Abstract
This work aimed to identify the mechanisms by which taurine exerts its anti-obesity effects in the C57BL/6J ob/ob mice model and determine if taurine supplementation increases the amelioration of inflammation and lipogenesis linked genes in the adipose and liver tissues. Three groups of C57BL/6J mice were fed a standard chow diet for a period of 10 weeks the C57BL/6J normal group, the C57BL/6J ob/ob negative control group with no taurine intake and C57BL/6J ob/ob taurine group with taurine intake. Real time PCR was used to examine the gene expression profile in the experimental groups intrascapular brown adipose tissue (BAT), inguinal white adipose tissue (WAT) and liver. TNF-alpha, Ccl2, Adgre and illb genes that are associated with inflammation were found to have varying level of expression in the three tissues. In comparison to BAT and liver these genes were expressed at a much lower level in WAT, with enhanced serum adiponectin levels.
Keywords: Fatty acid synthetase, Inflammation, Obesity, Fatty liver, Lipogenesis
Highlights
-
•
Investigated taurine's anti-obesity effects in C57BL/6J ob/ob mice.
-
•
Taurine reduced obesity by lowering inflammation, lipid synthesis, and increasing adiponectin levels.
-
•
Further research required to uncover mechanisms and therapeutic potential.
1. Introduction
The most abundant free amino acid discovered in mammalian cells is taurine, a noteworthy amino acid with a sulfur group that appears in millimolar amounts [1,2]. Taurine is a crucial component of many physiological and biological processes, including osmoregulation, calcium modulation, salt conjugation, membrane stabilization, immunomodulation, and anti-oxidation. Its concentration in cells can range from 2 to 20 mol/g of wet weight in different tissues, including the brain, heart, and skeletal muscles [3]. The prime organs for synthesizing taurine endogenously by utilizing cysteine and methionine are the liver and kidneys where it is responsible for the critical role of bile acid conjugation and maintenance of normal osmotic pressure respectively [4,5]. The enzymes responsible for the critical role of taurine synthesis, cysteine dioxygenase (CDO) and cysteine sulfinic acid decarboxylase (CSDA) are found in subtle amounts in other tissues thus producing lower amount of taurine compared to the liver and kidneys where there excess amount retains [6,7]. Surprisingly, the white adipose tissues around the rats kidneys and liver -the perirenal and epididymal regions-have higher levels of the CDO enzyme and thus higher level of taurine synthesis [8,9]. It is possible that taurine plays a crucial physiological role in the adipose tissues, liver, and kidneys because active taurine synthesis in the white adipose tissues tends to decrease in obese state thus suggesting a positive link between taurine deficit state and obese conditions [9].
Animal studies have shown that taurine has a strong anti-obesity impact, but the dosages employed to treat obese animals are not suitable for human intake as a nutritional supplement [10]. The fact that clinical research on humans has not been able to conclusively demonstrate taurine's ability to reduce obesity can be due to the lack of physiologically practical dose. Due to their hereditary propensity to become obese and in response to consuming a high fat diet (HFD), C57BL/6J ob/ob mouse models are utilized to imitate obese conditions [10,11]. These ob/ob mice frequently experience pre-diabetic symptoms such as obesity, elevated blood triglycerides levels, and altered glucose homeostasis [11,12]. Therefore, the ob/ob mouse model offers a promising way to study the positive effects of taurine of taurine on obesity and its associated diseases.
The anti-obesity impact of taurine has been highlighted and studied in both human clinical trials and animal models, besides other positive effects [[10], [11], [13]]. It was discovered that taurine intake was inversely associated to Body mass index (BMI) and slowed down the onset of obesity via several mechanisms [14,15]. By consuming taurine, oxidative stress and inflammatory indicators brought on by obesity tend to decline in adipocytes [16]. Previous research describing the minute levels of taurine in human neutrophils, where it combines with the enzyme myeloperoxidase to generate taurine chloride (TauCl), which regulates the synthesis of various pro-inflammatory cytokines, have shed light n the anti-inflammatory benefits of taurine [17,18]. The ability of adipocytes, which are specialized lipids-rich cells found in adipose tissues, to store lipids was formerly thought to be their exclusive function, but now are believed to function as an endocrine organ and produce a variety of active compounds [19,20]. Adipose tissue secretes several adipokines, like adiponectin, which regulates the metabolism of nutrients and has anti-diabetic, anti-hypertensive, anti-atherogenic, anti-inflammatory, and stress response capabilities [21]. Adenosine monophosphate-activated protein kinase (AMPK) activation, elevated fatty acids translocase,acyl-CoA oxidase, and uncoupling protein 2 (UCP 2) levels have all been linked to increased adiponectin levels boosting insulin sensitivity [22]. It is widely known that as obesity develops, adipocytes exhibit hypertrophy, which compromises the production and secretion of adiponectin and contributes to the pathogenesis of obesity, diabetes, endoplasmic reticulum (ER) stress, and lipotoxicity [23]. Chronic inflammation associated with increased macrophage infiltration and pro-inflammatory cytokines production (Tumor necrosis factor-alpha (TNF-alpha), interleukin-6 (IL-6), monocyte chemoattractant protein-1 (MCP-1)) are characteristics of the obese state, which in turn encourages the recruitment of extra immune cells to the site of inflammation [[24], [25], [26], [27], [28], [29], [30]].
Obesity is recognized to play a significant role in the development of non-alcoholic fatty liver disorders (NAFLD), in addition to other chronic diseases [31]. Beginning with the buildup of triglyceride (TG) content, NAFLD develops to produce liver inflammation, fibrosis, hepatocellular carcinoma, cirrhosis, and non-alcoholic steatohepatitis (NASH) [31,32]. In rodents, TG buildup in the hepatocytes is the primary cause of hepatic steatosis [33]. The elevated serum free fatty acids produced by lipolysis in the adipose tissue, excessive dietary fatty acids intake, elevated DE novo lipid synthesis, impaired mitochondrial beta oxidation, or a slower rate of very low-density lipoprotein (VLDL) clearance may all be responsible for the increased hepatic fat content [34]. In order to cure NAFLD, it may be possible to target the modification of important transcriptional factors, enzymes, and genes involved in hepatic lipid metabolism [35]. To reduce obesity without creating any negative side effects, increasing the WAT bulk or its activity has therefore emerged as a promising technique. As a result, many scientists have tried to identify dietary substances that lessen the formation of lipids and triglycerides as well as inflammation. However, the effects of dietary taurine on obesity and its associated comorbidities are yet to be explored in ob/ob mice. Our study for the first time explores the link among taurine intake, adipose tissue inflammation, lipogenesis, and alleviation of fatty liver via regulation of genes in the adipose tissues thus highlighting the mechanism behind the crucial role of adipocytes in causing obesity induced fatty liver diseases.
2. Materials and methods
2.1. Animal and diets
Twenty-four male, one-week old C57BL/6J mice were purchased from Shizuoka Laboratory Center (Shizuoka, Japan) and were randomly subdivided into three groups C57BL/6J ob/ob mice (n = 16) and C57BL/6J mice (n = 8) as normal control, housed in a specific pathogen free (SPF) facility with a 12h light/dark cycle, and given chow diet (Purina Lab diets Louis, MO, USA) access to food and water. The first group, which consisted of C57BL/6J mice was called the Normal group (n = 8). The second group, which consisted of leptin knockout ob/ob mice, was called the Negative control group (n = 8). The third group, which consisted of ob/ob mice that were given 2 g/bodyweight of taurine as a supplement, was called the Taurine group (T = 8). Mice in the taurine group received oral taurine (2 g/BW) from Sigma Aldric (St. Louis, MO, USA) for 10 weeks Saline water containing taurine was provided as oral tubing for the taurine supplemented group and normal saline water for the normal and negative control groups. The study protocol was approved by the Institutional Animal Care and Use committee of WOOJUNGBIO institute (Gyeonggi-do, Seoul) under registration number 20190225-1-23.
