Abstract
Chronic kidney disease (CKD) is a major public health concern in the Middle East and Africa (MEA) region and a leading cause of death in patients with type 2 diabetes mellitus (T2DM) and hypertension. Early initiation of sodium-glucose cotransporter - 2 inhibitors (SGLT-2i) and proper sequencing with renin-angiotensin-aldosterone system inhibitors (RAASi) in these patients may result in better clinical outcomes due to their cardioprotective properties and complementary mechanisms of action. In this review, we present guideline-based consensus recommendations by experts from the MEA region, as practical algorithms for screening, early detection, nephrology referral, and treatment pathways for CKD management in patients with hypertension and diabetes mellitus. This study will help physicians take timely and appropriate actions to provide better care to patients with CKD or those at high risk of CKD.
Keywords: chronic kidney disease, nephrology referral, screening, sodium-glucose cotransporter-2 inhibitors
Introduction
Chronic kidney disease (CKD) is a leading cause of morbidity and mortality worldwide.1 The global prevalence of CKD is predicted to be 13.4% (11.7% to 15.1%), with 4.9–7.1 million individuals requiring kidney replacement therapy.2 In the majority of the Middle East, diabetes mellitus (DM) and hypertension account for 45%–74% of end-stage kidney disease (ESKD).3,4
Controlling hyperglycemia,5 hypertension,5 hyperlipidemia,6 and obesity7,8 along with lifestyle modification9 and the use of cardiorenal protective medications10 are the mainstays of treatment for slowing CKD progression. A post-hoc analysis of the Nephropathy in Diabetes type-2 (NID-2) trial showed that multifactorial intensive treatment (a pre-specified algorithm for managing hypertension, hyperglycemia, and dyslipidemia) resulted in an increased number of well-managed risk factors compared to standard of care that gradually improved the cardiovascular prognosis in patients with type 2 diabetes mellitus (T2DM) and albuminuria.11 Mima et al, suggested that enhancing the endogenous protective factors, such as insulin, glucagon-like peptide-1, and others may neutralize the adverse effects of hyperglycemia and prevent diabetic nephropathy.12 Enriching of these endogenous factors involves life style modifications, the reduction in oxidative stress, and application of targeted therapies that can modulate the activity of protein kinase C-β and nuclear factor κB.12,13 Among the available therapeutic arsenals, the cardioprotective effects of sodium-glucose cotransporter - 2 inhibitors (SGLT-2i) in patients with T2DM are now well proven.14,15 The results of relative risk reduction of kidney and heart failure (HF) outcomes have been demonstrated in patients with HF/CKD with and without T2DM.16–20 Recent breakthroughs in therapeutic and preventive approaches for CKD management,21 training and advocacy for this cause, and inclusion of CKD in national non-communicable disease policies are strategies that could be implemented to improve the management of CKD in low- and middle-income countries.3,22
Methodology
A panel of 12 key external experts (KEEs) from the Middle East and Africa (MEA) region (Egypt [n=2], Iraq [n=1], Jordan [n=1], Kingdom of Saudi Arabia [n=1], Kuwait [n=1], Lebanon [n=1], Morocco [n=1], Turkey [n=2], and United Arab Emirates [n=2]) congregated and discussed the current unmet need for the management of CKD, along with the use of SGLT-2i in CKD management in the MEA region. This manuscript is an outcome of a literature review, KEE group discussion, and consensus recommendations to provide an action plan for the screening, early detection, and management of CKD in the MEA region.
Screening and Early Diagnosis of CKD in MEA
Up to 90% of individuals with CKD stage 3 are undiagnosed in primary care settings; the majority are diagnosed at the advanced stage of the disease with the availability of limited treatment options for either dialysis or organ transplantation.23 Early screening, diagnosis, and management of patients with CKD by primary care physicians (PCPs) are crucial owing to the associated poor clinical outcomes (ESKD, cardiovascular disease, and increased mortality) with progressive CKD.24,25 Hypertension is another risk factor that is undertreated in most Middle Eastern countries such as Morocco; only 17.1% of the patients had controlled hypertension with a history of hypertension and received antihypertensive medications and/or lifestyle and dietary advice.26 Furthermore, the use of a single threshold of estimated glomerular filtration rate (eGFR; < 60 mL/min/1.73 m2) in classifying CKD stages 3 to 5 may lead to underdiagnoses of CKD in young adults with eGFR > 60 mL/min/1.73 m2 with or without proteinuria or other markers of kidney damage.27
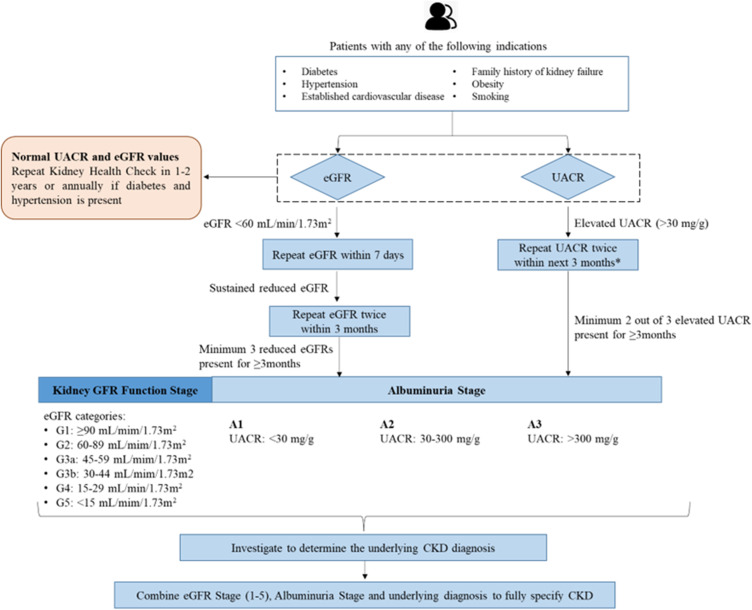
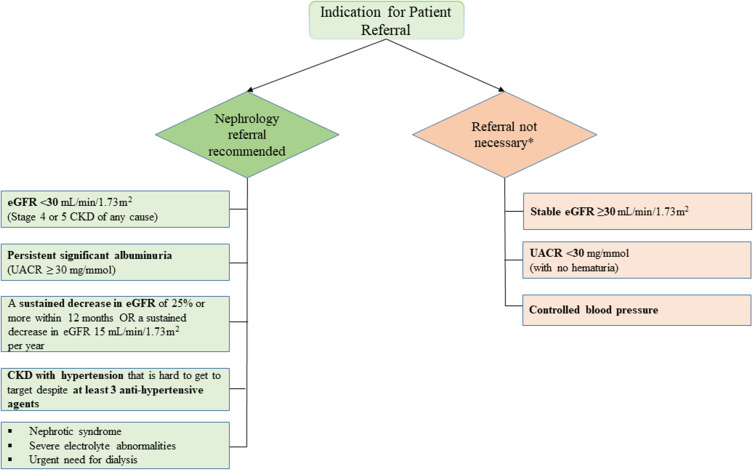
Several studies have shown the cost-effectiveness of population screening for CKD, considering the incidence of ESKD.28–30 The American Diabetes Association released its 2023 Standards of Medical Care in Diabetes, which suggested that eGFR should be measured twice a year to guide their treatment in patients with DM with a urine albumin-creatinine ratio (UACR) of ≥300 mg/g and/or an eGFR of 30 to 60 mL/min/1.73 m2.31 Table 1 presents the summary of international guidelines for CKD management.31–35 In view of this, our panel suggested recommendations for screening, diagnosis, and a nephrology referral pathway to provide a better and more standardized service to the patients (Figures 1 and 2).
