Abstract
Gastrointestinal cancers are a huge worldwide health concern, which includes a wide variety of digestive tract cancers. Circular RNAs (circRNAs), a kind of non-coding RNA (ncRNAs), are a family of single-stranded, covalently closed RNAs that have become recognized as crucial gene expression regulators, having an impact on several cellular functions in cancer biology. The gut microbiome, which consists of several different bacteria, actively contributes to the regulation of host immunity, inflammation, and metabolism. CircRNAs and the gut microbiome interact significantly to greatly affect the growth of GI cancer. Several studies focus on the complex functions of circRNAs and the gut microbiota in GI cancers, including esophageal cancer, colorectal cancer, gastric cancer, hepatocellular cancer, and pancreatic cancer. It also emphasizes how changed circRNA expression profiles and gut microbiota affect pathways connected to malignancy as well as how circRNAs affect hallmarks of gastrointestinal cancers. Furthermore, circRNAs and gut microbiota have been recommended as biological markers for therapeutic targets as well as diagnostic and prognostic purposes. Targeting circRNAs and the gut microbiota for the treatment of gastrointestinal cancers is also being continued to study. Despite significant initiatives, the connection between circRNAs and the gut microbiota and the emergence of gastrointestinal cancers remains poorly understood. In this study, we will go over the most recent studies to emphasize the key roles of circRNAs and gut microbiota in gastrointestinal cancer progression and therapeutic options. In order to create effective therapies and plan for the future gastrointestinal therapy, it is important to comprehend the functions and mechanisms of circRNAs and the gut microbiota.
Keywords: Circular RNAs, Gut microbes, Gastrointestinal (GI) cancers, Therapeutic target
1. Introduction
Gastrointestinal (GI) cancers refer to a variety of malignancies that can appear throughout the digestive system, including the organs that aid in digestion [1]. GI malignancies, such as esophagus cancer [2], gastric cancer [3], hepatocellular cancer [4], colorectal cancer [5], and pancreatic cancer [6], are only a few of the numerous GI malignancies that seriously endanger world health. Research in recent years has revealed that two new variables play a role in the onset and spread of GI malignancies: the circular circRNAs [7] and the gut microbiome [8].
Circular RNAs (circRNAs) are closed and single-stranded RNA molecules and do not have poly (A) tails or 5′–3′ ends [9]. They are more stable and may have a long lifespan within cells because they can withstand the destruction caused by exonucleases [10]. CircRNAs have attracted attention recently due to their variety of roles in gene regulation, like serving as microRNA sponges [11,12], regulating RBPs [13], and even producing functional peptides [14]. They can affect the growth of cancer by controlling important signaling pathways, which play a role in cell differentiation, proliferation, EMT, metastasis, and cell death [[15], [16], [17]].
The gut microbiota has also been found to be a significant contributor to GI malignancies, which refers to the millions of bacteria that survive in the gastrointestinal system of humans [18,19]. The host immune system [20], food metabolism [21], and a variety of bioactive metabolites produced by the gut microbiota all interact with one another to influence the gut microenvironment and general health [22]. Dysbiosis, an imbalance in the gut's microbial population, has been connected to several gastrointestinal illnesses, including esophageal cancer [23], gastric cancer [24], hepatocellular cancer [25], pancreatic cancer [26], and colorectal cancer [27]. The gut microbiota can either directly or indirectly influence tumor development and therapeutic response in GI malignancies [19]. For instance, some gut bacteria can create genotoxic byproducts like nitrosamines or secondary bile acids that can cause DNA damage and encourage the development of cancer [28].
In the context of GI malignancies, the interaction between circRNAs and the gut microbiota has become an exciting topic of investigation, affording unique insights into both disease causes and prospective therapeutic approaches [29]. It may be possible to modify oncogenic pathways by targeting particular circRNAs, and probiotics and dietary changes that aim to alter the gut microbiota may help lower the risk of cancer and improve the effectiveness of current cancer treatments [30,31].
The relationship underlying circRNAs, gut microbiota, and the development of gastrointestinal malignancies is remained unclear, despite significant studies in this direction. In this review, we will discuss the most up-to-date research that focuses on the significance of circRNAs and the gut microbiota in the development and therapeutic interventions of gastrointestinal cancer.
2. Role of circRNAs in GI malignancies
The significance of circRNAs in gastrointestinal malignancies is becoming increasingly obvious. Through "sponging" on various miRNAs and altering various signaling pathways, they alter the expression of several classes of genes. Some of these alterations contribute to the development and spread of GI cancer, whereas others do the opposite. The oncogenic circ 0000654 in esophageal squamous-cell carcinomas (ESCCs) promotes ESCC proliferation by sponging miR-149-5p and activating the IL-6/STAT3 signaling cascade [32]. Similarly, He et al. in their in vivo study have shown that circ 0006282, by sponging miR-155, enhances FBXO22 expression and the advancement of GC [33]. Further, Zhang and his team proved that the circTMEM45A expression is increased and acts as an oncogene in hepatocellular cancer (HCC). By mechanically sponging of miR-665 expression, circTMEM45A promotes the upregulation of IGF2 and speeds up the development of HCC [34].
Although circRNAs are involved in many processes, some may even serve as oncogenes in gastrointestinal malignancies. They play key roles in cancer-related activities including tumor formation and metastasis (Table 1). Through several specific mechanisms oncogenic circRNAs has the ability to control gene expression, such as competing endogenous RNAs (ceRNAs) [35]. CeRNA activity promotes carcinogenesis by elevating carcinogenic mRNAs that miRNAs would typically target and suppress [36]. For instance, in ESCC, Shi et al. revealed that circ_LRP6 sponges miR-198 to induce the invasion, migration, and metastasis of EC [37]. By sponging miR-582-3p, circSHKBP1 was able to upregulate HUR expression, which in turn improved VEGF mRNA stability. Furthermore, circSHKBP1 directly attached to HSP90 and impeded the engagement of STUB1 with HSP90, which in turn inhibited the ubiquitination process of HSP90 and increased the growth of GCs in vitro and in vivo [38]. Similarly, Lu and his colleagues found that circ-RanGAP1 controls the expression of VEGFA by sponging miR-877–3p to promote invasion and metastasis of the GC [39]. Likewise, in CRC cells, Zhang et al. showed that circ-0084615 is an oncogenic circRNA that competes with endogenous RNA to control the expression of DNMT3A by miR-599 sponging [40]. Moreover, Xing et al. confirmed that the circular RNA ADAM9 promotes malignant behaviors in pancreatic cancer by sponging miR-217 and elevating PRSS3 expression [41]. In addition, Guo et al. reported that circBFAR enhanced the proliferation of PDAC cells by sponging miR-34b-5p, which increased MET expression and then activated the downstream phosphorylation of Akt (Ser 473) [42]. Furthermore, Liu et al. observed that PC development is aided by the modification of the miR-96-5p/KRAS/MAPK axis, which suggests that the specifically overexpressed has-circ-0006117 may be a potential target for PC therapy [43] (Fig. 1). In CRC, circSPARC was shown to be upregulated in both the plasma and tissues of CRC patients. Mechanistically, circSPARC sponged miR-485-3p to increase JAK2 expression, which in turn helped phosphorylated (p)-STAT3 to be accumulate. Thus, by controlling the JAK/STAT pathway, the circSPARC promotes CRC migration and proliferation [44]. Moreover, Zhang and his colleagues demonstrated that circNRIP1 sponges miR-149-5p to modify AKT1 expression levels and ultimately functions as a tumor promoter in GC. By modulating the AKT1/mTOR axis, circNRIP1 can change metabolism and autophagy and facilitate tumor spread through exosome communication [45].
Table 1.
Oncogenic roles of various circRNAs in GI tumorigenesis through regulation of target genes and signaling pathways (↑: upregulated, ↓: downregulated).
| Cancer types | CircRNAs | Clinical studies | Animal studies | Cell line studies | Target genes/signaling pathways | Clinicopathological characteristics | Description | Ref. |
|---|---|---|---|---|---|---|---|---|
| Esophageal cancer | LPAR3 | ESCC = 52 case | BALB/c nude mice | ECA109, HET-1A, TE-13, Kyse150, Kyse510, Kyse450 | -miR-198, MET -RAS/MAPK and PI3K/Akt axis |
Clinical stage and LNM | ↑ LPAR3, ↓ miR-198, ↑ MET, ↑ RAS/MAPK and PI3K/Akt signaling: ↑ Metastasis | [37] |
| CircAKT3 | EC = 82 case | Nude mice | KYSE-150, HEK-293T, TE-10, TE-1 | -miR-17-5p, RHOC, STAT3 | Tumor size, clinical tumor node metastasis staging, and lymphatic metastasis | ↑ CircAKT3, ↓ miR-17-5p, ↑ RHOC, STAT3: ↑ Cell proliferation, and metastasis | [51] | |
| Circ_LRP6 | ESCC = 78 pairs | Nude mice | TE-1, EC109 | -miR-182, Myc | Larger tumor size, later TNM stage | ↑Circ-LRP6, ↓ miR-182, ↑ Myc: ↑ Tumor progression | [52] | |
| hsa_circ_0067934 | EC = 51 pairs | – | TE-13, KYSE-410 | – | T stage, tumor differentiation, TNM stage | ↑hsa_circ_0067934: ↑ Cell proliferation | [53] | |
| hsa_circ_0000654 | ESCC = 55 case | BALB/c athymic nude mice | TE-1, HEEC, KYSE410, TE-13, KYSE45, ECA-109 | -miR-149-5p, IL-6, STAT3 | Higher T stage, local LNM | ↑hsa_circ_0000654, ↓ miR-149-5p, ↑ IL-6/STAT3: ↑ Cell proliferation, and metastasis | [32] | |
| Circ_0006168 | -EC at phase I + II = 17 case - EC at phase III + IV = 20 case |
– | ECA-109, HET-1A, KYSE-510 | miR-384, RBBP7 | – | ↑Circ_0006168, ↓miR-384, ↑RBBP7: ↑Cell proliferation, metastasis, and glycolysis | [54] | |
| CircFNDC3B | EC tissues = 23 pairs | – | ECA109, KYSE150 | FNDC3B | – | ↑CircFNDC3B, ↑FNDC3B: ↑Proliferation and metastasis | [55] | |
| hsa_circ_0000277 | ESCC tissues = 92 pairs | BALB/c nude mice | ECA109, Het1A, KYSE-410, EC9706, TE-1, KYSE-150, TE-10 | miR-4766-5p, LAMA1 | Advanced TNM stage, dismal prognosis | ↑hsa_circ_0000277, ↓miR-4766-5p, ↑LAMA1: ↑ ESCC progression | [56] | |
| CircNTRK2 | ESCC tissues = 56 pairs | BALB/c nude mice | Eca-109, Het-1A, EC-9706, TE-1, KYSE-150, KYSE-30 | miR-140-3p, NRIP1 | Advanced TNM stage, LNM | ↑CircNTRK2, ↓ miR-140-3p, ↑ NRIP1: ↑ ESCC development | [57] | |
| Gastric cancer | CircPVT1 | – | – | MGC-803, AGS | miR-125 family | Sex, age, tumor site, tumor size, differentiation grade, lymph node status, distant metastasis, TNM stage, T stage, lymphatic invasion, nervous invasion | ↑ CircPVT1, ↓ miR-125 family: ↑ Cell proliferation. | [58] |
| hsa_circ_0000745 | GC = 60 pairs | – | – | – | Lymphatic metastasis, differentiation, TNM stage, sex, age | ↓hsa_circ_0000745: ↑Gastric growth | [59] | |
| Circ_RanGAP1 | -GC tissue = 97 pairs. - GC plasma exosome = 30 case |
BALB/c (nu/nu) mice | HGC-27, AGS, MGC-803, GES-1, MKN45, KATOIII, BGC-823 |
VEGFA, miR-877-3p | Advanced TNM stage, LNM, and worse survival | ↑Circ-RanGAP1, ↓ miR-877-3p, ↑ VEGFA: ↑ Metastasis | [39] | |
| CiRS-7 | -GC tissue = 102 pairs. | BALB/c nude mice | HGC-27, MGC-803, GES-1 | -miR-7 -PTEN/PI3K/AKT signaling |
Tumor stages, distant metastasis, lymph node involvement, overall survival | ↑CiRS-7, ↓ miR-7, ↓PTEN: ↑ GC progression. | [60] | |