2.2. Food intake and metabolic parameters
For a period of 10 weeks, mice body weight was recorded each day. Intake of food, drink consumption, and activity were averaged individually during the light and dark hours. The rodents were fed a regular chow diet, were housed in 12-h light-dark cycles and had unlimited access to water.
2.3. Body composition and biochemical parameters
The liver, white adipose tissue (WAT), and brown adipose tissue (BAT) of the mice were collected for histopathological investigation using hematoxylin and eosin (H & E) staining after fixation in 10 % formalin when the mice were 80+ days old and had fasted for 8 h. Fat tissues were first sliced into pieces after being fixed in 10 % neutral buffered formalin for a day. After that, the slices were fixed in paraffin for histological examination. The samples were then cut into slices using an Accu-cut SRM microtome from Sakura (Japan), mounted on d-polyline coated slides from Thermo Fisher Scientific (USA), deparaffinized in xylene, and stained with H &E to measure adipocyte size. Using Image J software (NIH), the cross-sectional area of 100 adipocytes from eight mice in each group was assessed to determine the size of the adipocytes. Then, the adipocyte cells area was determined using the Image J program's float morphology (https://rsb.info.nih.gov/ij/). The mice's body weight was checked every week for 80 days during this time. For analysis, the mice were not constrained in the animal holder to allow for unfettered movement. Additionally, masses of subcutaneous, epididymal, mesenteric, and peritoneal fat were gathered and weighed. To assess adipokine levels, lipid profiles, and fasting plasma glucose and insulin levels, blood samples were taken. A commercial kit (Asan Pharmaceutical Co., Seoul, Korea) was used to measure plasma glucose levels. To measure the levels of insulin, adipokines, and pro-inflammatory cytokines, the blood was centrifuged at 10,000 g for 10 min at 4°. The serum that was collected was then tested using ELISA kit. Using commercial kits (Asan Pharmaceuticals Co., Seoul, Korea), the levels of plasma triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C) were examined. The data was displayed as serum mg/dL. Insulin resistance was calculated by Homeostatic Model Assessment for Insulin Resistance using formula HOMA-IR = [fasting plasma insulin (ng/ml) = [fasting plasma insulin (ng/mL) × fasting plasma glucose (mg/dL)]/405. The formula [(TC-HDL-C)/HDL-C] was also used to determine the atherogenic index (AI). Using commercial kits from Abcam, UK, the plasma concentrations of heme oxygenase-1 (HO-1), transferrin, and ferritin were measured. High sensitivity C-reactive protein (hs-CRP; Mybiosource, San Diego, CA, USA) and IL-6 (Invitrogen, CA, USA) were also assessed. Plasma insulin and adiponectin levels were determined using Alpco kits (Alpco diagnostics, Windham, NH). The manufacturer's instructions were followed when using each kit.
2.4. Tissue harvesting from mice
The heart, kidneys, intestine, and liver tissues were removed from euthanized mice by cervical dislocation, immediately frozen in liquid nitrogen, and maintained at-80° for further examination. Trizol (Thermo Fisher Scientific Korea, Seoul, Korea) was used to extract total RNA from the collected WAT, BAT, and liver tissues.
2.5. Evaluation of liver function
By examining the levels of total fat, TG, and total cholesterol in the liver tissues as well as the serum alanine aminotransferase (ALT) and aspartate amino transferase (AST) activities, liver function was assessed. Using commercial kits and following the manufacturer's instructions, serum ALT and AST levels were measured using spectrophotometry (Asan Pharmaceuticals Co., Seoul, Korea). The expression of genes involved in the metabolism of cholesterol, such as 3-hydroxy-3-methylglutaryl-CoA reductase (Hmgcr), low-density lipoprotein receptor (Ldlr), sterol regulatory element binding protein 2 (Sreb2), lipogenesis (sterol regulatory element binding protein (Srebf1), fatty acid synthetase (Fasn) and lipid oxidation related genes carnitine palmitoyl transferase 1A (Cpt1a), peroxisomal acyl-coenzyme A oxidase 1 (Acox1), and peroxisome proliferator-activated receptor gamma coactivator 1 alpha (Ppargc1a) were measured. The tissue samples extracted were immediately frozen in liquid nitrogen at −80° for later examination.
2.6. Quantitative real-time PCR
Tissue samples (WAT, BAT and liver) were processes for total RNA extraction using a Bandelin tissue homogenizer (Bandelin electrical GmbH, Berlin, Germany), and total RNA was isolated according to the manufacturers protocol. Additionally, using a thermal cycler (Takara PCR Thermal cycler SP. Takara Shuzo, Shiga, Japan), total RNA was translated using MMLV reverse transcriptase (Invitrogen Corp. CA, USA) in accordance with the protocol. With SYBR green fluorescence dye (Thermo Fisher Scientific) standardized by the Rplp0 gene, quantitative real time PCR (RT-PCR) was carried out utilizing a BIORAD CFX96 Real time PCR equipment (C1000 Thermal Cycler, USA) to measure mRNA expression. Supporting Table 1 provides a description of each primer sequence.
2.7. Western blotting
The tissues were extracted and homogenized using a lysis solution containing 1 % SDS,50 mM Tris-HCl (pH = 6.8), 10 mM DTT,10 % glycerol,0.002 % bromophenol blue, 1 mM sodium fluoride (Sigma),1 mM sodium orthovanadate (Sigma), and a protease inhibitor combination (Roche Applied Science). SDS-PAGE was used to separate identical amounts of proteins, which were then transferred to a polyvinylidene difluoride membrane (Millipore Corp. Bedford, MA), Membranes were blocked for 1 h at 20 °C using 5 % skim milk in Tris-buffered saline with 0.1 % Tween-20 (TBS-T). They were then incubated with the primary antibodies listed below for an additional night at 4 °C, purchased from Thermo Fisher: TNF-alpha (cat. no. PA1-40281; 1:1000), Interleukin 1 beta (Il1b; cat. no. P420B; 1:1000), Uncoupling protein 1 (UCP1 cat. no. pa5-29575; 1:1000), Adhesion G-protein receptor (Adgre ca. t no.14-4801-82; 1:1000), CCL2 (cat. no. pa5-46954; 1:1000), and β-actin: (cat. no. ab8226; 1:1000). Membranes were incubated for 1 h at room temperature with goat anti-rabbit immunoglobulin G (1:5000; cat. no. CL7802AP; Cedarlane Laboratories, Burlington, Canada) conjugated to horseradish peroxidase. Membranes were prepared for autoradiography using enhanced chemiluminescence (Pierce; Thermo Fisher Scientific, Inc.) after five TBS-T washes. GE Healthcare Life Sciences, Little Chalfont, UK, used Image Quant (version LAS-4000) for densitometric analysis. Triples of each experiment were run.
