Abstract
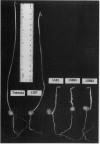
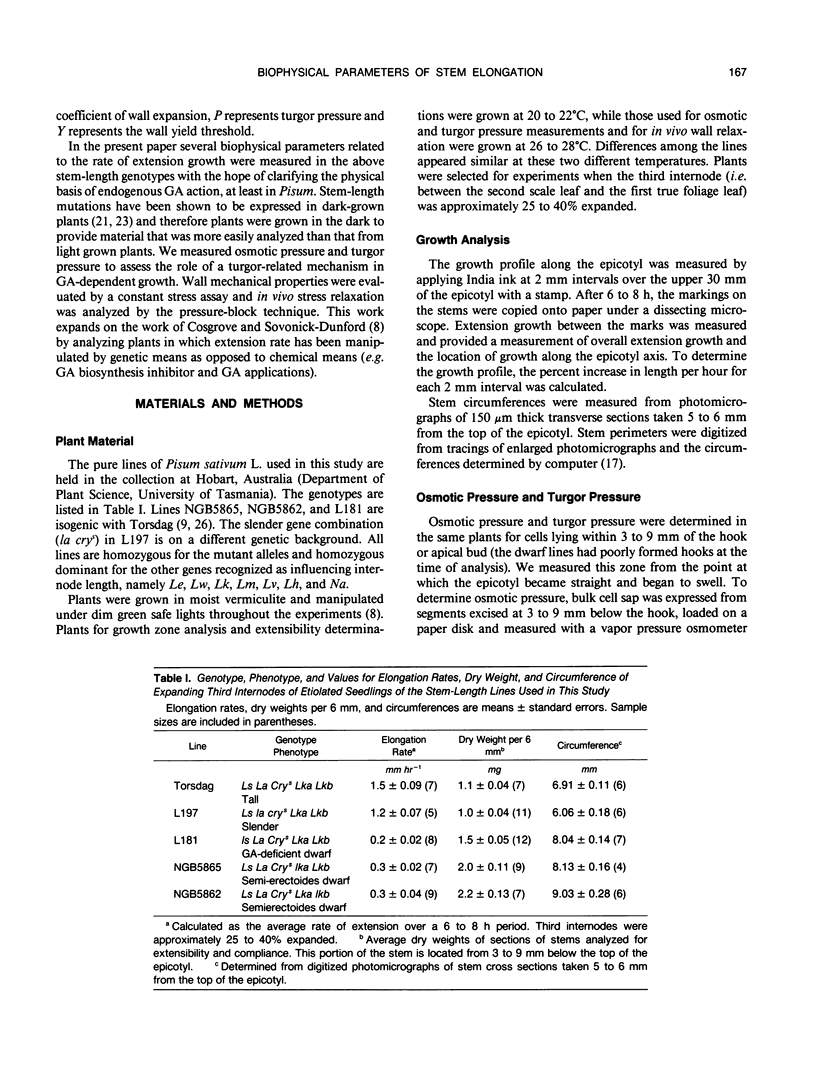
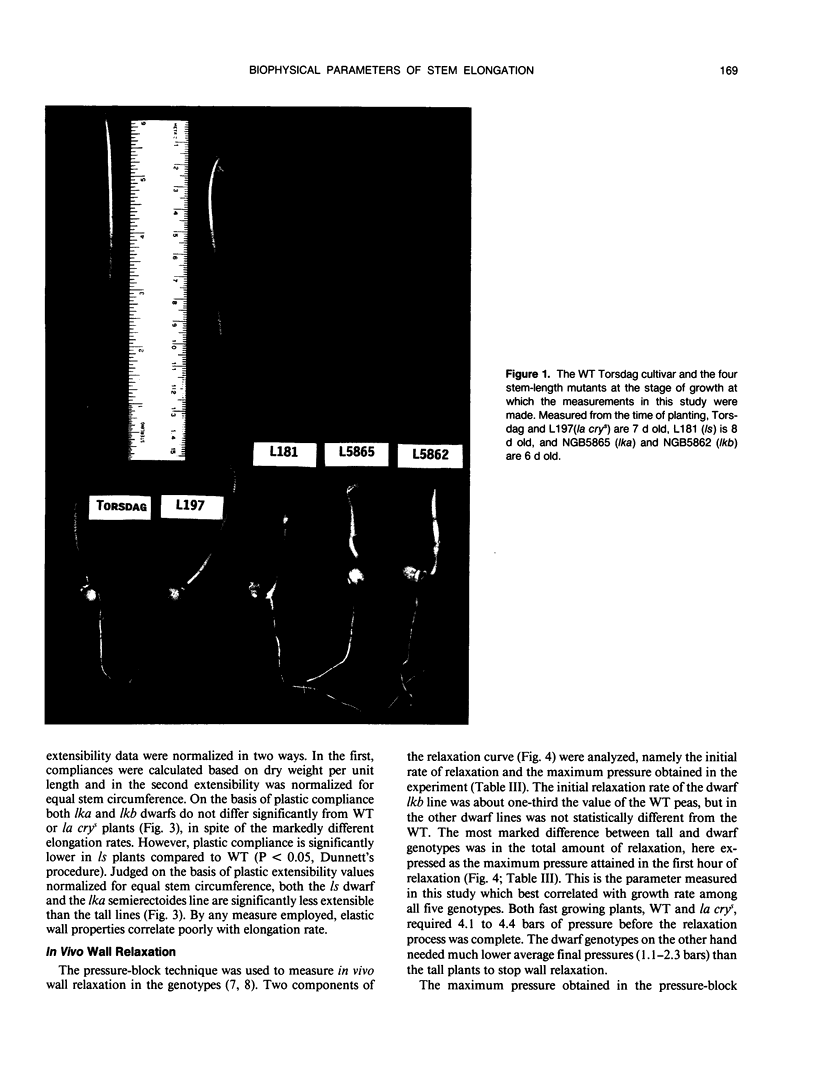
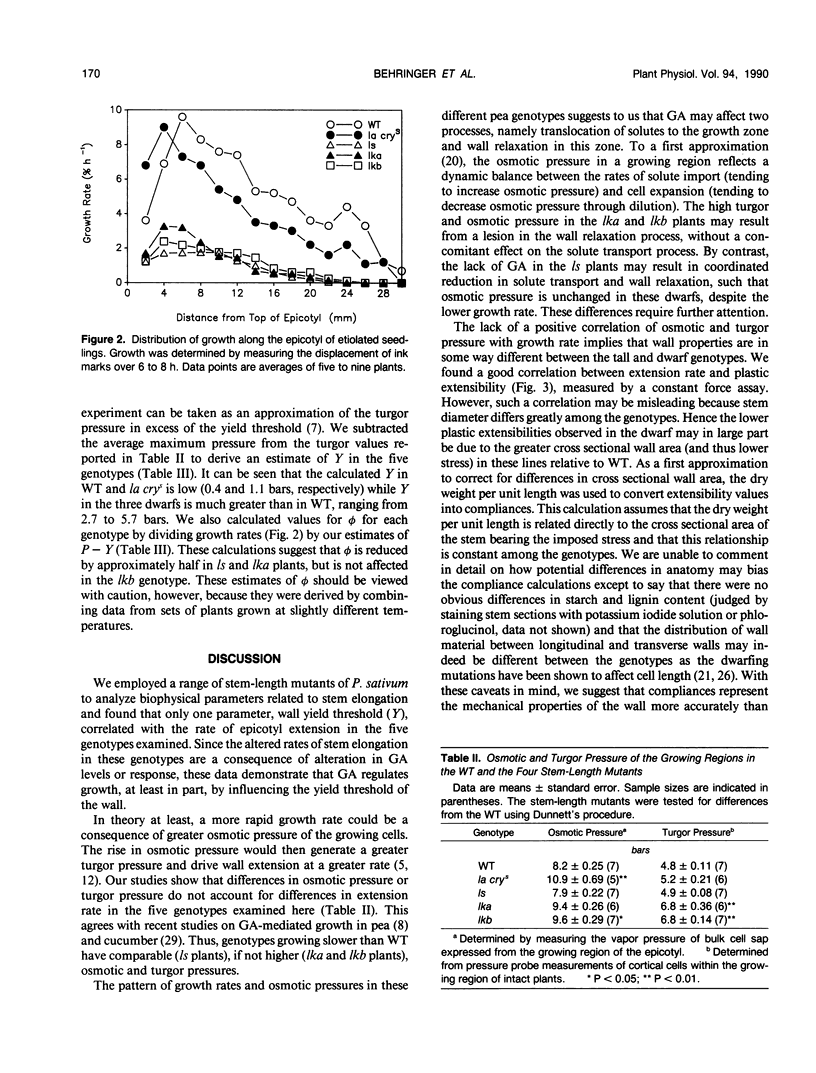
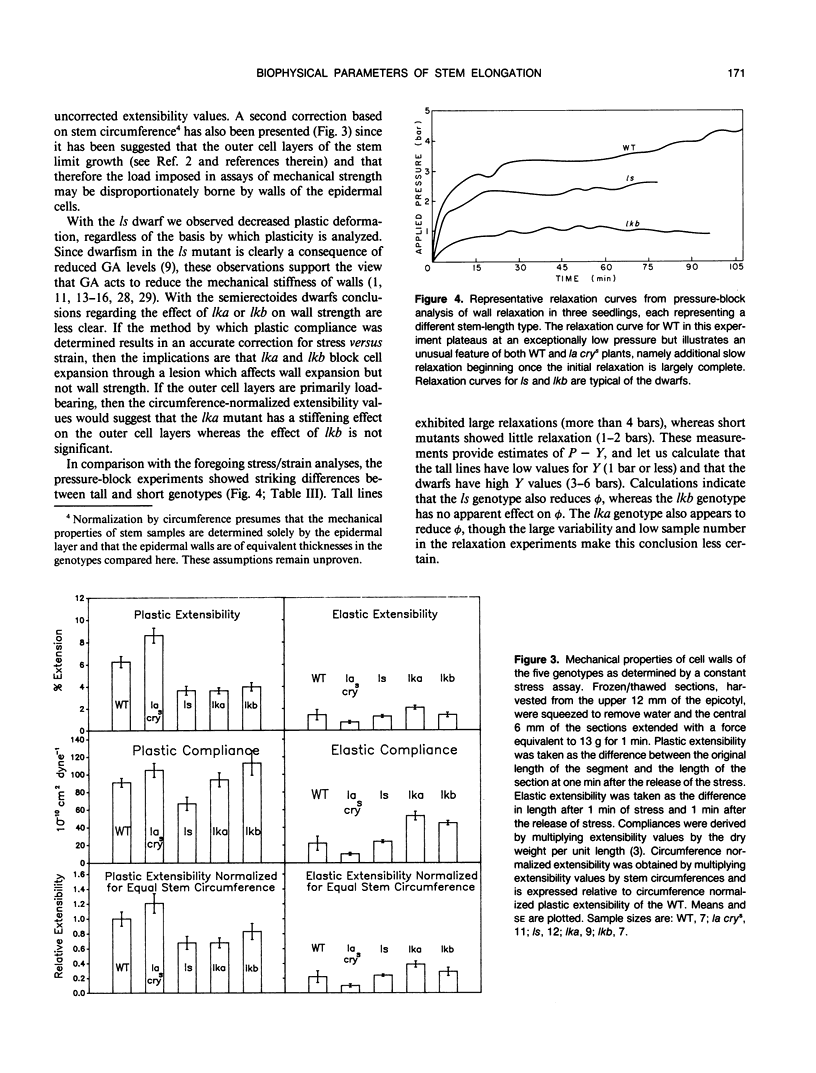
Biophysical parameters related to gibberellin (GA)-dependent stem elongation were examined in dark-grown stem-length genotypes of Pisum sativum L. The rate of internode expansion in these genotypes is altered due to recessive mutations which affect either the endogenous levels of, or response to, GA. The GA deficient dwarf L181 (ls), two GA insensitive semierectoides dwarfs NGB5865 and NGB5862 (Ika and Ikb, respectively) and the `slender' line L197 (la cry[ill]), which is tall regardless of GA content, were compared to the wild-type tall cultivar, Torsdag. Osmotic pressure, estimated by vapor pressure osmometry, and turgor pressure, measured directly with a pressure probe, did not correlate with the differences in growth rate among the genotypes. Mechanical wall properties of frozen-thawed tissue were measured using a constant force assay. GA deficiency resulted in increased wall stiffness judged both on the basis of plastic compliance and plastic extensibility