| CircAKT3 | GC tissues = 149 (cohorts 1, 2) | BALB/c nude mice | SGC7901, BGC823 | PIK3R1, miR-198 | Clinical stage, tumor size, histological grade | ↑CircAKT3, ↓miR-198, ↑PIK3R1: ↑ resistance to CDDP | [61] | |
| CircDLG1 | GC tissues = 126 case | BALB/c nude mice, C57BL/6 mice | HGC27, HEK293 T, BGC823, MFC, MKN45, MKN28, GES-1, SGC7901, AGS | miR-141-3p, CXCL12 | Age, Lauren's classification, sex, tumor size, peritoneal metastasis, tumor cell differentiation | ↑CircDLG1, ↓miR-141-3p, ↑CXCL12: ↑ GC progression and anti-PD-1 resistance | [62] | |
| CircNRIP1 | Tissue samples | BALB/c nude mice | BGC-823, GES-1, SGC-7901, HGC-27, MGC-803, AGS, MKN-45 | -miR-149-5p -AKT1/mTOR signaling |
Lymphatic invasion, tumor size, | ↑CircNRIP1, ↓miR-149-5p, ↑AKT1: ↑ Proliferation, invasion, and migration | [45] | |
| hsa_circ_0008035 | GC = 30 case | – | BGC-823, SGC-7901, GES-1, MGC-803, AGS | miR-375, YBX1 | – | ↑hsa_circ_0008035, ↓miR-375, ↑YBX1: ↑ Tumorigenesis | [63] | |
| Circ_0006282 | Tissue samples | BALB/c nude mice | BGC-823, GES-1, MKN-45, HGC-27, AGS | miR-155, FBXO22 | Tumor size, TNM stage, lymph node metastasis | ↑Circ_0006282, ↓miR-155, ↑FBXO22: ↑ GC progression | [33] | |
| hsa_circ_0001368 | GC tissue = 68 pairs | BALB/c (nu/nu) mice | HGC-27, HEK 293T, MGC-803, GES1, AGS, NUGC-3, | miR-6506–5p, FOXO3 | – | ↓hsa_circ_0001368, ↓miR-6506–5p, ↑FOXO3: ↓ GC progression | [64] | |
| Colorectal cancer | Circ_0084615 | CRC = 50 case | BALB/c athymic nude mice | HCT116, FHC, SW480, RKO, DLD1 | miR-599, DNMT3A | Age, gender, TNM stage, CEA, CA19-9, differentiation | ↑Circ_0084615, ↓ miR-599, ↑DNMT3A: ↑ CRC proliferation and metastasis | [40] |
| CircSPARC | CRC = 84 case | BALB/c female nude mice | HCT116, LoVo, SW620, HT-29, SW480, DLD1 | -miR-485-3p, p-STAT3 -JAK/STAT signaling |
Advanced TNM stage, poor survival, and LNM | ↑CircSPARC, ↓miR-485-3p, ↑JAK2: ↑ CRC proliferation and migration | [44] | |
| CircGLIS2 | CRC tissue = 3 pairs | – | NCM460, HCT-15, DLD1, HT-29, HCT-8, HCT116, RKO, | - miR-671 -NF-κB signaling |
– | ↑CircGLIS2, ↓miR-671, ↑ NF-κB signaling: ↑ CRC pro-metastasis microenvironment | [65] | |
| CircVAPA | CRC tissue = 60 pairs | – | HEK-293T, RKO, SW480, LoVo, SW620, HT29, HCT116, | miR-101 | Gender, age, tumor site, tumor size, lymphovascular invasion, differentiation, TNM stage, LNM | ↑CircVAPA, ↓miR-101: ↑ CRC progression | [66] | |
| hsa_circRNA_102958 | CRC = 58 case | BALB/c nude mice | LoVo, FHC, SW480, HCT116, SW620, HCT8, HT29 | miR-585/CDC25B | Tumor stage, LNM, differentiation, survival rate | ↑hsa_circRNA_102958, ↓miR-585, ↑CDC25B: ↑ Tumorigenesis | [67] | |
| hsa_circ_0007142 | Tissue sample | – | HT-29, HCT-116, LoVo, HCO | miR-103a-2-5p | Lymphatic metastasis and poor differentiation | ↑hsa_circ_0007142, ↓miR-103a-2-5p: ↑ CRC proliferation and migration | [68] | |
| CircSEMA5A | – | Nude mice | LoVo, NCM460, SW620, SW480, Caco-2 | miR-195-5p, CCNE1 | – | ↑CircSEMA5A, ↓miR-195-5p, ↑CCNE1: ↑ CRC development | [69] | |
| Circ-METTL9 | – | Nude mice | – | miR-551b-5p, CDK6 | – | ↑Circ-METTL9, ↓miR-551b-5p, ↑CDK6: ↑ CRC development | [70] | |
| CircLDLR | CRC tissue = 5 pairs | BALB/c nude mouse | HEK293T, Caco-2, HCT116, HT29, SW480, RKO, LoVo, SW620, HCT8, | miR-30a-3p/SOAT1 | TNM stage, overall survival | ↑CircLDLR, ↓miR-30a-3p, ↑SOAT1: ↑ CRC progression | [71] | |
| Hepatocellular carcinoma | CircRHOT1 | HCC tissue = 100 pairs | Nude mice | Huh7, Hep3B | NR2F6 | Overall survival | ↑CircRHOT1, ↑NR2F6: ↑ HCC progression | [72] |
| CircTMEM45A | HCC tissue = 68 pairs | BALB/c nude mice | LO2, Hep3B, HLE, Huh7, HCCLM6, BEL7402, HCCLM3, MHCC97H, SMCC7721, MHCC97L | miR-665, IGF2 | Poor prognosis, age, gender, HBsAg, cirrhosis, tumor size, tumor number, TNM stage, vascular invasion | ↑CircTMEM45A, ↓miR-665, ↑IGF2: ↑ HCC progression | [34] | |
| hsa_circ_0016788 | HCC tissue = 63 pairs | BALB/c nude mice | HepG2, LO2, Hep3B, MHCC97L, HCCLM3, Huh7 | miR-486, CDK4 | – | ↑hsa_circ_0016788, ↓miR-486, ↑CDK4: ↑ HCC tumorigenesis | [73] | |
| Circ-BIRC6 | HCC tissue = 55 pairs | BALB/c nude mice | HepG2, L02, Bel-7402, Rockville, Huh-7, MD, SMMC-7721, USA | miR-3918, Bcl2 | Age, serum alpha-fetoprotein level, gender, tumor size, TNM stage, vascular invasion | ↑Circ-BIRC6, ↓miR-3918, ↑Bcl2: ↑ HCC progression | [74] | |
| hsa_circRNA_103809 | HCC = 60 case | BALB/c nude mice | LO2, HCCLM3, MHCC97L, Hep3B, SK‐HEP‐1, Huh7 |
miR-377-3p, FGFR1, ERK, cyclin D1, CDK4, CDK6 | Age, AFP, HBsAg, cirrhosis, tumor size, Edmonson grade | ↑hsa_circRNA_103809, ↓miR-377-3p, ↑FGFR1, ↑ERK: ↑ HCC development | [75] | |
| CircMAT2B | – | Nude mice | Huh7, HepG2 | miR-338 -3p, PKM2 | Tumor size, Edmonson stage, vascular invasion, TNM stage, tumor encapsulation, tumor multiplicity |
↑CircMAT2B, ↓miR-338-3p, ↑PKM2: ↑ HCC glycolysis and malignancy | [76] | |
| hsa_circ_0000673 | HCC = 51 case | BALB/c (nu/nu) mice | Huh7, Hep3B | miR-767-3p, SET | Overall survival | ↑hsa_circ_0000673, ↓miR-767-3p, ↑SET: ↑ HCC malignancy | [77] | |
| Circ_0000517 | HCC = 45 case | BALB/c nude mice | THLE-2, HCCLM3, Huh7 | miR-326, IGF1R | – | ↑Circ_0000517, ↓miR-326, ↑IGF1R: ↑ HCC development | [78] | |
| CircMAST1 | HCC = 39 case | BALB/c nude mice | HepG2, SK-HepG1, L02, HCCLM3, Huh7 | miR-1299, CTNND1 | – | ↑CircMAST1, ↓miR-1299, ↑CTNND1: ↑HCC proliferation and migration | [79] | |
| hsa_circ_0056836 | Nude mice | HUH7, SK-HEP-1, SNU449, HEPG2 | miR-766-3p, FOSL2 | – | ↑hsa_circ_0056836, ↓miR-766-3p, ↑FOSL2: ↑ HCC proliferation and migration | [80] | ||
| Pancreatic cancer | Circ-PDE8A | PDAC = 93 case | Nude mice | BxPC-3, HEK-293, Capan-1, Aspc-1, Hs 766T, Hs 766T | -miR-338, MACC1 -MET signaling pathway |
TNM stage, lymphatic invasion, poor survival rate | ↑Circ-PDE8A, ↓miR-338, ↑MACC1, ↑MET/AKT, and ERK pathway: ↑ PDAC development and invasion | [81] |
| Circ-ADAM9 | PC tissue = 58 pairs | BALB/c nude mice | PANC1, HPDE, MiaPaca2, BxPC3, SW1990 | miR-217, PRSS3 | TNM stage, lymph node status | ↑Circ-ADAM9, ↓miR-217, ↑PRSS3: ↑ PC malignancy and behavior | [41] | |
| ciRS-7 | PDAC tissue = 41 pairs | – | PANC-1, BXPC-3, HPC-Y5 | -miR-7 - EGFR/STAT3 signaling |
Venous invasion, LNM | ↑ciRS-7, ↓miR-7, ↑EGFR/STAT3 signaling: ↑ PC proliferation and metastasis | [82] | |
| Circ CDR1as | PC = 27 case | BALB/c nude mice | PC-3, HPDE6-C7, PANC1, BXPC-3, ASPC1, MIApaCa-2, CFPAC-1 | miR-432-5p, E2F3 | – | ↑CircCDR1as, ↓miR-432-5p, ↑E2F3: ↑ PC progression | [83] | |
| hsa_circ_0006117 | PC = 20 case | – | SW1990, PaCa-2, BxPC-3, PANC-1, MIA, AsPC-1 | -miR-96-5p, -KRAS/MAPK Signaling | – | ↑hsa_circ_0006117, ↓miR-96-5p, ↑KRAS/MAPK Signaling: ↑ PC progression | [43] | |
| CircBFAR | PDAC = 208 case | SCID mice | BxPC-3, hTERT-HPNE, MIA PaCa-2, PANC-1, CFPAC-1 | -miR-34b-5p - MET/PI3K/Akt signaling |
Gender, age, BMI, smoking, tumor size, differentiation, T stage, TNM stage, Ki67 expression, neoadjuvant chemotherapy, lymphatic metastasis, adjuvant chemotherapy | ↑ CircBFAR, ↓ miR-34b-5p, ↑MET/PI3K/Akt signaling: ↑ PDAC progression | [42] | |
| hsa_circ_0014784 | PC = 10 case | Nude mice | SW1990, HPDE6-C7, PANC-1, AsPC-1, BxPC3, Capan-2 | miR-214-3p, YAP1 | – | ↑hsa_circ_0014784, ↓miR-214-3p, ↑YAP1: ↑ PC progression | [84] | |
| hsa_circ_0007367 | Frozen PC tissue = 25 pairs | BALB/c nude mice | AsPC-1, HPDE, BxPC-3, SW1990, Capan-1, PANC-1 | miR-6820-3p, YAP1 | LNM, advanced histological grade | ↑hsa_circ_0007367, ↓ miR-6820-3p, ↑ YAP1: ↑ PDAC progression | [85] |
PC pancreatic cancer, HCC hepatocellular cancer, GC gastric cancer, EC esophageal cancer, LPAR3 lysophosphatidic acid receptor 3, Circ_LRP6 circular RNA-lipoprotein receptor 6, CircPVT1 circular RNA plasmacytoma variant translocation 1, Circ_RanGAP1 circular RNA_ran GTPase-activating protein 1, CiRS-7 circular RNA sponge for miR-7, CircDLG1 circular RNA discs large homolog 1, CircSPARC circular RNA secreted protein acidic and rich in cysteine, CircSEMA5A circular RNA semaphorin 5A, Circ-METTL9 circular RNA methyltransferase like 9, CircLDLR circular RNA low-density lipoprotein receptor, CircTMEM45A circular RNA transmembrane protein 45A, CircMAST1 circular RNA microtubule associated serine/threonine kinase 1, Circ-PDE8A circular RNA phosphodiesterase 8A, Circ-ADAM9 circular RNA a disintegrin and metalloproteinase domain 9, MET mesenchymal epithelial transition, RHOC as homolog gene family member C, RBBP7 retinoblastoma-binding protein 7, VEGFA vascular endothelial growth factor A, DNMT3A DNA methyltransferase 3 alpha, JAK Janus kinase, STAT signal transducer and activator of transcription, NF-κB nuclear factor kappa-light-chain-enhancer of activated B cells, CDC25B cell division cycle 25B, CCNE1 cyclin E1, CDK6 cyclin dependent kinase 6, SOAT1 sterol o-acyltransferase 1, NR2F6 nuclear receptor subfamily 2 group F member 6, CDK4 cyclin dependent kinase 4, Bcl2 B-cell lymphoma 2, PKM2 pyruvate kinase muscle 2, CTNND1 catenin delta 1, MACC1 metastasis-associated in colon cancer 1, PRSS3 protease, serine 3, KRAS kirsten rat sarcoma viral oncogene homolog, YAP1 yes-associated protein 1.
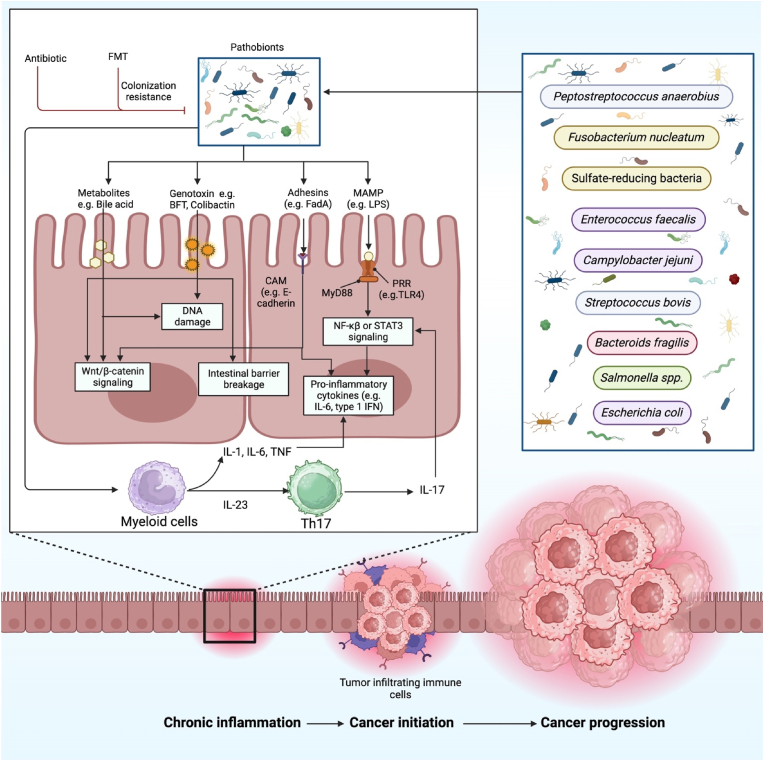
Fig. 1.
The schematic illustration represents the connection between colorectal cancer's sequential progression and the gut microbiome. Interactions between the host and the microbe lead to activate pro-carcinogenic signaling pathways, which in turn cause molecular changes that speed up CRC. In the colorectal epithelium, certain gut microbes, including Peptostreptococcus anaerobius, Fusobacterium nucleatum, sulfate-reducing bacteria, Enterococcus faecalis, Campylobacter jejune, Streptococcus bovis, Bacteroids fragilis, Salmonella spp., and Escherichia coli, cause persistent inflammation. Through changes in several signaling pathways and the production of proinflammatory cytokines, these microbial elements damage DNA and break down the barrier in the gut. Nevertheless, several treatments, such as antibiotics and FMT, inhibit the growth of microbes and colonization.
In contrast, in GI cancers, circRNAs have been found to inhibit tumor growth and offer great promise as therapeutic targets. These circRNAs act as tumor suppressors and have been found to be crucial in the development of chemoresistance in GI malignancies. They do this by influencing crucial signaling networks, affecting cellular processes, and regulating gene expression. For instance, circ-Foxo3 was shown to be reduced in ESCC cell lines and tissues, which suppresses ESCC growth via miR‐23a sponging and controls the PTEN gene expression [46]. Likewise, Fang et al. discovered that circFAT1(e2) controls tumor suppressor RUNX1 expression in GC cells by acting as a sponge for miR-548 g, which in turn, by interacting with YBX1 in the nucleus and targeting miR-548 g in the cytoplasm, suppresses the growth of GC [47]. Similarly, according to Chen et al., circRHOBTB3 expression is markedly downregulated in CRC tissues as well as cell lines, and it reduces the aggressiveness of the tumor through working with the HuR/PTBP1 axis [48]. Moreover, Zhong et al. demonstrated that circC3P1 works as a tumor suppressor by increasing PCK1 expression via miR-4641 sponging in HCC [49]. Furthermore, Shi et al. confirmed that circa ANAPC7 is a new tumor suppressor that prevents muscle atrophy and tumor growth in PC by reducing cyclin D1 and TGF through the CREB-miR-373-PHLPP2 axis [50] (Fig. 2). Therefore, circRNAs could be identified and characterized to shed light on the molecular causes of GI cancer and possibly enhance therapeutic approaches. Table 2 lists the numerous circRNAs' tumor-suppressing functions in the development of GI tumors through controlling their target genes and signaling networks.
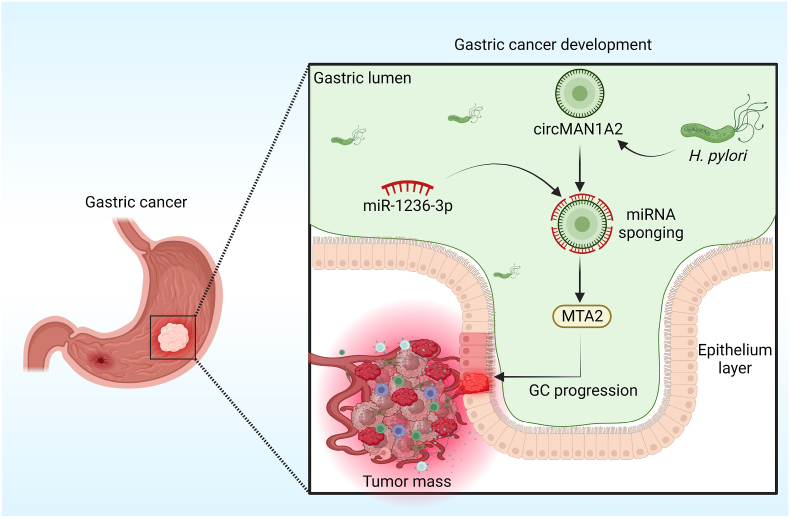
Fig. 2.
The diagram illustrates the interplay of circRNA and gut microbes in the progression of GC. CircMAN1A2 could induce GC progression, which was induced by H. pylori by miR-1236-3p sponging to regulate the expression of the MTA2 gene.
Table 2.
