Abstract
Background and Objective
Peptic ulcer is a high incidence gastrointestinal disease in China. Berberine (BBR) is a natural product isolated from the Chinese herb Coptis chinensis Franch that has protective effects in digestive diseases. We aimed to evaluate the ability of BBR to attenuate acute gastric ulcer induced by one-time administration of ethanol in the rat.
Methods
Tissue pathological morphology, macroscopic score, ulcer healing rate, and serum levels of the inflammatory cytokines nitric oxide (NO), interleukin-6 (IL-6), and prostaglandin E2 (PGE2), and anti-inflammatory interleukin-10 (IL-10) were used to determine the efficacy of BBR and evaluated to identify the optimal dosage. Subsequently, transcriptome and metabolome sequencing were conducted in Control, Model, and optimal dosage groups to explore the pathogenesis of the disease and the mechanism of action of the drug. The levels of malondialdehyde (MDA), myeloperoxidase (MPO), as well as those of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) were determined by enzyme-linked immunosorbent assay to verify the results of transcriptomics and metabolomics analyses.
Results
BBR significantly improved the pathological morphology of gastric ulcers, increased the macroscopic score and healing rate, decreased serum levels of NO, IL-6, and PGE2, and increased serum levels of IL-10, thus effectively alleviating gastric ulcer severity. Transcriptome results showed that the therapeutic effect of BBR was mainly mediated by the arachidonic acid metabolism pathway at the gene level, which is closely associated with inflammation and increased levels of reactive oxygen species (ROS). The differentially accumulated metabolite prostaglandin E1, which is a negative regulator of ROS, was significantly up-regulated after BBR administration. The validation results indicated that BBR pretreatment increased SOD and GSH-Px enzyme activities, while reducing levels of the oxidative products MDA and MPO.
Conclusion
This study demonstrated that BBR exerts a protective effect on acute gastric ulcer by promoting tricarboxylic acid cycle-mediated arachidonic acid metabolism.
Keywords: Berberine, acute gastric ulcer, transcriptome, untargeted metabolomics
Graphical Abstract
Introduction
As one of the most common gastrointestinal diseases, peptic ulcer seriously affects quality of life and health with a long duration and high recurrence rate. The incidence of peptic ulcer is reported to be 2.4% in Western countries and 6.1% in China.1 The main causes of peptic ulcers include physical stress, poor dietary habits, smoking, alcohol or caffeine, excessive use of non-steroidal anti-inflammatory drugs, and Helicobacter pylori infection. Long-term gastric ulcer leads to gastrointestinal perforation, gastrointestinal bleeding, pyloric obstruction, and even gastric cancer.2 At present, the commonly used drugs for treatment of gastric ulcers include proton pump inhibitors, antibiotics, and gastric mucosal protective drugs.2,3
However, due to side effects, microbial resistance and other factors, the primary drugs used for management of gastric ulcers, such as antacids and proton pump inhibitors, turned out to be ineffective.4 Several studies have demonstrated that sustained inflammatory cell infiltration and oxidative stress during H pylori infection are the main players in the pathogenesis of stomach ulcers.5,6 In addition, research has indicated that H pylori infection results in persistent reactive oxygen species (ROS) production. Excessive ROS levels can disrupt stomach glandular epithelial cell signal transduction and other biological processes.7 Thus, it is critical to identify anti-ulcer drugs that decrease the deleterious effects of excessive ROS. It has been reported that many natural products derived from medicinal plants, with broad and varied biological activities, as well as good efficacy and safety characteristics, have been used as anti-ulcer treatments to provide more choice for patients.8
Berberine (BBR) is an alkaloid component of Scutellaria baicalensis Georgi, Phellodendron amurense Rupr, and Coptis chinensis Franch, that has excellent anti-bacterial and anti-diarrheal effects with few side effects.9,10 According to the literature, BBR exerts anti-gastric ulcer activity by modulating H+/K+-ATPase activity and the dynamic balance of pro-oxidant and antioxidant systems.11–13 However, the underlying mechanisms mediating BBR activity on peptic ulcers remain to be elucidated, limiting its clinical application. Thus, this study thoroughly investigated the effect of BBR in a rat model of acute gastric ulcers. Our results will enrich the mechanistic understanding and support the use of BBR as a promising agent for treatment of acute gastric ulcers.
Materials and Methods
Drugs and Reagents
BBR and omeprazole were provided by Shanghai Macklin Biochemical Technology Co, Ltd (Shanghai, China) (CAS No 2086-83-1, product code: B875003 and CAS No 73590-58-6, product code: O815322, respectively). Ethanol was purchased from Innochem (Beijing) Technology Co, Ltd (Beijing, China) (CAS No. 64-17-5, product code: A60719). Saline (0.9%) was provided by Shandong Kelun Pharmaceutical Co, Ltd (Shandong, China) (product code: B22082402A).
Experimental Animals, Grouping and Administration
Thirty-six clean-grade male Sprague Dawley rats (10–12 weeks old), were obtained from Beijing Vital River Laboratory Animal Technology Co, Ltd (Beijing, China), license number SCXK (Beijing) 2021-0011. The temperature of the feeding room was 25 ± 2 °C, the relative humidity was 50%–60%, and light and dark periods were alternated every 12 h. Animals had free access to food and water.
The rats were divided into six groups: Control, Model, Positive, BBR-Low, BBR-Mid, and BBR-High (n = 6 per group). The rat model of acute gastric ulcer was prepared by intragastric administration of absolute ethanol (5 mL/kg). Rats were given omeprazole (20 mg/kg) as a positive control, based on published data.14 Rats in the BBR-Low, Mid and High groups (25, 50, or 100 mg/kg, respectively) and the omeprazole group were dosed by intragastric administration for 14 consecutive days. Absolute ethanol was then given orally 1 h before sacrifice to induce acute gastric ulcers. Rats were anesthetized with isoflurane, blood samples were collected, and gastric tissues were immediately removed. After centrifuging the blood samples, the supernatants were stored at −80 °C. The collected gastric tissues were cut into three equal parts. A third of the tissues were kept in 4% paraformaldehyde for hematoxylin-eosin staining, while the remaining tissues were stored at −80 °C for untargeted metabolomics and transcriptome analysis.