2.8. Statistical analysis
The outcomes are displayed as mean SEM. One-way analysis of variance (ANOVA) and Duncan's post hoc test (P < 0.05) were used to evaluate the data using SPSS 26.0 software (SPSS, San Diego, CA). For statistical analysis and graphing, Prism software v.5 (GraphPad Software, San Diego, CA, USA) was employed. At P < 0.05, differences were deemed statistically significant.
3. Results
3.1. Food intake analysis and anti-obesity effects of taurine
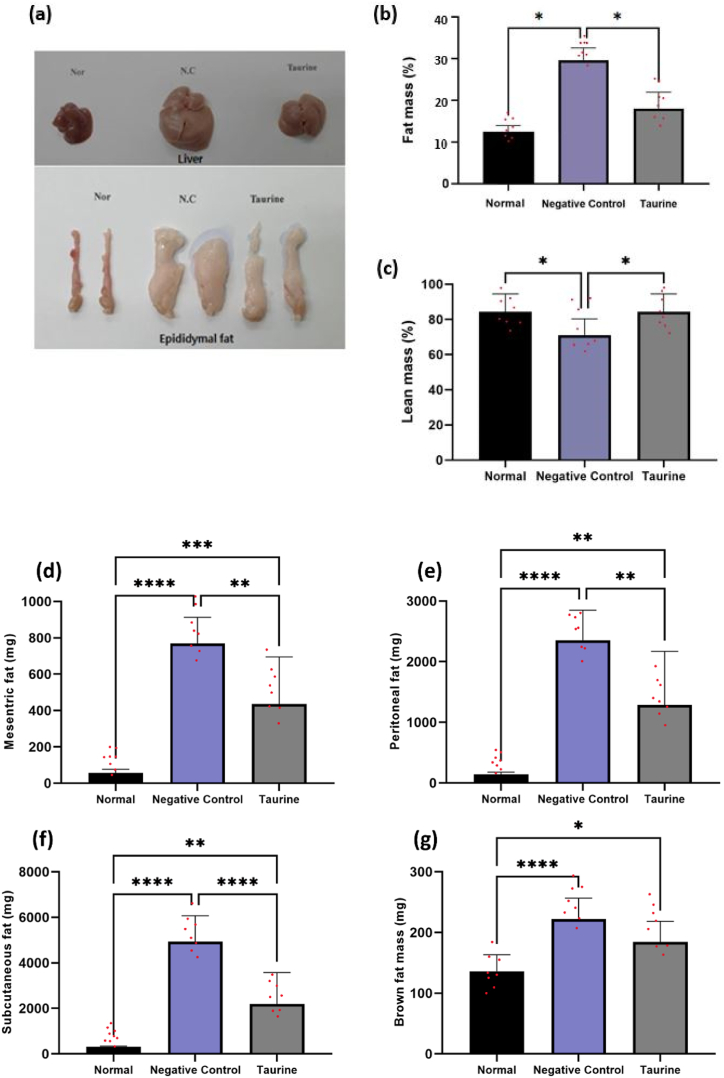
Leptin knockout, C57BL/6J ob/ob mice for a period of twelve weeks were used to replicate human obesity. In contrast to the normal and taurine supplemented group, our findings indicated that these negative control ob/ob mice had significantly increased bodyweight throughout the course of 10 weeks. As seen in Fig. 1 (a), throughout the course of 10 weeks, the taurine group considerably reduced the gain in bodyweight. The food intake data demonstrated that feed intake rose each day in the negative control ob/ob mice relative to that in the normal group, and that it decreased with taurine supplementation (Fig. 1 b). In the ob/ob negative control group mice, the body and fat pad weight tended to rise, which was slowed by taurine intake, as depicted in Fig. 1 (c). The mean body weights (mean ± SEM) of the negative control ob/ob mice and normal mice were 48.2 ± 5.4 g and 29.8 ± 2.7 g, respectively. Taurine supplementation to the ob/ob mice induced weight loss in taurine group ob/ob mice compared to the negative control ob/ob mice (40.6 ± 9.6 g vs 48.2 ± 5.4 g). In accordance with the body weight change (Fig. 1 (d)), body composition analysis showed that the fat mass of negative control ob/ob mice significantly increased to 14.6 ± 3.8 % of body weight from 8.1 ± 1.8 % of the normal group mice (Fig. 2(a–i). Taurine supplementation reversed the fat mass buildup to 7.0 ± 7.1 %. According to the change in bodyweight, body composition analysis revealed that the fat mass (subcutaneous, epididymal, mesenteric, and peritoneal WAT and BAT tissues) and liver weight increased significantly in the negative control group. This change in body composition was significantly reversed by taurine supplementation in the taurine-treated group. Together, these results imply that taurine administration may cause loss of fat mass in ob/ob mice.
Fig. 1.
Effect of taurine on food intake and body weight. Food intakes, body weights (BW), weights of C57BL/6J ob/ob mice treated without taurine (negative control ob/ob group) or with taurine (taurine ob/ob group) for 10 weeks and compared with the normal control group (Normal diet). Values are Mean ± SD. Differences among groups were assessed by one-way ANOVA with a Duncan's test. Means that do not share a common letter are significantly different at P < 0.05. Initial BW, initial body weight; Final BW, final body weight.
Fig. 2.
Body organ weight of the three groups was analyzed using digital balance after 10 weeks. Differences among groups were assessed by one-way ANOVA with Duncan's test. Means that do not share a common letter are significantly different at P < 0.05.
3.2. Effect of taurine on adipocyte cellularity
Our research revealed a substantial difference in total bodyweight and fat mass between our negative control ob/ob mice treated with taurine; hence, we intended to investigate the adiposity dependent mechanism of taurine in reducing adipose cellularity (Fig. 3 (b)). Hematoxylin and eosin staining of adipose and liver tissues was done and it was demonstrated that taurine supplementation reduced adipocytes size overall. Fig. 3 (a) shows that the taurine-treated ob/ob mice group had cells with the same size as control group and were smaller than the negative control ob/ob mice. Additionally, taurine therapy decreased the number of large and medium-sized adipocytes (50–100 μm2 and 40-50-102μm2). Fig. 3 (c) shows that taurine supplementation also decreased the number of large sized fat cells in the liver of mice.
Fig. 3.
Histological changes in adipocyte cell size upon taurine supplementation in C57BL/6J ob/ob mice. Representative images of hematoxylin and eosin–stained sections from epididymal adipose tissue (b) and liver (c) are shown—quantification of mean cross-sectional adipocyte area from epididymal adipose tissue has been shown in Fig. 3 (a).