Tumor suppressor roles of various circRNAs in GI tumorigenesis through regulation of target genes and signaling pathways (↑: upregulated, ↓: downregulated).
| Cancer types | circRNAs | Clinical studies | Animal studies | Cell line studies | Target genes/signaling pathways | Clinicopathological characteristics | Description | Ref. |
|---|---|---|---|---|---|---|---|---|
| Esophageal cancer | Circ-Foxo3 | ESCC tissues = 94 pairs | BALB/c nude mice | KYSE510, ECA109, TE‐1, TE‐13 | miR-23a, PTEN | Age, TNM stage, gender, tumor size, histological grade | ↓Circ-Foxo3, ↓ miR-23a, ↑PTEN: ↓ ESCC progression | [46] |
| Circ-TNRC6B | -ESCC tissues = 53 - Healthy tissue = 48 |
– | TE-1, KYSE-170, KYSE-30, KYSE-150 | miR-452-5p, DAG1 | T stage | ↓Circ-TNRC6B, ↓miR-452-5p, ↑DAG1: ↓ ESCC invasion and proliferation | [86] | |
| Circ_0007624 | Tissue sample | – | – | -miR-224-5p, CPEB3 - EGFR/PI3K/AKT pathway |
Poor prognosis | ↓Circ_0007624, ↓miR-224-5p, ↑CPEB3, ↓ EGFR/PI3K/AKT pathway: ↓ ESCC development | [87] | |
| Gastric cancer | CircFAT1(e2) | GC = 38 case | BALB/c mice | GSE-1, SGC-7901, AGS, BGC-823, MKN-28, MGC-803, MKN-45 | miR-548 g/RUNX1 | Overall survival | ↓ CircFAT1(e2), ↓ miR-548 g, ↑ RUNX1: ↓ Tumor progression | [47] |
| CircRHOBTB3 | GC tissue = 75 pairs | BALB/C nude mice | AGS, MKN45, HGC27 | -miR-654-3p - p21 signaling |
Tumor stage | ↓CircRHOBTB3, ↓miRNA-654-3p, ↑p21 signaling: ↓ GC growth | [88] | |
| CircRTN4 | Tissue sample | – | – | miR-424-5p, LATS2 | Low survival rate | ↑CircRTN4, ↓miR-424-5p, ↓LATS2: ↓ GC development | [89] | |
| circ-HN1 | GC tissue = 30 pairs | – | GES-1, MKN-28, HGC-27, SGC-7901, AGS | miR-485-5p, GSK3A | – | ↓Circ-HN1, ↓miR-485-5p, ↑GSK3A: ↓ GC progression | [90] | |
| CircEIF4G3 | -GC tissue = 103 pairs - Serum = 120 from GC individuals - Serum = 50 from gastritis individuals - Serum = 120 normal controls |
BALB/c nude mice | AGS, GSE-1, HGC-27, MKN-45, HEK-293 T, SGC-7901 | miR-4449, SIK1, β-catenin pathway | TNM stage, venous invasion | ↑CircEIF4G3, ↓miR-4449, ↑SIK1, ↓β-catenin pathway: ↓ GC development and metastasis | [91] | |
| hsa_circ_0026344 | – | – | – | miR-590-5p, PDCD4 | Tumor size, TNM stage, LNM | ↓hsa_circ_0026344, ↓miR-590-5p, ↑PDCD4: ↓GC progression | [92] | |
| CircPFKP | GC tissue = 25 pairs | Nude mice | HGC-27, GES-1, MKN45, NCI–N87, SGC-7901, AGS | miR-644, ADAMTSL5 | – | ↑CircPFKP, ↓ miR-644, ↑ADAMTSL5: ↓ GC metastasis and proliferation | [93] | |
| Colorectal cancer | CircRHOBTB3 | CRC = 83 pairs | BALB/c nude mice | RKO, NCM460, HCT116, FHC, SW480, HT29, SW620, DLD-1, HCE8693, Colo320 | HuR/PTBP1 | Advanced clinical stages and greater risk of metastases | ↓CircRHOBTB3, ↓ PTBP1: ↓ metastasis | [48] |
| Circ0104103 | Tissue samples | BALB/c nude mice | NCM460, CACO2, HCT116, HT29, DLD1, HCT8, SW620, SW480 | LACTB, miR‐373‐5p | TNM stage, tumor invasion depth | ↓Circ0104103, ↓miR‐373‐5p, ↑LACTB: ↓ CRC progression | [94] | |
| CircCUL2 | – | Nude mice | HT290, FHC, HCT116, SW620, SW480 | miR-208a-3p, PPP6C | TNM stage and distant metastasis | ↓CircCUL2, ↓miR-208a-3p, ↑PPP6C: ↓ CRC proliferation | [95] | |
| CircITGA7 | – | Mice | SW480, FHC, RKO, DLD1, Caco-2, HCT116, LoVo, SW620 | -miR-370-3p, ITGA7, RREB1 -Ras signaling |
TNM stage, tumor size, distant metastasis, lymph metastasis | ↓CircITGA7, miR-370-3p, ↑ITGA7, ↓ RREB1, ↓ Ras signaling: ↓CRC development and metastasis | [96] | |
| Hepatocellular carcinoma | CircDLC1 | HCC = 110 case | BALB/c nude mice | Huh-7, SNU449, Hep3B, SK-Hep1, HepG2 | MMP1 | AFP level, BCLC stage, TNM stage, macrovascular invasion, microvascular invasion, OS, RFS | ↓CircDLC1, ↓MMP1: ↓ HCC progression | [97] |
| CircMTO1 | HCC tissue = 289 pairs | Nude mice | HepG2, SK-Hep1, QGY-7701, SMMC-7721, | miR-9, p21 | – | ↓CircMTO1, ↓miR-9, ↑p21: ↓ HCC progression | [98] | |
| cSMARCA5 | HCC tissue = 208 pairs | BALB/c nude mice | HCCLM3, Huh7, Hep3B, SMMC-7721, MHCC97H | miR-17-3p, miR-181b-5p, TIMP3 | Poorer tumor differentiation, microvascular invasion, advanced tumor stage, tumor size, OS | ↓cSMARCA5, ↓miR-17-3p and miR-181b-5p, ↑TIMP3: ↓ HCC development and metastasis | [99] | |
| CircC3P1 | HCC tissue = 47 pairs | BALB/c nude mice | BEL7402, HL-7702, Hep3B, MHCC97-L, HuH7 | miR-4641, PCK1 | Age, gender, AFP, size, TNM, vascular invasion | ↓CircC3P1, ↓miR-4641, ↑PCK1: ↓ HCC development and metastasis | [49] | |
| CircTRIM33–12 | HCC = 150 case | Nude mice | SMMC-7721, HCCLM3, Huh 7, HepG2, MHCC97L | miR-191, TET1 | Poor prognosis | ↓CircTRIM33–12, ↓miR-191, ↑TET1: ↓ HCC progression and metastasis | [100] | |
| Pancreatic cancer | CircANAPC7 | – | Athymic nude mice | AsPC-1, HPDE, MIA PaCa-2, CFPAC-1, Panc-1, BxPC-3 | CREB, miR-373, PHLPP2 | – | ↑CircANAPC7, ↓miR-373, ↓CREB, ↓PHLPP2: ↓ PC tumor growth and muscle wasting | [50] |
| Circ_0047744 | Tissue samples | – | HPDE6-c7, PANC-1 | miR-21, SOCS5 | LNM, positively correlated with OS | ↓circ_0047744, ↓miR-21, ↑SOCS5: ↓PDAC metastasis | [101] | |
| Circ-STK39 | Tissue sample | – | – | miR-140-3p, TRAM2 | – | ↓Circ-STK39, ↓ ↓miR-140-3p, ↓TRAM2: ↓ PC progression | [102] | |
| hsa_circRNA_001587 | PC tissue = 67 pairs | BALB/C nude mice | HPDE, PC-3, AsPC-1, COLO357, PANC-1 | miR-223, SLC4A4, MMP-2, MMP-9 | Tumor differentiation, LNM | ↓hsa_circRNA_001587, ↓miR-223, ↑SLC4A4: ↓ PC angiogenesis, migration and invasion | [103] |
Circ-Foxo3 circular RNA forkhead box O3, Circ-TNRC6B circular RNA trinucleotide repeat containing adaptor 6B, CircRTN4 circular RNA reticulon 4, CircEIF4G3 circular RNA enhancer of eIF4G3, CircITGA7 circular RNA integrin subunit alpha 7, CircMTO1 circular RNA mitochondrial translation optimization 1 homolog, Circ-STK39 circular RNA serine/threonine kinase 39, PTEN phosphatase and tensin homolog, PI3K phosphoinositide 3-kinase, RUNX1 runt-related transcription factor 1, SIK1 salt inducible kinase 1, PTBP1 polypyrimidine tract-binding protein 1, LACTB lactamase beta, PPP6C protein phosphatase 6 catalytic subunit, ITGA7 integrin subunit alpha 7, MMP1 matrix metallopeptidase 1, TIMP3 tissue inhibitor of metalloproteinases-3, PCK1 phosphoenolpyruvate carboxykinase 1, TET1 tet methylcytosine dioxygenase 1, TRAM2 translocating chain-associated membrane protein 2.
3. Role of gut microbiome in GI cancers
The gut microbiota may affect GI cancer progression through several mechanisms, including alteration of immune function, modification of gut barrier activity, and production of carcinogenic metabolites [19]. Several GI malignancies, including EC, CRC, GC, HCC, and PC, have been linked to an elevated risk because of modifications in the structure and activity of the gut microbiota [104]. The efficiency of anticancer treatments like chemotherapy and immunotherapy may also be influenced by the gut microbiota [105].
Moreover, FMT stands for a prospective cancer treatment plan through improving bile acid metabolism, reestablishing the gut microbiota, and adjusting the effectiveness of immunotherapy [106]. Gut dysbiosis can be brought on by a variety of causes, including host genetics, nutrition, antibiotics, and stress [107]. Through the activation of tumorigenic pathways, generating inflammation, and harming host DNA, microbial dysbiosis and specific bacteria in the gut might influence the growth and progression of cancer [108]. Certain bacterial products, such as the CagA protein of Helicobacter pylori [109], the FadA toxin of Fusobacterium nucleatum [110], the AvrA protein of S.enterica Typhi [111], and BFT from Enterotoxigenic Bacteroides fragilis, may encourage E-cadherin and β-catenin separation, which can result in β-catenin activation and aid in tumor development [112]. Microbial dysbiosis also results in a reduction in the beneficial component of bacterial metabolites such as short-chain fatty acids (SCFAs) [113]. The microbe-associated molecular patterns (MAMPs), which activate TLRs in macrophages and dendritic cells, play a role in intestinal dysbiosis's potential for bacterial translocation and exert a pro-inflammatory consequence [114]. TLR signaling encourages the expression of pro-inflammatory molecules like IL-23, TNF, and IL-1, which promote the growth of cancer [115]. Moreover, numerous microbial metabolites can directly or indirectly harm host DNA, which promotes tumorigenesis. Special microbial toxins, such as CDT and colibactin, may cause DNA damage directly [116]. In addition, DNA is also indirectly harmed by gut bacteria via polyamines, DCA, RNS, ROS, and H2S [117] (Fig. 1).
BFT bacteroides fragilis toxin; FadA fusobacterium adhesin A; CAM cell adhesion molecule; IFN interferon; LPS lipopolysaccharide; NF-κB nuclear factor-κB; MAMP microbe-associated molecular pattern; STAT3 signal transducer and activator of transcription 3; PRR pattern recognition receptor; TLR4 Toll-like receptor 4.
4. The interplay between circular RNAs and microbiota in gastrointestinal cancer
The circRNAs and the microbiota interaction in GI cancer is a growing area of research that might provide information on the intricate pathways that underlie the development of cancer. Helicobacter pylori is recognized as a major contributor to the development of stomach cancer [118]. In GC, Guo et al. found that independent of CagA, Helicobacter pylori could cause the overexpression of circMAN1A2 in AGS and BGC823 cells to induce GC tumorigenesis [119]. They showed that Helicobacter pylori might cause CagA-independent overexpression of circ_MAN1A2 in GC cells. Furthermore, in vivo, the growth of xenograft tumors was decreased by circ_MAN1A2 knockdown, and the advancement of GC was linked to circ_MAN1A2 overexpression (Fig. 2). Thus, circ_MAN1A2 may be a new GC therapeutic target and diagnostic marker.
Similarly, Diling et al., found that the gut microbiota and brain circRNA sequence profiles of AD-like animals were altered due to circNF1-419's ability to improve autophagy and treat senile dementia. According to their findings, over-expression of circNF1-419 in the brain altered the composition of the gut microbiota, including Candidatus arthromitus, Lachnospiraceae FCS020 group, Lachnospiraceae UCG-006 group, and [Eubacterium] Xylanophilum group, as well as intestinal homeostasis and physiology, and even the progression of the gut microbiota in newborn mice [120].
In contrast, tumor metastasis is controlled by gut microbiota through the circRNA and miRNA pathway. For example, it was proved that the specified pathogen-free (SPF) mice treated with ABX showed increased lung metastases. Lung metastases was dramatically reduced after fecal microbiota transplantation (FMT) from SPF mice or Bifidobacterium into germ-free mice. In order to control the concentrations of oncogenic miRNAs, the gut microbiota influences the expression of circRNA such as mmu_circ_0000730 [121]. In particular, these modifications in the gut microbiota suppress the expression of IL-11. Mutual suppression between mmu circ_0000730 and mmu-miR-466i-3p was identified using bioinformatics tools and luciferase reporter studies. Internal homeostasis and appropriate protection against microbial infection and malignancy are two examples of how circRNAs contribute to human immunity (Table 3).
Table 3.
The circRNAs and the microbiota interaction in GI malignancies.
| CircRNA | Associated microbes | Targes | Cell line | Animal | Clinical sample | Findings | Ref. |
|---|---|---|---|---|---|---|---|
| CircMAN1A2 | Helicobacter pylori | miR-1236-3p, MTA2 | GES-1, AGS, BGC823 | BALB/c nude mice | Hp− human gastritis = 52, Hp + human gastritis = 47 | By sponging miR-1236-3p to control the expression of MTA2, circMAN1A2 could accelerate the development of GC brought on by H. pylori. | [119] |
| CircNF1-419 | Candidatus Arthromitus, Lachnospiraceae UCG-006, Lachnospiraceae FCS020 group, and [Eubacterium] xylanophilum group | - | - | SAMP8 mice | - | In AD-like mice, CircNF1-419 enhances gut microbiota structure and function. | [120] |
| ciRS-7 | Cryptosporidium parvum | NF–B signaling pathway | HCT-8 | - | - | CiRS-7 might facilitate the spread of C. parvum by controlling the miR-1270/relA axis and the NF–B pathway. | [122] |
| mmu_circ_0000730 | - | mmu-miR-466i-3p, mmu-miR-466 f-3p, SOX9, IL-11 | LLC, B16–F10 | C57BL/6 mice | - | Through the IL-11/circRNA/miRNA/SOX9 axis, gut microbiota controls the development and metastasis of cancer. | [121] |
| circHIPK2 | Firmicutes, Proteobacteria, Bacteroidetes | - | - | NLRP3 KO mice | - | NLRP3-deficient mice's gut microbiome reduces depressive-like behaviors through controlling astrocyte dysfunction via circ HIPK2 | [123] |
However, understanding the complex relationships between these two variables may lead to the development of new therapeutic approaches and biomarkers, ultimately improving the detection, diagnosis, and treatment of GI malignancies.
5. Gastrointestinal cancer and therapeutic targets
GI malignancies are a group of tumors that generally affect numerous digestive system organs. Numerous therapeutic targets for GI tumors have been discovered over time, and targeted medicines are now a crucial component of cancer treatment. Here are several treatment approaches for GI malignancies that are frequently researched.
6. Current treatment strategies for gastrointestinal cancers
According to the specific kind and stage of the tumor, many therapeutic approaches are used for GI malignancies [124]. Surgery, chemotherapy, radiation therapy, targeted treatments, and immunotherapy are frequently used as treatment options [125,126].
For localized GI malignancies, surgery is frequently the first line of treatment [127]. To stop the spread of cancer cells, the tumor and adjacent lymph nodes must be removed [128]. If the cancer is advanced or has spread to distant organs, surgery may not always be an option [129]. Similarly, chemotherapy is a systemic therapy that employs medications to either kill or limit the growth of cancer cells. When surgery is not an option, chemotherapy is frequently employed before or after surgery to reduce tumor size [130]. GI malignancies are routinely treated with combination chemotherapy regimens [131]. Likewise, to eradicate cancerous cells and reduce tumors, radiation treatment employs high-energy photons. It can be used as the main course of treatment for localized GI malignancies, in conjunction with surgery or chemotherapy, or to treat advanced cases of the disease's symptoms [132]. Moreover, drugs used in targeted therapy precisely target certain molecules or pathways thought to be essential in the progression and spread of tumors [133]. For instance, HER2-targeted treatments in PC [134], VEGF inhibitors [135], and EGFR inhibitors [136] are utilized to treat particular GI malignancies. In addition, through immunotherapy, cancer cells are recognized and attacked by the patient's immune system. Some GI malignancies have responded well to the use of immune checkpoint inhibitors, particularly in patients with advanced or metastatic illness [137]. Examples of these agents are PD-1 and PD-L1 inhibitors [138]. Furthermore, neoadjuvant therapy is another therapeutic approach used to reduce tumors before surgery and enhance its results [139]. Following surgery, adjuvant therapy is administered to lower the possibility of a cancer recurrence [140].
Despite that, healthcare is a crucial part of treatment for those with advanced or metastatic GI malignancies [141]. Through the control of symptoms, pain, and other side effects of malignant therapy, healthcare aims to increase the quality of life [142].