Ethics Statement
All animal experiments and procedures were approved by the Institutional Animal Care and Use Committee and Animal Ethics Committee of the Institute of Chinese Materia Medica (approval #2023B018). All applied procedures followed the Chinese guidelines for the welfare of laboratory animals (GB/T 35823–2018).
Macroscopic Assessment of Gastric Ulcers
The degree of gastric mucosal injury was determined according to a published procedure.15 In detail, gastric tissues were cut along the arcus major ventriculi with the mucosa turned outside, and then washed with 0.9% saline. The tissues were observed under a stereomicroscope at ×10 magnification and the macroscopic score was counted according to the Guth grade standard. The macroscopic score was reported as mean ± standard deviation. The ulcer healing rate was calculated by:
Observation of Gastric Tissue Pathological Morphology by Hematoxylin-Eosin Staining
The gastric tissues were submerged in 4% paraformaldehyde at room temperature for 24 h, embedded in paraffin, sectioned, and dewaxed for staining. The tissues were then examined under a microscope and scanned for specific analysis.
Biochemical Analysis of Serum by Enzyme-Linked Immunosorbent Assay (ELISA)
The ELISA kits for inflammatory cytokines (nitric oxide (NO), interleukin-6 (IL-6), prostaglandin E2 (PGE2), and interleukin-10 (IL-10)) and oxidative stress indicators (malondialdehyde (MDA), myeloperoxidase (MPO), superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px)) were obtained from Shanghai Enzyme-linked Biotechnology Co, Ltd (product codes: ml102828, ml059000, ml077384, ml003036, ml077384, ml059387, ml003250, and ml097316, respectively).
RNA-Seq and Data Analysis
Total RNA collected from gastric tissues of rats in each group (n = 6 per group) was subjected to Illumina TruSeq RNA library construction. The sequencing libraries were barcoded and the sequencing reaction was performed using Illumina NovaSeq 6000. A cut-off of 2-fold change and p < 0.05 were used to define the threshold of significance, identifying differentially expressed genes. The R programming language was used for Gene Ontology (GO) functional enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis.
Untargeted Metabolome Data Analysis
The remaining gastric tissue samples (n = 6 per group) were prepared and evaluated using the metabolome analysis protocol of Novogene Co, Ltd (Beijing, China). Principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) were used for data visualization. Variable importance (VIP) ≥ 1, p < 0.05 and fold change ≥ 1.2 were regarded as the conditions for determination of differential accumulated metabolites. The human metabolome database (HMDB, https://hmdb.ca/) and mzCloud database were used to check the first-order accurate molecular weight and the second-order mass spectrometry fragments after comparison to identify the differential metabolites. Finally, the data were presented as heat maps and the relevant pathways were analyzed by KEGG.
Statistical Analysis
All results are reported as the mean ± standard deviation and processed using GraphPad Prism 9 (GraphPad Software, USA). One-way analysis of variance (ANOVA) was used to examine the degree of data difference. Welch and Brown-Forsythe tests were used to assess the normality but not the homogeneity of variance. Non-parameter tests were used if normality was not met. Results were considered significant when p < 0.05.
Results
BBR Alleviated the Gastric Pathological Changes Induced by Absolute Ethanol
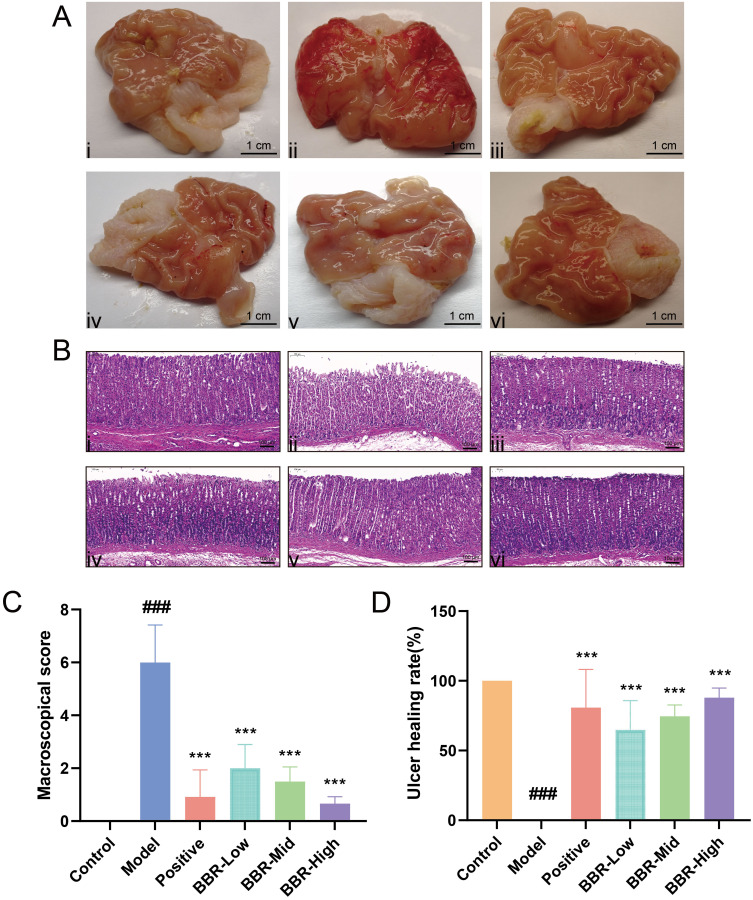
The surface of normal gastric tissue was bright, the structure was clear, and the gastric mucosa was pink without thickening (Figure 1A). Gastric tissue from rats in the Model group exhibited punctate ulcers in the form of obvious craters, part of the mucosal folds was eroded and thickened, and hyperemia was indicated by dark red coloration. After dosing with omeprazole, the congestion and swelling of the gastric mucosa were reduced, but some mucosal folds were still eroded. Compared to the Model group, BBR pretreatment decreased the severity of the gastric ulcers caused by absolute ethanol, and significantly reduced mucosal congestion and swelling.
Figure 1.