Table 1.
Summary of Recommendations from International Guidelines for Screening, Diagnosis and Referral of Patients with CKD
| Guidelines | Screening Population | Screening/Diagnosis | Referral to Nephrologist |
|---|---|---|---|
| KDIGO28,29 |
|
Estimate eGFR and ACR
|
|
| ADA26 | Patients with
|
Estimate eGFR and spot UACR at least yearly once
|
|
| Kidney Health Australia27 | Patients with
|
Estimate eGFR and spot UACR every 1 to 2 years in individuals at high-risk of CKD |
|
| KDOQI30 | Patients with
|
Estimate eGFR and ACR
|
|
Notes: GFR categories: G1, eGFR of ≥90 mL/min/1.73 m2; G2, eGFR of 60 to 89 mL/min/1.73 m2; G3a, eGFR of 45 to 59 mL/min/1.73 m2; G3b, eGFR of 30 to 44 mL/min/1.73 m2; G4, eGFR of 15 to 29 mL/min/1.73 m2; G5, <15 mL/min/1.73 m2. Albuminuria categories: A1, UACR of <30 mg/g; A2, UACR of 30 to 300 mg/g; A3, UACR of >300 mg/g. *In case kidney structural abnormalities. **If this is a stable isolated finding, formal referral might not be required.
Abbreviations: ADA, American Diabetes Association; ACR, albumin-creatinine ratio; AKI, acute kidney injury; BMI, body mass index; CKD, chronic kidney disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; KDIGO, Kidney Disease: Improving Global Outcomes; KDOQI, Kidney Disease Outcomes Quality Initiative; RBC, red blood cell; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; UACR, urine albumin-creatinine ratio; UAER, urine albumin excretion rate; US, United States.
Figure 1.
Recommended algorithm for screening and diagnosis of CKD.
Note: *Prefer first morning void.
Abbreviations: CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; UACR, urine albumin-creatinine ratio.
Figure 2.
Recommended nephrology referral pathway.
Note: *In the absence of other referral indicators.
Abbreviations: CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; UACR, urine albumin-creatinine ratio.
Implementation of an early screening program and appropriate treatment by specialists can delay CKD progression, prevent ESKD, and improve patient outcomes. One such initiative is the “SEARCH” program for Screening Early Renal Complications in High-risk patients. This program is an ongoing project implemented in Europe and other emerging countries. The goal of the SEARCH program is to identify awareness among PCPs about early CKD consequences in high-risk patients (DM or hypertension).36 The involvement of PCPs or general practitioners is crucial for its success. However, PCPs need expert guidance to initiate screening. Population screening, data mining, and disease awareness campaigns are three fundamental strategies for early CKD screening. Al-Ghamdi et al, suggested that national renal associations, nephrology conferences, and memorial days such as World Kidney Day can be used to raise awareness at patients and PCPs levels about novel therapies and the importance of early detection and management of CKD.37
Evidence Supporting the Use of SGLT-2i for Management of Population at High-Risk of CKD
Several real-world studies and randomized controlled trials have shown the renoprotective effect of SGLT-2i.16,38,39 The Japan Chronic Kidney Disease Database registry demonstrated a renoprotective effect of SGLT-2i, with no evidence of a decline in the rate of kidney function decline or the presence of proteinuria.39 Table 2 presents an overview of major randomized controlled trials for SGLT-2i.10,14,19,40–44 Canagliflozin, dapagliflozin, and empagliflozin have shown frequent regression of albuminuria and reduced risk of kidney-specific outcomes and death from kidney-related causes.14,40–42 The DAPA-CKD trial showed that the dapagliflozin group had a significantly lower occurrence of a composite of a sustained decline in the eGFR of at least 50%, ESKD, or death from kidney or cardiovascular causes (9.2% vs 14.5%; hazard ratio [HR], 0.61; 95% confidence interval [CI], 0.51, 0.72; p<0.001) compared to placebo.16 Results of the CVD-REAL 3 (Comparative Effectiveness of Cardiovascular Outcomes in New Users of SGLT-2 Inhibitors) study demonstrated that initiation of SGLT-2i was associated with a slower decline in eGFR rate compared to other antidiabetic drugs.38 Another large registry-based cohort study using nationwide data from routine clinical practice in Sweden, Denmark, and Norway showed that SGLT-2i lowered the risk of kidney events compared with dipeptidyl peptidase 4 inhibitor.45
Table 2.
