Abstract
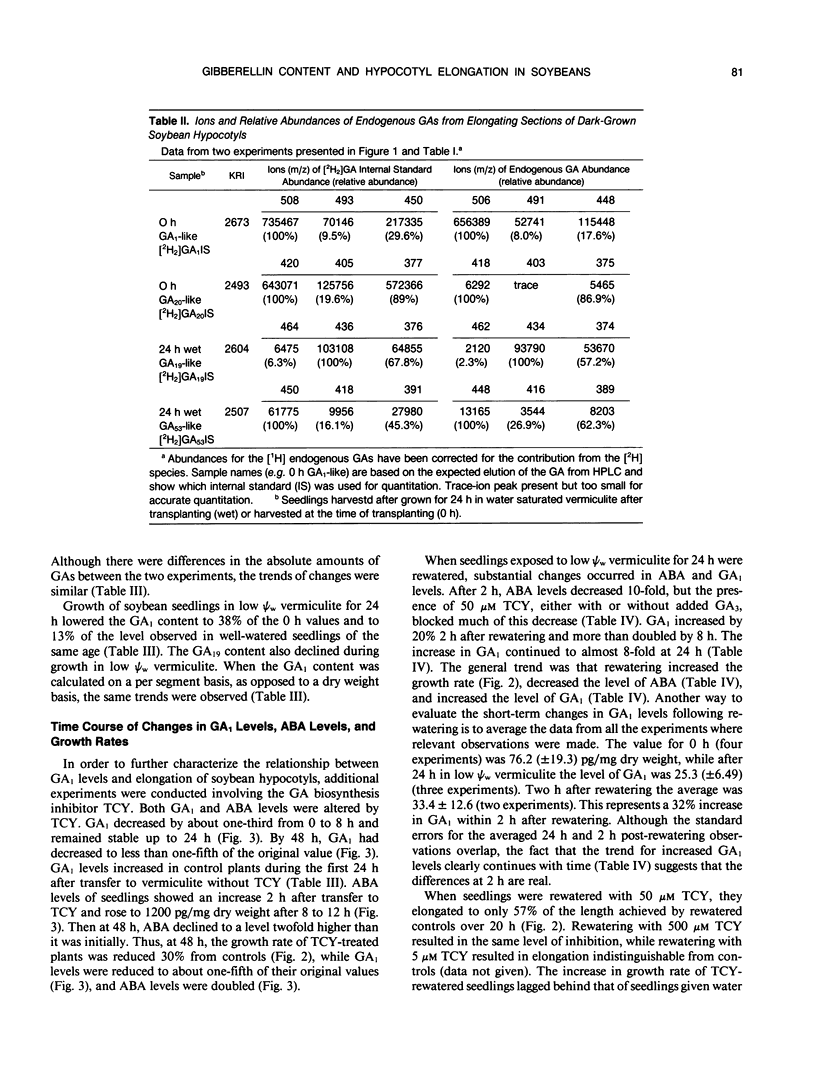
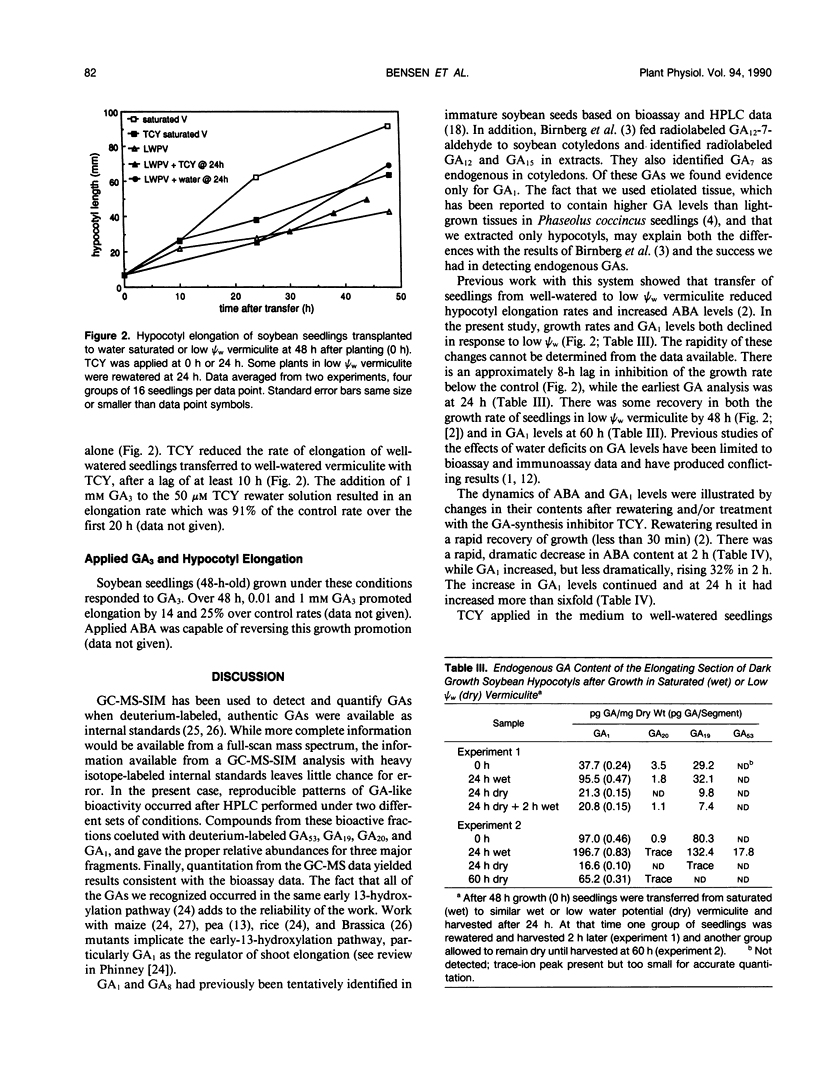
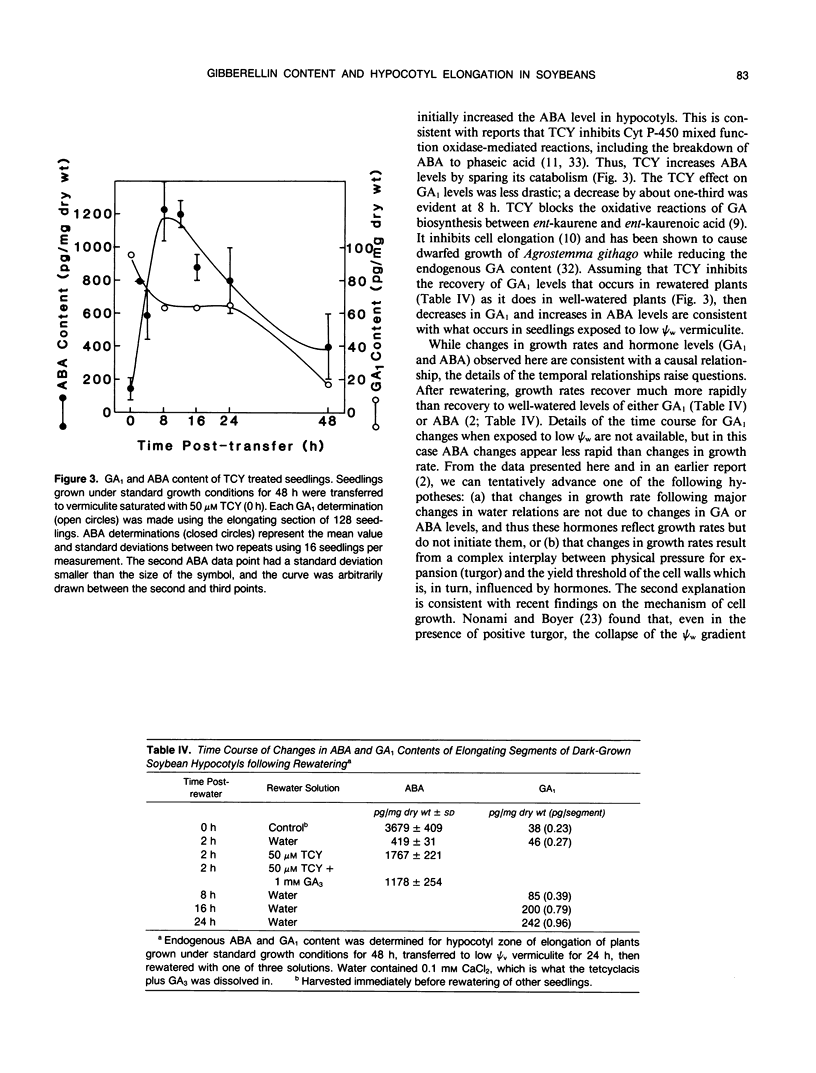
Four gibberellins, GA53, GA19, GA20, and GA1, were detected by bioassay, chromatography in two HPLC systems, and combined gas chromatography-mass spectroscopy-selected ion monitoring (GC-MS-SIM) in etiolated soybean (Glycine max [L.] Merr.) hypocotyls. GC-MS-SIM employed [2H2]-labeled standards for each endogenous gibberellin detected, and quantities estimated from bioassays and GC-MS-SIM were similar. This result plus the tentative detection of GA44 and GA8 (standards not available) indicates that the early-C-13-hydroxylation pathway for gibberellin biosynthesis predominates in soybean hypocotyls. Other gibberellins were not detected. Growth rates decreased after transfer to low water potential (ψw) vermiculite and were completely arrested 24 hours after transfer. The GA1 content in the elongating region of hypocotyls had declined to 38% of the 0 time value at 24 hours after transfer to low ψw vermiculite, a level which was only 13% of the GA1 content in control seedlings at the same time (24 hours posttransfer). Rewatering seedlings following 24 hours growth in low ψw vermiculite resulted in a complete recovery in elongation rate, an increase in GA1 (20% at 2 hours, two-fold at 8 hours, eightfold at 24 hours), and a decrease in ABA levels (tenfold at 2 hours). Treatment of well-watered seedlings with the GA-synthesis inhibitor