normalized for equal stem circumference. Plastic compliance was not reduced in the GA insensitive dwarfs, though Ika reduced circumference-normalized plasticity. In contrast, in vivo wall relaxation, determined by the pressure-block technique, differed among genotypes in a manner which did correlate with extension rates. The wall yield threshold was 1 bar or less in the tall lines, but ranged from 3 to 6 bars in the dwarf genotypes. The results with the ls mutant indicate that GA enhances stem elongation by both decreasing the wall yield threshold and increasing the wall yield coefficient. In the GA-insensitive mutants, Ika and Ikb, the wall yield threshold is substantially elevated. Plants possessing Ika may also possess a reduced wall yield coefficient.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. A., Montague M. J., Tepfer M., Rayle D. L., Ikuma H., Kaufman P. B. Effect of gibberellic Acid on the plasticity and elasticity of Avena stem segments. Plant Physiol. 1975 Dec;56(6):757–760. doi: 10.1104/pp.56.6.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagshaw S. L., Cleland R. E. Wall extensibility and gravitropic curvature of sunflower hypocotyls: correlation between timing of curvature and changes in extensibility. Plant Cell Environ. 1990;13:85–89. doi: 10.1111/j.1365-3040.1990.tb01303.x. [DOI] [PubMed] [Google Scholar]
