a Determination of Non‐Research from the VA Greater Los Angeles Healthcare System Institutional Review Board.
2.2. Dependent variables
Our dependent measures were hypertension control and diabetes control. Hypertension control was defined as blood pressure of 140/90 mmHg or less. Diabetes control was defined as a glycosylated hemoglobin of 9% or less. These quality metrics were adopted from the Healthcare Effectiveness Data and Information Set, a common performance improvement tool for U.S. healthcare. 36 Trained EPRP reviewers abstracted data from charts of the sampled patients to determine if diabetes or hypertension control had been achieved.
2.3. Independent variable
Our primary predictor of interest was patient race and ethnicity. Patient race and ethnicity were obtained from the CDW, and for those with missing CDW race or ethnicity, from the VA's Observational Medical Outcomes Partnership data model. Race and ethnicity data from these sources are typically self‐reported and are missing for 3.2% overall. 37 Race and ethnicity were assessed separately, and then combined into one measure where individuals who identified as Hispanic ethnicity were categorized as Hispanic, and non‐Hispanic individuals were categorized by race. Patient race and ethnicity were categorized as AIAN, Asian, Black, Hispanic, Multi‐Race, Native Hawaiian or Other Pacific Islander (NHOPI), and non‐Hispanic White.
2.4. Mediator and moderator variable
The mediator and moderator variable of interest was the patient's perception of access to their PCMH. In the SHEP‐PCMH survey, there are three questions designed to assess access: (1) In the last 6 months, when you contacted this provider's office to get an appointment for care you needed right away, how often did you get an appointment as soon as you needed? (2) In the last 6 months, when you made an appointment for a check‐up or routine care with this provider, how often did you get an appointment as soon as you needed? (3) In the last six months, when you contacted this provider's office during regular office hours, how often did you get an answer to your medical question that same day? These three questions align with the dimension of “availability and accommodation” in the Levesque framework. 7 The survey has the following response scale: never, sometimes, usually, always. The score for access for each respondent was then calculated as the percentage of responses that fall in the “always” category (the most positive category), in accordance with the calculation of patient experience domain scores in the Consumer Assessment of Healthcare Providers (CAHPS) surveys. 38 If a respondent did not respond to a component question, this question would be removed from the denominator, so that scores were calculated based on only the questions answered.
2.5. Covariates
We assessed for covariates that aligned with upstream determinants of access in the Levesque framework. 7 These included the domains of “health care needs” and “perceptions of needs and desire for care,” and patients' “abilities to perceive” and “seek” healthcare. We incorporated urban/rural residential status (additionally associated with the domain of “availability”), socioeconomic status based on VA enrollment priority group income thresholds for copayment, 13 highest education achieved (less than eighth grade, some high school, some/2 year college, college graduation, post‐graduate degree), Gagne comorbidity score, 39 and self‐rated physical health and mental health (excellent, very good, good, fair, or poor), in addition to standard demographic covariates of age (categorized as 18–44, 45–64, 65 and older) and sex. To account for the fact that our comorbidity score depends on medical record documentation which may vary independently by race and ethnicity group, and ratings of physical and mental health may be independently associated with chronic disease control, we performed sensitivity analyses excluding, then including, these covariates.
2.6. Statistical analysis
First, we calculated weighted descriptive statistics on the analytic sample. The distributions of covariates were compared between racial/ethnic groups by chi‐square test for categorical variables or t‐test for continuous variables. We performed weighted unadjusted linear regression models to compare access scores between different racial and ethnic groups.
Next, we determined if race and ethnicity were associated with our primary outcomes of interest by testing weighted logistic regression models to compare rates of diabetes and hypertension control among different racial and ethnic groups, initially in unadjusted models and then while adjusting for the above covariates as well as patient‐perceived access.
2.7. Tests of mediation by perceived access
Through our mediation analysis, we decomposed the total effects of race and ethnicity on diabetes and hypertension control into the direct effects of race and ethnicity and the indirect effects of race and ethnicity mediated by patient perception of access. This was achieved using the following technique. We first estimated the total effects of race and ethnicity on diabetes and hypertension control through logistic regression models controlling for the above covariates and the mediator variable of patient perception of access. Next, we estimated the direct and indirect effects of race and ethnicity on diabetes and hypertension control using the Tchetgen Tchetgen's inverse odds weighting (IOW) approach. 40 , 41 IOWs were derived from polytomous regression models estimating the relationship between race and ethnicity and patient‐perceived access with covariate adjustment by using the regression coefficients to determine an IOW for each racial and ethnic minoritized patient observation. Each White patient observation was given an IOW of 1, thus designating it as a “control.” We then fitted IOW logistic regression models to estimate the direct effect of race and ethnicity on diabetes and hypertension control while controlling for the above covariates. Next, we calculated indirect effects by subtracting the direct effect coefficients from the total effects coefficients for the racial and ethnic minoritized groups and used bootstrapping methods to derive standard errors. We considered any indirect effects at p < 0.05 as evidence of mediation.
2.8. Tests of moderation by perceived access
To determine if the racial and ethnic group disparities in hypertension control and diabetes control differed across levels of perceived access, we fitted separate logistic regression models with product terms for race and ethnicity‐by‐access score, controlling for the previously identified covariates We considered product terms for each race and ethnicity category to be significant at p < 0.05. To estimate the magnitude of potential moderation by access, we report differences between minoritized and White racial and ethnic groups in transformed estimates of hypertension and diabetes control for those at a hypothetical perceived access score of 0%, and differences between minoritized and White groups in hypertension and diabetes control for those at a hypothetical perceived access score of 100%.
3. RESULTS
Overall, there were 34,233 patients included in the hypertension sample and 23,039 patients included in the diabetes sample (13,799 patients were present in both samples). The demographic and health characteristics of the sample are displayed in Tables 1 and 2. In both samples, White patients tended to be older and have higher Gagne comorbidity scores than minoritized patients. Asian patients were most likely to live in urban areas and had the highest rates of college or post‐graduate degrees.