3.3. Biochemical analysis
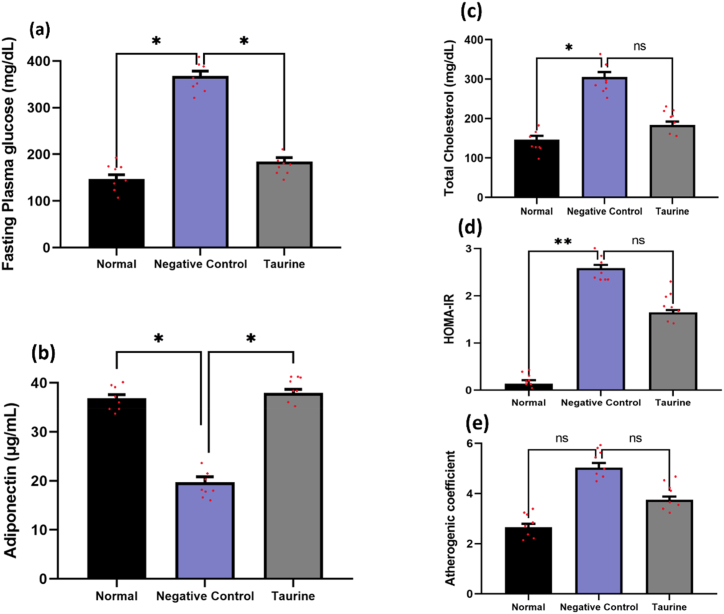
Compared to the normal group, the fasting negative control ob/ob group depicted hyperglycemia. Fig. 4 (a) demonstrates that taurine supplementation significantly decreased the fasting blood glucose levels (P < 0.05). Additionally, when compared to the negative control group, taurine supplementation significantly raised serum adiponectin levels in the taurine group (P < 0.05) Fig. 4 (b). A decrease in the trend of total cholesterol, HOMA-IR, and atherogenic coefficient was also seen after taurine supplementation as compared to the negative control mice group Fig. 4(c–e).
Fig. 4.
Effect of taurine on glycemic indices and lipids profile in mice. Values are expressed in mean ± SD. Differences among groups were assessed using one-way ANOVA with Duncan's test. Significantly different values are considered at P < 0.05. HOMA-IR, homeostatic model assessment-insulin resistance; TG, triglyceride; TC, total cholesterol.
3.4. Effect of taurine on the inflammatory enzymes in liver of C57BL/6J mice
The impact of taurine on inflammation related enzymes in liver was assessed. Plasma AST levels were elevated in the negative control ob/ob group (162 ± 65.2 IU/L) and significantly decreased with taurine supplementation in the taurine ob/ob group (102.8 ± 69.7 IU/L) Fig. 6 (a). In addition, as seen in Fig. 6 (b), taurine supplementation reduced serum IL-6 levels in comparison to the negative control group. Hence these results suggest that taurine intake can lead to the suppression of inflammatory enzymes.
Fig. 6.
Effect of taurine on liver functions and inflammatory status in mice. Values are expressed in mean ± SD. Differences among groups were assessed using one-way ANOVA with Duncan's test. Significantly different values are considered at P < 0.05. AST, aspartate aminotransferase; IL-6, Interleukin-6.
3.5. Effect of taurine on lipid oxidation and lipogenesis related genes in fat and liver tissues of C57BL/6J mice
The Mrna expression level of Cpt1a, Acox1, Ppargc1a, Srebf1, Scd1, Nr1h2, and Nr1h3 were evaluated in the WAT, BAT, and liver to determine whether taurine influences the expression of genes linked to lipid oxidation and lipogenesis. As shown in Fig. 7(a–i), in the liver of taurine treated ob/ob group the expression of genes involved in DE novo lipogenesis such as sterol regulatory element binding protein (Srebf1) showed a reducing trend compared to the negative control group. Similar decreasing trend was observed in case of Srebf1 in WAT only. When compared to the untreated and normal groups, the taurine treated group, though not significant, had the higher levels of the hepatic Mrna expression of lipid peroxidation enzyme peroxisome proliferator-activated receptor gamma coactivator 1-alpha (Ppargc1a). The increased expression of this gene in negative control ob/ob mice can be explained by the elevated level of triglycerides and lipids caused by the obese state, which can result in an enhanced supply of the lipids for oxidation purposes. The gene also showed enhanced expression in the WAT compared to normal group. While no such trend was observed in BAT. The acyl-CoA oxidase 1 (Acox1), Stearoyl CoA desaturase 1 (SCD1), Nuclear receptor subfamily (Nr1h3), Nuclear receptor subfamily (Nr1h2), Diacylglycerol O-acyltransferase 2 (Dgat2), MLX interacting protein-like (Mlxipl), genes involved in lipids synthesis and oxidation showed increased expression pattern in WAT compared to the liver and BAT where it showed no such expression. All these findings point to taurine's potential to increase lipid peroxidation and decrease lipids synthesis in the WAT of ob/ob mice by stimulating the expression of these genes.
Fig. 7.
The mRNA expression levels of the lipid oxidation and lipogenesis-related genes in WAT, BAT, and liver of the three groups were compared to investigate the effects of taurine supplementation on gene expression. The three groups included: N group, negative control group of ob/ob mice (n = 8), and ob/ob mice group supplemented with 2 % taurine in drinking water (n = 8). Differences among groups were assessed by one-way ANOVA with a Duncan's test. Means that do not share a common letter are significantly different at P < 0.05. The P values of the two groups were included to show the expression difference of thermogenesis-related genes. Statistical significance was considered at p < 0.05 (*), p < 0.01 (**), and not significant (ns).
3.6. Effect of taurine on expression of fatty acids synthesis genes in fat of C57BL/6J mice
To investigate if taurine effects the expression of fatty acids synthesis associated genes, the basal expression level of relevant genes including 3-hydroxy-3-methylglutaryl-CoA reductase (Hmgcr), low- density lipoprotein receptor (Ldlr), sterol regulator element binding protein 2 (Srebf2), solute carrier family 27 member 2 (Slc27A2), Diacyl glycerol O-acyltransferase 1 (Dgat1), and cluster of differentiation 36 (CD36) were analyzed in three tissues Fig. 8(a–f). Except for hmgcr decreased expression in liver and Slc27A2, Dgat 1, and CD36 increased expression in WAT of the taurine supplemented ob/ob mice group, other genes were expressed at comparable levels in the three tissues. Taurine did not greatly affect the expression of these genes in the liver and especially in the WAT. Furthermore, the Fasn gene expression was shown to be significantly decreased in the WAT of taurine treated ob/ob mice, but not in liver or BAT. These findings infer that taurine supplementation may contribute to the anti-obesity effect in part by decreasing the production of fatty acids in WAT but not in BAT or liver Fig. 8 (g).
Fig. 8.
The mRNA expression levels of the Fatty acid synthesis and uptake -related genes in WAT, BAT, and liver of the three groups were compared to investigate the effects of taurine supplementation on gene expression. The three groups included: N group, negative control group of ob/ob mice (n = 8), and ob/ob mice group supplemented with 2 % taurine in drinking water (n = 8). Differences among groups were assessed by one-way ANOVA with Duncan's test. Means that do not share a common letter are significantly different at P < 0.05. The P values of the two groups were included to show the expression difference of thermogenesis-related genes. Statistical significance was considered at P < 0.05 (*), P < 0.01 (**), and not significant (ns).
3.7. Effect of taurine on the inflammation related genes in fat tissues of C57BL/6J mice
First, the transcriptional levels of a number of inflammatory genes, including Illb, Adgre, Ccl2, and TNF-alpha were examined and compared in three tissues: white adipose tissue (WAT), brown adipose tissue (BAT), and liver tissues. All of the genes had high level of mRNA expression, as seen in Fig. 9(a–d). These genes may be linked to hypertrophic adipocyte-derived inflammation and the exacerbation of fatty liver disease via inflammation, attributed to the WAT and liver's more vivid expression of these genes. Since increased Fasn gene expression has been associated with adipocyte inflammation, hepatic steatosis, and the emergence of insulin resistance, we primarily focused on the inflammation related genes in the WAT, liver and BAT. The basal transcriptional levels of the expression of tumor necrosis factor (TNF-alpha), adhesion G protein couple receptor (Adgre), and chemokine ligand 2 (Ccl2), were significantly lower in the ob/ob mice group receiving taurine supplementation than in ob/ob negative control mice group, as shown in Fig. 9 (P < 0.05). The protein level expression was also decreased as shown in Fig. 9 (e). This suggests that increased fatty acid synthesis was accompanied by enhanced inflammation in the WAT in contrast no such effect was observed in the liver or BAT. This indirectly suggests that taurine supplementation inhibits the increase of fatty acids synthesis and inflammation in the WAT more than liver and BAT.