Thus, the specifics of the tumor, the cancer's stage, and the patient's general health all play a role in determining the course of treatment for GI malignancies. For each patient, multidisciplinary teams of oncologists, surgeons, radiation oncologists, and other professionals collaborate need to develop the most efficient and individualized course of therapy.
7. Strategies to target circRNAs
In recent years, significant advancements have been achieved in the research of circRNA. A growing body of research has confirmed that circRNAs can be therapeutically targeted in both vivo and in vitro using a range of methods, including exosome-mediated transport, RNA interference (RNAi), ASOs, and circRNA modification. Even though research in this area is still ongoing, here are some potential strategies that researchers are taking into consideration.
A strategy based on siRNAs makes use of the endogenous RNAi process, in which double-stranded RNA (dsRNA) molecules cause post-transcriptional silencing [143]. Short interfering RNA (siRNA) or short hairpin RNA are frequently used in RNAi to knock down circRNAs. By complementary pairing, siRNAs, which are 21–23 nt in dsRNA length, target circRNAs and add them to the RISC, where they will be cleaved [30]. ShRNAs are converted into siRNAs after being processed and are distinguished by their loops and base-paired stems [144]. The back-splice junction particular to circRNAs is typically targeted to reduce circRNAs without impacting their corresponding linear mRNA. Further, through complementary pairing, antisense oligonucleotides (AON) can also target circRNAs [145]. Despite their effectiveness at blocking protein interaction sites on circRNAs, their length prevents them from being used to specifically target the back-splice junction and knockdown of circRNAs. In addition, the most practical approach to knocking down circRNAs in vivo currently involves the use of siRNA and shRNA delivered in lipid-based polymers. Nevertheless, RNAi molecules have some drawbacks that need to be addressed. These include their quick destruction by nucleases [146], inefficient cellular delivery [147], lack of cell selectivity [148], immunogenicity [149], and off-target consequences [150].
Moreover, direct biogenesis and purification allow circRNAs to be overexpressed [151]. Many different methods can be used to circularize RNA [152]. Splint ligation can be used to cycle single-stranded linear RNA that has been chemically or in vitro transcribed [153]. This approach produces exceedingly pure circRNA molecules that can be given directly to target cells [154]. Also, a successful in vitro miRNA sponge has been produced using this technique [155].
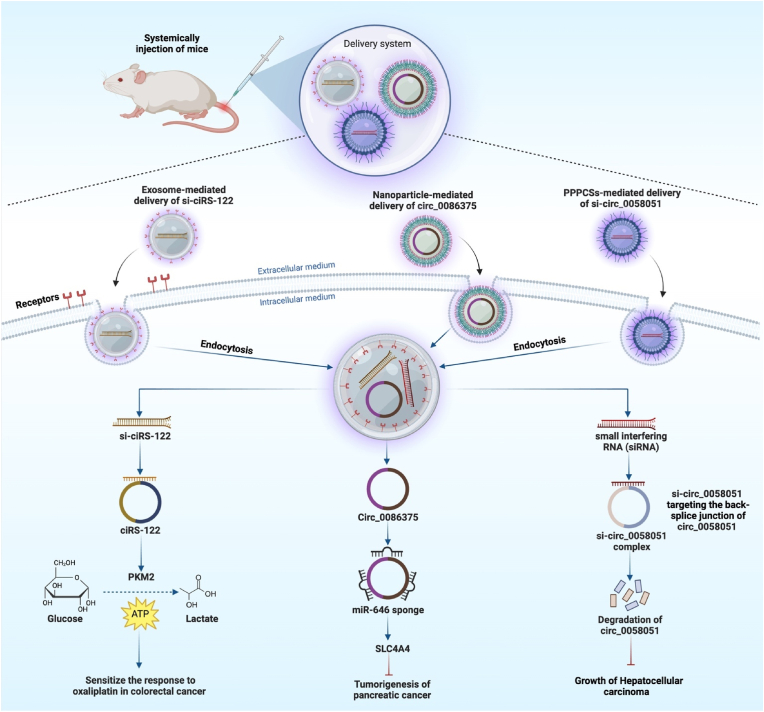
By employing nanoparticles as carriers, circRNA molecules can be efficiently delivered to specific cells or tissues while simultaneously being protected, resolving several concerns with conventional therapies [156]. After being released into the tumor target cells, circRNA-based medicines can start to work in several different ways [157]. They can act as powerful regulators, influencing signaling pathways, changing gene expression, or absorbing microRNAs, all of which have the potential to have therapeutic effects [158]. For instance, Wang et al. proved that using nanoparticles loaded with circ 0086375 to target the miR-646/SLC4A4 axis can prevent PC from getting worse. In which circ 0086375 was identified as miR-646's verified target, it worked as a miR-646 sponge to elevate the expression of SLC4A4 [159]. Similarly, You et al. showed that has-circ-0058051 may function as an oncogene that induces HCC cell proliferation and migration. They developed a safe and effective magnetic nanoparticle-mediated delivery strategy for transporting circ-0058051-siRNA under an external MF to silence circ-0058051 in HCC. Their outcomes demonstrated that si-circ-0058051 is protected effectively by PEG-PCL-PEI-C14-SPIONs (PPPCSs) against degradation by serum and tissue enzymes. As a result, circ_0058051 was silenced in vitro and in vivo by the PPPCSs/si-circ_0058051 complex, which greatly reduced carcinogenesis and HCC development [160].
In addition to nanoparticles, exosomes, which are small extracellular vesicles made by cells, can be used to naturally transport circRNAs [161]. When exosomes are taken up by the recipient cells, the circRNAs are sent straight to the cytoplasm [162]. The circRNAs can either interact with RBPs or act as sponges for miRNAs to influence gene expression and signaling pathways linked to drug resistance or other pathogenic events [163]. For instance, Wang et al. found that CRC cell exosomes contained an enriched form of ciRS-122, which contributed to therapeutic resistance by miR-122 sponging and overexpressing PKM2. The increased PKM2 led to higher glycolysis and ATP production, allowing cells to expel drugs effectively. The chemoresistant CRC cells transferred si-ciRS-122 via exosomes to nonresistant cells to sensitize the response to oxaliplatin in CRC by preventing glycolysis [164] (Fig. 3).
Fig. 3.
The illustration shows common ways to target circRNAs in vivo as a therapeutic approach to knocking down circRNAs, such as exosome-induced delivery of siRNA and nanoparticle-induced delivery of siRNA.
Further, CRISPR/Cas gene-editing system is a cutting-edge tool for precisely targeting and silencing circular RNA molecules through a process called CRISPR/Cas-mediated circRNA knockdown [165]. The ability to knock down circRNAs with CRISPR/Cas is a powerful method for studying the roles of these non-coding RNAs in disease [166]. To achieve circRNA knockdown, the CRISPR/Cas system should be designed to target the back-splice junction region of the circular RNA molecule [167]. CRISPR/Cas is a particularly useful tool for circRNA knockdown because of its high specificity and flexibility, which allow precise targeting of individual circRNAs in a cell- or tissue-specific way [168].
8. Targeting the gut microbiome in GI cancer therapy
A developing area of inquiry that holds promise as a potential therapeutic method is targeting the gut microbiota in the treatment of GI [169]. The microbiome of the gut is an important component in the processes that keep gut health, digestion, and immunity in good standing [170,171]. Growing evidence suggests that the gut microbiota influences cancer progression and the efficacy of cancer therapy [172]. Various studies have shown that the presence or absence of certain gut bacteria can promote or inhibit tumor growth, as well as influence how well chemotherapy and immunotherapy work [173].
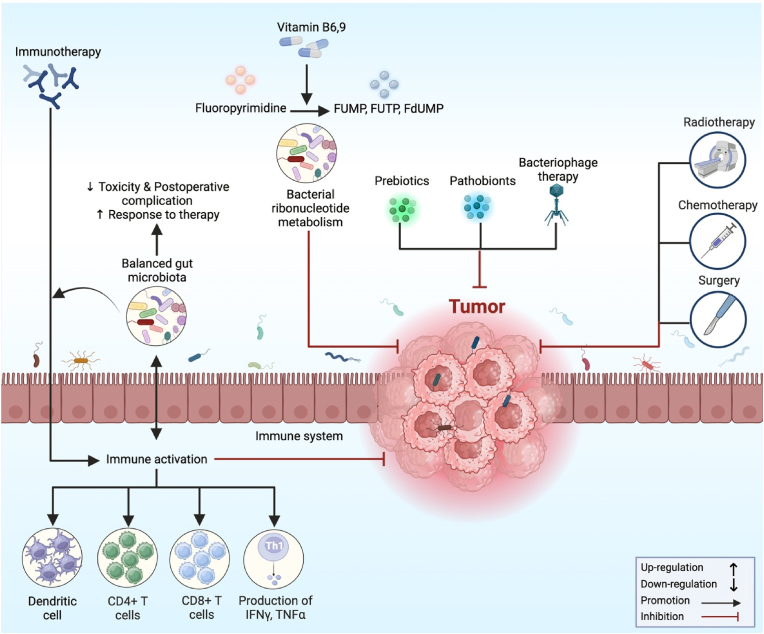
Scientists are looking at the potential benefits of gut microbiota in the diagnosis and intervention of gastrointestinal cancer. For instance, oncologists may be able to personalize cancer treatment strategies by having a better understanding of a patient's gut microbiota [174]. By analyzing a patient's gut microbiota, medical professionals may be able to predict the patient's response to certain drugs [175]. Moreover, in CRC, numerous strategies have been demonstrated to target and alter the composition of the gut microbiota, considering both microbial physiology and/or their metabolites that directly or indirectly lead to CRC [176]. These strategies include dietary interventions, antibiotic treatments, probiotics, prebiotics, and postbiotics, as well as FMT [177] (Fig. 4). For instance, through several processes, including microbial-derived elements such as metabolites or genotoxins, the gut microbiota can affect the growth of CRC [178]. Antibiotic use is effective at eliminating pathobionts, but because it also kills beneficial bacteria for health, it hurts gut homeostasis and should therefore be used less frequently in CRC care [179].
Fig. 4.
The schematic diagram illustrates the multifaceted mechanisms of microbiota modulation in response to therapy aimed at suppressing tumor growth. These complex mechanisms entail the gut microbiota's dynamic interaction with the host's immune system, therapeutic drugs, and the tumor environment.
Probiotic development is encouraged by prebiotic activity. Probiotics have a variety of anti-cancerogenic effects, including the prevention of pathogenic bacteria from colonizing the body [180], improving barrier function by increasing the production of mucin and tight junction proteins [181], encouraging homeostatic immune responses by encouraging the growth of anti-inflammatory responses by Treg cells [182], modulating pro-inflammatory cytokine release [183], and encouraging cancer cell apoptosis. Additionally, postbiotics cause selective cytotoxicity against tumor cells as well as the regulation of tumor cell proliferation [184].
Furthermore, FMT has the potential to be employed in the treatment of GI cancers by preventing the colonization of pathogenic bacteria [185]. This method has demonstrated promise in treating specific digestive disorders, such as recurrent Clostridium difficile infections [186,187]. The growing interest in the potential of FMT for the treatment of cancer is sparked by the observation that cancer patients often have intestinal dysbiosis [188]. Thus, to develop novel and individualized therapeutic strategies in the fight against GI malignancies, additional study is required to better understand the complex relationships between the gut microbiome and cancer.
9. Microbiota-targeted circRNA therapies
Microbiota-targeted circRNA therapies are an innovative approach to personalized medicine that hopes to focus on the connections between the gut microbiota and human health. Recent studies have revealed how circRNAs affect host-microbiota crosstalk, which affects physiological processes and the onset of illness [29,189]. Through circRNA-based interventions, these treatments aim to modify the structure and function of the gut microbiota. Additionally, bacteria have emerged as a possible cancer treatment tool due to their natural affinity for hypoxic tumor environments and their potential for genetic engineering as a vector for gene and medication therapy [190].
Microbiota-targeted circRNA treatments work by identifying and modifying certain circRNAs that serve as brokers of host-microbiota interactions [191]. These circRNAs may serve as signaling pathway regulators, binding partners, or molecular scaffolds in the homeostasis of the microbiota [192,193]. Understanding the intricate interactions between circRNAs and the gut microbiota will allow researchers to create artificial circRNAs or alter endogenous ones to precisely control the structure and activity of the microbiota. Innovative delivery methods, such as nanoparticles, synthetic bacteria, or even genetically altered probiotics, are used to deliver circRNA therapeutics to the gut [194,195]. In the gut environment, where they may exert their regulatory effects on the microbiota, these vehicles ensure the exact release of circRNAs. By using this method, circRNA treatments may be able to reduce inflammation, dysbiosis, and other conditions that are impacted by the microbiome.
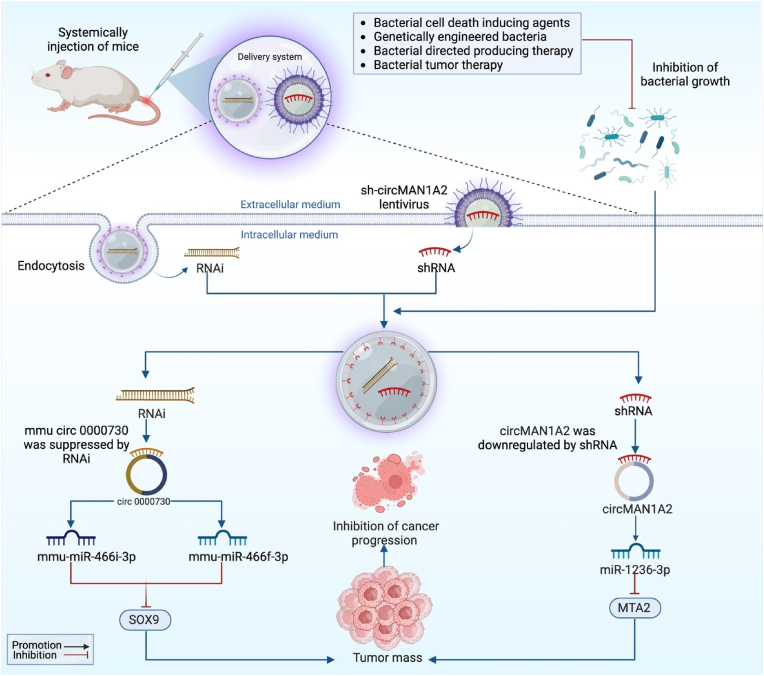
For instance, expression of mmu-miR-466i-3p and mmu-miR-466f-3p was dramatically elevated when mmu circ 0000730 was suppressed by RNAi. Through modulation of the gut microbiota SOX9 or mmu circ 0000730 dramatically downregulated and decreased cancer cell invasion. Thus, mmu_circ_0000730 targets mmu-miR-466i-3p and inhibits cancer progression by decreasing SOX9 expression and blocking the STAT3 signaling pathway [121]. Similarly, in gastric cancer, Guo et al. revealed that downregulation of circMAN1A2 prevents the growth of gastric tumor cells. Therefore, to determine how circMAN1A2 affected the development of gastric cancer in vivo, they used a xenograft mouse model. By using sh-circMAN1A2 lentivirus and inhibiting H. pylori growth, they reduced circMAN1A2 expression in BGC823 cells. Downregulating circMAN1A2 expression significantly slowed tumor development, weight, and volume as compared to the control group by sponging miR-1236-3p to regulate MTA2 expression. These findings imply that downregulating circMAN1A2 can prevent gastric cancer cells from proliferating in vivo [119] (Fig. 5).
Fig. 5.
The schematic illustration represents the microbiota-targeted circRNA therapies. Through the systematic administration of RNAi and shRNA, and the inhibition of microbial growth, circ000730 and circMAN1A2, as well as their corresponding target genes SOX9 and MTA2, are downregulated, which inhibits the advancement of cancer.
However, exploring combination therapies that simultaneously target the gut microbiome and circRNAs may have synergistic effects and open up new therapeutic possibilities for the treatment of GI cancer.
10. CircRNAs and gut microbiota as indicators for GI cancer prognosis and diagnosis
The gut microbiota and circRNAs play significantly important roles in the landscape of GI malignancies as indications for prognosis and diagnosis [29]. CircRNAs, which were once thought to be splicing artifacts, have emerged as powerful molecular markers as a result of their unique characteristics, such as stability and tissue-specific expression [196]. These characteristics make them promising candidates for prognostic assessment, as changed expression profiles of particular circRNAs have been linked with various disease stages, recurrence, and overall patient survival [197].