TABLE 1.
Patient characteristics for hypertension sample (n = 34,233), by race and ethnicity group.
| Characteristic (%) | AIAN (n = 288) | Asian (n = 222) | Black (n = 4265) | Hispanic (n = 1484) | Multi‐Race (n = 275) | NHOPI (n = 287) | White (n = 26,099) | p‐value |
|---|---|---|---|---|---|---|---|---|
| Age | <0.001 | |||||||
| 18–44 | 14.6 | 18.6 | 8.9 | 12.6 | 3.3 | 6.9 | 4.4 | |
| 45–64 | 45.4 | 46.4 | 59.2 | 44.4 | 54.7 | 63.3 | 35.4 | |
| 65+ | 40.0 | 32.0 | 31.8 | 43.1 | 42.0 | 29.8 | 60.2 | |
| Female | 15.5 | 21.1 | 29.3 | 11.5 | 23.3 | 19.0 | 12.7 | <0.001 |
| Urban (vs. Rural) | 53.1 | 90.3 | 82.7 | 83.1 | 67.1 | 74.2 | 56.0 | <0.001 |
| SES | <0.001 | |||||||
| High | 8.4 | 5.3 | 7.6 | 8.0 | 9.2 | 9.2 | 14.1 | |
| Low | 19.7 | 7.0 | 19.3 | 19.5 | 18.4 | 17.8 | 19.7 | |
| Indeterminate | 71.9 | 87.8 | 73.1 | 72.6 | 72.5 | 73.0 | 66.2 | |
| Education | <0.001 | |||||||
| 8th grade or less | 0.7 | 0.0 | 0.5 | 2.3 | 0.2 | 0.3 | 1.3 | |
| Some HS | 2.9 | 0.1 | 3.9 | 1.5 | 1.7 | 1.4 | 4.0 | |
| HS grad/GED | 25.2 | 12.3 | 25.8 | 4.4 | 23.6 | 24.5 | 30.5 | |
| Some college | 46.6 | 47.4 | 46.3 | 46.9 | 47.6 | 46.3 | 43.7 | |
| College grad | 11.6 | 17.4 | 11.4 | 11.3 | 10.6 | 14.4 | 10.4 | |
| Post‐grad deg | 13.1 | 22.8 | 12.2 | 11.6 | 16.4 | 13.3 | 10.1 | |
| Self‐rated physical health | <0.001 | |||||||
| Excellent | 3.5 | 6.1 | 4.3 | 7.1 | 1.9 | 2.6 | 3.4 | |
| Very Good | 13.0 | 8.5 | 14.5 | 13.2 | 16.2 | 7.6 | 15.3 | |
| Good | 34.0 | 32.7 | 35.6 | 35.1 | 42.5 | 31.5 | 38.5 | |
| Fair | 36.2 | 36.7 | 37.1 | 33.0 | 32.6 | 38.1 | 32.5 | |
| Poor | 13.4 | 16.0 | 8.5 | 11.7 | 6.8 | 20.3 | 10.3 | |
| Self‐rated mental health | <0.001 | |||||||
| Excellent | 10.7 | 15.9 | 11.5 | 11.6 | 13.3 | 15.2 | 16.0 | |
| Very Good | 15.9 | 13.2 | 17.7 | 17.7 | 16.1 | 16.8 | 23.6 | |
| Good | 27.3 | 27.5 | 26.2 | 27.2 | 30.4 | 22.8 | 29.6 | |
| Fair | 40.6 | 29.3 | 31.1 | 31.2 | 29.9 | 29.2 | 23.7 | |
| Poor | 5.6 | 14.1 | 14.6 | 12.3 | 10.3 | 16.1 | 7.3 | |
| Gagne score, mean (SE) | 0.66 (0.16) | 0.40 (0.12) | 0.69 (0.042) | 0.47 (0.072) | 0.82 (0.22) | 0.62 (0.14) | 0.83 (0.018) | <0.001 |
| HTN control | 79.2 | 81.3 | 75.9 | 80.2 | 76.7 | 78.0 | 78.7 | |
| Access score, mean (SE) a | 53.49 (4.36) | 46.18 (5.06) | 49.70 (1.03) | 53.31 (1.97) | 44.97 (4.12) | 43.06 (4.12) | 54.29 (0.42) | <0.001 |
Note: Unknown race or ethnicity for 1313 individuals.
Abbreviations: AIAN, American Indian or Alaska Native; GED, general educational development; HS, high school; HTN, hypertension; NHOPI, Native Hawaiian or Other Pacific Islander; SES, socioeconomic status.
Access score range 0–100, with higher scores indicating greater perceived access.
TABLE 2.
Patient characteristics for diabetes sample (n = 23,039), by race and ethnicity group.