tetcyclacis (TCY) resulted in lowered GA1 levels and increased ABA levels. When seedlings grown 24 hours in low ψw vermiculite were rewatered with TCY, recovery of the elongation rate was delayed and reduced, and the decline in ABA levels was slowed. Addition of GA3 restored the elongation rate inhibited by TCY. Seedlings were growth responsive to exogenous GA3, and this GA3-promoted growth was inhibited by exogenous ABA. The data are consistent with the hypothesis that changes in GA1 and ABA levels play a role in adjusting hypocotyl elongation rates. However, the changes observed are not of sufficient magnitude nor do they occur rapidly enough to suggest they are the primary regulators of elongation rate responses to rapidly changing plant water status.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharoni N., Blumenfeld A., Richmond A. E. Hormonal activity in detached lettuce leaves as affected by leaf water content. Plant Physiol. 1977 Jun;59(6):1169–1173. doi: 10.1104/pp.59.6.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensen R. J., Boyer J. S., Mullet J. E. Water deficit-induced changes in abscisic Acid, growth, polysomes, and translatable RNA in soybean hypocotyls. Plant Physiol. 1988 Oct;88(2):289–294. doi: 10.1104/pp.88.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnberg P. R., Brenner M. L., Mardaus M. C., Abe H., Pharis R. P. Metabolism of gibberellin a(12)-7-aldehyde by soybean cotyledons and its use in identifying gibberellin a(7) as an endogenous gibberellin. Plant Physiol. 