Effects of BBR on macromorphology and micromorphology in rats with acute gastric ulcer. (A) Gastric mucosa macroscopic morphology of gastric tissues from rats in different groups. Scale bar = 1 cm. Ai–Avi show the Control, Model, Positive drug, and BBR-Low/Mid/High groups, respectively. (B) Hematoxylin–eosin staining of gastric tissues from rats in different groups (Scale bar = 100 µm; magnification ×100). Bi–Bvi show the Control, Model, Positive drug, and BBR-Low/Mid/High groups, respectively. (C) Macroscopic scores in rats from each group. (D) Gastric ulcer healing rates in each group. All values are expressed as mean ± standard deviation, n = 6. ###p < 0.001 vs Control; ***p < 0.001 vs Model.
Hematoxylin-eosin staining showed that the structure of normal gastric mucosa was complete and the glands were evenly arranged. In addition, the gastric layer was normal, without signs of deterioration. In the Model group, severe congestion and inflammatory cell infiltration occurred in the submucosa and the ulcer surface of the gastric mucosa (Figure 1B). Rats in the BBR pretreatment groups at different doses showed fewer signs of inflammation and the mucosa was partially repaired. Moreover, BBR significantly decreased macroscopic scores and markedly increased ulcer healing rates, as shown in Figures 1C and D. Similar results were obtained in the omeprazole-treated group with only mild signs of inflammation and significant improvement in pathological severity. These results demonstrate the efficacy of BBR in alleviating gastric ulcer injury.
BBR Significantly Inhibited Inflammation in the Rat Gastric Ulcer Model
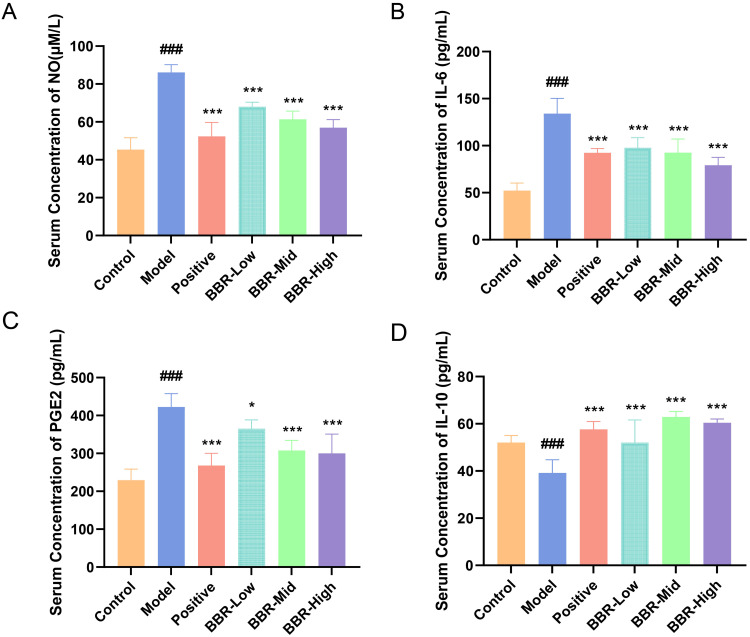
In this study, the serum levels of inflammatory factors including NO, IL-6, and PGE2, as well as the anti-inflammatory factor IL-10, were determined to evaluate the anti-inflammatory effect of BBR acting on gastric ulcers. As shown in Figure 2A–C, the serum concentrations of NO, IL-6, and PGE2 were markedly increased in rats with acute gastric ulcer (p < 0.001). Compared with the Model group, these levels in both the omeprazole and BBR groups were significantly decreased (p < 0.001). Levels of IL-10 were significantly decreased in the Model group compared with those in normal rats (p < 0.001) and were increased in both the omeprazole and BBR groups (p < 0.001, Figure 2D). The highest dose of BBR gave the best results, so samples from the BBR-High group, together with those from the Control and Model groups, were selected for transcriptome analysis and lipid metabolomics.
Figure 2.
Effect of BBR on serum inflammatory factors in rats with acute gastric ulcer. (A–D) Serum concentrations of NO, IL-6, PGE2, and IL-10, respectively, in rats from each group. All values are expressed as mean ± standard deviation, n = 6. ###p < 0.001 vs Control; *p < 0.05, ***p < 0.001 vs Model.