| Characteristic (%) | AIAN (n = 206) | Asian (n = 176) | Black (n = 2902) | Hispanic (n = 1223) | Multi‐Race (n = 189) | NHOPI (n = 239) | White (n = 17,269) | p‐value |
|---|---|---|---|---|---|---|---|---|
| Age | <0.001 | |||||||
| 18–44 | 21.0 | 23.7 | 7.3 | 11.2 | 1.5 | 9.0 | 3.8 | |
| 45–64 | 41.4 | 43.8 | 57.7 | 46.0 | 50.9 | 57.3 | 37.9 | |
| 65+ | 37.7 | 32.6 | 35.0 | 42.9 | 47.6 | 33.7 | 58.4 | |
| Female | 20.7 | 8.4 | 19.3 | 15.2 | 15.8 | 16.3 | 8.1 | <0.001 |
| Urban (vs. Rural) | 57.9 | 88.2 | 82.9 | 84.6 | 70.5 | 79.1 | 56.0 | <0.001 |
| SES | <0.001 | |||||||
| High | 6.5 | 6.0 | 8.3 | 6.6 | 10.5 | 10.5 | 11.8 | |
| Low | 10.4 | 7.7 | 18.8 | 17.3 | 15.3 | 15.1 | 17.8 | |
| Indeterminate | 83.1 | 86.3 | 72.8 | 76.1 | 74.2 | 74.4 | 70.4 | |
| Education | <0.001 | |||||||
| 8th grade or less | 0.6 | 0.0 | 0.2 | 1.0 | 0.0 | 0.3 | 1.0 | |
| Some HS | 1.8 | 0.4 | 3.3 | 3.3 | 1.0 | 1.0 | 3.6 | |
| HS grad/GED | 27.5 | 13.9 | 27.9 | 26.9 | 29.6 | 20.3 | 31.1 | |
| Some college | 44.6 | 45.7 | 47.3 | 47.9 | 46.7 | 48.7 | 44.7 | |
| College grad | 7.9 | 24.7 | 11.7 | 9.7 | 12.0 | 12.7 | 9.8 | |
| Post‐grad deg | 17.5 | 15.3 | 9.6 | 11.2 | 10.6 | 17.0 | 9.8 | |
| Self‐rated physical health | <0.001 | |||||||
| Excellent | 4.0 | 5.1 | 5.1 | 5.0 | 3.0 | 3.3 | 2.5 | |
| Very Good | 7.7 | 8.0 | 12.3 | 9.6 | 10.2 | 10.3 | 12.7 | |
| Good | 29.2 | 33.3 | 33.6 | 36.0 | 40.8 | 29.3 | 37.8 | |
| Fair | 39.9 | 40.6 | 39.2 | 35.8 | 38.3 | 34.9 | 35.7 | |
| Poor | 19.3 | 13.1 | 9.9 | 13.5 | 7.8 | 22.2 | 11.3 | |
| Self‐rated mental health | <0.001 | |||||||
| Excellent | 11.1 | 15.6 | 13.1 | 13.7 | 12.0 | 16.6 | 16.0 | |
| Very Good | 15.7 | 13.3 | 19.2 | 16.8 | 17.8 | 18.3 | 23.4 | |
| Good | 22.1 | 25.6 | 25.2 | 24.9 | 27.1 | 23.1 | 30.2 | |
| Fair | 38.8 | 26.2 | 31.1 | 31.9 | 30.7 | 26.2 | 23.8 | |
| Poor | 12.3 | 19.2 | 11.5 | 12.7 | 12.5 | 15.8 | 6.5 | |
| Gagne score, mean (SD) | 0.86 (0.17) | 0.96 (0.20) | 1.06 (0.057) | 0.93 (0.075) | 1.23 (0.31) | 0.78 (0.19) | 1.12 (0.021) | 0.016 |
| DM control, % | 81.0 | 86.4 | 80.8 | 78.6 | 87.7 | 81.2 | 86.2 | <0.001 |
| Access score %, mean (SE) a | 48.23 (6.88) | 47.40 (6.77) | 49.02 (1.26) | 51.20 (2.43) | 39.20 (4.62) | 43.07 (4.57) | 53.69 (0.51) | <0.001 |
Note: Unknown race or ethnicity for 835 individuals.
Abbreviations: AIAN, American Indian or Alaska Native; GED, general educational development; HS, high school; NHOPI, Native Hawaiian or Other Pacific Islander; SES, socioeconomic status.
Access score range 0–100, with higher scores indicating greater perceived access.
In the hypertension sample, compared to White patients, Black patients had a significantly lower unadjusted rate of disease control (Table 1, Appendix Table 1). In the diabetes sample, compared to White patients, Black and Hispanic patients had significantly lower unadjusted rates of disease control (Table 2, Appendix Table 1).
Mean perceived access scores range from 43.1% to 54.3% in the hypertension sample and from 39.2% to 53.7% in the diabetes sample (Tables 1 and 2). They were highest among White patients in both samples. In both samples, compared to White patients, perceived access scores were significantly lower among Black, Multi‐Race, and NHOPI patients.
3.1. Racial and ethnic differences in hypertension and diabetes control
Adjusted rates of hypertension and diabetes control by race and ethnicity are displayed in Table 3. Compared to White patients, Black patients had statistically significant lower rates of hypertension (75.5% vs. 78.8%, rate difference −3.4 percentage points [pp], p = 0.0022) and diabetes control (81.8% vs. 85.9%, rate difference − 4.0 pp, p = 0.0011). For Hispanic patients, the rate difference compared to White patients was statistically significant for the diabetes sample (80.4% vs. 85.9%, rate difference −5.4 pp, p = 0.0064). Otherwise, differences between White and other racial and ethnic groups were not statistically significant. Associations between covariates and chronic disease control are displayed in Appendix Tables 3 and 4.
TABLE 3.