Interestingly, circRNAs have the potential to be used as a diagnostic tool, as their abnormal patterns of expression can be used to differentiate between malignant tissues and their healthy counterparts or normal tissues [198]. For instance, Wang et al. demonstrated that circSLIT2 RNA accumulation was higher in GC tissues compared to non-tumor tissues and was associated with distant tumor metastases, and plasma circSLIT2 was only found in GC patients. Plasma circSLIT2 positively correlated with circSLIT2 in GC tissues, distinguishing GC patients from other disease groups and HC patients [199]. Similarly, Wang with his colleagues confirmed that tissue and plasma expression levels of circ_0071662 were found to be decreased in HCC patients compared to HCs and other patient groups. This decreased expression was associated with poor survival, tumor metastasis, and tumor size, and was only observed in the radioresistance group after radiotherapy [200]. Likewise, in CRC, Li et al. demonstrated circGAPVD1's diagnostic efficacy in plasma exosomes. They expected that the highly expressed circGAPVD1 will serve as a novel CRC diagnostic marker [201].
Moreover, the GI tract's complex ecosystem, the gut microbiota, has become recognized as a major factor in processes that are connected to cancer [202]. The complex interactions between the host's health and the gut microbiota have made it a valuable source of predictive data [203]. Patients with GI cancer have had a range of outcomes, and variations in microbial compositions have been linked to these outcomes, offering information on the course of the disease and the survival of the patient [204]. These microbial signatures may be used as prognostic markers, assisting in the customization of treatment plans based on unique patient profiles. Additionally, the gut microbiota's impact on immune responses and metabolism affects fecal metabolites and immunological markers, paving the way for non-invasive diagnostic techniques that could completely transform the early identification of cancer [205]. According to Guo et al. study, Fusobacterium nucleatum may contribute to microbiota dysbiosis by secreting compounds that are hostile to probiotics. Furthermore, they was discovered that the ratio of Fusobacterium nucleatum to the crucial probiotics Faecalibacterium prausnitzii and Bifidobacterium is a useful biomarker for early CRC screening [206]. Similarly, in GC, salivary Fusobacterium nucleatum abundance shows promise as a biomarker for the diagnosis of GC, and Fusobacterium nucleatum infection may accelerate the EMT process and cause GC metastases [207]. CircRNAs and gut microbiota are expanding as promising biomarker for GI cancer prognosis and diagnosis. However, despite their enormous potential, many strategies are still being researched and might not have been widely used in clinical practice yet.
11. Conclusion
GI malignancies' relationship with circRNAs and the gut microbiota has become a novel field of study with important implications for comprehending carcinogenesis and identifying possible treatment targets. CircRNAs, a type of ncRNA, have been discovered to have essential roles in controlling many cellular processes associated with the initiation and development of GI malignancies. Additionally, it is now widely acknowledged that the trillions of microbes that survive in the gastrointestinal system and make up the gut microbiome have an essential role in controlling host physiology, immunological responses, and even cancer development.
The discovery of prospective treatment targets has been made possible by a better understanding of the involvement of circRNAs and the gut microbiome as well as their interactions in GI malignancies. It may be possible to develop new methods for cancer treatment and detection by focusing on particular circRNAs linked to carcinogenesis. Despite that, using probiotics, prebiotics, or FMT to modify the gut microbiome may provide creative strategies to affect circRNA-mediated cancer-related pathways.
A promising route for expanding our knowledge of cancer biology and creating new therapeutic approaches is the investigation of circRNAs and the gut microbiota in GI malignancies. Future advancements in cancer treatment and patient outcomes could result from continued study in this field. However, it's important to recognize that this area of study is still in its early stage and further study is needed to fully understand the complex mechanisms behind the interaction between circRNAs and the gut microbiota in GI malignancies.
Ethics approval and consent to participant
Not applicable.
Consent of publication
Not applicable.
Availability of data and materials
Not applicable.
Competing interest
The authors declare they have no conflict of interest.
Funding
Not applicable.
CRediT authorship contribution statement
Sara Tharwat Abdullah: Data curation, Investigation, Visualization. Snur Rasool Abdullah: Data curation, Methodology, Supervision. Bashdar Mahmud Hussen: Data curation, Validation. Yousif Mohammed Younis: Investigation, Methodology, Writing – original draft. Mohammed Fatih Rasul: Validation, Writing – original draft, Writing – review & editing. Mohammad Taheri: Investigation, Resources, Supervision.
Acknowledgement
Not applicable.
Contributor Information
Bashdar Mahmud Hussen, Email: bashdar.hussen@hmu.edu.krd.
Mohammad Taheri, Email: Mohammad.Taheri@uni-jena.de.
Abbreviation
- Bcl2
B-cell lymphoma 2
- CD274
Cluster of Differentiation 274
- CDC25B
Cell division cycle 25B
- CDK6
Cyclin-dependent kinase 6
- c-Myc
Cellular-myelocytomatosis oncogene
- CREB
cAMP-response element binding protein
- CTNND1
Catenin delta 1
- DNMT3A
DNA methyltransferase 3 alpha
- FBXO22
F-box protein 22
- FGFR1
Fibroblast growth factor receptor 1
- FNDC3B
Fibronectin type III domain containing 3B
- FOXO3
Forkhead box O3
- HK2
Hexokinase 2
- IGF2
Insulin-like growth factor 2
- IL-10
Interleukin 10
- ITGA7
Integrin subunit alpha 7
- JAK
Janus kinase
- KRAS
Kirsten rat sarcoma viral oncogene homolog
- LACTB
Lactamase beta
- MMP-2
Matrix metalloproteinase-2
- MMP-9
Matrix metalloproteinase −9
- mTOR
echanistic target of rapamycin
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NR2F6
Nuclear receptor subfamily 2 group F member 6
- PCK1
Phosphoenolpyruvate carboxykinase 1
- PIK3R1
Phosphoinositide-3-kinase regulatory subunit 1
- PKM2
Pyruvate kinase muscle 2
- PPP6C
Protein phosphatase 6 catalytic subunit
- PTBP1
Polypyrimidine tract-binding protein 1
- PTEN
Phosphatase and tensin homolog
- RBBP7
Retinoblastoma-binding protein 7
- RREB1
Ras-responsive element-binding protein 1
- SLC4A4
Solute carrier family 4-member 4
- SOCS5
Suppressor of cytokine signaling 5
- SPARCL1
SPARC-like protein 1
- STAT3
Signal transducer and activator of transcription 3
- TCF7
Transcription factor 7
- TRAM2
Translocating chain-associated membrane protein 2
- VEGFA
Vascular endothelial growth factor-A
- YAP1
Yes-associated protein 1
References
- 1.Vajihinejad M. A systematic review of clinic pathology and survival in gastrointestinal stromal tumors. Int. J. Network. Commun. 2023;10(1) [Google Scholar]
- 2.Allo G., Bürger M., Chon S.-H., Gülcicegi D., Krämer L., Goeser T., et al. Efficacy of endoscopic therapy and long-term outcomes of upper gastrointestinal tumor bleeding in patients with esophageal cancer. Scand. J. Gastroenterol. 2023:1–7. doi: 10.1080/00365521.2023.2199439. [DOI] [PubMed] [Google Scholar]
- 3.Pihlak R., Fong C., Starling N. Targeted therapies and developing precision medicine in gastric cancer. Cancers. 2023;15(12):3248. doi: 10.3390/cancers15123248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sethi G., Rath P., Chauhan A., Ranjan A., Choudhary R., Ramniwas S., et al. Apoptotic mechanisms of quercetin in liver cancer: recent trends and advancements. Pharmaceutics. 2023;15(2):712. doi: 10.3390/pharmaceutics15020712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sedlak J.C., Yilmaz Ö.H., Roper J. Metabolism and colorectal cancer. Annu. Rev. Pathol. 2023;18:467–492. doi: 10.1146/annurev-pathmechdis-031521-041113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halbrook C.J., Lyssiotis C.A., di Magliano M.P., Maitra A. Pancreatic cancer: advances and challenges. Cell. 2023;186(8):1729–1754. doi: 10.1016/j.cell.2023.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J., Xu Q., Huang Z-j, Mao N., Lin Z-t, Cheng L., et al. CircRNAs: a new target for the diagnosis and treatment of digestive system neoplasms. Cell Death Dis. 2021;12(2):205. doi: 10.1038/s41419-021-03495-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ağagündüz D., Cocozza E., Ö Cemali, Bayazıt A.D., Nanì M.F., Cerqua I., et al. Understanding the role of the gut microbiome in gastrointestinal cancer: a review. Front. Pharmacol. 2023;14 doi: 10.3389/fphar.2023.1130562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hussen B.M., Abdullah S.R., Hama Faraj G.S., Rasul M.F., Salihi A., Ghafouri-Fard S., et al. Exosomal circular RNA: a signature for lung cancer progression. Cancer Cell Int. 2022;22(1):378. doi: 10.1186/s12935-022-02793-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tao M., Zheng M., Xu Y., Ma S., Zhang W., Ju S. CircRNAs and their regulatory roles in cancers. Mol. Med. 2021;27(1):1–22. doi: 10.1186/s10020-021-00359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo L., Jia L., Luo L., Xu X., Xiang Y., Ren Y., et al. Critical roles of circular RNA in tumor metastasis via acting as a sponge of miRNA/isomiR. Int. J. Mol. Sci. 2022;23(13):7024. doi: 10.3390/ijms23137024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghafouri-Fard S., Taheri M., Hussen B.M., Vafaeimanesh J., Abak A., Vafaee R. Function of circular RNAs in the pathogenesis of colorectal cancer. Biomed. Pharmacother. 2021;140 doi: 10.1016/j.biopha.2021.111721. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda Y., Morikawa S., Nakashima M., Yoshikawa S., Taniguchi K., Sawamura H., et al. CircRNAs and RNA-binding proteins involved in the pathogenesis of cancers or central nervous system disorders. Non-coding RNA. 2023;9(2):23. doi: 10.3390/ncrna9020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi Y., Jia X., Xu J. The new function of circRNA: translation. Clin. Transl. Oncol. 2020;22:2162–2169. doi: 10.1007/s12094-020-02371-1. [DOI] [PubMed] [Google Scholar]
- 15.Lin G.R., Chen W.R., Zheng P.H., Chen W.S., Cai G.Y. Circular RNA circ_0006089 promotes the progression of gastric cancer by regulating the miR‐143‐3p/PTBP3 axis and PI3K/AKT signaling pathway. Journal of Digestive Diseases. 2022;23(7):376–387. doi: 10.1111/1751-2980.13116. [DOI] [PubMed] [Google Scholar]
- 16.Wu H.-B., Huang S.-S., Lu C.-G., Tian S.-D., Chen M. CircAPLP2 regulates the proliferation and metastasis of colorectal cancer by targeting miR-101-3p to activate the Notch signalling pathway. Am. J. Tourism Res. 2020;12(6):2554. [PMC free article] [PubMed] [Google Scholar]
- 17.Hussen B.M., Mohamadtahr S., Abdullah S.R., Hidayat H.J., Rasul M.F., Hama Faraj G.S., et al. Exosomal circular RNAs: new player in breast cancer progression and therapeutic targets. Front. Genet. 2023;14 doi: 10.3389/fgene.2023.1126944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Domingues C., Cabral C., Jarak I., Veiga F., Dourado M., Figueiras A. The debate between the human microbiota and immune system in treating aerodigestive and digestive tract cancers: a review. Vaccines. 2023;11(3):492. doi: 10.3390/vaccines11030492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadrekarimi H., Gardanova Z.R., Bakhshesh M., Ebrahimzadeh F., Yaseri A.F., Thangavelu L., et al. Emerging role of human microbiome in cancer development and response to therapy: special focus on intestinal microflora. J. Transl. Med. 2022;20(1):1–20. doi: 10.1186/s12967-022-03492-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gunec C. The role of intestinal flora in the immune system. Microenviron Microecol Res. 2022;4:14. [Google Scholar]
- 21.Zhang P. Influence of foods and nutrition on the gut microbiome and implications for intestinal health. Int. J. Mol. Sci. 2022;23(17):9588. doi: 10.3390/ijms23179588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luu M., Schütz B., Lauth M., Visekruna A. The impact of gut microbiota-derived metabolites on the tumor immune microenvironment. Cancers. 2023;15(5):1588. doi: 10.3390/cancers15051588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tustumi F., Arienzo V.P., Sunye I.R., Lucas P.F.S., Colonno B.B., Quintas J.G., et al. Esophageal dysbiosis in achalasia and cancer development: a critical review. Genes. 2023;14(8):1521. doi: 10.3390/genes14081521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liatsos C., Papaefthymiou A., Kyriakos N., Galanopoulos M., Doulberis M., Giakoumis M., et al. Helicobacter pylori, gastric microbiota and gastric cancer relationship: unrolling the tangle. World J. Gastrointest. Oncol. 2022;14(5):959. doi: 10.4251/wjgo.v14.i5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Said I., Ahad H., Said A. Gut microbiome in non-alcoholic fatty liver disease associated hepatocellular carcinoma: current knowledge and potential for therapeutics. World J. Gastrointest. Oncol. 2022;14(5):947. doi: 10.4251/wjgo.v14.i5.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chai Y., Huang Z., Shen X., Lin T., Zhang Y., Feng X., et al. Microbiota regulates pancreatic cancer carcinogenesis through altered immune response. Microorganisms. 2023;11(5):1240. doi: 10.3390/microorganisms11051240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh S., Sharma P., Sarma D.K., Kumawat M., Tiwari R., Verma V., et al. Implication of obesity and gut microbiome dysbiosis in the etiology of colorectal cancer. Cancers. 2023;15(6):1913. doi: 10.3390/cancers15061913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou C.-B., Fang J.-Y. The regulation of host cellular and gut microbial metabolism in the development and prevention of colorectal cancer. Crit. Rev. Microbiol. 2018;44(4):436–454. doi: 10.1080/1040841X.2018.1425671. [DOI] [PubMed] [Google Scholar]
- 29.Fardi F., Khasraghi L.B., Shahbakhti N., Naseriyan A.S., Najafi S., Sanaaee S., et al. An interplay between non-coding RNAs and gut microbiota in human health. Diabetes Res. Clin. Pract. 2023 doi: 10.1016/j.diabres.2023.110739. [DOI] [PubMed] [Google Scholar]
- 30.He A.T., Liu J., Li F., Yang B.B. Targeting circular RNAs as a therapeutic approach: current strategies and challenges. Signal Transduct. Targeted Ther. 2021;6(1):185. doi: 10.1038/s41392-021-00569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seidel D.V., Azcárate-Peril M.A., Chapkin R.S., Turner N.D., editors. Seminars in Cancer Biology. Elsevier; 2017. Shaping functional gut microbiota using dietary bioactives to reduce colon cancer risk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu Z., Tie X., Li N., Yi Z., Shen F., Zhang Y. Circular RNA hsa_circ_0000654 promotes esophageal squamous cell carcinoma progression by regulating the miR‐149‐5p/IL‐6/STAT3 pathway. IUBMB Life. 2020;72(3):426–439. doi: 10.1002/iub.2202. [DOI] [PubMed] [Google Scholar]