BBR Markedly Regulated the Arachidonic Acid Metabolism Pathway
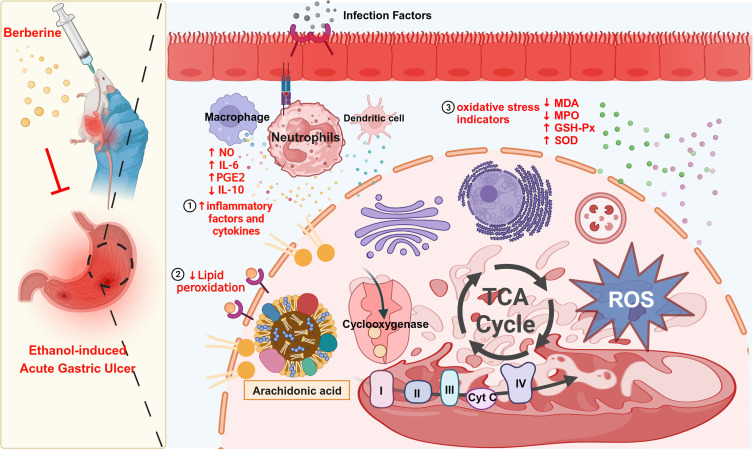
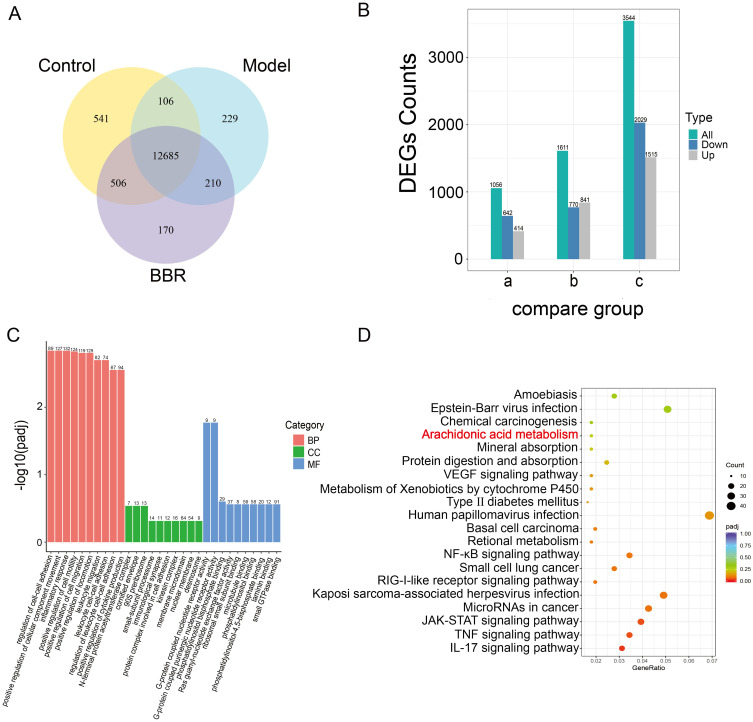
As displayed in the transcriptomics data (Figure 3A and B), there were differentially expressed genes (DEGs) among the different groups. There were 12,685 genes common to the three groups. There were 3544 DEGs that were significantly different between the Model and Control groups, including 1515 that were up-regulated and 2029 that were down-regulated. There were 1611 DEGs that were markedly different between the BBR and Model groups, including 840 that were up-regulated and 770 that were down-regulated. The DEGs from the BBR treatment group were mainly enriched in pathways associated with biological processes such as inflammatory response, leukocyte cell-cell adhesion, and leukocyte migration (Figure 3C). We then conducted KEGG enrichment analysis of the DEGs from the BBR vs Model groups, which indicated that “arachidonic acid metabolism” was a significant signaling pathway (Figure 3D). As has been reported,16–18 macrophages and neutrophils release large amounts of polyunsaturated fatty acids (PUFAs), such as arachidonic acid (AA), during the inflammatory response. Arachidonic acid and its metabolites are susceptible to attack by reactive oxygen species, producing lipid ROS. Lipid accumulation can lead to disturbances of pro-oxidant and antioxidant systems, and arachidonic acid metabolism plays an important role in inflammation. It is likely, therefore, that BBR exerts its gastric protective effects by inhibiting the inflammatory response via regulation of the arachidonic acid metabolism pathway.
Figure 3.
Transcriptomics profiling of gastric tissues from different groups. (A) Venn diagram showing co-expressed genes among the Control, Model and BBR groups. (B) Bar chart showing DEGs of the compared groups. a. BBR vs Control; b. BBR vs Model; c. Model vs Control. (C and D) GO functional enrichment and KEGG pathway enrichment results for DEGs in BBR vs Model.
Analysis of Differentially Accumulated Metabolites
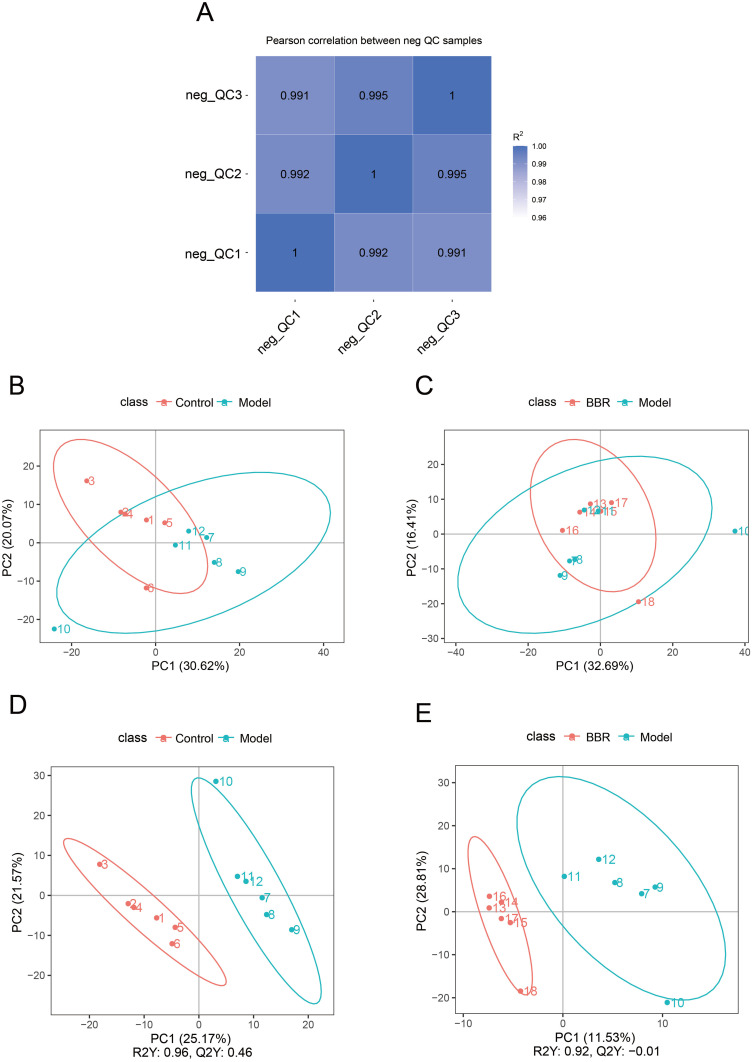
As shown in Figure 4A, the R2 correlation coefficients of the quality control (QC) samples were close to 1, indicating the stability of the whole detection process. The differentially accumulated metabolites (DAMs) of Model vs Control and BBR vs Model were analyzed by PCA and PLS-DA. Metabolites of Model vs Control as well as those of BBR vs Model exhibited significant differences (Figure 4B and C). Model vs Control and BBR vs Model showed significantly different distributions in the PLS-DA graphs (Figure 4D and E).
Figure 4.
Metabolomics analysis of gastric tissues from different groups. (A) QC correlations of the three compared groups. (B and C) PCA score plots of Model vs Control. and BBR vs Model. (D and E) PLS-DA score plots of Model vs Control and BBR vs Model.