Fig. 9.
(a–d) Basal mRNA expression levels of inflammatory-related genes (I11b, Adgre, Ccl2, and Tnf-alpha) were compared among three different tissues (white adipose tissue (WAT), brown adipose tissue (BAT), and liver) in the normal diet group (N, n = 8). (e) The mRNA protein level expression levels of the inflammatory and lipogenesis-related genes in WAT were compared to investigate the effects of taurine supplementation on gene expression Fig. S1 (Supplementary data). Differences among groups were assessed by one-way ANOVA with a Duncan's test. Means that do not share a common letter are significantly different at P < 0.05. Statistical significance was considered at P < 0.05 (*), P < 0.01 (**), and not significant (ns).
3.8. Effect of taurine on thermogenesis in BAT of C57BL/6J mice
The expression of beta 3 adrenergic receptor and uncoupling-protein 1 was examined in the BAT tissues to see if taurine influences the expression of genes associated to thermogenesis. As shown in Fig. 10, the UCP-1 gene was enhanced in the BAT of the taurine supplemented group even though its expression was minimal, indicating that taurine may have the ability to promote thermogenesis in the BAT by upregulating the expression of the UCP-1 gene (Fig. 9 (e)).
Fig. 10.
(a) Shows the beta 3 adrenergic receptor and (b) uncoupling-protein 1 expression levels in BAT tissue among the three groups: N normal diet group (n = 8), negative control ob/ob group (n = 8), and ob/ob taurine supplemented group (2 % in drinking water) group (n = 8). Differences among groups were assessed by one-way analysis of variance with Duncan's test. Means that do not share a common letter are significantly different at P < 0.05. Statistically significant differences were defined as P < 0.05, with ** representing P < 0.01, * representing P < 0.05, and ns representing not significant.
4. Discussion
According to earlier research, taurine supplementation prevents the development of obesity in the HFD-induced mice model [[19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [36], [37], [38]]. Taurine's anti-obesity properties were credited with reducing chronic inflammation in white adipose tissue [39,40]. The development of obesity causes adipocytes to grow and accumulate lipids, which disrupts paracrine communication in the adipose tissue and stimulates the release of pro-inflammatory cytokines [41]. Expanded adipocyte-derived inflammatory mediators have been implicated in the pathophysiology of NAFLD. TNF-alpha increases insulin resistance, which aids in the development of NAFLD. Our research revealed that the expression of genes that produce pro-inflammatory cytokines was increased in ob/ob mice. In the ob/ob mice, there was considerable increase in the expression of Adgre, Ccl2, Illb and TNF-alpha which contributes to chronic inflammation by attracting macrophages and releasing pro-inflammatory cytokine mediators. Our work demonstrated that this increase was completely mitigated by taurine supplementation. The current study also showed a decrease in the expression of the lipogenesis-related fatty acid synthetase (Fasn) gene in white adipose tissue of ob/ob mice. In line with earlier research by Zhu et al. [42] our findings demonstrated a reduction in the expression of the fatty acid synthetase gene in the white adipose tissue of mice receiving taurine supplements. As previously suggested by Berndt et al. [31], an increase in the expression of the Fasn gene in adipose tissue is linked to an increase in visceral fat accumulation, impaired insulin sensitivity, increased levels of circulating fasting insulin, andIL-6, indicating its critical role in the lipogenic pathways and the emergence of obesity. Our findings revealed a reduction in Fasn gene expression in the adipocytes of taurine treated group, indicating that this substance may possibly be a contributing factor in the reduction of fat storage, and ultimately, the reduction of obesity.
NAFLD occurs when the hepatic DE novo fatty acid synthesis precedes the excretion or oxidation process of these fatty acids in the form of VLDL [[32], [33], [34]]. As a treatment strategy for NAFLD, weight loss therapy and increased physical activity are insufficient to reverse this metabolic syndrome. In this study, we used C57BL/6J ob/ob mice as an experimental model and found that the mice efficiently developed obesity, insulin resistance and the characteristic NAFLD hallmark, i.e., increase in liver mass. Previous research on NAFLD patients and experimental rodents revealed raised blood ALT, AST and IL-6 activity, as well as increased expression of lipogenic genes and enzymes such as Srebp-1c, Acc-1, and Fasn [[35], [43], [44]]., all of which were reduced by taurine [[45], [46], [47]]. Similar to these investigations, we discovered elevated serum levels of AST, ALT and IL-6 by taurine intake. Also, the Srebf1 gene, which is involved in sterol biosynthesis, and the Fasn gene, which determines the hepatic capacity to produce fatty acids through DE novo lipogenesis, both showed a lowering trend in the taurine treated group, even though decrease was not statistically significant.
The C57BL/6J ob/ob mice used in this study were one week old at the start of supplementation and the taurine supplementation for 12 weeks dramatically increased insulin sensitivity and decreased serum glucose levels. These findings imply that taurine supplementation was able to reduce hyperglycemic conditions, which may have a significant positive health effect for subjects with diabetes. Because adipokines are involved in energy homeostasis, the regulation of glucose metabolism, neuroendocrine functions, immunity, and cardiovascular functions, we looked at serum adiponectin levels in the ob/ob mice in order to better understand the physiological mechanisms by which taurine exerts its therapeutic effects on glucose levels [48,49]. Adenosine monophosphate-activated protein kinase (AMPK) activation and the inhibition of acetyl-CoA carboxylase (ACC) in the liver and muscle cells are two mechanisms by which adiponectin exerts its anti-inflammatory effects [50,51]; increases insulin sensitivity; and produces anti-atherogenic effects [52,53]. Adiponectin tends to boost insulin-induced signal transduction and subsequently improve insulin sensitivity through augmentation of fatty acids and energy combustion process [54,55]. As adiponectin levels were much higher in the taurine group in the current investigation, we hypothesize that this may have contributed to the taurine group's improved glucose tolerance and insulin sensitivity.
Although the pathogenesis of NAFLD is poorly understood, data from earlier studies suggests that inflammation, oxidative stress, and the disruption of lipid metabolism are the driving factors behind the pathogenesis of NAFLD [56,57]. Taurine's capacity to lower TG and cholesterol is well established to be a crucial mechanism underlying both its physiological and pharmacological actions [58,59]. Therefore, we concentrated on the expression of genes for lipogenesis, lipid oxidation, and cholesterol metabolism and assessed taurine's impact on hepatic steatosis. Our findings demonstrated that taurine reduced liver weight, which is directly related to the maintenance of inflammation and lipogenesis in the hepatic tissues or indirectly through white adipose tissue (WAT), which is the site for producing and transporting fatty acids for storage in the liver [60]. Animal models of obesity resulting from high fat diets are extensively used to study NAFLD and hepatic steatosis [61]. After 12 weeks of consuming a HFD, the rodents developed a considerable increase in total body weight, cholesterol levels, and hepatic triglycerides deposits. Additionally, the consumption of HFD was observed to dramatically increase the enzymatic activity of ALT and AST enzymes, the indicators of liver damage, suggesting hepatic dysfunction [62,63]. In our research, we found that the negative control group had elevated IL-6 activity as well as increased ALT and AST enzymes activity. The current investigation showed that taurine intake prevented the development of hepatic steatosis that was produced in ob/ob mice by its relieving effect of triglycerides accumulation and lowering the serum activity inflammatory enzymes. Several organs, including the liver and skeletal muscles, receive fatty acids transport. The primary source of hepatic triglycerides accumulation is the lipolysis of triglycerides released by white adipose cells. Due to taurine's advantageous impact on lipogenesis in the WAT, the alleviation of hepatic steatosis in our study can thus partly be explained.