Adjusted chronic disease control rates by patient race and ethnicity, with comparison to rates for White patients.
| Race or ethnicity | HTN Control | DM Control | ||||
|---|---|---|---|---|---|---|
| Adjusted rate (95% CI) | Rate difference compared to White (pp) (95% CI) | Rate difference p‐value | Adjusted rate (95% CI) | Rate difference compared to White (pp) (95% CI) | Rate difference p‐value | |
| AIAN | 79.4 (71.1, 87.7) | +0.5 (−7.8, 8.9) | 0.90 | 81.2 (69.0, 93.4) | −4.7 (−16.9, 7.6) | 0.46 |
| Asian | 82.0 (74.2, 89.8) | +3.1 (−4.8, 11.1) | 0.44 | 88.7 (79.8, 97.6) | +2.8 (−6.1, 11.8) | 0.54 |
| Black | 75.5 (73.6, 77.4) | −3.4 (−5.5, −1.2) | 0.0022 | 81.8 (79.6, 84.1) | −4.0 (−6.5, −1.6) | 0.0011 |
| Hispanic | 80.0 (76.5, 83.4) | +1.1 (−2.4, 4.7) | 0.53 | 80.4 (76.6, 84.2) | −5.4 (−9.3, −1.5) | 0.0064 |
| Multi‐Race | 77.4 (69.7, 85.1) | −1.4 (−9.2, 6.3) | 0.72 | 87.9 (82.1, 93.8) | +2.1 (−3.8, 8.0) | 0.49 |
| NHOPI | 79.3 (72.3, 86.3) | +0.4 (−6.6, 7.5) | 0.90 | 82.2 (73.8, 90.6) | −3.7 (−12.1, 4.8) | 0.39 |
| White | 78.8 (78.0, 79.7) | ‐ | ‐ | 85.9 (85.0, 86.7) | ‐ | ‐ |
Note: Models adjusted for age, sex, socioeconomic status, urban/rural status, education, self‐rated physical and mental health, Gagne comorbidity score, and access. 95% CI derived from standard errors clustered at healthcare system level.
Abbreviations: AIAN, American Indian or Alaska Native; DM, diabetes mellitus; HTN, hypertension; NHOPI, Native Hawaiian or Other Pacific Islander; pp, percentage point.
3.2. Tests of mediation by perceived access
While perceived access scores and chronic disease control were significantly lower among Black, compared to White, patients, there was no evidence that the disparities in hypertension and diabetes control were mediated by access. In the hypertension sample, the tests of the indirect effects indicated that there was no evidence that the Black/White disparity in hypertension control was mediated by perceived access. There was also no evidence that the Black/White and Hispanic/White disparities in diabetes control were mediated by access.
3.3. Tests of moderation by perceived access
There was no evidence of modification of the association of Black/White or Hispanic/White race or ethnicity and disease control by perceived access (Table 4).
TABLE 4.
Adjusted moderation of access on chronic disease control for racial and ethnic groups compared to White patients.
| Race or ethnicity × access score product term p‐value | Estimated disease control rate with access = 0% (95% CI) | Delta control rate compared to White at access = 0% (pp) (95% CI) | Estimated control rate with access = 100% (95% CI) | Delta control rate compared to White at access = 100% (pp) (95% CI) | |
|---|---|---|---|---|---|
| HTN Control | |||||
| AIAN | 0.51 | 75.2 (58.8, 91.5) | −2.7 (−19.1, 13.7) | 83.2 (75.3, 91.0) | 3.4 (−4.5, 11.3) |
| Asian | 0.59 | 83.2 (73.7, 92.7) | 5.4 (−4.2, 15.0) | 80.2 (66.2, 94.2) | 0.4 (−13.6, 14.5) |
| Black | 0.60 | 75.0 (72.0. 77.9) | −2.9 (−6.2, 0.4) | 75.9 (73.2, 78.5) | −3.9 (−6.8, −1.0) |
| Hispanic | 0.11 | 75.5 (69.2, 81.8) | −2.3 (−8.8, 4.2) | 83.9 (79.9, 87.9) | 4.1 (−0.1, 8.3) |
| Multi‐Race | 0.36 | 72.8 (59.5, 86.2) | −5.0 (−18.4, 8.4) | 82.4 (74.0, 90.8) | 2.6 (−5.9, 11.1) |
| NHOPI | 0.23 | 82.2 (72.8, 91.6) | 4.4 (−5.2, 13.9) | 75.1 (64.5, 85.7) | −4.7 (−15.3, 6.0) |
| White | Ref | 77.8 (76.6, 79.1) | ‐ | 79.8 (78.6, 80.9) | ‐ |
| DM Control | |||||
| AIAN | 0.11 | 71.9 (48.5, 95.2) | −13.2 (−36.6, 10.2) | 91.5 (84.7, 98.4) | 4.9 (−2.1, 11.8) |
| Asian | 0.28 | 83.9 (68.0, 99.8) | −1.2 (−17.1, 14.8) | 93.6 (87.4, 99.9) | 7.0 (0.5, 13.4) |
| Black | 0.64 | 80.2 (76.6, 83.8) | −4.8 (−8.7, −1.0) | 83.4 (80.7, 86.1) | −3.3 (−6.3, −0.3) |
| Hispanic | 0.82 | 78.9 (72.7, 85.0) | −6.2 (−12.4, 0.1) | 81.9 (76.8, 87.1) | −4.7 (−10.0, 0.5) |
| Multi‐Race | 0.98 | 87.3 (78.7, 95.8) | 2.2 (−6.4, 10.9) | 88.5 (79.1, 97.8) | 1.8 (−7.6, 11.3) |
| NHOPI | 0.31 | 85.4 (74.4, 96.4) | 0.4 (−10.7, 11.5) | 77.6 (63.3, 91.8) | −9.1 (−23.4, 5.2) |
| White | Ref | 85.0 (83.7, 86.3) | ‐ | 86.7 (85.5, 87.8) | ‐ |
Note: Models adjusted for age, sex, socioeconomic status, urban/rural status, education, self‐rated physical and mental health, Gagne comorbidity score, and access.
Abbreviations: AIAN, American Indian or Alaska Native; DM, diabetes mellitus; HTN, hypertension, NHOPI, Native Hawaiian or Other Pacific Islander; pp, percentage points.
3.4. Sensitivity analyses
We tested the sensitivity of our results to the inclusion and exclusion of health status variables in the model specification (including self‐rated physical and mental health and Gagne comorbidity score) by removing these variables from the chronic disease control, mediation, and moderation models, and rerunning the analyses. The results of these analyses did not change notably with the removal of these covariates (Appendix Tables 2 and 5).