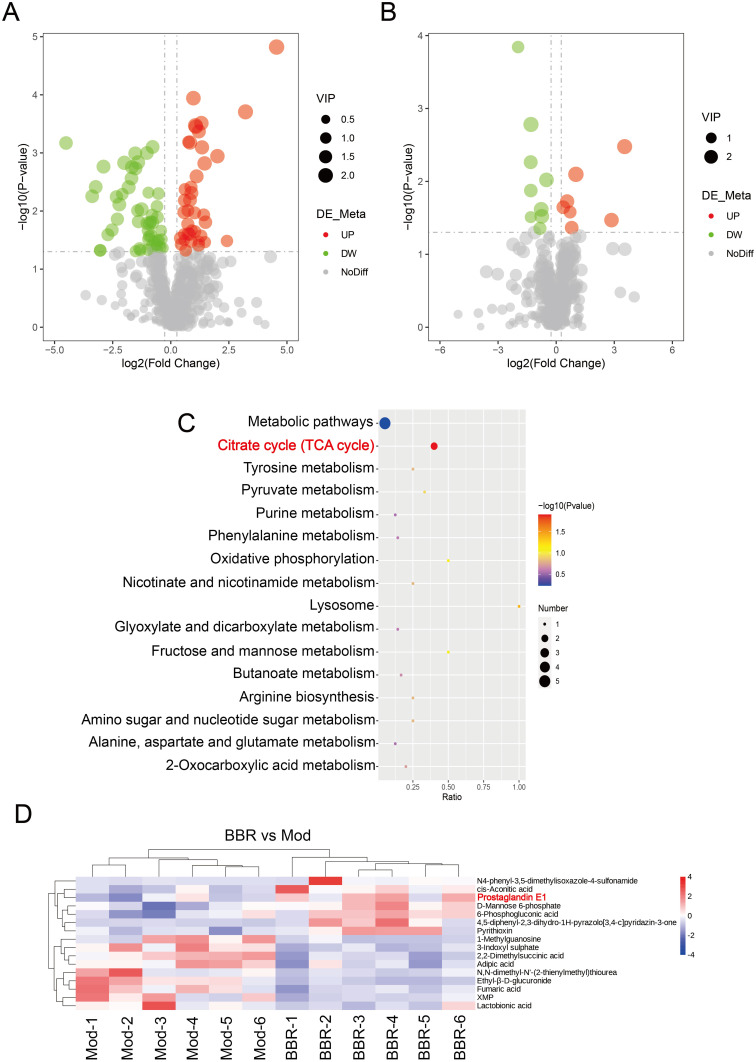
According to the standards, there were 91 significant DAMs from Model vs Control, including 39 that were up-regulated and 52 that were down-regulated. There were 16 DAMs identified from BBR vs Model, including 7 that were up-regulated and 9 that were down-regulated (Figure 5A and B). KEGG enrichment analysis of DAMs from BBR vs Model found that “tricarboxylic acid (TCA) cycle” was a significantly correlated metabolic pathway (Figure 5C). In addition, we found that prostaglandin E1 (PGE1) was significantly up-regulated in the cluster heatmap of BBR vs Model (Figure 5D). These findings indicated that BBR may regulate PGE1 and the TCA cycle.
Figure 5.
DAMs from BBR vs Model and associated important pathways. (A) Volcano map of DAMs from Model vs Control. (B) Volcano map of DAMs from BBR vs Model. (C) KEGG analysis of DAMs from BBR vs Model. (D) Cluster heatmap of DAMs from BBR vs Model.
BBR Significantly Regulated Oxidative Stress Indicators in Serum
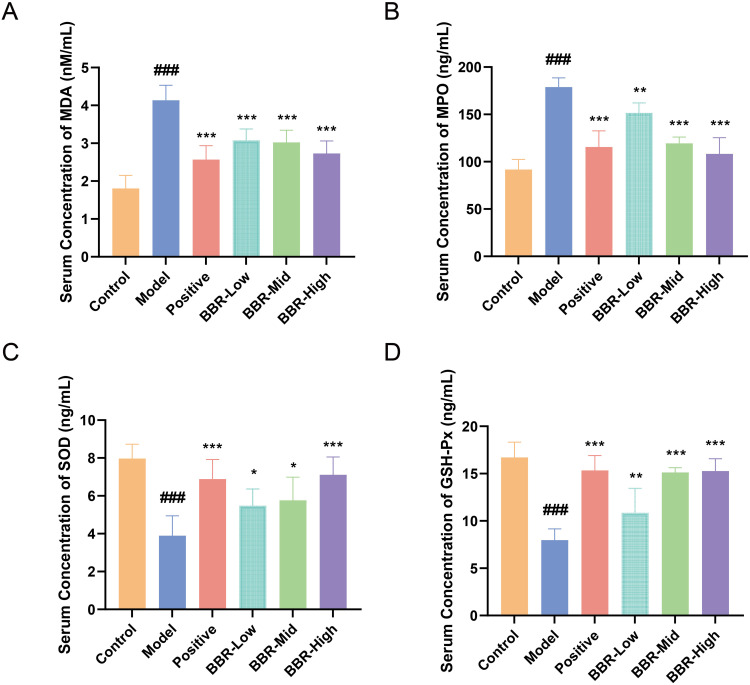
Considering both the transcriptomics and metabolomics data, we found that the genes or metabolites modulated by BBR were enriched in the regulation of arachidonic acid or tricarboxylic acid cycle, pathways that are involved in oxidative stress and maintaining the balance between pro-oxidant and antioxidant activity. To validate these results, we analyzed serum oxidative stress indicators. Compared with the Control group, the serum levels of MPO and the oxidative metabolite MDA in rats from the Model group were markedly increased (p < 0.001, Figure 6A and B), while the levels of SOD and GSH-Px were reduced (p < 0.001, Figure 6C and D). To sum up, BBR pretreatment increased the level of antioxidant enzymes and reduced the accumulation of oxidative metabolites.
Figure 6.
Influence of BBR on serum oxidative stress indicators. (A–D) Serum concentrations of MDA, MPO, SOD, and GSH-Px, respectively, in rats from each group. All values are expressed as mean ± standard deviation, n = 6. ###p < 0.001 vs Control; *p < 0.05, **p < 0.01, ***p < 0.001 vs Model.