However, there are certain limitations as large-scale studies are required to confirm the relationship and distinction between Fasn, Ccl2, TNF, Adgre, Illb and obesity derived fatty liver because the size of the study animals is relatively modest. Second, since Fasn gene's regulatory mechanism is yet unknown, additional research on the impact of inflammatory genes and Fasn on obesity is required. Understanding the potential involvement of Fasn and inflammatory genes variation in obesity may be aided by current research. However, future studies are needed to thoroughly elucidate the mechanisms of lipogenesis and other diseases happening during an obese state to uncover probable pathways of obesity-induced diseases.
In other words, the Fasn gene may act as genetic marker for obesity susceptibility in C57BL/6J ob/ob mice through its relationship with the metabolic syndrome and its components. Fasn serves as a possible target for the diagnosis, prognosis, and therapy of the obese state, which leads to the development of fatty liver from obesity. A comparative study of Fasn along with inflammation related genes, including functional genomics and epigenetics analysis, may be helpful to understand the key mechanisms mediated by Fasn gene in obesity related diseases (Fig. 5).
Fig. 5.
The relative expression of genes in the negative control and taurine treated group has been shown in the figure where TNF-alpha (NC) depicts the expression of tumor necrosis factor-alpha (TNF-alpha) in the negative control group whereas TNF-alpha (T) shown the relative expression of TNF-alpha in the taurine supplemented group. Similarly, the expression of adhesion protein G coupled receptors (Adgre), Interleukin-1 beta (il1b), CC motif Chemokine ligand 2 (CCL2), Fatty acid synthetase (Fasn) differences in both the NC and T group have been compared. Genes including Uncoupling protein 1 (UCP1), Cluster of differentiation 36 (CD36), Nuclear receptor subfamily 1 group H member 2 (Nr1h2), Nuclear receptor subfamily 1 group H member 3 (Nr1h3),Sterol regulatory element binding transcription factor 1 and 2 (Srebf1 and Srebf2 respectively), 3-Hydroxy-3-Methylglutaryl-CoA reductase (Hmgcr),Diacylglycerol O-acyltransferase 1 and 2 (Dgat 1 and Dgat 2 respectively) showed no significant differences among the negative control and taurine group so their relative expression in the both groups have been depicted as an average.
5. Conclusions
The liver and skeletal muscles are just two of the organs that fatty acids are being delivered to. The lipolysis of TG from WAT is the main source of hepatic TG accumulation. Therefore, the positive impact of taurine on lipogenesis in WAT can help to explain some of the improvements in hepatic steatosis as observed in our study.
Funding
This research was funded by convergence research financial program for instructors, graduate students, and professors in 2022,and NRF grant funded by the Korea government (NRF-2017R1D1A1B03036108).
Data availability
Data will be made available upon request.
Credit authorship contribution statement
Kainat Ahmed: Writing - review & editing, Writing - original draft, Validation, Methodology, Investigation, Formal analysis, Data curation. Ha-Neul Choi: Investigation, Conceptualization. Ji-sook Park: Investigation, Formal analysis. Yu-gyeong Kim: Software, Methodology. Min kyung Bae: Methodology, Investigation, Formal analysis, Data curation. Jung-Eun Yim: Writing - review & editing, Supervision, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e23288.
Contributor Information
Min Kyung Bae, Email: mkbae@changwon.ac.kr.
Jung-Eun Yim, Email: jeyim@changwon.ac.kr.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Rais N., Ved A., Shadab M., Ahmad R., Shahid M. Taurine, a non-proteinous essential amino acid for human body systems: an overview. Arab Gulf J. Sci. Res. 2023;41(1):48–66. [Google Scholar]
- 2.Karpowicz S.J. Kinetics of taurine biosynthesis metabolites with reactive oxygen species: Implications for antioxidant-based production of taurine. Biochim. Biophys. Acta Gen. Subj. 2022;1866(6) doi: 10.1016/j.bbagen.2022.130131. [DOI] [PubMed] [Google Scholar]
- 3.Mezzomo N.J. Universidade Federal de Santa Maria; 2020. Efeitos do tipo ansiolítico e antiestresse da taurina em peixe-zebra (Danio rerio) [Google Scholar]
- 4.Surai P.F., Earle-Payne K., Kidd M.T. Taurine as a natural antioxidant: from direct antioxidant effects to protective action in various toxicological models. Antioxidants. 2021;10(12):1876. doi: 10.3390/antiox10121876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gregor A. 2022. Impact of Caloric Restriction on the Gastrointestinal Tract and the Role of Taurine and Bile Acids. [Google Scholar]
- 6.Ueki I., Roman H.B., Hirschberger L.L., Junior C., Stipanuk M.H. Extrahepatic tissues compensate for loss of hepatic taurine synthesis in mice with liver-specific knockout of cysteine dioxygenase. Am. J. Physiol. Endocrinol. Metabol. 2012;302(10):E1292–E1299. doi: 10.1152/ajpendo.00589.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ueki I., Stipanuk M.H. Enzymes of the taurine biosynthetic pathway are expressed in rat mammary gland. J. Nutr. 2007;137(8):1887–1894. doi: 10.1093/jn/137.8.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ueki I., Stipanuk M.H. 3T3-L1 adipocytes and rat adipose tissue have a high capacity for taurine synthesis by the cysteine dioxygenase/cysteinesulfinate decarboxylase and cysteamine dioxygenase pathways. J. Nutr. 2009;139(2):207–214. doi: 10.3945/jn.108.099085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Latorre J., Mayneris-Perxachs J., Oliveras-Cañellas N., Ortega F., Comas F., Fernández-Real J.M., et al. Adipose tissue cysteine dioxygenase type 1 is associated with an anti-inflammatory profile, impacting on systemic metabolic traits. EBioMedicine. 2022;85 doi: 10.1016/j.ebiom.2022.104302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bae M., Ahmed K., Yim J.E. Beneficial effects of taurine on metabolic parameters in animals and humans. 2022;31(2):134–146. doi: 10.7570/jomes21088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan L., Miao P. The effects of taurine supplementation on obesity, blood pressure and lipid profile: a meta-analysis of randomized controlled trials. Eur. J. Pharmacol. 2020;885 doi: 10.1016/j.ejphar.2020.173533. [DOI] [PubMed] [Google Scholar]
- 12.Doulberis M., Papaefthymiou A., Polyzos S.A., Katsinelos P., Grigoriadis N., Srivastava D.S., Kountouras J. Rodent models of obesity. Minerva Endocrinol. 2020;45(3):243–263. doi: 10.23736/S0391-1977.19.03058-X. [DOI] [PubMed] [Google Scholar]