4. DISCUSSION
In our study, we sought to expand upon prior research demonstrating racial and ethnic disparities in hypertension and diabetes control among VA beneficiaries who receive VA‐based primary care 32 , 42 and to explore the role of patient‐perceived access in these disparities. We found that Black patients experienced lower rates of hypertension control, and Black and Hispanic patients experienced lower rates of diabetes control, compared to White patients. We found no evidence that patient‐perceived access mediated these disparities or modified the magnitudes of these disparities. Overall, our findings suggest that patient perception of access minimally affects Black and Hispanic versus White disparities in hypertension control and diabetes control among VA primary care users.
To our knowledge, this is among the first studies to examine the role of patient‐perceived access in racial and ethnic disparities in chronic disease control in the VA. We found no evidence that patient‐perceived access was a mediator or moderator of the association between race or ethnicity and hypertension and diabetes control for Black and Hispanic patients. This does not support our hypothesis that racial and ethnic disparities in access might lie on the causal pathway between race or ethnicity and chronic disease control. It is possible that with the high “ceiling” of access that the VA provides its beneficiaries, any marginal improvement in access, from PACT models or other interventions, will have limited ability to impact disparities in chronic disease control. However, we did note differences by race and ethnicity in patient‐perceived access scores, though the absolute differences were small and of unknown clinical significance. This is consistent with prior literature suggesting lower perceived access to outpatient care among Black and Hispanic VA beneficiaries. 22 This is also similar to findings of disparities in perceived access outside the VA, 23 , 24 , 25 , 29 , 30 despite the fact that the VA paradigm for care delivery is inherently designed to reduce barriers to access. 31 It is possible that patient‐perceived access plays a role in mediating the association between race, ethnicity, and chronic disease control in non‐VA‐delivered care. Additional studies could use a similar approach to investigate this research question and compare the magnitude of these associations between VA and non‐VA care.
In reference to the Levesque conceptual framework of access to healthcare, the questions in the SHEP‐PCMH survey related to access correspond with the dimension of “availability and accommodation”. 7 Therefore, the use of the SHEP‐PCMH derived measures of access may have limited utility in assessing other dimensions of accessibility and the ability of populations to interact with these dimensions. For example, the dimensions of “approachability” and “acceptability” are not directly assessed in the SHEP‐PCMH survey. We attempted to control for the “affordability”/“ability to pay” dimensions of access by studying our question in the VA, which generally offers reduced financial obstacles to eligible beneficiaries, especially those at lower socioeconomic status. 31 , 32 However, it is possible that these other dimensions of access do play a role in mediating racial and ethnic disparities in hypertension and diabetes control for minoritized groups in the VA. There may also be unmeasured additional mediators that operate simultaneously with access or sequentially following access that mediate the association between race and ethnicity and chronic disease control along with perceived access. Future research into the role of healthcare access in racial and ethnic disparities in hypertension and diabetes control could use the Levesque framework to design measurement instruments that interrogate specific dimensions of access. 16
As was the case in prior similar studies, 32 , 42 we found that implementation of PACT models at the VA did not eliminate Black/White and Hispanic/White disparities in diabetes control. It is possible that aspects of the PACT model beyond access (e.g., embedded pharmacists, nursing support for complex patients) have made positive contributions toward narrowing these disparities. In a study by Leung et al., the authors used effect decomposition analytic techniques to determine measured (e.g., by socioeconomic status, comorbidity) versus unmeasured factors contributing to Black and Hispanic versus White disparities in hypertension and diabetes control and found that most disparities were due to unmeasured factors. 32 Leung et al. hypothesized these unmeasured factors could include differential community and healthcare experiences, such as discrimination, implicit bias, communication disparities, or potential experience with access. Experience with access may have been compromised by an overall increase in the volume of patients that may have overwhelmed the staffing capabilities of PACTs and reduced PACT access. 42 The findings from our mediation analysis suggest that perceived “availability”, 7 as measured by the access components of the SHEP‐PCMH survey, does not contribute to these unexplained contributors.
In our study, we did not find evidence of disparities in hypertension and diabetes control in smaller racial and ethnic groups in the VA, including AIAN, Asian, Multi‐Race, and NHOPI individuals. Given the small number of participants included in our samples, we had limited power to detect differences. There is a dearth of research on these understudied racial and ethnic groups in the VA, with some studies suggesting lower quality of care and worse outcomes, 13 , 42 , 43 , 44 , 45 , 46 and others suggesting improved outcomes 13 , 45 , 46 for these patients. Reporting the outcomes of quality improvement interventions by race and ethnicity is warranted to ensure that progress is made toward health equity in these understudied groups.