Discussion
BBR has pharmacological activity that protects against digestive diseases. It has been reported to inhibit toxins, Helicobacter pylori and other bacteria, to protect the intestinal epithelial barrier from damage, and to ameliorate gastric injury.19 BBR has been found to protect gastric mucosa from ethanol, and its anti-ulcer activity may be due to modulation of oxidant/antioxidant balance.12,20 However, the mechanism underlying the anti-ulcer effect of BBR remains unclear. In this study, transcriptomics, metabolomics and experimental verification were used to analyze the protective effect of BBR toward acute gastric ulcer induced by anhydrous alcohol in rats, and to explore potential mechanisms.
Absolute ethanol was used to establish a rat model of acute gastric ulcer, which mimics the human ulcerative condition. In particular, absolute ethanol triggers typical pathological changes of alcohol injury such as linear hemorrhagic lesions and inflammatory cell infiltration.21,22 The reported effects of ethanol are consistent with those observed in our study, and BBR pretreatment significantly reduced these changes. We first confirmed the therapeutic effect of BBR on ethanol-induced acute gastric ulcer. The high dose was found to be optimal, so was selected for further mechanistic analysis.
The inflammatory response of gastric ulcer is characterized by monocyte infiltration of the ulcer area and damage to the gastric mucosa.23 During the inflammatory process, migrating macrophages release IL-6, PGE2, and NO, stimulating neutrophil infiltration of the gastritis area, stimulating mucosal microcirculation of the gastric ulcer and delaying recovery.20 Therefore, we measured serum levels of the proinflammatory cytokines NO, IL-6, and PGE2, and the anti-inflammatory cytokine IL-10, in rats. We discovered that absolute ethanol administration induced inflammatory responses with evidence of significant increases in serum levels of IL-6, PGE2, and NO, together with a marked decrease of IL-10 serum concentration. Pretreatment with BBR significantly reversed the above phenomena.
Subsequently, based on the transcriptome sequencing results, we focused on the arachidonic acid metabolism pathway, which is closely involved in inflammation.24,25 As one of the most abundant fatty acids released during inflammation, arachidonic acid (AA) has proinflammatory and pro-oxidant effects. Oxidation products of AA react with cyclooxygenases (COX), which can further increase ROS production.26,27 Saeed et al stated that BBR, as a natural modulator of inflammatory signaling pathways, plays an anti-inflammatory role through three major inflammatory signaling pathways: NF-κB, JAK/STAT, and MAPK.28 Moreover, previous studies have shown that the gastroprotective mechanism of BBR might involve antioxidant and anti-inflammatory activity.12,13,29 However, there are few reports on the involvement of the arachidonic acid metabolism pathway in the BBR treatment of gastric ulcers. Our results provide evidence for the first time that BBR regulates the arachidonic acid metabolism pathway under gastric ulcer conditions.
Furthermore, BBR vs Model differential metabolites were significantly enriched in TCA cycle pathways. It has been reported that regulatory T cells and M2 macrophages, which inhibit inflammation, prioritize the use of fatty acid oxidation as the main source of energy generation through the TCA cycle.30 Inflammatory signals increase ROS production and the accumulation of TCA intermediates by influencing mitochondrial function. Treatment with anti-inflammatory cytokines such as IL-4 could reverse this situation and switch to more oxidative metabolism.31 Interestingly, BBR has been reported to have proinflammatory effects on Th17 cells, but anti-inflammatory effects on Treg cells.32 Transcriptome and metabolome analysis data in this study were consistent with previous studies. BBR, as an anti-inflammatory molecule, regulates AA metabolism through the TCA cycle pathway, thereby delaying progression of gastric ulcer. More interestingly, we noticed that prostaglandin E1 was significantly up-regulated in the BBR vs Model cluster heatmap. PGE1 is able to resist inflammation, regulate vascular reactivity, and alleviate liver injury by reducing oxidative stress damage.33–35 Gezginci et al demonstrated that the protective effect of PGE1 on renal ischemia/reperfusion injury (IRI)-induced gastric damage was mediated by reducing ROS and MDA levels.36 The above results are consistent with a mechanism involving increased production of PGE1 and decreased production of ROS from the TCA cycle, which has a protective effect on acute gastric ulcers.
It has been reported that ROS generated by neutrophils during inflammation play a critical role in gastric mucosal injury.37 The accumulation of ROS further causes oxidative damage. To verify the inhibitory effect of BBR on oxidative damage, several indexes related to oxidative stress were assessed. Our study indicated that serum concentrations of MDA and MPO were increased in rats with gastric ulcer, while levels of SOD and GSH-Px were decreased. BBR pretreatment reversed these changes and maintained a state of oxidative balance. Our study is consistent with a previous study38 demonstrating that the natural product BBR has a potential gastro-protective effect on ethanol-induced gastric ulcer through suppression of oxidative stress and gastric inflammation.
Conclusions
This study demonstrates that BBR protects against ethanol-induced acute gastric ulcer in rats. The potential mechanism underlying the protective effect of BBR on acute gastric ulcer is promotion of the tricarboxylic acid cycle-mediated arachidonic acid metabolism pathway.
Funding Statement
The authors gratefully acknowledge the financial support from, the National Natural Science Foundation of China (No. 82104480), the Fundamental Research Funds for the Central Public Welfare Research Institutes (Nos. ZZ14-YQ-060, and ZZ16-ND-10-19), and the Young Elite Scientists Sponsorship Program by CAST (NO.2021-QNRC2-B29), and Science and Technology Innovation Project of China Academy of Chinese Medical Sciences (CI2021A04313).