- 13.Murakami S. Role of taurine in the pathogenesis of obesity. Mol. Nutr. Food Res. 2015;59(7):1353–1363. doi: 10.1002/mnfr.201500067. [DOI] [PubMed] [Google Scholar]
- 14.Savini I., Catani M.V., Evangelista D., Gasperi V., Avigliano L. Obesity-associated oxidative stress: strategies finalized to improve redox state. Int. J. Mol. Sci. 2013;14(5):10497–10538. doi: 10.3390/ijms140510497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamori Y., Liu L., Mori M., Sagara M., Murakami S., Nara Y., et al., editors. Taurine as the Nutritional Factor for the Longevity of the Japanese Revealed by a World-wide Epidemiological Survey. Springer; 2009. Taurine 7. [DOI] [PubMed] [Google Scholar]
- 16.Haber C.A., Lam T.K., Yu Z., Gupta N., Goh T., Bogdanovic E., et al. N-acetylcysteine and taurine prevent hyperglycemia-induced insulin resistance in vivo: possible role of oxidative stress. Am. J. Physiol. Endocrinol. Metabol. 2003;285(4):E744–E753. doi: 10.1152/ajpendo.00355.2002. [DOI] [PubMed] [Google Scholar]
- 17.Kim D.G., Kwon Y.M., Kang I.S., Kim C. Taurine chloramine selectively regulates neutrophil degranulation through the inhibition of myeloperoxidase and upregulation of lactoferrin. 2020;52(8):1191–1199. doi: 10.1007/s00726-020-02886-5. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y., Xu T., Zhao H., Gu C., Li Z. Effect of taurine in muscle damage markers and inflammatory cytokines in running exercise. Front. Physiol. 2022:1916. doi: 10.3389/fphys.2022.1008060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parra-Peralbo E., Talamillo A., Barrio R. Origin and development of the adipose tissue, a key organ in physiology and disease. Front. Cell Dev. Biol. 2021;9:3636. doi: 10.3389/fcell.2021.786129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang P., Mariman E., Renes J., Keijer J. The secretory function of adipocytes in the physiology of white adipose tissue. J. Cell. Physiol. 2008;216(1):3–13. doi: 10.1002/jcp.21386. [DOI] [PubMed] [Google Scholar]
- 21.Siriwardhana N., Kalupahana N.S., Cekanova M., LeMieux M., Greer B., Moustaid-Moussa N. Modulation of adipose tissue inflammation by bioactive food compounds. J. Nutr. Biochem. 2013;24(4):613–623. doi: 10.1016/j.jnutbio.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 22.Yoon M.J., Lee G.Y., Chung J.J., Ahn Y.H., Hong S.H., Kim J.B. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor alpha. Diabetes. 2006;55(9):2562–2570. doi: 10.2337/db05-1322. [DOI] [PubMed] [Google Scholar]
- 23.Tripathi Y.B., Pandey V. Obesity and endoplasmic reticulum (ER) stresses. Front. Immunol. 2012;3:240. doi: 10.3389/fimmu.2012.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maurizi G., Della Guardia L., Maurizi A., Poloni A. Adipocytes properties and crosstalk with immune system in obesity‐related inflammation. J. Cell. Physiol. 2018;233(1):88–97. doi: 10.1002/jcp.25855. [DOI] [PubMed] [Google Scholar]
- 25.Engin A. The pathogenesis of obesity-associated adipose tissue inflammation. Obesity and lipotoxicity. 2017:221–245. doi: 10.1007/978-3-319-48382-5_9. [DOI] [PubMed] [Google Scholar]
- 26.Xu M., Ji Y. Immunoregulation of synovial macrophages for the treatment of osteoarthritis. Open Life Sci. 2023;18(1) doi: 10.1515/biol-2022-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim K.S., Jang M.J., Fang S., Yoon S.G., Kim I.Y., Seong J.K., et al. Anti-obesity effect of taurine through inhibition of adipogenesis in white fat tissue but not in brown fat tissue in a high-fat diet-induced obese mouse model. Amino Acids. 2019;51(2):245–254. doi: 10.1007/s00726-018-2659-7. [DOI] [PubMed] [Google Scholar]
- 28.Rosen E.D., Hsu C.-H., Wang X., Sakai S., Freeman M.W., Gonzalez F.J., et al. C/EBPα induces adipogenesis through PPARγ: a unified pathway. Gene Dev. 2002;16(1):22–26. doi: 10.1101/gad.948702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bargut T.C.L., Aguila M.B., Mandarim-de-Lacerda C.A. Brown adipose tissue: updates in cellular and molecular biology. Tissue Cell. 2016;48(5):452–460. doi: 10.1016/j.tice.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Aboouf M.A., Armbruster J., Thiersch M., Gassmann M., Gödecke A., Gnaiger E., et al. Myoglobin, expressed in brown adipose tissue of mice, regulates the content and activity of mitochondria and lipid droplets. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2021;1866(12) doi: 10.1016/j.bbalip.2021.159026. [DOI] [PubMed] [Google Scholar]
- 31.Berndt J., Kovacs P., Ruschke K., Klöting N., Fasshauer M., Schön M., et al. Fatty acid synthase gene expression in human adipose tissue: association with obesity and type 2 diabetes. Diabetologia. 2007;50(7):1472–1480. doi: 10.1007/s00125-007-0689-x. [DOI] [PubMed] [Google Scholar]
- 32.Grünig D., Szabo L., Marbet M., Krähenbühl S. Valproic acid affects fatty acid and triglyceride metabolism in HepaRG cells exposed to fatty acids by different mechanisms. Biochem. Pharmacol. 2020;177 doi: 10.1016/j.bcp.2020.113860. [DOI] [PubMed] [Google Scholar]
- 33.Hijmans B.S., Grefhorst A., Oosterveer M.H., Groen A.K. Zonation of glucose and fatty acid metabolism in the liver: mechanism and metabolic consequences. Biochimie. 2014;96:121–129. doi: 10.1016/j.biochi.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Meyer L.R.F. Lipid metabolism in fatty liver disease. HEPATIC STEATOSIS. 2014;63 [Google Scholar]
- 35.Higuchi N., Kato M., Shundo Y., Tajiri H., Tanaka M., Yamashita N., et al. Liver X receptor in cooperation with SREBP‐1c is a major lipid synthesis regulator in nonalcoholic fatty liver disease. Hepatol. Res. 2008;38(11):1122–1129. doi: 10.1111/j.1872-034X.2008.00382.x. [DOI] [PubMed] [Google Scholar]
- 36.Murakami S. Role of taurine in the pathogenesis of obesity. Mol. Nutr. Food Res. 2015;59(7):1353–1363. doi: 10.1002/mnfr.201500067. [DOI] [PubMed] [Google Scholar]
- 37.Tsuboyama-Kasaoka N., Shozawa C., Sano K., Kamei Y., Kasaoka S., Hosokawa Y., et al. Taurine (2-aminoethanesulfonic acid) deficiency creates a vicious circle promoting obesity. Endocrinology. 2006;147(7):3276–3284. doi: 10.1210/en.2005-1007. [DOI] [PubMed] [Google Scholar]
- 38.Kim K.S., Jang M.J., Fang S., Yoon S.G., Kim I.Y., Seong J.K., et al. Anti-obesity effect of taurine through inhibition of adipogenesis in white fat tissue but not in brown fat tissue in a high-fat diet-induced obese mouse model. Amino Acids. 2019;51(2):245–254. doi: 10.1007/s00726-018-2659-7. [DOI] [PubMed] [Google Scholar]