4.1. Limitations
First, we relied on patient‐perceived access to care as our primary mediator variable, rather than a behavioral measure of healthcare access (e.g., number of primary care visits). However, patient‐perceived access is considered to be a reliable proxy measure for access to the healthcare services that a patient requires as per accepted frameworks for healthcare access. 7 , 28 , 47 , 48 Further, using a behavioral measure, such as the number of PACT visits, would be imprecise as we could not determine the indication for such visits in our data. Feasibly, a beneficiary could easily access care at their PACT providers for certain conditions (e.g., urgent care, mental health care) but have challenges accessing care to discuss chronic disease control. Second, the overall response rate to the survey was 38%, which is similar to other national patient experience surveys. 49 , 50 This raises the concern for possible non‐response bias, especially among smaller subpopulations (e.g., racial and ethnic groups with lower representation in the VA population). However, all analyses incorporated design/non‐response weights to account for potential non‐response bias. Third, we did not account for access to healthcare outside of the VA, which is common for beneficiaries, especially older beneficiaries who are dually enrolled in Medicare. 51 , 52 Access to care outside the VA may confound the relationship between patient‐perceived access to PCMH and hypertension and diabetes control (e.g., if it was easier for a beneficiary to access non‐VA care compared to VA care, they may perceive access to their PACT as lower). Further research should investigate the role of non‐VA care in patient perception of access to their VA PACT and whether racial and ethnic disparities in access to non‐VA care for VA beneficiaries may impact this association. Fourth, we assume that the EPRP and SHEP measures we use are accurate representations of the populations from which they are derived. However, since SHEP and EPRP include data from sample of VA users who recently received primary care services, our sample may represent VA users with greater realized access than the general VA user population. It is possible that for VA users who do not often seek primary care services due to other access barriers, access plays a greater role in mediating or moderating chronic disease control. This could have biased our findings toward the null. Finally, as with any study involving VA beneficiaries, the generalizability of these findings to the general U.S. population may be limited as VA users are older, mostly male, and have greater comorbidity. 53
Balancing these limitations are several key strengths. By linking data from the SHEP survey and EPRP by beneficiary, we were able to determine the role of patient‐perceived access in mediating racial and ethnic differences in chronic disease control at an individual, rather than population, level. Additionally, we included data from smaller, understudied racial and ethnic groups, which are important to study as the VA strives toward equitable care for all subpopulations.
4.2. Conclusions
In our study to examine racial and ethnic disparities in hypertension and diabetes control among VA primary care users, we found evidence of persistent disparities in hypertension control among Black Veterans and in diabetes control among Black and Hispanic Veterans, compared to White Veterans. We did not find evidence that patient‐perceived access to their PACT in the access dimension of “availability and accommodation” mediated or moderated this association. Our findings suggest that reducing racial and ethnic disparities in hypertension and diabetes control requires interventions beyond those that focus on this aspect of access. The contribution of gaps in other aspects of access and in social determinants of health associated with the exogenous construct of race and ethnicity toward disparities in chronic disease control should be explored and tested.
Supporting information
Appendix S1. Supporting Information.
ACKNOWLEDGMENTS
This work was supported by the VHA Office of Health Equity and VHA Quality Enhancement Research Initiative (QUERI) through grant no. PEC‐15‐239 to the Health Equity‐QUERI National Partnered Evaluation Center, and by VA HSR&D grant no. IIR‐17‐189. External Peer Review Program (EPRP) data and SHEP‐PCMH data were obtained through a data use agreement with the VHA Office of Quality and Patient Safety–Analytics and Performance Integration (QPS‐API 10A8). We would like to acknowledge the editorial review and feedback of Chloe Bird, PhD, Director of Center for Health Equity Research, Tufts Medical Center, Boston, MA. Her time was supported through the VA Greater Los Angeles HSR&D Center for the Study of Healthcare Innovation, Implementation, and Policy (Project no. CIN 13‐417). The views expressed in this paper are those of the authors and do not necessarily reflect the position or policy of the U.S. Department of Veterans Affairs or the United States government.
Shannon EM, Steers WN, Washington DL. Investigation of the role of perceived access to primary care in mediating and moderating racial and ethnic disparities in chronic disease control in the veterans health administration. Health Serv Res. 2024;59(1):e14260. doi: 10.1111/1475-6773.14260
[Correction added on 13 December 2023, after first online publication: the Funding information section has been corrected.]
REFERENCES
- 1. Dong L, Fakeye OA, Graham G, Gaskin DJ. Racial/ethnic disparities in quality of care for cardiovascular disease in ambulatory settings: a review. Med Care Res Rev. 2018;75(3):263‐291. doi: 10.1177/1077558717725884 [DOI] [PubMed] [Google Scholar]
- 2. Danaei G, Rimm EB, Oza S, Kulkarni SC, Murray CJ, Ezzati M. The promise of prevention: the effects of four preventable risk factors on national life expectancy and life expectancy disparities by race and county in the United States. PLoS Med. 2010;7(3):e1000248. doi: 10.1371/journal.pmed.1000248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fiscella K, Sanders MR. Racial and ethnic disparities in the quality of health care. Annu Rev Public Health. 2016;37:375‐394. doi: 10.1146/annurev-publhealth-032315-021439 [DOI] [PubMed] [Google Scholar]