Abbreviations
AA, arachidonic acid; ANOVA, analysis of Variance; BBR, berberine; COX, cyclooxygenases; CYP, cytochrome P450s; DAMs, Differentially Accumulated Metabolites; DEGs, differentially expressed genes; ELISA, enzyme linked immunosorbent assay; GO, Gene Ontology; GSH-Px, glutathione peroxidase; HE, hematoxylin-eosin; HMDB, human metabolome database; IL-4, interleukin-4; IL-6, interleukin-6; IL-10, interleukin-10; JAK, janus kinase; KEGG, Kyoto Encyclopedia of Genes and Genomes; LOX, lipoxygenases; MAPK, mitogen-activated protein kinase; MDA, malondialdehyde; MPO, myeloperoxidase; NF-κB, nuclear factor kappa B; NO, nitric oxide; NSAIDs, non-steroidal anti-inflammatory drugs; PCA, principal components analysis; PGs, prostaglandins; PGE1, prostaglandin E1; PLS-DA, partial least squares discriminant analysis; PUFA, polyunsaturated fatty acid; QC, quality control; R2, R squared; RNA, ribonucleic acid; ROS, reactive oxygen species; SD, standard deviation; SOD, superoxide dismutase; STAT, signal transducer and activator of transcription; TCA cycle, reactive oxygen species; Th17, T helper cell 17; TXA2, thromboxane A2.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee and Animal Ethics Committee of the Institute of Chinese Materia Medica, with approval number 2023B018. All the applied procedures followed the Chinese guidelines for the welfare of the laboratory animals (GB/T 35823-2018).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. All authors have read and approved the final submitted manuscript.
Disclosure
The authors declare that they have no competing interest.
References
- 1.Xie L, Guo Y-L, Chen Y-R, et al. A potential drug combination of omeprazole and patchouli alcohol significantly normalizes oxidative stress and inflammatory responses against gastric ulcer in ethanol-induced rat model. Int Immunopharmacol. 2020;85:106660. doi: 10.1016/j.intimp.2020.106660 [DOI] [PubMed] [Google Scholar]
- 2.Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet. 2009;374(9699):1449–1461. doi: 10.1016/s0140-6736(09)60938-7 [DOI] [PubMed] [Google Scholar]
- 3.Kamada T, Satoh K, Itoh T, et al. Evidence-based clinical practice guidelines for peptic ulcer disease 2020. J Gastroenterol. 2021;56(4):303–322. doi: 10.1007/s00535-021-01769-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Wajeeh NS, Hajrezaie M, Al-Henhena N, et al. The antiulcer effect of Cibotium barometz leaves in rats with experimentally induced acute gastric ulcer. Drug Des Devel Ther. 2017;11:995–1009. doi: 10.2147/dddt.S107018 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Fagundes FL, Pereira QC, Zarricueta ML, et al. Malvidin protects against and repairs peptic ulcers in mice by alleviating oxidative stress and inflammation. Nutrients. 2021;13(10). doi: 10.3390/nu13103312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Sun L, Lai X, et al. Gastroprotective effects of extract of Jasminum grandiflorum L. flower in HCl/EtOH-induced gastric mucosal ulceration mice. Biomed Pharmacother. 2021;144:112268. doi: 10.1016/j.biopha.2021.112268 [DOI] [PubMed] [Google Scholar]
- 7.Wu S, Chen Y, Chen Z, et al. Reactive oxygen species and gastric carcinogenesis: the complex interaction between Helicobacter pylori and host. Helicobacter. 2023:e13024. doi: 10.1111/hel.13024 [DOI] [PubMed] [Google Scholar]
- 8.Dinat S, Orchard A, Van Vuuren S. A scoping review of African natural products against gastric ulcers and Helicobacter pylori. J Ethnopharmacol. 2023;301:115698. doi: 10.1016/j.jep.2022.115698 [DOI] [PubMed] [Google Scholar]
- 9.Wang K, Feng X, Chai L, et al. The metabolism of berberine and its contribution to the pharmacological effects. Drug Metab Rev. 2017;49(2):139–157. doi: 10.1080/03602532.2017.1306544 [DOI] [PubMed] [Google Scholar]
- 10.Liang S, Kuang Y, Ma F, et al. A sensitive spectrofluorometric method for detection of berberine hydrochloride using Ag nanoclusters directed by natural fish sperm DNA. Biosens Bioelectron. 2016;85:758–763. doi: 10.1016/j.bios.2016.05.070 [DOI] [PubMed] [Google Scholar]
- 11.Zhang SL, Li H, He X, et al. Alkaloids from Mahonia bealei posses anti-H⁺/K⁺-ATPase and anti-gastrin effects on pyloric ligation-induced gastric ulcer in rats. Phytomedicine. 2014;21(11):1356–1363. doi: 10.1016/j.phymed.2014.07.007 [DOI] [PubMed] [Google Scholar]
- 12.Pan LR, Tang Q, Fu Q, et al. Roles of nitric oxide in protective effect of berberine in ethanol-induced gastric ulcer mice. Acta Pharmacol Sin. 2005;26(11):1334–1338. doi: 10.1111/j.1745-7254.2005.00186.x [DOI] [PubMed] [Google Scholar]
- 13.Luo C, Chen H, Wang Y, et al. Protective effect of coptisine free base on indomethacin-induced gastric ulcers in rats: characterization of potential molecular mechanisms. Life Sci. 2018;193:47–56. doi: 10.1016/j.lfs.2017.12.004 [DOI] [PubMed] [Google Scholar]
- 14.Chen X, Zhao Y, Liu K, et al. Lycopene aggravates acute gastric injury induced by ethanol. Front Nutr. 2021;8:697879. doi: 10.3389/fnut.2021.697879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahman Z, Dwivedi DK, Jena GB. Ethanol-induced gastric ulcer in rats and intervention of tert-butylhydroquinone: involvement of Nrf2/HO-1 signalling pathway. Hum Exp Toxicol. 2020;39(4):547–562. doi: 10.1177/0960327119895559 [DOI] [PubMed] [Google Scholar]