- 33.He Y., Wang Y., Liu L., Liu S., Liang L., Chen Y., et al. Circular RNA circ_0006282 contributes to the progression of gastric cancer by sponging miR-155 to upregulate the expression of FBXO22. OncoTargets Ther. 2020:1001–1010. doi: 10.2147/OTT.S228216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang T., Jing B., Bai Y., Zhang Y., Yu H. Circular RNA circTMEM45A acts as the sponge of MicroRNA-665 to promote hepatocellular carcinoma progression. Mol. Ther. Nucleic Acids. 2020;22:285–297. doi: 10.1016/j.omtn.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khalilian S., Mohajer Z., Tabari M.A.K., Ghobadinezhad F., Ghafouri-Fard S. circGFRA1: a circular RNA with important roles in human carcinogenesis. Pathol. Res. Pract. 2023 doi: 10.1016/j.prp.2023.154588. [DOI] [PubMed] [Google Scholar]
- 36.Zeng X., Xiao J., Bai X., Liu Y., Zhang M., Liu J., et al. Research progress on the circRNA/lncRNA–miRNA–mRNA axis in gastric cancer. Pathol. Res. Pract. 2022 doi: 10.1016/j.prp.2022.154030. [DOI] [PubMed] [Google Scholar]
- 37.Shi Y., Fang N., Li Y., Guo Z., Jiang W., He Y., et al. Circular RNA LPAR3 sponges microRNA‐198 to facilitate esophageal cancer migration, invasion, and metastasis. Cancer Sci. 2020;111(8):2824–2836. doi: 10.1111/cas.14511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie M., Yu T., Jing X., Ma L., Fan Y., Yang F., et al. Exosomal circSHKBP1 promotes gastric cancer progression via regulating the miR-582-3p/HUR/VEGF axis and suppressing HSP90 degradation. Mol. Cancer. 2020;19(1):112. doi: 10.1186/s12943-020-01208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu J., Wang Y-h, Yoon C., Huang X-y, Xu Y., Xie J-w, et al. Circular RNA circ-RanGAP1 regulates VEGFA expression by targeting miR-877–3p to facilitate gastric cancer invasion and metastasis. Cancer Lett. 2020;471:38–48. doi: 10.1016/j.canlet.2019.11.038. [DOI] [PubMed] [Google Scholar]
- 40.Zhang B., Yang S., Wang J. Circ_0084615 is an oncogenic circular RNA in colorectal cancer and promotes DNMT3A expression via repressing miR-599. Pathol. Res. Pract. 2021;224 doi: 10.1016/j.prp.2021.153494. [DOI] [PubMed] [Google Scholar]
- 41.Xing C., Ye H., Wang W., Sun M., Zhang J., Zhao Z., et al. Circular RNA ADAM9 facilitates the malignant behaviours of pancreatic cancer by sponging miR-217 and upregulating PRSS3 expression. Artif. Cell Nanomed. Biotechnol. 2019;47(1):3920–3928. doi: 10.1080/21691401.2019.1671856. [DOI] [PubMed] [Google Scholar]
- 42.Guo X., Zhou Q., Su D., Luo Y., Fu Z., Huang L., et al. Circular RNA circBFAR promotes the progression of pancreatic ductal adenocarcinoma via the miR-34b-5p/MET/Akt axis. Mol. Cancer. 2020;19:1–18. doi: 10.1186/s12943-020-01196-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu T., Zhou L., He Z., Chen Y., Jiang X., Xu J., et al. Circular RNA hsa_circ_0006117 facilitates pancreatic cancer progression by regulating the miR-96-5p/KRAS/MAPK signaling pathway. Journal of Oncology. 2021;2021 doi: 10.1155/2021/9213205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J., Zhang Y., Song H., Yin H., Jiang T., Xu Y., et al. The circular RNA circSPARC enhances the migration and proliferation of colorectal cancer by regulating the JAK/STAT pathway. Mol. Cancer. 2021;20(1):81. doi: 10.1186/s12943-021-01375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang X., Wang S., Wang H., Cao J., Huang X., Chen Z., et al. Circular RNA circNRIP1 acts as a microRNA-149-5p sponge to promote gastric cancer progression via the AKT1/mTOR pathway. Mol. Cancer. 2019;18(1):1–24. doi: 10.1186/s12943-018-0935-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xing Y., Zha W.J., Li X.M., Li H., Gao F., Ye T., et al. Circular RNA circ‐Foxo3 inhibits esophageal squamous cell cancer progression via the miR‐23a/PTEN axis. J. Cell. Biochem. 2020;121(3):2595–2605. doi: 10.1002/jcb.29481. [DOI] [PubMed] [Google Scholar]
- 47.Fang J., Hong H., Xue X., Zhu X., Jiang L., Qin M., et al. A novel circular RNA, circFAT1 (e2), inhibits gastric cancer progression by targeting miR-548g in the cytoplasm and interacting with YBX1 in the nucleus. Cancer Lett. 2019;442:222–232. doi: 10.1016/j.canlet.2018.10.040. [DOI] [PubMed] [Google Scholar]
- 48.Chen J., Wu Y., Luo X., Jin D., Zhou W., Ju Z., et al. Circular RNA circRHOBTB3 represses metastasis by regulating the HuR-mediated mRNA stability of PTBP1 in colorectal cancer. Theranostics. 2021;11(15):7507. doi: 10.7150/thno.59546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhong L., Wang Y., Cheng Y., Wang W., Lu B., Zhu L., et al. Circular RNA circC3P1 suppresses hepatocellular carcinoma growth and metastasis through miR-4641/PCK1 pathway. Biochem. Biophys. Res. Commun. 2018;499(4):1044–1049. doi: 10.1016/j.bbrc.2018.03.221. [DOI] [PubMed] [Google Scholar]
- 50.Shi X., Yang J., Liu M., Zhang Y., Zhou Z., Luo W., et al. Circular RNA ANAPC7 inhibits tumor growth and muscle wasting via PHLPP2–AKT–TGF-β signaling Axis in pancreatic cancer. Gastroenterology. 2022;162(7):2004–2017. doi: 10.1053/j.gastro.2022.02.017. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zang H.-L., Ji F.-J., Ju H.-Y., Tian X.-F. Circular RNA AKT3 governs malignant behaviors of esophageal cancer cells by sponging miR-17-5p. World J. Gastroenterol. 2021;27(3):240. doi: 10.3748/wjg.v27.i3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang J., Zhu W., Tao G., Wang W. Circular RNA circ-LRP6 facilitates Myc-driven tumorigenesis in esophageal squamous cell cancer. Bioengineered. 2020;11(1):932–938. doi: 10.1080/21655979.2020.1809922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xia W., Qiu M., Chen R., Wang S., Leng X., Wang J., et al. Circular RNA has_circ_0067934 is upregulated in esophageal squamous cell carcinoma and promoted proliferation. Sci. Rep. 2016;6(1) doi: 10.1038/srep35576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xie Z.-F., Li H.-T., Xie S.-H., Ma M. Circular RNA hsa_circ_0006168 contributes to cell proliferation, migration and invasion in esophageal cancer by regulating miR-384/RBBP7 axis via activation of S6K/S6 pathway. Eur. Rev. Med. Pharmacol. Sci. 2020;24(1) doi: 10.26355/eurrev_202001_19906. [DOI] [PubMed] [Google Scholar]
- 55.Luo G., Li R., Li Z. CircRNA circFNDC3B promotes esophageal cancer progression via cell proliferation, apoptosis, and migration regulation. Int. J. Clin. Exp. Pathol. 2018;11(8):4188. [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou P.L., Wu Z., Zhang W., Xu M., Ren J., Zhang Q., et al. Circular RNA hsa_circ_0000277 sequesters miR-4766-5p to upregulate LAMA1 and promote esophageal carcinoma progression. Cell Death Dis. 2021;12(7):676. doi: 10.1038/s41419-021-03911-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen X., Jiang J., Zhao Y., Wang X., Zhang C., Zhuan L., et al. Circular RNA circNTRK2 facilitates the progression of esophageal squamous cell carcinoma through up-regulating NRIP1 expression via miR-140-3p. J. Exp. Clin. Cancer Res. 2020;39(1):1–14. doi: 10.1186/s13046-020-01640-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen J., Li Y., Zheng Q., Bao C., He J., Chen B., et al. Circular RNA profile identifies circPVT1 as a proliferative factor and prognostic marker in gastric cancer. Cancer Lett. 2017;388:208–219. doi: 10.1016/j.canlet.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 59.Huang M., He Y.-R., Liang L.-C., Huang Q., Zhu Z.-Q. Circular RNA hsa_circ_0000745 may serve as a diagnostic marker for gastric cancer. World J. Gastroenterol. 2017;23(34):6330. doi: 10.3748/wjg.v23.i34.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pan H., Li T., Jiang Y., Pan C., Ding Y., Huang Z., et al. Overexpression of circular RNA ciRS‐7 abrogates the tumor suppressive effect of miR‐7 on gastric cancer via PTEN/PI3K/AKT signaling pathway. J. Cell. Biochem. 2018;119(1):440–446. doi: 10.1002/jcb.26201. [DOI] [PubMed] [Google Scholar]
- 61.Huang X., Li Z., Zhang Q., Wang W., Li B., Wang L., et al. Circular RNA AKT3 upregulates PIK3R1 to enhance cisplatin resistance in gastric cancer via miR-198 suppression. Mol. Cancer. 2019;18(1):1–20. doi: 10.1186/s12943-019-0969-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen D.-L., Sheng H., Zhang D.-S., Jin Y., Zhao B.-T., Chen N., et al. The circular RNA circDLG1 promotes gastric cancer progression and anti-PD-1 resistance through the regulation of CXCL12 by sponging miR-141-3p. Mol. Cancer. 2021;20(1):1–18. doi: 10.1186/s12943-021-01475-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang S., Zhang X., Guan B., Sun P., Hong C.T., Peng J., et al. A novel circular RNA hsa_circ_0008035 contributes to gastric cancer tumorigenesis through targeting the miR-375/YBX1 axis. Am. J. Tourism Res. 2019;11(4):2455. [PMC free article] [PubMed] [Google Scholar]
- 64.Lu J., Zhang P-y, Li P., Xie J-w, Wang J-b, Lin J-x, et al. Circular RNA hsa_circ_0001368 suppresses the progression of gastric cancer by regulating miR-6506–5p/FOXO3 axis. Biochem. Biophys. Res. Commun. 2019;512(1):29–33. doi: 10.1016/j.bbrc.2019.02.111. [DOI] [PubMed] [Google Scholar]
- 65.Chen J., Yang X., Liu R., Wen C., Wang H., Huang L., et al. Circular RNA GLIS2 promotes colorectal cancer cell motility via activation of the NF-κB pathway. Cell Death Dis. 2020;11(9):788. doi: 10.1038/s41419-020-02989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li X.-N., Wang Z.-J., Ye C.-X., Zhao B.-C., Huang X.-X., Yang L. Circular RNA circVAPA is up-regulated and exerts oncogenic properties by sponging miR-101 in colorectal cancer. Biomed. Pharmacother. 2019;112 doi: 10.1016/j.biopha.2019.108611. [DOI] [PubMed] [Google Scholar]
- 67.Li R., Wu B., Xia J., Ye L., Yang X. Circular RNA hsa_circRNA_102958 promotes tumorigenesis of colorectal cancer via miR-585/CDC25B axis. Cancer Manag. Res. 2019:6887–6893. doi: 10.2147/CMAR.S212180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu C-l, Sha X., Wang Y., Li J., Zhang M-y, Guo Z-y, et al. Circular RNA hsa_circ_0007142 is upregulated and targets miR-103a-2-5p in colorectal cancer. Journal of Oncology. 2019;2019 doi: 10.1155/2019/9836819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ye Q., Liu S., Lin S., Xie W. Circular RNA circSEMA5A facilitates colorectal cancer development by regulating microRNA-195-5p to target CCNE1 axis. Cell. Signal. 2023;107 doi: 10.1016/j.cellsig.2023.110649. [DOI] [PubMed] [Google Scholar]
- 70.Li M., Zhi Z., Jiang X., Duan G.-C., Zhu W.-N., Pang Z., et al. METTL9 derived circular RNA circ-METTL9 sponges miR-551b-5p to accelerate colorectal cancer progression by upregulating CDK6. Carcinogenesis. 2023 doi: 10.1093/carcin/bgad031. [DOI] [PubMed] [Google Scholar]
- 71.Wang R., Wang J., Chen Y., Chen Y., Xi Q., Sun L., et al. Circular RNA circLDLR facilitates cancer progression by altering the miR-30a-3p/SOAT1 axis in colorectal cancer. Cell Death Discovery. 2022;8(1):314. doi: 10.1038/s41420-022-01110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang L., Long H., Zheng Q., Bo X., Xiao X., Li B. Circular RNA circRHOT1 promotes hepatocellular carcinoma progression by initiation of NR2F6 expression. Mol. Cancer. 2019;18(1):1–12. doi: 10.1186/s12943-019-1046-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guan Z., Tan J., Gao W., Li X., Yang Y., Li X., et al. Circular RNA hsa_circ_0016788 regulates hepatocellular carcinoma tumorigenesis through miR‐486/CDK4 pathway. J. Cell. Physiol. 2019;234(1):500–508. doi: 10.1002/jcp.26612. [DOI] [PubMed] [Google Scholar]
- 74.Yang G., Wang X., Liu B., Lu Z., Xu Z., Xiu P., et al. circ-BIRC6, a circular RNA, promotes hepatocellular carcinoma progression by targeting the miR-3918/Bcl2 axis. Cell Cycle. 2019;18(9):976–989. doi: 10.1080/15384101.2019.1601477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhan W., Liao X., Chen Z., Li L., Tian T., Yu L., et al. Circular RNA hsa_circRNA_103809 promoted hepatocellular carcinoma development by regulating miR‐377‐3p/FGFR1/ERK axis. J. Cell. Physiol. 2020;235(2):1733–1745. doi: 10.1002/jcp.29092. [DOI] [PubMed] [Google Scholar]
- 76.Li Q., Pan X., Zhu D., Deng Z., Jiang R., Wang X. Circular RNA MAT2B promotes glycolysis and malignancy of hepatocellular carcinoma through the miR‐338‐3p/PKM2 axis under hypoxic stress. Hepatology. 2019;70(4):1298–1316. doi: 10.1002/hep.30671. [DOI] [PubMed] [Google Scholar]
- 77.Jiang W., Wen D., Gong L., Wang Y., Liu Z., Yin F. Circular RNA hsa_circ_0000673 promotes hepatocellular carcinoma malignance by decreasing miR-767-3p targeting SET. Biochem. Biophys. Res. Commun. 2018;500(2):211–216. doi: 10.1016/j.bbrc.2018.04.041. [DOI] [PubMed] [Google Scholar]
- 78.He S., Yang J., Jiang S., Li Y., Han X. Circular RNA circ_0000517 regulates hepatocellular carcinoma development via miR-326/IGF1R axis. Cancer Cell Int. 2020;20:1–12. doi: 10.1186/s12935-020-01496-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 79.Yu X., Sheng P., Sun J., Zhao X., Zhang J., Li Y., et al. The circular RNA circMAST1 promotes hepatocellular carcinoma cell proliferation and migration by sponging miR-1299 and regulating CTNND1 expression. Cell Death Dis. 2020;11(5):340. doi: 10.1038/s41419-020-2532-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li Z., Liu Y., Yan J., Zeng Q., Hu Y., Wang H., et al. Circular RNA hsa_circ_0056836 functions an oncogenic gene in hepatocellular carcinoma through modulating miR-766-3p/FOSL2 axis. Aging. 2020;12(3):2485. doi: 10.18632/aging.102756. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 81.Li Z., Yanfang W., Li J., Jiang P., Peng T., Chen K., et al. Tumor-released exosomal circular RNA PDE8A promotes invasive growth via the miR-338/MACC1/MET pathway in pancreatic cancer. Cancer Lett. 2018;432:237–250. doi: 10.1016/j.canlet.2018.04.035. [DOI] [PubMed] [Google Scholar]
- 82.Liu L., Liu F.-B., Huang M., Xie K., Xie Q.-S., Liu C.-H., et al. Circular RNA ciRS-7 promotes the proliferation and metastasis of pancreatic cancer by regulating miR-7-mediated EGFR/STAT3 signaling pathway. Hepatobiliary Pancreat. Dis. Int. 2019;18(6):580–586. doi: 10.1016/j.hbpd.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 83.Xiong X., Feng J., Yang X., Li H., Shi Q., Tao J., et al. Circular RNA CDR1as promotes tumor progression by regulating miR-432-5p/E2F3 axis in pancreatic cancer. Cancer Cell Int. 2021;21(1):1–10. doi: 10.1186/s12935-021-01812-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu B., Gong Y., Jiang Q., Wu S., Han B., Chen F., et al. Hsa_circ_0014784-induced YAP1 promoted the progression of pancreatic cancer by sponging miR-214-3p. Cell Cycle. 2023:1–15. doi: 10.1080/15384101.2023.2222519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang H., Ma X., Wang L., Li X., Feng D., Liu M., et al. Circular RNA hsa_circ_0007367 promotes the progression of pancreatic ductal adenocarcinoma by sponging miR-6820-3p and upregulating YAP1 expression. Cell Death Dis. 2022;13(8):736. doi: 10.1038/s41419-022-05188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu R., Ding P., Zhao X., Li Z., Liu F., Gu L., et al. Circular RNA circ‐TNRC6B inhibits the proliferation and invasion of esophageal squamous cell carcinoma cells by regulating the miR‐452‐5p/DAG1 axis. Mol. Oncol. 2023;17(7):1437. doi: 10.1002/1878-0261.13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cheng J., Ma H., Yan M., Zhang Z., Xing W. Circ_0007624 suppresses the development of esophageal squamous cell carcinoma via targeting miR-224-5p/CPEB3 to inactivate the EGFR/PI3K/AKT signaling. Cell. Signal. 2022;99 doi: 10.1016/j.cellsig.2022.110448. [DOI] [PubMed] [Google Scholar]