Clinical Trials Describing the Kidney-Protective Outcome of SGLT-2i
| Name of Study | Design | Objective | Population Characteristics | Number of Patients | Treatment Modality | Outcome |
|---|---|---|---|---|---|---|
| CANVAS and CANVAS-R9 | Randomized, placebo-controlled study | Effect of canagliflozin on CV, kidney, and safety outcomes | Patients with
|
10,142 (CANVAS:4330, CANVAS-R:5812) |
CANVAS (1:1:1) – canagliflozin (300 mg), canagliflozin (100 mg), or matching placebo CANVAS-R (1:1) – canagliflozin: 100 mg with an optional increase to 300 mg or matched placebo |
|
| CREDENCE37 | Randomized, double-blind, event-driven, placebo-controlled, multicenter study | Effects of canagliflozin on kidney and CV outcomes in participants with diabetic nephropathy | Patients with
|
4401 | Canagliflozin 100 mg versus placebo |
|
| DAPA-CKD11 | International, multicenter, randomized, double-blind, parallel group, placebo-controlled study | Effect of dapagliflozin on kidney outcomes and CV mortality in patients with CKD | Patients with
|
4304 | Dapagliflozin 10 mg versus placebo |
|
| DECLARE-TIMI 5834,35 | Randomized, double-blind, multinational, placebo-controlled study | Effect of dapagliflozin on CV outcomes | Patients with established ASCVD-40.6% | 17,160 | Dapagliflozin 10 mg versus matched placebo (1:1) | DECLARE-TIMI 58, 2019
DECLARE-TIMI 58, 2021
|
| EMPA-REG38 | Randomized, double-blind, placebo-controlled study | Long-term kidney effects of empagliflozin | Patients with
|
4124 | Empagliflozin 10 mg or 25 mg versus placebo |
|
| EMPEROR-Reduced14 | Randomized, event-driven double-blind, parallel-group, placebo-controlled study | Efficacy and safety of once daily empagliflozin | Patients with chronic HFrEF | 3730 | Empagliflozin 10 mg versus placebo |
|
| VERTIS36 | Randomized, double-blind, placebo-controlled multicentric study | CV outcomes with ertugliflozin in subjects with T2DM and established atherosclerotic cardiovascular disease | Patients with
|
8246 | Ertugliflozin 5 mg versus ertugliflozin 15 mg versus matched placebo once daily (1:1:1) |
|
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; CI, confidence interval; CKD, chronic kidney disease; CV, cardiovascular; ESKD, end-stage kidney disease; eGFR, estimated glomerular filtration rate; HbA1C, glycated haemoglobin; HHF, hospitalization for heart failure; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; MI, myocardial infarction; SBP, systolic blood pressure; SGLT-2i, sodium-glucose cotransporter 2 inhibitor; T2DM, type 2 diabetes mellitus.
Practical Considerations for the Usage of SGLT-2i in CKD Management
Although initiation of SGLT-2i may initially decrease the glomerular filtration rate (GFR) and increase serum creatinine level, these changes are usually short-lived and may occur even with improvement in patient outcomes, and long-term use of SGLT-2i may slow the process of worsening kidney function.10,19 SGLT-2i can provide renal protection by improving mitochondrial dynamics and reducing oxidative stress and metabolic burden in proximal tubular renal cells. SGLT-2i also decrease albuminuria, delay the progression of nephropathy, and defer the initiation of renal replacement therapy.46–49 A post hoc analysis from the CREDENCE trial revealed that in patients with T2DM and CKD for each 30% decrease in urinary albumin-creatinine ratio during the initial 26 weeks of treatment, SGLT-2i (canagliflozin) use was associated with a 29% (HR, 0.71; 95% CI, 0.67, 0.76) reduced risk of kidney outcomes compared to placebo.50 Although canagliflozin resulted in early and sustained reductions in albuminuria, residual albuminuria was noted that was associated with kidney and cardiovascular events. These findings highlight the importance of monitoring albuminuria during canagliflozin treatment to effectively address both renal and cardiovascular prognoses.50
According to the European Society of Cardiology (ESC) guidelines, SGLT-2i, renin-angiotensin-aldosterone system inhibitor (RAASi), or angiotensin receptor-neprilysin inhibitors should not be promptly interrupted due to a transient decrease in renal function. An increase in serum creatinine of <50% above baseline, as long as it is <266 µmol/L (3 mg/dL), or a decrease in eGFR of <10% from baseline, provided eGFR is >25 mL/min/1.73 m2 can be considered as acceptable.51 Furthermore, there are few adverse events reported with dapagliflozin in patients with and without T2DM.15,52 Despite being a rare adverse event, diabetic ketoacidosis is more common in patients with T2DM in the dapagliflozin arm compared with the placebo arm (0.3% versus 0.1%; p=0.02).15 Results from the DAPA-CKD trial showed that SGLT-2i (dapagliflozin) safely reduced kidney and cardiovascular events independent of baseline diabetes and glycemic status. Dapagliflozin and placebo arms have similar overall incidence of adverse events and serious adverse events in patients with normoglycemia or prediabetes.16,53 In addition, no events of diabetic ketoacidosis and severe hypoglycemia were observed in patients without T2DM.16 There were fewer occurrences of hyperkalemia and kidney-related adverse events were observed.53,54
Complementary Mechanism of Action of SGLT-2i and RAASi
Despite the availability of RAASi, there is a considerable risk of CKD progression with current therapeutic agents due to underdiagnosis, treatment with kidney-protective agents (SGLT-2i), or lower effectiveness of RAASi compared to the combined use of RAASi and SGLT-2i to slowing CKD progression.55–57 A United States-based retrospective observational study reported that 12% to 20% of patients with albuminuria and T2DM were untreated with any relevant recommended treatment within 6 months following the index diagnosis. Moreover, these patients are at greater risk of disease progression (microalbuminuric cohort: HR 1.31; 95% CI 1.08, 1.60; macroalbuminuric cohort: HR 1.44; 95% CI 1.21, 1.72; p <0.05) than those with normoalbuminuria, suggesting a need for early intervention to prevent or delay disease progression.58 The use of RAASi (hindering the excretion of potassium) along with impaired GFR, higher dietary potassium intake, and extracellular shift of potassium result in hyperkalemia being frequently reported among the users.59–61 A retrospective study by Luo et al, reported U-shaped relationships between serum potassium levels and mortality and major adverse cardiovascular events.62
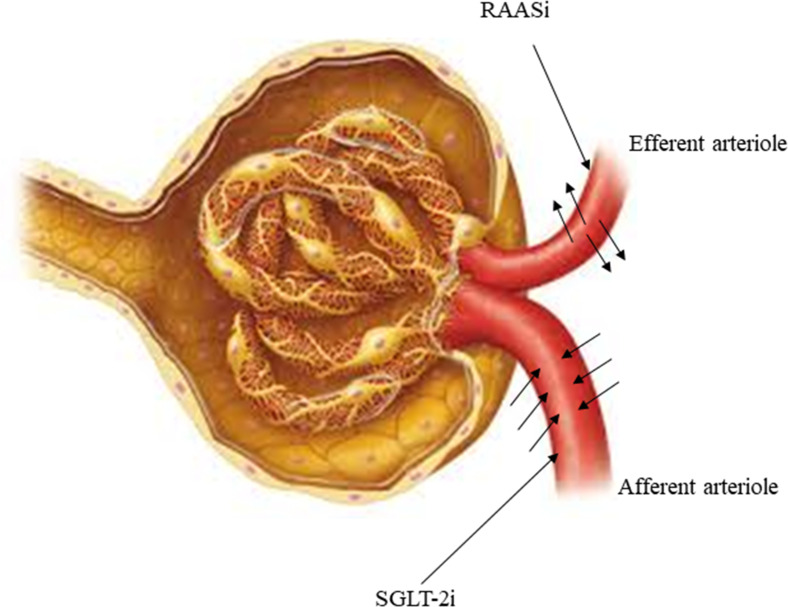
SGLT-2i such as dapagliflozin, offers a complementary pathway to the renin-angiotensin-aldosterone system inhibition to lower intra-glomerular pressure and protect nephrons.63 Figure 3 presents the mechanism of action of RAASi and SGLT-2i. RAASi dilates efferent arterioles by decreasing the net filtration pressure, whereas dapagliflozin constricts the afferent arteriole,55,64 thus complementing the RAASi mechanism of action.