- 16.Wen J, Aili A, Yan YX, et al. OIT3 serves as a novel biomarker of hepatocellular carcinoma by mediating ferroptosis via regulating the arachidonic acid metabolism. Front Oncol. 2022;12:977348. doi: 10.3389/fonc.2022.977348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beavers WN, Monteith AJ, Amarnath V, et al. Arachidonic Acid Kills Staphylococcus aureus through a Lipid Peroxidation Mechanism. mBio. 2019;10(5). doi: 10.1128/mBio.01333-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhuge A, Li S, Yuan Y, et al. Microbiota-induced lipid peroxidation impairs obeticholic acid-mediated antifibrotic effect towards nonalcoholic steatohepatitis in mice. Redox Biol. 2023;59:102582. doi: 10.1016/j.redox.2022.102582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song D, Hao J, Fan D. Biological properties and clinical applications of berberine. Front Med. 2020;14(5):564–582. doi: 10.1007/s11684-019-0724-6 [DOI] [PubMed] [Google Scholar]
- 20.Li W, Wang X, Zhang H, et al. Anti-ulcerogenic effect of cavidine against ethanol-induced acute gastric ulcer in mice and possible underlying mechanism. Int Immunopharmacol. 2016;38:450–459. doi: 10.1016/j.intimp.2016.06.016 [DOI] [PubMed] [Google Scholar]
- 21.Hossen MA, Reza A, Ahmed AMA, et al. Pretreatment of Blumea lacera leaves ameliorate acute ulcer and oxidative stress in ethanol-induced Long-Evan rat: a combined experimental and chemico-biological interaction. Biomed Pharmacother. 2021;135:111211. doi: 10.1016/j.biopha.2020.111211 [DOI] [PubMed] [Google Scholar]
- 22.Lin K, Wang Y, Gong J, et al. Protective effects of total flavonoids from Alpinia officinarum rhizoma against ethanol-induced gastric ulcer in vivo and in vitro. Pharm Biol. 2020;58(1):854–862. doi: 10.1080/13880209.2020.1803370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mousa AM, El-Sammad NM, Hassan SK, et al. Antiulcerogenic effect of Cuphea ignea extract against ethanol-induced gastric ulcer in rats. BMC Complement Altern Med. 2019;19(1):345. doi: 10.1186/s12906-019-2760-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seeds MC, Bass DA. Regulation and metabolism of arachidonic acid. Clin Rev Allergy Immunol. 1999;17(1–2):5–26. doi: 10.1007/bf02737594 [DOI] [PubMed] [Google Scholar]
- 25.Xu M, Wang X, Li Y, et al. Arachidonic acid metabolism controls macrophage alternative activation through regulating oxidative phosphorylation in PPARγ dependent manner. Front Immunol. 2021;12:618501. doi: 10.3389/fimmu.2021.618501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma Y, Zheng L, Wang Y, et al. Arachidonic acid in follicular fluid of PCOS induces oxidative stress in a human ovarian granulosa tumor cell line (KGN) and upregulates GDF15 expression as a response. Front Endocrinol. 2022;13:865748. doi: 10.3389/fendo.2022.865748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Badimon L, Vilahur G, Rocca B, et al. The key contribution of platelet and vascular arachidonic acid metabolism to the pathophysiology of atherothrombosis. Cardiovasc Res. 2021;117(9):2001–2015. doi: 10.1093/cvr/cvab003 [DOI] [PubMed] [Google Scholar]
- 28.Haftcheshmeh SM, Abedi M, Mashayekhi K, et al. Berberine as a natural modulator of inflammatory signaling pathways in the immune system: focus on NF-κB, JAK/STAT, and MAPK signaling pathways. Phytother Res. 2022;36(3):1216–1230. doi: 10.1002/ptr.7407 [DOI] [PubMed] [Google Scholar]
- 29.Li B, Shang JC, Zhou QX. Study of total alkaloids from Rhizoma Coptis Chinensis on experimental gastric ulcers. Chin J Integr Med. 2005;11(3):217–221. doi: 10.1007/bf02836508 [DOI] [PubMed] [Google Scholar]
- 30.Soto-Heredero G, Gómez de Las Heras MM, Gabandé-Rodríguez E, et al. Glycolysis - a key player in the inflammatory response. Febs j. 2020;287(16):3350–3369. doi: 10.1111/febs.15327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stifel U, Wolfschmitt EM, Vogt J, et al. Glucocorticoids coordinate macrophage metabolism through the regulation of the tricarboxylic acid cycle. Mol Metab. 2022;57:101424. doi: 10.1016/j.molmet.2021.101424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li YH, Xiao HT, Hu DD, et al. Berberine ameliorates chronic relapsing dextran sulfate sodium-induced colitis in C57BL/6 mice by suppressing Th17 responses. Pharmacol Res. 2016;110:227–239. doi: 10.1016/j.phrs.2016.02.010 [DOI] [PubMed] [Google Scholar]
- 33.Qin Z, Kong B, Zheng J, et al. Alprostadil injection attenuates coronary microembolization-induced myocardial injury through GSK-3β/Nrf2/HO-1 signaling-mediated apoptosis inhibition. Drug Des Devel Ther. 2020;14:4407–4422. doi: 10.2147/dddt.S272877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang M, Cai XF, Zhang SM, et al. Alprostadil alleviates liver injury in septic rats via TLR4/NF-κB pathway. Eur Rev Med Pharmacol Sci. 2021;25(3):1592–1599. doi: 10.26355/eurrev_202102_24869 [DOI] [PubMed] [Google Scholar]
- 35.Levin G, Duffin KL, Obukowicz MG, et al. Differential metabolism of dihomo-gamma-linolenic acid and arachidonic acid by cyclo-oxygenase-1 and cyclo-oxygenase-2: implications for cellular synthesis of prostaglandin E1 and prostaglandin E2. Biochem J. 2002;365(Pt 2):489–496. doi: 10.1042/bj20011798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gezginci-Oktayoglu S, Orhan N, Bolkent S. Prostaglandin-E1 has a protective effect on renal ischemia/reperfusion-induced oxidative stress and inflammation mediated gastric damage in rats. Int Immunopharmacol. 2016;36:142–150. doi: 10.1016/j.intimp.2016.04.021 [DOI] [PubMed] [Google Scholar]
- 37.Lu H, Lin J, Xu C, et al. Cyclosporine modulates neutrophil functions via the SIRT6-HIF-1α-glycolysis axis to alleviate severe ulcerative colitis. Clin Transl Med. 2021;11(2):e334. doi: 10.1002/ctm2.334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdel-Kawi SH, Hashem KS, Saad MK, et al. The ameliorative effects of cinnamon oil against ethanol-induced gastric ulcer in rats by regulating oxidative stress and promoting angiogenesis. J Mol Histol. 2022;53(3):573–587. doi: 10.1007/s10735-022-10072-y [DOI] [PubMed] [Google Scholar]