- 88.Deng G., Mou T., He J., Chen D., Lv D., Liu H., et al. Circular RNA circRHOBTB3 acts as a sponge for miR-654-3p inhibiting gastric cancer growth. J. Exp. Clin. Cancer Res. 2020;39:1–16. doi: 10.1186/s13046-019-1487-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li X., Wang T., Chen Q., Fu Q., Cui Y., Pan H. CircRTN4 inhibits the progression of gastric cancer by sponging miR-424-5p and regulating LATS2 expression. Trop. J. Pharmaceut. Res. 2023;22(5):967–974. [Google Scholar]
- 90.Zhang M., Jiang Y. Downregulation of circular RNA circ-HN1 suppressed the progression of gastric cancer through the miR-485-5p/GSK3A pathway. Bioengineered. 2022;13(3):5675–5684. doi: 10.1080/21655979.2021.1987124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zang X., Jiang J., Gu J., Chen Y., Wang M., Zhang Y., et al. Circular RNA EIF4G3 suppresses gastric cancer progression through inhibition of β-catenin by promoting δ-catenin ubiquitin degradation and upregulating SIK1. Mol. Cancer. 2022;21(1):141. doi: 10.1186/s12943-022-01606-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lv L., Du J., Wang D., Yan Z. Circular RNA hsa_circ_0026344 suppresses gastric cancer cell proliferation, migration and invasion via the miR-590-5p/PDCD4 axis. J. Pharm. Pharmacol. 2022;74(8):1193–1204. doi: 10.1093/jpp/rgac032. [DOI] [PubMed] [Google Scholar]
- 93.Kong S., Tian S., Wang Z., Shi Y., Zhang J., Zhuo H. Circular RNA circPFKP suppresses the proliferation and metastasis of gastric cancer cell via sponging miR-644 and regulating ADAMTSL5 expression. Bioengineered. 2022;13(5):12326–12337. doi: 10.1080/21655979.2022.2073001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tan P., Sun H., Xu M., Liu X., Qin J., Nie J., et al. Circular RNA circ0104103 inhibits colorectal cancer progression through interactions with HuR and miR‐373‐5p. Cancer Sci. 2023;114(4):1396. doi: 10.1111/cas.15695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang B.-L., Liu G.-Q., Li P., Li X.-H. Circular RNA CUL2 regulates the development of colorectal cancer by modulating apoptosis and autophagy via miR-208a-3p/PPP6C. Aging (Albany NY) 2022;14(1):497. doi: 10.18632/aging.203827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li X., Wang J., Zhang C., Lin C., Zhang J., Zhang W., et al. Circular RNA circITGA7 inhibits colorectal cancer growth and metastasis by modulating the Ras pathway and upregulating transcription of its host gene ITGA7. J. Pathol. 2018;246(2):166–179. doi: 10.1002/path.5125. [DOI] [PubMed] [Google Scholar]
- 97.Liu H., Lan T., Li H., Xu L., Chen X., Liao H., et al. Circular RNA circDLC1 inhibits MMP1-mediated liver cancer progression via interaction with HuR. Theranostics. 2021;11(3):1396. doi: 10.7150/thno.53227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Han D., Li J., Wang H., Su X., Hou J., Gu Y., et al. Circular RNA circMTO1 acts as the sponge of microRNA‐9 to suppress hepatocellular carcinoma progression. Hepatology. 2017;66(4):1151–1164. doi: 10.1002/hep.29270. [DOI] [PubMed] [Google Scholar]
- 99.Yu J., Xu Q-g, Wang Z-g, Yang Y., Zhang L., Ma J-z, et al. Circular RNA cSMARCA5 inhibits growth and metastasis in hepatocellular carcinoma. J. Hepatol. 2018;68(6):1214–1227. doi: 10.1016/j.jhep.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 100.Zhang P.-F., Wei C.-Y., Huang X.-Y., Peng R., Yang X., Lu J.-C., et al. Circular RNA circTRIM33–12 acts as the sponge of MicroRNA-191 to suppress hepatocellular carcinoma progression. Mol. Cancer. 2019;18(1):1–15. doi: 10.1186/s12943-019-1031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xie H., Zhao Q., Yu L., Lu J., Peng K., Xie N., et al. Circular RNA circ_0047744 suppresses the metastasis of pancreatic ductal adenocarcinoma by regulating the miR-21/SOCS5 axis. Biochem. Biophys. Res. Commun. 2022;605:154–161. doi: 10.1016/j.bbrc.2022.03.082. [DOI] [PubMed] [Google Scholar]
- 102.Li C., Cai J., Liu W., Gao Z., Li G. Downregulation of circ-STK39 suppresses pancreatic cancer progression by sponging mir-140-3p and regulating TRAM2-mediated epithelial-mesenchymal transition. Apoptosis. 2023:1–11. doi: 10.1007/s10495-023-01813-9. [DOI] [PubMed] [Google Scholar]
- 103.Zhang X., Tan P., Zhuang Y., Du L. hsa_circRNA_001587 upregulates SLC4A4 expression to inhibit migration, invasion, and angiogenesis of pancreatic cancer cells via binding to microRNA-223. Am. J. Physiol. Gastrointest. Liver Physiol. 2020;319(6):G703–G717. doi: 10.1152/ajpgi.00118.2020. [DOI] [PubMed] [Google Scholar]
- 104.Al-Ishaq R.K., Koklesova L., Kubatka P., Büsselberg D. Immunomodulation by gut microbiome on gastrointestinal cancers: focusing on colorectal cancer. Cancers. 2022;14(9):2140. doi: 10.3390/cancers14092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chrysostomou D., Roberts L.A., Marchesi J.R., Kinross J.M. Gut microbiota modulation of efficacy and toxicity of cancer chemotherapy and immunotherapy. Gastroenterology. 2022;164(2):198–213. doi: 10.1053/j.gastro.2022.10.018. [DOI] [PubMed] [Google Scholar]
- 106.Kaźmierczak-Siedlecka K., Daca A., Fic M., van de Wetering T., Folwarski M., Makarewicz W. Therapeutic methods of gut microbiota modification in colorectal cancer management–fecal microbiota transplantation, prebiotics, probiotics, and synbiotics. Gut Microb. 2020;11(6):1518–1530. doi: 10.1080/19490976.2020.1764309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cuevas-Sierra A., Ramos-Lopez O., Riezu-Boj J.I., Milagro F.I., Martinez J.A. Diet, gut microbiota, and obesity: links with host genetics and epigenetics and potential applications. Adv. Nutr. 2019;10(suppl_1):S17–S30. doi: 10.1093/advances/nmy078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Irrazábal T., Belcheva A., Girardin S.E., Martin A., Philpott D.J. The multifaceted role of the intestinal microbiota in colon cancer. Mol. Cell. 2014;54(2):309–320. doi: 10.1016/j.molcel.2014.03.039. [DOI] [PubMed] [Google Scholar]
- 109.Yong X., Tang B., Li B.-S., Xie R., Hu C.-J., Luo G., et al. Helicobacter pylori virulence factor CagA promotes tumorigenesis of gastric cancer via multiple signaling pathways. Cell Commun. Signal. 2015;13(1):1–13. doi: 10.1186/s12964-015-0111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rubinstein M.R., Baik J.E., Lagana S.M., Han R.P., Raab W.J., Sahoo D., et al. Fusobacterium nucleatum promotes colorectal cancer by inducing Wnt/β‐catenin modulator Annexin A1. EMBO Rep. 2019;20(4) doi: 10.15252/embr.201847638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gagnaire A., Nadel B., Raoult D., Neefjes J., Gorvel J.-P. Collateral damage: insights into bacterial mechanisms that predispose host cells to cancer. Nat. Rev. Microbiol. 2017;15(2):109–128. doi: 10.1038/nrmicro.2016.171. [DOI] [PubMed] [Google Scholar]
- 112.Lee C.-G., Hwang S., Gwon S.-Y., Park C., Jo M., Hong J.-E., et al. Bacteroides fragilis toxin induces intestinal epithelial cell secretion of interleukin-8 by the e-Cadherin/β-Catenin/NF-κB dependent pathway. Biomedicines. 2022;10(4):827. doi: 10.3390/biomedicines10040827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kovács T., Mikó E., Ujlaki G., Sári Z., Bai P. The microbiome as a component of the tumor microenvironment. Tumor Microenvironment: Recent Advances. 2020:137–153. doi: 10.1007/978-3-030-35727-6_10. [DOI] [PubMed] [Google Scholar]
- 114.Louis P., Hold G.L., Flint H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014;12(10):661–672. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- 115.Taniguchi K., Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nat. Rev. Immunol. 2018;18(5):309–324. doi: 10.1038/nri.2017.142. [DOI] [PubMed] [Google Scholar]
- 116.Grasso F., Frisan T. Bacterial genotoxins: merging the DNA damage response into infection biology. Biomolecules. 2015;5(3):1762–1782. doi: 10.3390/biom5031762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Payne C.M., Bernstein C., Dvorak K., Bernstein H. Hydrophobic bile acids, genomic instability, Darwinian selection, and colon carcinogenesis. Clin. Exp. Gastroenterol. 2008:19–47. doi: 10.2147/ceg.s4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guo Y., Cao X.-S., Zhou M.-G., Yu B. Gastric microbiota in gastric cancer: different roles of Helicobacter pylori and other microbes. Front. Cell. Infect. Microbiol. 2023;12:1975. doi: 10.3389/fcimb.2022.1105811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Guo R., Cui X., Li X., Zang W., Chang M., Sun Z., et al. CircMAN1A2 is upregulated by Helicobacter pylori and promotes development of gastric cancer. Cell Death Dis. 2022;13(4):409. doi: 10.1038/s41419-022-04811-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Diling C., Longkai Q., Yinrui G., Yadi L., Xiaocui T., Xiangxiang Z., et al. CircNF1-419 improves the gut microbiome structure and function in AD-like mice. Aging (Albany NY) 2020;12(1):260. doi: 10.18632/aging.102614. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 121.Zhu Z., Huang J., Li X., Xing J., Chen Q., Liu R., et al. Gut microbiota regulate tumor metastasis via circRNA/miRNA networks. Gut Microb. 2020;12(1) doi: 10.1080/19490976.2020.1788891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yin Y.-L., Liu T.-L., Yao Q., Wang Y.-X., Wu X.-M., Wang X.-T., et al. Circular RNA ciRS-7 affects the propagation of Cryptosporidium parvum in HCT-8 cells by sponging miR-1270 to activate the NF-κB signaling pathway. Parasites Vectors. 2021;14(1):1–12. doi: 10.1186/s13071-021-04739-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang Y., Huang R., Cheng M., Wang L., Chao J., Li J., et al. Gut microbiota from NLRP3-deficient mice ameliorates depressive-like behaviors by regulating astrocyte dysfunction via circHIPK2. Microbiome. 2019;7(1):1–16. doi: 10.1186/s40168-019-0733-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cummings D., Wong J., Palm R., Hoffe S., Almhanna K., Vignesh S. Epidemiology, diagnosis, staging and multimodal therapy of esophageal and gastric tumors. Cancers. 2021;13(3):582. doi: 10.3390/cancers13030582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Joshi S.S., Badgwell B.D. Current treatment and recent progress in gastric cancer. CA A Cancer J. Clin. 2021;71(3):264–279. doi: 10.3322/caac.21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li K., Zhang A., Li X., Zhang H., Zhao L. Advances in clinical immunotherapy for gastric cancer. Biochim. Biophys. Acta Rev. Canc. 2021;1876(2) doi: 10.1016/j.bbcan.2021.188615. [DOI] [PubMed] [Google Scholar]
- 127.Schaefer I.-M., DeMatteo R.P., Serrano C. The GIST of advances in treatment of advanced gastrointestinal stromal tumor. American Society of Clinical Oncology Educational Book. 2022;42:885–899. doi: 10.1200/EDBK_351231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.de Oliveira Ferreira F., Akaishi E.H., Cavarsan F. Springer; 2023. Principles of Surgical Oncology. Oncodermatology: an Evidence-Based, Multidisciplinary Approach to Best Practices; pp. 179–202. [Google Scholar]
- 129.Zhang Z., Lin H., Li H., Wang X. A case report of surgical resection treatment for complete remission after chemotherapy for advanced pancreatic cancer. Front. Oncol. 2023;13 doi: 10.3389/fonc.2023.1155233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Puhr H.C., Reiter T.J., Preusser M., Prager G.W., Ilhan-Mutlu A. Recent advances in the systemic treatment of localized gastroesophageal cancer. Cancers. 2023;15(6):1900. doi: 10.3390/cancers15061900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Eso Y., Seno H. Current status of treatment with immune checkpoint inhibitors for gastrointestinal, hepatobiliary, and pancreatic cancers. Therapeutic advances in gastroenterology. 2020;13 doi: 10.1177/1756284820948773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ji Z., Ren L., Liu L., Zhu J., Yin L., Ji G., et al. 2023. Comparison of Long-Term Survival between Surgery-Plus-Chemotherapy and Surgery-Plus-Chemoradiotherapy for High-Grade Gastrointestinal Neuroendocrine Tumors: a SEER-Based Study. [Google Scholar]
- 133.Yan T., Yu L., Zhang N., Peng C., Su G., Jing Y., et al. The advanced development of molecular targeted therapy for hepatocellular carcinoma. Cancer Biology & Medicine. 2022;19(6):802. doi: 10.20892/j.issn.2095-3941.2021.0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Han S.-H., Ryu K.H., Kwon A.-Y. The prognostic impact of HER2 genetic and protein expression in pancreatic carcinoma—HER2 protein and gene in pancreatic Cancer. Diagnostics. 2021;11(4):653. doi: 10.3390/diagnostics11040653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mimori K. Novel and classic approaches for managing gastrointestinal cancers. Annals of Gastroenterological Surgery. 2023;7(2):196. doi: 10.1002/ags3.12670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gomes-Silva W., Vechiato-Filho A.J., Luiz A.C., Guollo A., de Oliveira M.C.Q., Gomes M.N., et al. Clinical characterization of stomatitis cases with an EGFR inhibitor in metastatic colorectal cancer patients: a study of 7 cases and literature review. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology. 2023;136(2):162–172. doi: 10.1016/j.oooo.2023.01.004. [DOI] [PubMed] [Google Scholar]
- 137.Erman A., Ignjatović M., Leskovšek K., Miceska S., Lampreht Tratar U., Bošnjak M., et al. The prognostic and predictive value of human gastrointestinal microbiome and exosomal mRNA expression of PD-L1 and IFNγ for immune checkpoint inhibitors response in metastatic melanoma patients: protocol trial. Biomedicines. 2023;11(7):2016. doi: 10.3390/biomedicines11072016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu W., Huo G., Chen P. Efficacy of PD-1/PD-L1 inhibitors in advanced gastroesophageal cancer based on characteristics: a meta-analysis. Immunotherapy. 2023;(0) doi: 10.2217/imt-2022-0305. [DOI] [PubMed] [Google Scholar]
- 139.Chamseddine S., LaPelusa M., Kaseb A.O. Systemic neoadjuvant and adjuvant therapies in the management of hepatocellular carcinoma—a narrative review. Cancers. 2023;15(13):3508. doi: 10.3390/cancers15133508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kotani D., Oki E., Nakamura Y., Yukami H., Mishima S., Bando H., et al. Molecular residual disease and efficacy of adjuvant chemotherapy in patients with colorectal cancer. Nat. Med. 2023;29(1):127–134. doi: 10.1038/s41591-022-02115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Taieb J., Abdallah R., Thibault R., Pessaux P., Artru P., Marchal T., et al. Nutrition support in pancreatic cancer: an expert statement on practical implementation of French guidelines. Clinics and Research in Hepatology and Gastroenterology. 2023 doi: 10.1016/j.clinre.2023.102153. [DOI] [PubMed] [Google Scholar]