- 4. Thorlby R, Jorgensen S, Siegel B, Ayanian JZ. How health care organizations are using data on patients' race and ethnicity to improve quality of care. Milbank Q. 2011;89(2):226‐255. doi: 10.1111/j.1468-0009.2011.00627.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McWilliams JM, Meara E, Zaslavsky AM, Ayanian JZ. Differences in control of cardiovascular disease and diabetes by race, ethnicity, and education: U.S. trends from 1999 to 2006 and effects of medicare coverage. Ann Intern Med. 2009;150(8):505‐515. doi: 10.7326/0003-4819-150-8-200904210-00005 [DOI] [PubMed] [Google Scholar]
- 6. Derose KP, Gresenz CR, Ringel JS. Understanding disparities in health care access‐and reducing them‐through a focus on public health. Health Aff (Millwood) 2011;30(10):1844–1851. doi: 10.1377/hlthaff.2011.0644 [DOI] [PubMed] [Google Scholar]
- 7. Levesque JF, Harris MF, Russell G. Patient‐centred access to health care: conceptualising access at the interface of health systems and populations. Int J Equity Health. 2013;12(18). doi: 10.1186/1475-9276-12-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O'Malley AS. Current evidence on the impact of continuity of care. Curr Opin Pediatr. 2004;16(6):693‐699. doi: 10.1097/01.mop.0000142488.67171.02 [DOI] [PubMed] [Google Scholar]
- 9. Rust G, Ye J, Baltrus P, Daniels E, Adesunloye B, Fryer GE. Practical barriers to timely primary care access: impact on adult use of emergency department services. Arch Intern Med. 2008;168(15):1705‐1710. doi: 10.1001/archinte.168.15.1705 [DOI] [PubMed] [Google Scholar]
- 10. Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005;83(3):457‐502. doi: 10.1111/j.1468-0009.2005.00409.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fortney JC, Maciejewski ML, Warren JJ, Burgess JF Jr. Does improving geographic access to VA primary care services impact patients' patterns of utilization and costs? Inquiry. 2005;42(1):29‐42. doi: 10.5034/inquiryjrnl_42.1.29 [DOI] [PubMed] [Google Scholar]
- 12. Washington DL, Bean‐Mayberry B, Riopelle D, Yano EM. Access to care for women veterans: delayed healthcare and unmet need. J Gen Intern Med. 2011;26(Suppl 2):655‐661. doi: 10.1007/s11606-011-1772-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wong MS, Steers WN, Hoggatt KJ, Ziaeian B, Washington DL. Relationship of neighborhood social determinants of health on racial/ethnic mortality disparities in US veterans‐mediation and moderating effects. Health Serv Res. 2020;55(Suppl 2):851‐862. doi: 10.1111/1475-6773.13547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wong MS, Upchurch DM, Steers WN, Haderlein TP, Yuan AT, Washington DL. The role of community‐level factors on disparities in COVID‐19 infection among American Indian/Alaska native veterans. J Racial Ethn Health Disparities. 2022;9(5):1861‐1872. doi: 10.1007/s40615-021-01123-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Penchansky R, Thomas JW. The concept of access: definition and relationship to consumer satisfaction. Med Care. 1981;19(2):127‐140. doi: 10.1097/00005650-198102000-00001 [DOI] [PubMed] [Google Scholar]
- 16. Cu A, Meister S, Lefebvre B, Ridde V. Assessing healthcare access using the Levesque's conceptual framework—a scoping review. Int J Equity Health. 2021;20(1):116. doi: 10.1186/s12939-021-01416-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.In: Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. The National Academies Press; 2003. [PubMed] [Google Scholar]
- 18. Baumgarter JCC, Sara R, Radley DC. Racial and Ethnic Inequities in Health Care Coverage and Access, 2013–2019. The Commonwealth Fund; 2021. Accessed August 30, 2022. https://www.commonwealthfund.org/publications/issue‐briefs/2021/jun/racial‐ethnic‐inequities‐health‐care‐coverage‐access‐2013‐2019 [Google Scholar]
- 19. Radley DCB, Jesse C, Collins SR, Laure Z, Schneider EC. Achieving Racial and Ethnic Equity in U.S. Health Care: A Scorecare of State Performace. The Commonwealth Fund; 2021. Accessed August 30, 2022. https://www.commonwealthfund.org/publications/scorecard/2021/nov/achieving‐racial‐ethnic‐equity‐us‐health‐care‐state‐performance#8 [Google Scholar]
- 20. Bailey ZD, Krieger N, Agenor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389(10077):1453‐1463. doi: 10.1016/S0140-6736(17)30569-X [DOI] [PubMed] [Google Scholar]
- 21. Fenton AT, Burkhart Q, Weech‐Maldonado R, et al. Geographic context of black‐white disparities in Medicare CAHPS patient experience measures. Health Serv Res. 2019;54(Suppl 1):275‐286. doi: 10.1111/1475-6773.13091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hausmann LR, Gao S, Mor MK, Schaefer JH Jr, Fine MJ. Understanding racial and ethnic differences in patient experiences with outpatient health care in veterans affairs medical centers. Med Care. 2013;51(6):532‐539. doi: 10.1097/MLR.0b013e318287d6e5 [DOI] [PubMed] [Google Scholar]
- 23. Hunt KA, Gaba A, Lavizzo‐Mourey R. Racial and ethnic disparities and perceptions of health care: does health plan type matter? Health Serv Res. 2005;40(2):551‐576. doi: 10.1111/j.1475-6773.2005.00372.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lurie N, Zhan C, Sangl J, Bierman AS, Sekscenski ES. Variation in racial and ethnic differences in consumer assessments of health care. Am J Manag Care. 2003;9(7):502‐509. [PubMed] [Google Scholar]
- 25. Martino SC, Mathews M, Agniel D, et al. National racial/ethnic and geographic disparities in experiences with health care among adult Medicaid beneficiaries. Health Serv Res. 2019;54(Suppl 1):287‐296. doi: 10.1111/1475-6773.13106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anhang Price R, Elliott MN, Zaslavsky AM, et al. Examining the role of patient experience surveys in measuring health care quality. Med Care Res Rev. 2014;71(5):522‐554. doi: 10.1177/1077558714541480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zolnierek KB, Dimatteo MR. Physician communication and patient adherence to treatment: a meta‐analysis. Med Care. 2009;47(8):826‐834. doi: 10.1097/MLR.0b013e31819a5acc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kimerling R, Pavao J, Greene L, et al. Access to mental health care among women veterans: is VA meeting women's needs? Med Care. 2015;53(4 Suppl 1):S97‐S104. doi: 10.1097/MLR.0000000000000272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nguyen KH, Wilson IB, Wallack AR, Trivedi AN. Racial and ethnic disparities in patient experience of care among nonelderly Medicaid managed care enrollees. Health Aff (Millwood). 