Figure 3.
Mechanism of action of SGLT-2i and RAASi.
Abbreviations: RAASi, renin-angiotensin-aldosterone system inhibitor; SGLT-2i, sodium-glucose cotransporter-2 inhibitor.
Sequencing of SGLT-2i and RAASi
RAASi can be used to stabilize patients with CKD and hypertension according to the Kidney Disease Improving Global Outcomes (KDIGO) guidelines.65 In patients with T2DM who are at a high-risk of cardiovascular or kidney disease, initiation of SGLT-2i is recommended as per the KDIGO and ESC guidelines irrespective of glycemic status.51,65 The National Institute for Health and Care Excellence (NICE) guidelines also recommend the use of SGLT-2i as an add-on to RAASi, unless contraindicated, in individuals with eGFR of 25–75 mL/min/1.73 m2 with T2DM or UACR of ≥22.6 mg/mmol.66 If a patient does not have any high-risk conditions, both RAASi and SGLT-2i can be used together after educating the patient about hyperkalemia mainly due to RAASi treatment.
Recommended Treatment Algorithms
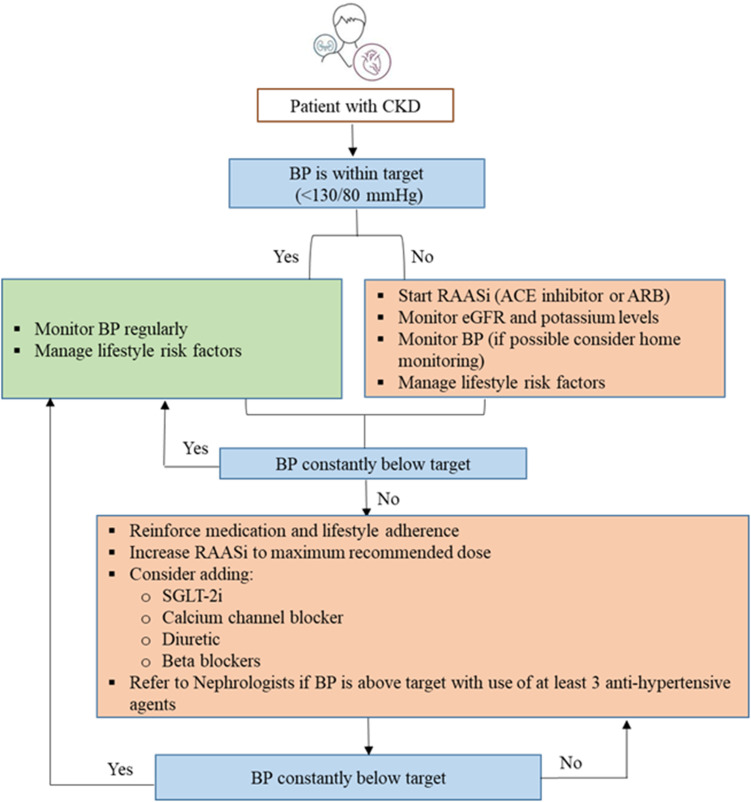
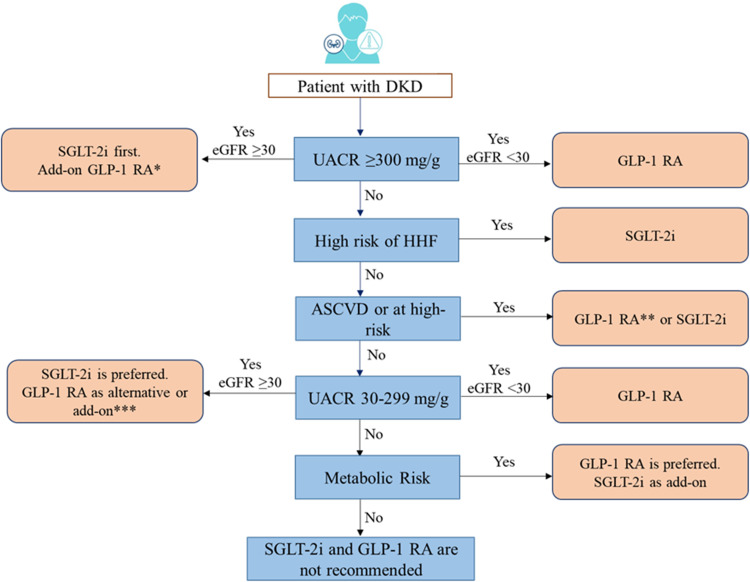
CKD is often associated with a high risk for major adverse cardiovascular events, HF, and all-cause mortality. This may have risk stratification implications for patients with T2DM, based on background CKD. Canagliflozin, dapagliflozin, empagliflozin, ertugliflozin, and sotagliflozin are recommended in patients with T2DM at risk of cardiovascular events to reduce hospitalization for HF, major cardiovascular events, ESKD, and cardiovascular death per 2021 ESC guidelines. Dapagliflozin, empagliflozin, or sotagliflozin are also recommended in patients with T2DM with and HF with reduced ejection fraction to reduce hospitalizations HF and cardiovascular death (2021 ESC guidelines).51 Based on cardiovascular outcome trials, KDIGO and NICE guidelines recommend the use of SGLT-2i in patients with CKD with and without diabetes.66,67 In patients with CKD with an eGFR ≥20 mL/min/1.73 m2 and albuminuria with or without diabetes, the KDIGO guidelines recommend starting SGLT-2i, such as canagliflozin, empagliflozin, or dapagliflozin. If the eGFR falls below 20 mL/min/1.73 m2 after initiating SGLT-2i, it may be continued for kidney protection unless it is not tolerated or kidney replacement therapy is initiated.65,68 Figures 4 and 5 present the algorithm for the management of patients with hypertension and diabetic kidney disease, respectively.
