Abstract
Endovascular strategies play a vital role in the treatment of peripheral arterial disease (PAD). However, luminal loss or restenosis after endovascular intervention remains a significant challenge. The main underlying mechanisms are negative vascular remodeling and elastic recoil in balloon angioplasty. During stenting, the main reason for this complex is neointimal proliferation. Endothelial cell injury due to endovascular intervention initiates a series of molecular events, such as overexpression of growth factors, cytokine secretion, and adhesion molecules. These induce platelet activation and inflammatory processes, which trigger the proliferation and migration of vascular smooth muscle cells into the intima, resulting in neointimal hyperplasia. During this process, PAD progression is mainly caused by chronic inflammation, in which macrophages play a central role. Of the current strategies, drug release interventions aim to suppress restenosis using antiproliferative drugs, such as sirolimus and paclitaxel, during drug release. These drugs inhibit vascular reendothelialization and reduce late in-stent restenosis. For this reason, immunotherapy can be considered an important alternative. Interventions that polarize macrophages to the M2 subtype are particularly important, as they shape the immune response in an anti-inflammatory direction and contribute to tissue repair. However, there are several challenges to overcome, such as localizing antiproliferative or polarizing agents only to areas of vascular injury. This review discusses, based on the early study observations, immunotherapeutic approaches to prevent restenosis after endovascular intervention for the treatment of PAD.
Keywords: Peripheral Arterial Disease, Stent, Stenosis, Immunotherapy, Macrophage, Phenotype
INTRODUCTION
Peripheral arterial disease (PAD) is a significant burden on healthcare systems, affecting more than 200 million people worldwide [1,2]. Clinically, it ranges from asymptomatic to severe life-threatening presentations [3]. If PAD patients are left untreated, cardiac events, such as myocardial infarction and events related to the central nervous system, such as stroke, are likely to develop [4].
Endovascular strategies have been the first-line therapy for symptomatic PAD, although there is no consensus on which method should be prioritized [4]. Unfortunately, luminal loss and restenosis after endovascular interventions has been a significant challenge [5]. The underlying mechanisms are complex; however, this phenomenon is mainly due to negative vascular remodeling and elastic recoil in the case of balloon angioplasty (BA) or neointimal proliferation in the case of stenting [6,7].
The occurrence of restenosis in the pre-stent period ranged 40–60% in percutaneous transluminal angioplasty [6]. It decreased to 17–41% during the period of bare metal stent, predominantly mitigating the effects of elastic recoil and negative remodeling. The formation of neointimal proliferation leading to in-stent restenosis (ISR) is a result of the response to injuries occurring after endovascular intervention. Although there is ISR due to neointimal proliferation after BA, this mechanism occurs more exaggeratedly in stent cases [6,8,9]. Drug-eluting stents (DES) have further reduced the rate of restenosis to < 10%, especially with the introduction of the second generation, a biodegradable and biocompatible polymer that provides controlled drug release, and a drug-coated balloon [10,11]. Although early elastic recoil and vascular remodeling were prevented to some extent by the stents, restenosis associated with neointimal hyperplasia could not be completely prevented in the long term [9]. The underlying mechanisms may induce factors associated with functional impairment of PAD, and a better understanding of these pathways may help guide new medical therapies for treatment [12]. Immunotherapeutic approaches are also being investigated for this reason [13,14].
This review aims to evaluate the role of immunotherapy in the prevention of restenosis after endovascular intervention in the treatment of PAD.
VASCULAR RESPONSE TO ENDOVASCULAR INTERVENTION
Despite promising technological advances in stent technology, managing the balance between revascularization of the target lesion and restenosis after endovascular intervention remains a highly complex issue owing to the underlying molecular mechanisms.
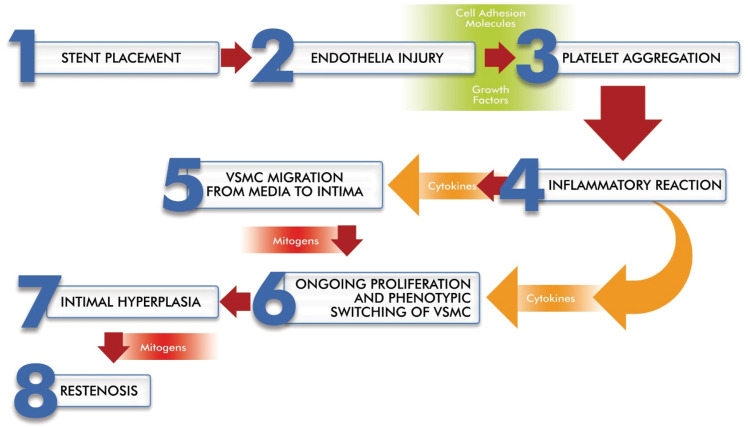
Both BA and stent placement disrupt the endothelial cell (EC) layer. EC damage affects not only the function of the vascular barrier, but also its secretory function [15]. This EC injury initiates a series of molecular events that induce platelet activation and aggregation, followed by infiltration of leukocytes and monocytes into the lesion site [16]. Platelets and inflammatory cells secrete growth factors, such as fibroblast growth factor 2 (FGF-2), epidermal growth factor (EGF), platelet-derived growth factor (PDGF), and insulin-like growth factor (IGF). These growth factors are responsible for initiating vascular smooth muscle cells (VSMCs) proliferation through tyrosine kinase receptors [16,17]. VSMCs switch from a quiescent contractile phenotype to a synthetic phenotype and migrate to the intima. The shifted VSMC migration into the intima and the accumulation of extracellular matrix are hallmarks of intimal hyperplasia [16]. Circulating mitogens, such as angiotensin II and plasmin, may be involved in VSMC proliferation and migration owing to overexposure based on endothelial denudation [18] (Fig. 1).