- Cleland R., Thompson M. L., Rayle D. L., Purves W. K. Difference in effects of gibberellins and auxins on wall extensibility of cucumber hypocotyls. Nature. 1968 Aug 3;219(5153):510–511. doi: 10.1038/219510a0. [DOI] [PubMed] [Google Scholar]
- Cosgrove D. J. Cell wall yield properties of growing tissue : evaluation by in vivo stress relaxation. Plant Physiol. 1985 Jun;78(2):347–356. doi: 10.1104/pp.78.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D. J., Sovonick-Dunford S. A. Mechanism of gibberellin-dependent stem elongation in peas. Plant Physiol. 1989;89:184–191. doi: 10.1104/pp.89.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D. J. Wall relaxation in growing stems: comparison of four species and assessment of measurement techniques. Planta. 1987;171:266–278. [PubMed] [Google Scholar]
- Ingram T. J., Reid J. B. Internode Length in Pisum: Gene na May Block Gibberellin Synthesis between ent-7alpha-Hydroxykaurenoic Acid and Gibberellin A(12)-Aldehyde. Plant Physiol. 1987 Apr;83(4):1048–1053. doi: 10.1104/pp.83.4.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes G., Sorrells M. E., Setter T. L. Gibberellic Acid Regulates Cell Wall Extensibility in Wheat (Triticum aestivum L.). Plant Physiol. 1990 Jan;92(1):242–245. doi: 10.1104/pp.92.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart J. A. Intracellular Mechanism of Growth Inhibition by Radiant Energy. Plant Physiol. 1960 Jan;35(1):129–135. doi: 10.1104/pp.35.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid J. B. Internode length in pisum: do the internode length genes effect growth in dark-grown plants? Plant Physiol. 1983 Jul;72(3):759–763. doi: 10.1104/pp.72.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart D. A., Jones R. L. Roles of Extensibility and Turgor in Gibberellin- and Dark-stimulated Growth. Plant Physiol. 1977 Jan;59(1):61–68. doi: 10.1104/pp.59.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A., Cosgrove D. J. Gibberellic Acid stimulation of cucumber hypocotyl elongation : effects on growth, turgor, osmotic pressure, and cell wall properties. Plant Physiol. 1989 Aug;90(4):1335–1340. doi: 10.1104/pp.90.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]