1986 Sep;82(1):241–246. doi: 10.1104/pp.82.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshioka M., Takeno K., Beall F. D., Pharis R. P. Purification and separation of plant gibberellins from their precursors and glucosyl conjugates. Plant Physiol. 1983 Oct;73(2):398–406. doi: 10.1104/pp.73.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonami H., Boyer J. S. Turgor and growth at low water potentials. Plant Physiol. 1989 Mar;89(3):798–804. doi: 10.1104/pp.89.3.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood S. B., Larsen K. M., Mander L. N., Abe H., Pharis R. P. Identification of endogenous gibberellins from sorghum. Plant Physiol. 1986 Sep;82(1):330–332. doi: 10.1104/pp.82.1.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood S. B., Pearce D., Williams P. H., Pharis R. P. A Gibberellin-Deficient Brassica Mutant-rosette. Plant Physiol. 1989 Feb;89(2):482–487. doi: 10.1104/pp.89.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A., Cosgrove D. J. Gibberellic Acid stimulation of cucumber hypocotyl elongation : effects on growth, turgor, osmotic pressure, and cell wall properties. Plant Physiol. 1989 Aug;90(4):1335–1340. doi: 10.1104/pp.90.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twente J. W., Twente J. A. Regulation of hibernating periods by temperature. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1044–1051. [PMC free article] [PubMed] [Google Scholar]