- 142.Li S., Zhu X., Zhang L., Huang C., Li D. The effect of pain-education nursing based on a mind map on postoperative pain score and quality of life in patients with colorectal cancer. Medicine. 2023;102(19) doi: 10.1097/MD.0000000000033562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kim D., Rossi J. RNAi mechanisms and applications. Biotechniques. 2008;44(5):613–616. doi: 10.2144/000112792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sheng P., Flood K.A., Xie M. Short hairpin RNAs for strand-specific small interfering RNA production. Front. Bioeng. Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Elcheva I.A., Spiegelman V.S. The role of cis- and trans-acting RNA regulatory elements in leukemia. Cancers. 2020;12(12) doi: 10.3390/cancers12123854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Whitehead K.A., Langer R., Anderson D.G. Knocking down barriers: advances in siRNA delivery. Nat. Rev. Drug Discov. 2009;8(2):129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kulkarni J.A., Witzigmann D., Chen S., Cullis P.R., van der Meel R. Lipid nanoparticle technology for clinical translation of siRNA therapeutics. Acc. Chem. Res. 2019;52(9):2435–2444. doi: 10.1021/acs.accounts.9b00368. [DOI] [PubMed] [Google Scholar]
- 148.Kozielski K.L., Tzeng S.Y., Green J.J. Bioengineered nanoparticles for siRNA delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2013;5(5):449–468. doi: 10.1002/wnan.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Robbins M., Judge A., MacLachlan I. siRNA and innate immunity. Oligonucleotides. 2009;19(2):89–102. doi: 10.1089/oli.2009.0180. [DOI] [PubMed] [Google Scholar]
- 150.Singh S., Narang A.S., Mahato R.I. Subcellular fate and off-target effects of siRNA, shRNA, and miRNA. Pharm. Res. (N. Y.) 2011;28(12):2996–3015. doi: 10.1007/s11095-011-0608-1. [DOI] [PubMed] [Google Scholar]
- 151.Obi P., Chen Y.G. The design and synthesis of circular RNAs. Methods. 2021;196:85–103. doi: 10.1016/j.ymeth.2021.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Müller S., Appel B. In vitro circularization of RNA. RNA Biol. 2017;14(8):1018–1027. doi: 10.1080/15476286.2016.1239009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sun M., Yang Y. Biological functions and applications of circRNA-next generation of RNA-based therapy. J. Mol. Cell Biol. 2023 doi: 10.1093/jmcb/mjad031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Malviya A., Bhuyan R. The recent advancements in circRNA research: from biogenesis to therapeutic interventions. Pathol. Res. Pract. 2023 doi: 10.1016/j.prp.2023.154697. [DOI] [PubMed] [Google Scholar]
- 155.Jost I., Shalamova L.A., Gerresheim G.K., Niepmann M., Bindereif A., Rossbach O. Functional sequestration of microRNA-122 from Hepatitis C Virus by circular RNA sponges. RNA Biol. 2018;15(8):1032–1039. doi: 10.1080/15476286.2018.1435248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mendes B.B., Conniot J., Avital A., Yao D., Jiang X., Zhou X., et al. Nanodelivery of nucleic acids. Nature reviews Methods primers. 2022;2 doi: 10.1038/s43586-022-00104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang Y., Luo J., Yang W., Ye W.-C. CircRNAs in colorectal cancer: potential biomarkers and therapeutic targets. Cell Death Dis. 2023;14(6):353. doi: 10.1038/s41419-023-05881-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ma B., Wang S., Wu W., Shan P., Chen Y., Meng J., et al. Mechanisms of circRNA/lncRNA-miRNA interactions and applications in disease and drug research. Biomed. Pharmacother. 2023;162 doi: 10.1016/j.biopha.2023.114672. [DOI] [PubMed] [Google Scholar]
- 159.Wang Y., Zhang M., Zhang L., Zhou M., Wang E. Nanoparticles loaded with circ_0086375 for suppressing the tumorigenesis of pancreatic cancer by targeting the miR-646/SLC4A4 axis. Clin. Exp. Metastasis. 2023;40(1):53–67. doi: 10.1007/s10585-022-10197-0. [DOI] [PubMed] [Google Scholar]
- 160.You S., Luo Z., Cheng N., Wu M., Lai Y., Wang F., et al. Magnetically responsive nanoplatform targeting circRNA circ_0058051 inhibits hepatocellular carcinoma progression. Drug Delivery and Translational Research. 2023;13(3):782–794. doi: 10.1007/s13346-022-01237-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang Y., Liu Q., Zhang X., Huang H., Tang S., Chai Y., et al. Recent advances in exosome-mediated nucleic acid delivery for cancer therapy. J. Nanobiotechnol. 2022;20(1):279. doi: 10.1186/s12951-022-01472-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shen B., Sun K. Exosomal circular RNAs: a new frontier in the metastasis of digestive system tumors. Oncol. Lett. 2021;22(6):1–13. doi: 10.3892/ol.2021.13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Vahabi A., Rezaie J., Hassanpour M., Panahi Y., Nemati M., Rasmi Y., et al. Tumor Cells-derived exosomal CircRNAs: novel cancer drivers, molecular mechanisms, and clinical opportunities. Biochem. Pharmacol. 2022;200 doi: 10.1016/j.bcp.2022.115038. [DOI] [PubMed] [Google Scholar]
- 164.Wang X., Zhang H., Yang H., Bai M., Ning T., Deng T., et al. Exosome‐delivered circRNA promotes glycolysis to induce chemoresistance through the miR‐122‐PKM2 axis in colorectal cancer. Mol. Oncol. 2020;14(3):539–555. doi: 10.1002/1878-0261.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Núñez-Muñoz L., Calderón-Pérez B., Ruiz-Medrano R., Xoconostle-Cázares B. Gene editing to improve drought tolerance. CABI Reviews. 2022;2022 [Google Scholar]
- 166.Kristensen L.S., Jakobsen T., Hager H., Kjems J. The emerging roles of circRNAs in cancer and oncology. Nat. Rev. Clin. Oncol. 2022;19(3):188–206. doi: 10.1038/s41571-021-00585-y. [DOI] [PubMed] [Google Scholar]
- 167.Gao X., Ma X.-K., Li X., Li G.-W., Liu C.-X., Zhang J., et al. Knockout of circRNAs by base editing back-splice sites of circularized exons. Genome Biol. 2022;23:1–22. doi: 10.1186/s13059-021-02563-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Palaz F., Kalkan A.K., Can O., Demir A.N., Tozluyurt A., Ozcan A., et al. CRISPR-Cas13 system as a promising and versatile tool for cancer diagnosis, therapy, and research. ACS Synth. Biol. 2021;10(6):1245–1267. doi: 10.1021/acssynbio.1c00107. [DOI] [PubMed] [Google Scholar]
- 169.Benech N., Sokol H. Targeting the gut microbiota in inflammatory bowel diseases: where are we? Curr. Opin. Microbiol. 2023;74 doi: 10.1016/j.mib.2023.102319. [DOI] [PubMed] [Google Scholar]
- 170.Ameen A., Amir A., Aziz M.N., Irshad A. Dysfunction of the gut microbiome and its onset progression of chronic and mental health disorders. Pakistan Journal of Medical & Health Sciences. 2023;17(5):425. [Google Scholar]
- 171.Dey P., Mukherjee S.K., Parai D. Springer; 2023. Association of Probiotics and Prebiotics with Human Microbiome and the Functioning of Immune System. Probiotics, Prebiotics, Synbiotics, and Postbiotics: Human Microbiome and Human Health; pp. 101–115. [Google Scholar]
- 172.Drommi F., Calabrò A., Vento G., Pezzino G., Cavaliere R., Omero F., et al. Crosstalk between ILC3s and microbiota: implications for colon cancer development and treatment with immune check point inhibitors. Cancers. 2023;15(11):2893. doi: 10.3390/cancers15112893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Han Z., Cheng S., Dai D., Kou Y., Zhang X., Li F., et al. The gut microbiome affects response of treatments in HER2‐negative advanced gastric cancer. Clin. Transl. Med. 2023;13(7):e1312. doi: 10.1002/ctm2.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Nierengarten M.B. Wiley Online Library; 2023. Helping Oncologists Utilize Genomics in Cancer Care: an Updated Definition of the Uses of Genetic Biomarkers in Cancer Diagnosis and Care Can Make a Difference in How Physicians Understand Their Use in the Clinic. [DOI] [PubMed] [Google Scholar]
- 175.Cammarota G., Ianiro G., Ahern A., Carbone C., Temko A., Claesson M.J., et al. Gut microbiome, big data and machine learning to promote precision medicine for cancer. Nat. Rev. Gastroenterol. Hepatol. 2020;17(10):635–648. doi: 10.1038/s41575-020-0327-3. [DOI] [PubMed] [Google Scholar]
- 176.Lozenov S., Krastev B., Nikolaev G., Peshevska-Sekulovska M., Peruhova M., Velikova T. Gut microbiome composition and its metabolites are a key regulating factor for malignant transformation, metastasis and antitumor immunity. Int. J. Mol. Sci. 2023;24(6):5978. doi: 10.3390/ijms24065978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Vieira A.T., Fukumori C., Ferreira C.M. New insights into therapeutic strategies for gut microbiota modulation in inflammatory diseases. Clinical & translational immunology. 2016;5(6):e87. doi: 10.1038/cti.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rebersek M. Gut microbiome and its role in colorectal cancer. BMC Cancer. 2021;21(1):1–13. doi: 10.1186/s12885-021-09054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dik V.K., van Oijen M.G., Smeets H.M., Siersema P.D. Frequent use of antibiotics is associated with colorectal cancer risk: results of a nested case–control study. Dig. Dis. Sci. 2016;61:255–264. doi: 10.1007/s10620-015-3828-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Azad M.A.K., Sarker M., Li T., Yin J. Probiotic species in the modulation of gut microbiota: an overview. BioMed Res. Int. 2018;2018 doi: 10.1155/2018/9478630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Maldonado Galdeano C., Cazorla S.I., Lemme Dumit J.M., Vélez E., Perdigón G. Beneficial effects of probiotic consumption on the immune system. Ann. Nutr. Metabol. 2019;74(2):115–124. doi: 10.1159/000496426. [DOI] [PubMed] [Google Scholar]
- 182.Marinelli L., Tenore G.C., Novellino E., editors. Seminars in Cancer Biology. Elsevier; 2017. Probiotic species in the modulation of the anticancer immune response. [DOI] [PubMed] [Google Scholar]
- 183.Sim A.A.X.H., Cheam J.Y., Law J.W.-F., Letchumanan V., Kumari Y., Ogawa S., et al. The ameliorative role of probiotics in 5-fluorouracil induced intestinal mucositis. Progress In Microbes & Molecular Biology. 2023;6(1) [Google Scholar]
- 184.Sarajar BO, Alizadeh A, Moradi M, Irannejad VS. Effects of Postbiotics from Food Probiotic and Protective Cultures on Proliferation and Apoptosis in HCT-116 Colorectal Cancer Cells..
- 185.Matsuo K., Haku A., Bi B., Takahashi H., Kamada N., Yaguchi T., et al. Fecal microbiota transplantation prevents Candida albicans from colonizing the gastrointestinal tract. Microbiol. Immunol. 2019;63(5):155–163. doi: 10.1111/1348-0421.12680. [DOI] [PubMed] [Google Scholar]
- 186.Gerardin Y., Timberlake S., Allegretti J.R., Smith M.B., Kassam Z. Beyond fecal microbiota transplantation: developing drugs from the microbiome. J. Infect. Dis. 2021;223(Supplement_3):S276–S282. doi: 10.1093/infdis/jiaa700. [DOI] [PubMed] [Google Scholar]
- 187.Goldenberg S.D., Batra R., Beales I., Digby-Bell J.L., Irving P.M., Kellingray L., et al. Comparison of different strategies for providing fecal microbiota transplantation to treat patients with recurrent Clostridium difficile infection in two English hospitals: a review. Infectious diseases and therapy. 2018;7:71–86. doi: 10.1007/s40121-018-0189-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chen D., Wu J., Jin D., Wang B., Cao H. Fecal microbiota transplantation in cancer management: current status and perspectives. Int. J. Cancer. 2019;145(8):2021–2031. doi: 10.1002/ijc.32003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ekine-Afolabi B.A., Njan A.A., Rotimi S.O., Ri A., Elbehi A.M., Cash E., et al. The impact of diet on the involvement of non-coding RNAs, extracellular vesicles, and gut microbiome-virome in colorectal cancer initiation and progression. Front. Oncol. 2020:2545. doi: 10.3389/fonc.2020.583372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sawant S.S., Patil S.M., Gupta V., Kunda N.K. Microbes as medicines: harnessing the power of bacteria in advancing cancer treatment. Int. J. Mol. Sci. 2020;21(20):7575. doi: 10.3390/ijms21207575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jia Z., An J., Liu Z., Zhang F. Non-coding RNAs in colorectal cancer: their functions and mechanisms. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.783079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kotelevets L., Chastre E. Rac1 signaling: from intestinal homeostasis to colorectal cancer metastasis. Cancers. 2020;12(3):665. doi: 10.3390/cancers12030665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Nokkeaw A., Thamjamrassri P., Tangkijvanich P., Ariyachet C. Regulatory functions and mechanisms of circular RNAs in hepatic stellate cell activation and liver fibrosis. Cells. 2023;12(3):378. doi: 10.3390/cells12030378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Suri K., D'Souza A., Huang D., Bhavsar A., Amiji M. Bacterial extracellular vesicle applications in cancer immunotherapy. Bioact. Mater. 2023;22:551–566. doi: 10.1016/j.bioactmat.2022.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Xu Y., Zhao C., Ma X., Zhang H. Life Sciences; 2022. Prospect of Bacteria for Tumor Diagnosis and Treatment. [DOI] [PubMed] [Google Scholar]
- 196.Huang S., Yang B., Chen B., Bliim N., Ueberham U., Arendt T., et al. The emerging role of circular RNAs in transcriptome regulation. Genomics. 2017;109(5–6):401–407. doi: 10.1016/j.ygeno.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 197.Zhang Z., Yang T., Xiao J. Circular RNAs: promising biomarkers for human diseases. EBioMedicine. 2018;34:267–274. doi: 10.1016/j.ebiom.2018.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Rajappa A., Banerjee S., Sharma V., Khandelia P. Circular RNAs: emerging role in cancer diagnostics and therapeutics. Front. Mol. Biosci. 2020;7 doi: 10.3389/fmolb.2020.577938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wang L., Xiao S., Zheng Y., Gao Z. CircRNA circSLIT2 is a novel diagnostic and prognostic biomarker for gastric cancer. Wien Klin. Wochenschr. 2023:1–6. doi: 10.1007/s00508-023-02155-x. [DOI] [PubMed] [Google Scholar]
- 200.Wang X., Zhang J., Luo F., Shen Y. Application of circular RNA Circ_0071662 in the diagnosis and prognosis of hepatocellular carcinoma and its response to radiotherapy. Dig. Dis. 2023;41(3):431–438. doi: 10.1159/000527696. [DOI] [PubMed] [Google Scholar]
- 201.Li T., Zhou T., Wu J., Lv H., Zhou H., Du M., et al. Plasma exosome-derived circGAPVD1 as a potential diagnostic marker for colorectal cancer. Translational Oncology. 2023;31 doi: 10.1016/j.tranon.2023.101652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Oliphant K., Allen-Vercoe E. Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome. 2019;7(1):1–15. doi: 10.1186/s40168-019-0704-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ussar S., Fujisaka S., Kahn C.R. Interactions between host genetics and gut microbiome in diabetes and metabolic syndrome. Mol. Metabol. 2016;5(9):795–803. doi: 10.1016/j.molmet.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Illiano P., Brambilla R., Parolini C. The mutual interplay of gut microbiota, diet and human disease. FEBS J. 2020;287(5):833–855. doi: 10.1111/febs.15217. [DOI] [PubMed] [Google Scholar]
- 205.Thomas A.M., Fidelle M., Routy B., Kroemer G., Wargo J.A., Segata N., et al. Gut OncoMicrobiome Signatures (GOMS) as next-generation biomarkers for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2023:1–21. doi: 10.1038/s41571-023-00785-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Guo S., Li L., Xu B., Li M., Zeng Q., Xiao H., et al. A simple and novel fecal biomarker for colorectal cancer: ratio of Fusobacterium nucleatum to probiotics populations, based on their antagonistic effect. Clin. Chem. 2018;64(9):1327–1337. doi: 10.1373/clinchem.2018.289728. [DOI] [PubMed] [Google Scholar]
- 207.Chen W.-D., Zhang X., Zhang M.-J., Zhang Y.-P., Shang Z.-Q., Xin Y.-W., et al. Salivary Fusobacterium nucleatum serves as a potential diagnostic biomarker for gastric cancer. World J. Gastroenterol. 2022;28(30):4120. doi: 10.3748/wjg.v28.i30.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.