2022;41(2):256‐264. doi: 10.1377/hlthaff.2021.01331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weech‐Maldonado R, Hall A, Bryant T, Jenkins KA, Elliott MN. The relationship between perceived discrimination and patient experiences with health care. Med Care. 2012;50(9 Suppl 2):S62‐S68. doi: 10.1097/MLR.0b013e31825fb235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Washington DL, Villa V, Brown A, Damron‐Rodriguez J, Harada N. Racial/ethnic variations in veterans' ambulatory care use. Am J Public Health. 2005;95(12):2231‐2237. doi: 10.2105/AJPH.2004.043570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leung LB, Steers WN, Hoggatt KJ, Washington DL. Explaining racial‐ethnic differences in hypertension and diabetes control among veterans before and after patient‐centered medical home implementation. PloS One. 2020;15(10):e0240306. doi: 10.1371/journal.pone.0240306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rosland AM, Nelson K, Sun H, et al. The patient‐centered medical home in the Veterans Health Administration. Am J Manag Care. 2013;19(7):e263‐e272. [PubMed] [Google Scholar]
- 34. Breland JY, Wong MS, Frayne SM, et al. Obesity and health care experiences among women and men veterans. Womens Health Issues. 2019;29(Suppl 1):S32‐S38. doi: 10.1016/j.whi.2019.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goulet JL, Erdos J, Kancir S, et al. Measuring performance directly using the veterans health administration electronic medical record: a comparison with external peer review. Med Care. 2007;45(1):73‐79. doi: 10.1097/01.mlr.0000244510.09001.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stiles RA, Bahl V, Bernstein SJ, Halman LJ, Harrison RV, Standiford CJ. Improving HEDIS measurement: linking managed care organization and health system ambulatory care data. Qual Manag Health Care. 2000;8(2):40‐48. doi: 10.1097/00019514-200008020-00005 [DOI] [PubMed] [Google Scholar]
- 37. Washington DL, ed. National Veteran Health Equity Report 2021: Focus on Veterans Health Administration Patient Experience and Health Care Quality. VHA Office of Health Equity; 2022. https://www.va.gov/HEALTHEQUITY/docs/NVHER_2021_Report_508_Conformant.pdf [Google Scholar]
- 38. Giordano LA, Elliott MN, Goldstein E, Lehrman WG, Spencer PA. Development, implementation, and public reporting of the HCAHPS survey. Med Care Res Rev. 2010;67(1):27‐37. doi: 10.1177/1077558709341065 [DOI] [PubMed] [Google Scholar]
- 39. Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749‐759. doi: 10.1016/j.jclinepi.2010.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nguyen QC, Osypuk TL, Schmidt NM, Glymour MM, Tchetgen Tchetgen EJ. Practical guidance for conducting mediation analysis with multiple mediators using inverse odds ratio weighting. Am J Epidemiol. 2015;181(5):349‐356. doi: 10.1093/aje/kwu278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tchetgen Tchetgen EJ. Inverse odds ratio‐weighted estimation for causal mediation analysis. Stat Med. 2013;32(26):4567‐4580. doi: 10.1002/sim.5864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Washington DL, Steers WN, Huynh AK, et al. Racial and ethnic disparities persist At veterans health administration patient‐centered medical homes. Health Aff (Millwood). 2017;36(6):1086‐1094. doi: 10.1377/hlthaff.2017.0029 [DOI] [PubMed] [Google Scholar]
- 43. Gatwood JD, Chisholm‐Burns M, Davis R, et al. Disparities in initial Oral antidiabetic medication adherence among veterans with incident diabetes. J Manag Care Spec Pharm. 2018;24(4):379‐389. doi: 10.18553/jmcp.2018.24.4.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lamprea‐Montealegre JA, Madden E, Tummalapalli SL, et al. Association of race and ethnicity with Prescription of SGLT2 inhibitors and GLP1 receptor agonists among patients with type 2 diabetes in the veterans health administration system. JAMA. 2022;328(9):861‐871. doi: 10.1001/jama.2022.13885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Peterson K, Anderson J, Boundy E, Ferguson L, McCleery E, Waldrip K. Mortality disparities in racial/ethnic minority groups in the veterans health administration: an evidence review and map. Am J Public Health. 2018;108(3):e1‐e11. doi: 10.2105/AJPH.2017.304246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wong MS, Hoggatt KJ, Steers WN, et al. Racial/ethnic disparities in mortality across the veterans health administration. Health Equity. 2019;3(1):99‐108. doi: 10.1089/heq.2018.0086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Augustine MR, Nelson KM, Fihn SD, Wong ES. Patient‐reported access in the patient‐centered medical home and avoidable hospitalizations: an observational analysis of the veterans health administration. J Gen Intern Med. 2019;34(8):1546‐1553. doi: 10.1007/s11606-019-05060-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fortney JC, Burgess JF Jr, Bosworth HB, Booth BM, Kaboli PJ. A re‐conceptualization of access for 21st century healthcare. J Gen Intern Med. 2011;26(Suppl 2):639‐647. doi: 10.1007/s11606-011-1806-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Elliott MN, Adams JL, Klein DJ, et al. Patient‐reported care coordination is associated with better performance on clinical care measures. J Gen Intern Med. 2021;36(12):3665‐3671. doi: 10.1007/s11606-021-07122-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jha AK, Orav EJ, Zheng J, Epstein AM. Patients' perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921‐1931. doi: 10.1056/NEJMsa0804116 [DOI] [PubMed] [Google Scholar]
- 51. Liu CF, Chapko M, Bryson CL, et al. Use of outpatient care in Veterans Health Administration and Medicare among veterans receiving primary care in community‐based and hospital outpatient clinics. Health Serv Res. 2010;45(5 Pt 1):1268‐1286. doi: 10.1111/j.1475-6773.2010.01123.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu CF, Manning WG, Burgess JF Jr, et al. Reliance on veterans affairs outpatient care by Medicare‐eligible veterans. Med Care. 2011;49(10):911‐917. doi: 10.1097/MLR.0b013e31822396c5 [DOI] [PubMed] [Google Scholar]
- 53. Rogers WH, Kazis LE, Miller DR, et al. Comparing the health status of VA and non‐VA ambulatory patients: the veterans' health and medical outcomes studies. J Ambul Care Manage. 2004;27(3):249‐262. doi: 10.1097/00004479-200407000-00009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.