- 39.Guo Y.-Y., Li B.-Y., Peng W.-Q., Guo L., Tang Q.-Q. Taurine-mediated browning of white adipose tissue is involved in its anti-obesity effect in mice. J. Biol. Chem. 2019;294(41):15014–15024. doi: 10.1074/jbc.RA119.009936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim K.S., Doss H.M., Kim H.-J., Yang H.-I. Taurine stimulates thermoregulatory genes in brown fat tissue and muscle without an influence on inguinal white fat tissue in a high-fat diet-induced obese mouse model. Foods. 2020;9(6):688. doi: 10.3390/foods9060688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson A.R., Justin Milner J., Makowski L. The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunol. Rev. 2012;249(1):218–238. doi: 10.1111/j.1600-065X.2012.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu L., Du W., Liu Y., Cheng M., Wang X., Zhang C., et al. Prolonged high‐glucose exposure decreased SREBP‐1/FASN/ACC in Schwann cells of diabetic mice via blocking PI3K/Akt pathway. J. Cell. Biochem. 2019;120(4):5777–5789. doi: 10.1002/jcb.27864. [DOI] [PubMed] [Google Scholar]
- 43.Ren L., Sun D., Zhou X., Yang Y., Huang X., Li Y., et al. Chronic treatment with the modified Longdan Xiegan Tang attenuates olanzapine-induced fatty liver in rats by regulating hepatic de novo lipogenesis and fatty acid beta-oxidation-associated gene expression mediated by SREBP-1c, PPAR-alpha and AMPK-alpha. J. Ethnopharmacol. 2019;232:176–187. doi: 10.1016/j.jep.2018.12.034. [DOI] [PubMed] [Google Scholar]
- 44.Chen X., Zhang C., Zhao M., Shi C.E., Zhu R.M., Wang H., et al. Melatonin alleviates lipopolysaccharide‐induced hepatic SREBP‐1c activation and lipid accumulation in mice. J. Pineal Res. 2011;51(4):416–425. doi: 10.1111/j.1600-079X.2011.00905.x. [DOI] [PubMed] [Google Scholar]
- 45.Saleh A.A.S., Shahin M.I., Kelada N.A. Hepatoprotective effect of taurine and coenzyme Q10 and their combination against acrylamide-induced oxidative stress in rats. Trop. J. Pharmaceut. Res. 2017;16(8):1849–1855. [Google Scholar]
- 46.Liu Y., Li F., Zhang L., Wu J., Wang Y., Yu H. Taurine alleviates lipopolysaccharide-induced liver injury by anti-inflammation and antioxidants in rats. Mol. Med. Rep. 2017;16(5):6512–6517. doi: 10.3892/mmr.2017.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abdel-Moneim A.M., Al-Kahtani M.A., El-Kersh M.A., Al-Omair M.A. Free radical-scavenging, anti-inflammatory/anti-fibrotic and hepatoprotective actions of taurine and silymarin against CCl4 induced rat liver damage. PLoS One. 2015;10(12) doi: 10.1371/journal.pone.0144509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahima R.S., Lazar M.A. Adipokines and the peripheral and neural control of energy balance. Mol. Endocrinol. 2008;22(5):1023–1031. doi: 10.1210/me.2007-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahima R.S., Osei S.Y. Adipokines in obesity. Obes. Metabol. 2008;36:182–197. doi: 10.1159/000115365. [DOI] [PubMed] [Google Scholar]
- 50.Fantuzzi G. Adiponectin and inflammation: consensus and controversy. J. Allergy Clin. Immunol. 2008;121(2):326–330. doi: 10.1016/j.jaci.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 51.Matsuzawa Y. Adiponectin: a key player in obesity related disorders. Curr. Pharmaceut. Des. 2010;16(17):1896–1901. doi: 10.2174/138161210791208893. [DOI] [PubMed] [Google Scholar]
- 52.Vazirian M., Nabavi S.M., Jafari S., Manayi A. Natural activators of adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK) and their pharmacological activities. Food Chem. Toxicol. 2018;122:69–79. doi: 10.1016/j.fct.2018.09.079. [DOI] [PubMed] [Google Scholar]
- 53.Martinez Cantarin M.P., Keith S.W., Waldman S.A., Falkner B. Adiponectin receptor and adiponectin signaling in human tissue among patients with end-stage renal disease. Nephrol. Dial. Transplant. 2014;29(12):2268–2277. doi: 10.1093/ndt/gfu249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamauchi T., Kamon J., Waki H., Terauchi Y., Kubota N., Hara K., et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001;7(8):941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 55.Chakraborti C.K. Role of adiponectin and some other factors linking type 2 diabetes mellitus and obesity. World J. Diabetes. 2015;6(15):1296. doi: 10.4239/wjd.v6.i15.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arroyave-Ospina J.C., Wu Z., Geng Y., Moshage H. Role of oxidative stress in the pathogenesis of non-alcoholic fatty liver disease: Implications for prevention and therapy. Antioxidants. 2021;10(2):174. doi: 10.3390/antiox10020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lim S., Kim J.-W., Targher G. Links between metabolic syndrome and metabolic dysfunction-associated fatty liver disease. Trends Endocrinol. Metabol. 2021;32(7):500–514. doi: 10.1016/j.tem.2021.04.008. [DOI] [PubMed] [Google Scholar]
- 58.Murakami S., Ono A., Kawasaki A., Takenaga T., Ito T. Taurine attenuates the development of hepatic steatosis through the inhibition of oxidative stress in a model of nonalcoholic fatty liver disease in vivo and in vitro. Amino Acids. 2018;50(9):1279–1288. doi: 10.1007/s00726-018-2605-8. [DOI] [PubMed] [Google Scholar]
- 59.Ahmadian M., Roshan V.D., Aslani E., Stannard S.R. Taurine supplementation has anti-atherogenic and anti-inflammatory effects before and after incremental exercise in heart failure. Therapeutic advances in cardiovascular disease. 2017;11(7):185–194. doi: 10.1177/1753944717711138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trayhurn P., Beattie J.H. Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc. Nutr. Soc. 2001;60(3):329–339. doi: 10.1079/pns200194. [DOI] [PubMed] [Google Scholar]
- 61.Lau J.K.C., Zhang X., Yu J. Animal models of non‐alcoholic fatty liver disease: current perspectives and recent advances. J. Pathol. 2017;241(1):36–44. doi: 10.1002/path.4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jung U.J., Cho Y.-Y., Choi M.-S. Apigenin ameliorates dyslipidemia, hepatic steatosis and insulin resistance by modulating metabolic and transcriptional profiles in the liver of high-fat diet-induced obese mice. Nutrients. 2016;8(5):305. doi: 10.3390/nu8050305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rahman M.M., Alam M.N., Ulla A., Sumi F.A., Subhan N., Khan T., et al. Cardamom powder supplementation prevents obesity, improves glucose intolerance, inflammation and oxidative stress in liver of high carbohydrate high fat diet induced obese rats. Lipids Health Dis. 2017;16(1):1–12. doi: 10.1186/s12944-017-0539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available upon request.