Figure 4.
Algorithm for management of CKD in patients with hypertension.
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BP, blood pressure; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; RAASi, renin-angiotensin-aldosterone system inhibitors; SGLT-2i, sodium-glucose cotransporter-2 inhibitor.
Figure 5.
Algorithm for management of patients with DKD.
Notes: *Add-on GLP-1RA for uncontrolled metabolic risk. **GLP-1RA is preferred with coexisting uncontrolled metabolic risks. ***As an alternative if SGLT-2i is contraindicated and as an add-on for uncontrolled metabolic risks.
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; DKD, diabetic kidney disease; GLP-1 RA, glucagon-like peptide-1 receptor agonist; HHF, hospitalization for heart failure; SGLT-2i, sodium-glucose cotransporter-2 inhibitor; UACR, urinary albumin-creatinine ratio.
In addition to RAASi and SGLT-2i, finerenone may also be administered to patients with CKD and T2DM. Data from the FIDELIO-DKD (Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease) trial showed a reduced risk of CKD progression (HR, 0.82; 95% CI, 0.73 to 0.93; p=0.001) and cardiovascular events (HR, 0.86; 95% CI, 0.75 to 0.99; p=0.03) compared with placebo.69 A systematic review and meta-analysis also demonstrated a similar result of lowering the risk of CKD progression (17.8% versus 21.1%) and cardiovascular risk (12.4% versus 14.2%) in patients with CKD and T2DM compared with placebo when finerenone was used as an add-on therapy to RAASi or SGLT-2i.70 Finerenone acts as one of the novel tools for CKD with T2DM and offers a unique approach to further delay the CKD progression in these patients. It also provides an alternative treatment approach for patients who are unable to tolerate RAASi or SGLT-2i.
Although the application of SGLT-2i have rapidly expanded, establishing them as a key therapeutic intervention in preventing CKD progression, an unmet need remains to reduce the residual risk in patients with renal or cardiovascular disease. Several studies have shown the efficacy of mineralocorticoid receptor antagonists and selective endothelin A receptor antagonists in patients with T2DM and CKD.71,72 Future studies are required to explore the clinical benefits of combination therapy with SGLT-2i for reducing residual albuminuria and mitigate cardiovascular risk.54,69 There is also scarcity of data on the efficacy and safety of SGLT-2i in transplant recipients and patients with ESKD. Other barriers for initiation of SGLT-2i are lower SGLT-2i prescription rate, presence of treatment inertia, unavailability, inaccessibility, unaffordability, time constraints, lack of continuity of care, and patients’ perceptions and preferences need to be overcome.73–76 Enhancing the knowledge of frontline primary care physicians and patients for cardiorenal benefits of SGLT-2i and risk control, addition of SGLT-2i by policymakers as crucial performance indicator along with glycemic control, adoption of multidisciplinary team-based care strategies, and providing access to financial assistance programs can improve the uptake of SGLT-2i for management of CKD.37,76,77
Conclusion
The burden of underdiagnosed and untreated CKD has assumed great proportions, primarily due to a lack of awareness of the condition among the general population and recent therapeutic advances in the healthcare community. DM and hypertension remain the most frequent causes, and patients with CKD often need to be treated for these comorbidities. SGLT-2i can play a significant role in CKD management in patients with or without DM because of its cardioprotective properties. Establishing nephrologist referral pathways, such as the SEARCH program, and creating guideline-based awareness about newer and novel therapeutic options in the healthcare community can help improve CKD management in MEA.
Acknowledgments
The authors would like to thank Dr. Dakshayani S and Dr. Soma Santra of Fortrea Scientific Pvt. Ltd. (formerly Labcorp Scientific Services & Solutions Pvt. Ltd) for medical writing support, which was funded by AstraZeneca FZ LLC in accordance with the Good Publication Practice 2022 guidelines.
Funding Statement
The preparation of this consensus manuscript and funding of the journal’s article processing charges were supported by AstraZeneca FZ, LLC. All authors had full access to all the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analyses.
Author Contributions
Ahmed Fathi ElKeraie conceptualized this study. All authors contributed equally to the literature review and the preparation of the first draft of the manuscript. All the authors contributed equally to study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, reviewing and editing the manuscript. All the authors have read and approved the final version of the manuscript to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Ahmed Fathi ElKeraie received speaker honoraria from AstraZeneca, Novartis, Boehringer Ingelheim, Sanofi, Johnson and Johnson, Amgen, Roche, Astellas Pharma, Inc., and Fresenius Kabi. Saeed Al-Ghamdi received speaker honoraria from AstraZeneca, Astellas Pharma Inc., AbbVie, and Vifor Pharmaceuticals. Ali K Abu-Alfa received speaking and consulting honoraria and/or research support from Astellas Pharma Inc., AstraZeneca, Baxter Healthcare, Boehringer Ingelheim, Fresenius Medical Care, Novartis and Sanofi-Aventis. His contribution to this publication was made in his individual capacity and not as part of his American University of Beirut or any other organization or institution duties or responsibilities. Mustafa Arici received speaker honoraria from Amgen, Astellas Pharma Inc., AstraZeneca, Bayer, Boehringer Ingelheim, Menarini, MSD, Novo Nordisk, Sandoz and Sanofi. He also received honoraria as an advisory board member for Astellas Pharma Inc., AstraZeneca, Bayer, Boehringer Ingelheim, MSD, and Novo Nordisk. Tevfik Ecder received speaker honoraria from Amgen, Astellas Pharma Inc., AstraZeneca, Bayer, Boehringer Ingelheim, Menarini, Recordati, Sandoz and Sanofi. The authors report no other conflicts of interest in this work.
References
- 1.Kaze AD, Ilori T, Jaar BG, Echouffo-Tcheugui JB. Burden of chronic kidney disease on the African continent: a systematic review and meta-analysis. BMC Nephrol. 2018;19(1):125. doi: 10.1186/s12882-018-0930-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lv JC, Zhang LX. Prevalence and disease burden of chronic kidney disease. Adv Exp Med Biol. 2019;1165:3–15. doi: 10.5414/cnp74s085 [DOI] [PubMed] [Google Scholar]
- 3.Shaheen FAM, Souqiyyeh MZ. Kidney health in the Middle East. Clin Nephrol. 2010;74(Suppl 1):S85–88. doi: 10.5414/cnp74s085 [DOI] [PubMed] [Google Scholar]
- 4.Al-Ghamdi SMG, Bieber B, AlRukhaimi M, et al. Diabetes prevalence, treatment, control, and outcomes among hemodialysis patients in the Gulf cooperation council countries. Kidney Int Rep. 2022;7(5):1093–1102. doi: 10.1016/j.ekir.2022.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong MG, Perkovic V, Chalmers J, et al. Long-term benefits of intensive glucose control for preventing end-stage kidney disease: ADVANCE-ON. Diabetes Care. 2016;39(5):694–700. doi: 10.2337/dc15-2322 [DOI] [PubMed] [Google Scholar]
- 6.Haynes R, Lewis D, Emberson J, et al. Effects of lowering LDL cholesterol on progression of kidney disease. J Am Soc Nephrol. 2014;25(8):1825–1833. doi: 10.1681/ASN.2013090965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mima A, Yasuzawa T, King GL, Ueshima S. Obesity‐associated glomerular inflammation increases albuminuria without renal histological changes. FEBS Open Bio. 2018;8(4):664–670. doi: 10.1002/2211-5463.12400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kovesdy CP, Furth SL, Zoccali C. Obesity and Kidney Disease. Can J Kidney Health Dis. 2017;4:2054358117698669. doi: 10.1177/2054358117698669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ricardo AC, Anderson CA, Yang W, et al. Healthy lifestyle and risk of kidney disease progression, atherosclerotic events, and death in CKD: findings from the chronic renal insufficiency cohort (CRIC) study. Am J Kidney Dis. 2015;65(3):412–424. doi: 10.1053/j.ajkd.2014.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heerspink HJL, Jongs N, Chertow GM, et al. Effect of dapagliflozin on the rate of decline in kidney function in patients with chronic kidney disease with and without type 2 diabetes: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021;9(11):743–754. doi: 10.1016/S2213-8587(21)00242-4 [DOI] [PubMed] [Google Scholar]
- 11.Sasso FC, Simeon V, Galiero R, et al. The number of risk factors not at target is associated with cardiovascular risk in a type 2 diabetic population with albuminuria in primary cardiovascular prevention. Post-hoc analysis of the NID-2 trial. Cardiovasc Diabetol. 2022;21(1):235. doi: 10.1186/s12933-022-01674-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mima A, Qi W, King GL. Implications of Treatment That Target Protective Mechanisms Against Diabetic Nephropathy. Semin Nephrol. 2012;32(5):471–478. doi: 10.1016/j.semnephrol.2012.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tabit CE, Shenouda SM, Holbrook M, et al. Protein Kinase-C Beta Contributes to Impaired Endothelial Insulin Signaling in Humans with Diabetes Mellitus. Circulation. 2013;127(1):86–95. doi: 10.1161/CIRCULATIONAHA.112.127514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 15.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. doi: 10.1056/NEJMoa1812389 [DOI] [PubMed] [Google Scholar]
- 16.Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–1446. doi: 10.1056/NEJMoa2024816 [DOI] [PubMed] [Google Scholar]
- 17.Staplin N, Roddick AJ, Emberson J, et al. Net effects of sodium-glucose co-transporter-2 inhibition in different patient groups: a meta-analysis of large placebo-controlled randomized trials. EClinicalMedicine. 2021;41:101163. doi: 10.1016/j.eclinm.2021.101163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. doi: 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 19.Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413–1424. doi: 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 20.Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–1461. doi: 10.1056/NEJMoa2107038 [DOI] [PubMed] [Google Scholar]
- 21.Shabaka A, Cases-Corona C, Fernandez-Juarez G. Therapeutic insights in chronic kidney disease progression. Front Med. 2021;8:645187. doi: 10.3389/fmed.2021.645187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ameh OI, Ekrikpo U, Bello A, Okpechi I. Current management strategies of chronic kidney disease in resource-limited countries. Int J Nephrol Renov Dis. 2020;13:239–251. doi: 10.2147/IJNRD.S242235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ravera M, Noberasco G, Weiss U, et al. CKD awareness and blood pressure control in the primary care hypertensive population. Am J Kidney Dis. 2011;57(1):71–77. doi: 10.1053/j.ajkd.2010.08.022 [DOI] [PubMed] [Google Scholar]
- 24.Nichols GA, Déruaz-Luyet A, Brodovicz KG, Kimes TM, Rosales AG, Hauske SJ. Kidney disease progression and all-cause mortality across estimated glomerular filtration rate and albuminuria categories among patients with vs. without type 2 diabetes. BMC Nephrol. 2020;21(1):167. doi: 10.1186/s12882-020-01792-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsushita K, Coresh J, Sang Y, et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3(7):514–525. doi: 10.1016/S2213-8587(15)00040-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El Achhab Y, Nazek L, Maalej M, Alami M, Nejjari C. Prevalence, control and risk factors related to hypertension among Moroccan adults: a multicentre study. East Mediterr Health J Rev Sante Mediterr Orient Al-Majallah Al-Sihhiyah Li-Sharq Al-Mutawassit. 2019;25(7):447–456. doi: 10.26719/emhj.18.057 [DOI] [PubMed] [Google Scholar]
- 27.Benghanem Gharbi M, Elseviers M, Zamd M, et al. Chronic kidney disease, hypertension, diabetes, and obesity in the adult population of Morocco: how to avoid “over”- and “under”-diagnosis of CKD. Kidney Int. 2016;89(6):1363–1371. doi: 10.1016/j.kint.2016.02.019 [DOI] [PubMed] [Google Scholar]
- 28.Thomas B, Matsushita K, Abate KH, et al. Global cardiovascular and renal outcomes of reduced GFR. J Am Soc Nephrol. 2017;28(7):2167–2179. doi: 10.1681/ASN.2016050562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kondo M, Yamagata K, Ling HS, et al. Cost-effectiveness of chronic kidney disease mass screening test in Japan. Clin Exp Nephrol. 2012;16(2):279–291. doi: 10.1007/s10157-011-0567-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Go DS, Kim SH, Park J, Ryu DR, Lee HJ, Jo MW. Cost-utility analysis of the National Health Screening Program for chronic kidney disease in Korea. Nephrol Carlton Vic. 2019;24(1):56–64. doi: 10.1111/nep.13203 [DOI] [PubMed] [Google Scholar]
- 31.American Diabetes Association Professional Practice Committee. Chronic Kidney Disease and Risk Management: standards of Medical Care in Diabetes—2023. Diabetes Care. 2021;46(Supplement_1):S191–S202. doi: 10.2337/dc23-S011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kidney Health Australia. Chronic Kidney Disease (CKD) Management in Primary Care; 2020. Available from: https://kidney.org.au/uploads/resources/CKD-Management-in-Primary-Care_handbook_2020.1.pdf. Accessed May 24, 2022.
- 33.KDIGO. Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease; 2012. Available from: https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf. Accessed August 2, 2022.
- 34.Shlipak MG, Tummalapalli SL, Boulware LE, et al. The case for early identification and intervention of chronic kidney disease: conclusions from a Kidney Disease: improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2021;99(1):34–47. doi: 10.1016/j.kint.2020.10.012 [DOI] [PubMed] [Google Scholar]
- 35.Inker LA, Astor BC, Fox CH, et al. KDOQI US Commentary on the 2012 KDIGO Clinical Practice Guideline for the Evaluation and Management of CKD. Am J Kidney Dis. 2014;63(5):713–735. doi: 10.1053/j.ajkd.2014.01.416 [DOI] [PubMed] [Google Scholar]
- 36.AstraZeneca Pharma India. Indian Society of Nephrology (ISN) and AstraZeneca India join hands this World Kidney Day to fulfil the theme of “Kidney Health for All”. Available from: https://www.prnewswire.com/in/news-releases/indian-society-of-nephrology-isn-and-astrazeneca-india-join-hands-this-world-kidney-day-to-fulfil-The-theme-of-kidney-health-for-all--893523744.html. Accessed August 22, 2022.
- 37.Al-Ghamdi S, Abu-Alfa A, Alotaibi T, et al. Chronic Kidney Disease Management in the Middle East and Africa: concerns, Challenges, and Novel Approaches. Int J Nephrol Renov Dis. 2023;16:103–112. doi: 10.2147/IJNRD.S363133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heerspink HJL, Karasik A, Thuresson M, et al. Kidney outcomes associated with use of SGLT2 inhibitors in real-world clinical practice (CVD-REAL 3): a multinational observational cohort study. Lancet Diabetes Endocrinol. 2020;8(1):27–35. doi: 10.1016/S2213-8587(19)30384-5 [DOI] [PubMed] [Google Scholar]
- 39.Nagasu H, Yano Y, Kanegae H, et al. Kidney outcomes associated with SGLT2 inhibitors versus other glucose-lowering drugs in real-world clinical practice: the Japan chronic kidney disease database. Diabetes Care. 2021;44(11):2542–2551. doi: 10.2337/dc21-1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mosenzon O, Wiviott SD, Cahn A, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7(8):606–617. doi: 10.1016/S2213-8587(19)30180-9 [DOI] [PubMed] [Google Scholar]
- 41.Cahn A, Raz I, Leiter LA, et al. renal, and metabolic outcomes of dapagliflozin versus placebo in a primary cardiovascular prevention cohort: analyses from DECLARE-TIMI 58. Diabetes Care. 2021;44(5):1159–1167. doi: 10.2337/dc20-2492 [DOI] [PubMed] [Google Scholar]
- 42.Cannon CP, Pratley R, Dagogo-Jack S, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383(15):1425–1435. doi: 10.1056/NEJMoa2004967 [DOI] [PubMed] [Google Scholar]
- 43.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. doi: 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- 44.Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–334. doi: 10.1056/NEJMoa1515920 [DOI] [PubMed] [Google Scholar]
- 45.Pasternak B, Wintzell V, Melbye M, et al. Use of sodium-glucose co-transporter 2 inhibitors and risk of serious renal events: Scandinavian cohort study. THE BMJ. 2020;369:m1186. doi: 10.1136/bmj.m1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mima A. Mitochondria-targeted drugs for diabetic kidney disease. Heliyon. 2022;8(2):e08878. doi: 10.1016/j.heliyon.2022.e08878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mima A. A Narrative Review of Diabetic Kidney Disease: previous and Current Evidence-Based Therapeutic Approaches. Adv Ther. 2022;39(8):3488–3500. doi: 10.1007/s12325-022-02223-0 [DOI] [PubMed] [Google Scholar]
- 48.Mima A. Sodium-Glucose Cotransporter 2 Inhibitors in Patients with Non-Diabetic Chronic Kidney Disease. Adv Ther. 2021;38(5):2201–2212. doi: 10.1007/s12325-021-01735-5 [DOI] [PubMed] [Google Scholar]
- 49.Mima A. Renal protection by sodium-glucose cotransporter 2 inhibitors and its underlying mechanisms in diabetic kidney disease. J Diabetes Complications. 2018;32(7):720–725. doi: 10.1016/j.jdiacomp.2018.04.011 [DOI] [PubMed] [Google Scholar]
- 50.Oshima M, Neuen BL, Li J, et al. Early Change in Albuminuria with Canagliflozin Predicts Kidney and Cardiovascular Outcomes: a Post Hoc Analysis from the CREDENCE Trial. J Am Soc Nephrol JASN. 2020;31(12):2925–2936. doi: 10.1681/ASN.2020050723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 52.Martinez FA, Serenelli M, Nicolau JC, et al. Efficacy and safety of dapagliflozin in heart failure with reduced ejection fraction according to age. Circulation. 2020;141(2):100–111. doi: 10.1161/CIRCULATIONAHA.119.044133 [DOI] [PubMed] [Google Scholar]
- 53.Persson F, Rossing P, Vart P, et al. Efficacy and safety of dapagliflozin by baseline glycemic status: a prespecified analysis from the DAPA-CKD trial. Diabetes Care. 2021;44(8):1894–1897. doi: 10.2337/dc21-0300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Provenzano M, Jongs N, Vart P, et al. The kidney protective effects of the sodium-glucose cotransporter-2 inhibitor, dapagliflozin, are present in patients with CKD treated with mineralocorticoid receptor antagonists. Kidney Int Rep. 2022;7(3):436–443. doi: 10.1016/j.ekir.2021.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heerspink HJL, Perkins BA, Fitchett DH, Husain M, Cherney DZI. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus. Circulation. 2016;134(10):752–772. doi: 10.1161/CIRCULATIONAHA.116.021887 [DOI] [PubMed] [Google Scholar]
- 56.Cherney DZI, Repetto E, Wheeler DC, et al. Impact of cardio-renal-metabolic comorbidities on cardiovascular outcomes and mortality in type 2 diabetes mellitus. Am J Nephrol. 2020;51(1):74–82. doi: 10.1159/000504558 [DOI] [PubMed] [Google Scholar]
- 57.Liu T, Li R, Wang X, Gao X, Zhang X. Benefits of SGLT2 inhibitors combining with renin-angiotensin-system blockers on cardiovascular outcomes in chronic kidney disease patients: a systemic review and meta-analysis. Med Clin. 2021;S0025-7753(21):647. doi: 10.1016/j.medcli.2021.09.031 [DOI] [PubMed] [Google Scholar]
- 58.Zhou Z, Chaudhari P, Yang H, et al. Healthcare resource use, costs, and disease progression associated with diabetic nephropathy in adults with type 2 diabetes: a retrospective observational study. Diabetes Ther Res Treat Educ Diabetes Relat Disord. 2017;8(3):555–571. doi: 10.1007/s13300-017-0256-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169(12):1156–1162. doi: 10.1001/archinternmed.2009.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kraft MD, Btaiche IF, Sacks GS, Kudsk KA. Treatment of electrolyte disorders in adult patients in the intensive care unit. Am J Health Syst Pharm. 2005;62(16):1663–1682. doi: 10.2146/ajhp040300 [DOI] [PubMed] [Google Scholar]
- 61.Dhondup T, Qian Q. Electrolyte and acid-base disorders in chronic kidney disease and end-stage kidney failure. Blood Purif. 2017;43(1–3):179–188. doi: 10.1159/000452725 [DOI] [PubMed] [Google Scholar]
- 62.Luo J, Brunelli SM, Jensen DE, Yang A. between serum potassium and outcomes in patients with reduced kidney function. Clin J Am Soc Nephrol. 2016;11(1):90–100. doi: 10.2215/CJN.01730215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hou YC, Zheng CM, Yen TH, Lu KC. Molecular mechanisms of SGLT2 inhibitor on cardiorenal protection. Int J Mol Sci. 2020;21(21):7833. doi: 10.3390/ijms21217833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dalal R, Bruss ZS, Sehdev JS. Physiology, Renal Blood Flow and Filtration. Treasure Island (FL): StatPearls Publishing; 2022. http://www.ncbi.nlm.nih.gov/books/NBK482248/. Accessed 25 May 2022. [PubMed] [Google Scholar]
- 65.Kidney Disease: Improving Global Outcomes (KDIGO). Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease; 2022. Available from: https://kdigo.org/wp-content/uploads/2022/03/KDIGO-2022-Diabetes-Management-GL_Public-Review-draft_1Mar2022.pdf. Accessed May 24, 2022.
- 66.The National Institute for Health and Care Excellence (NICE). Recommendations | dapagliflozin for treating chronic kidney disease | guidance. Available from: https://www.nice.org.uk/guidance/ta775/chapter/1-Recommendations. Accessed May 25, 2022.
- 67.Cheung AK, Chang TI, Cushman WC, et al. KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2021;99(3):S1–87. doi: 10.1016/j.kint.2020.11.003 [DOI] [PubMed] [Google Scholar]
- 68.Mende CW. Chronic kidney disease and SGLT2 Inhibitors: a review of the evolving treatment landscape. Adv Ther. 2022;39(1):148–164. doi: 10.1007/s12325-021-01994-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bakris GL, Agarwal R, Anker SD, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383(23):2219–2229. doi: 10.1056/NEJMoa2025845 [DOI] [PubMed] [Google Scholar]
- 70.Long A, Salvo M. Finerenone: a novel mineralocorticoid receptor antagonist for cardiorenal protection in CKD and T2DM. Ann Pharmacother. 2022;10600280211059576. doi: 10.1177/10600280211059577 [DOI] [PubMed] [Google Scholar]
- 71.Jiang X, Zhang Z, Li C, et al. Efficacy and Safety of Non-Steroidal Mineralocorticoid Receptor Antagonists in Patients With Chronic Kidney Disease and Type 2 Diabetes: a Systematic Review Incorporating an Indirect Comparisons Meta-Analysis. Front Pharmacol. 2022;13:896947. doi: 10.3389/fphar.2022.896947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heerspink HJL, Parving HH, Andress DL, et al. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet Lond Engl. 2019;393(10184):1937–1947. doi: 10.1016/S0140-6736(19)30772-X [DOI] [PubMed] [Google Scholar]
- 73.McCoy RG, Dykhoff HJ, Sangaralingham L, et al. Adoption of New Glucose-Lowering Medications in the U.S.—The Case of SGLT2 Inhibitors: nationwide Cohort Study. Diabetes Technol Ther. 2019;21(12):702–712. doi: 10.1089/dia.2019.0213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lam D, Shaikh A. Real-Life Prescribing of SGLT2 Inhibitors: how to Handle the Other Medications, Including Glucose-Lowering Drugs and Diuretics. Kidney360. 2021;2(4):742–746. doi: 10.34067/KID.0000412021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rivera AV, Wang XA, Grodsky M, Gandhi S, Karaisz F, Y LEV. 1499-PUB: increasing Utilization of SGLT2 Inhibitors for Cardiac Patients. Diabetes. 2022;71(Supplement_1):1499–PUB. doi: 10.2337/db22-1499-PUB [DOI] [Google Scholar]
- 76.Ng NM, Ng YS, Chu TK, Lau P. Factors affecting prescription of sodium-glucose co-transporter 2 inhibitors in patients with type 2 diabetes mellitus with established cardiovascular disease/ chronic kidney disease in Hong Kong: a qualitative study. BMC Prim Care. 2022;23(1):317. doi: 10.1186/s12875-022-01928-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Warden BA, Steiner J, Camacho A, et al. Optimizing sodium-glucose co-transporter 2 inhibitor use in patients with heart failure with reduced ejection fraction: a collaborative clinical practice statement. Am J Prev Cardiol. 2021;6:100183. doi: 10.1016/j.ajpc.2021.100183 [DOI] [PMC free article] [PubMed] [Google Scholar]