Figure 1. Basic steps of restenosis after stenting.
Importantly, matrix metalloproteinases (MMPs) are known to play a key role in the degradation of proteins such as collagen and elastin. In particular, MMP-2 and -9 appear to be clearly involved in the migration of SMCs to the intima and promote the development of intimal hyperplasia in in vivo models. Inhibition of MMP activity is envisioned as an approach in the treatment of cardiovascular diseases [19].
Although specific mechanisms are unclear, various factors, such as oxidized low-density lipoprotein (LDL), hyperglycemia, and reactive oxygen species, cause vascular endothelial damage. This damage, which can be attributed to abnormal signaling of cytokines and other molecules, results in alterations in gene expression and cellular behavior [20,21]. Endothelial progenitor cells (EPCs) play an important role in endothelial repair and angiogenesis by differentiating into mature ECs [16]. Due to this feature of EPC, 3 basic mechanisms are of great importance in the treatment of endothelial damage: transport of EPCs to endothelial injury sites, delivery of certain genes to EPCs, and use of certain drugs that can delay the aging of EPCs. Some drugs, such as LDL-cholesterol-lowering and anti-diabetic drugs, are already used to reduce adverse events in risky patients [22,23,24].
Remodulation of the imbalance between stimulatory growth factors/cytokines (such as PDGF, FGF, transforming growth factor [TGF]-β, and IGF-1) and inhibitory factors (endothelial-derived nitric oxide) that occurs as a result of injury can be envisioned as important therapeutic targets. In this context, vascular endothelial growth factor (VEGF, particularly VEGF-A), which acts nitric oxide-dependently, is one of the most extensively studied targets in preclinical studies [16,25].
MONOCYTE/MACROPHAGE RESPONSE FOLLOWING ENDOVASCULAR INTERVENTION
Stent implantation also induces immune cell migration by activating the expression of cell adhesion molecules, such as ICAM-1, PECAM-1, and VCAM-1, around the stent strut. Subsequently, monocytes that adhere to these cell adhesion molecules migrate to the subendothelial space and transform into macrophages, M1 or M2 depending on microenvironmental signals [26]. These significant changes in immune populations may explain the clinical phenotypes of restenosis and their variation in severity [5].
These cells produce pro-inflammatory cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-6, and interferon-γ. These cytokines contribute to the progression of the inflammatory process and, therefore, to stenosis [27] (Fig. 1). On the contrary, anti-inflammatory cytokines, such as IL-10 and TGF-β, also play a role in this process. The balance between pro-inflammatory and anti-inflammatory responses significantly determines the extent of disease progression [28].
Among these immune cells, macrophages play a predominant role in maintaining chronic inflammation [29]. However, macrophages do not exist as pure populations at the sites of vascular inflammation. Diversity and plasticity are the 2 distinguishing features of macrophages. Classically activated M1 macrophages are pro-inflammatory, associated with VSMC switching, and increase endothelial damage by secreting lytic enzymes. Alternatively, activated M2 macrophages are associated with anti-inflammatory reactions and tissue remodeling [14]. M2 macrophages can be further divided into subphenotypes: M2a (wound healing/anti-inflammatory), M2b (immune-mediated/pro-inflammatory), M2c (regulatory/anti-inflammatory), and M2d (tumor-associated/proangiogenic). However, in in vivo studies it is generally discussed as the broad M2 phenotype [30].
Following endovascular injury, monocytes initially adhere to adhesion molecules and differentiate into M1 macrophages, which sustain further endothelial damage and facilitate smooth muscle cell proliferation during restenosis [25]. M2 macrophages triggered by IL-4 and IL-13 contribute to tissue repair by secreting several molecules such as fibronectin and IGF-1. The M2 phenotype also secretes anti-inflammatory cytokines, primarily IL-10 and TGF-β [31]. However, the precise role of each subset is not yet known in the context of PAD [12]. The presence of CD68-positive and CD86-positive M1 macrophages on immunohistochemical examination suggests the presence of phagocytic inflammatory macrophages and inflammation in the neointima. IL-33 secreted by damaged ECs promotes M1 differentiation. IL-37, in contrast, promotes CD206-positive M2 and suppresses the M1 macrophage phenotypic switch [28].
ANTIPROLIFERATIVE EFFECTS OF CURRENT DEB/DES USE
Paclitaxel or sirolimus that is the most commonly used have an antiproliferative effect only during the elution period of the drug [32,33]. Furthermore, these agents are both nonspecific and cytotoxic, and significantly increase the risk of long-term morbidity and mortality due to their off-target effects, which are activity that differs from the targeted biological effect [32,34]. Due to the broader therapeutic index and lower risk of dose-related toxicity, less mortality outcomes are expected for sirolimus [35]. On the other hand, a meta-analysis study comparing paclitaxel-coated devices with control arms showed that the mortality rate gradually increased in patients treated with paclitaxel-coated devices at follow-up up to the 5th year, despite a similar mortality rate at first year [36]. To address these challenges, a new generation of biodegradable stents and cell-selective drugs are currently in development [8,37,38].
IMMUNOTHERAPY IN RESTENOSIS
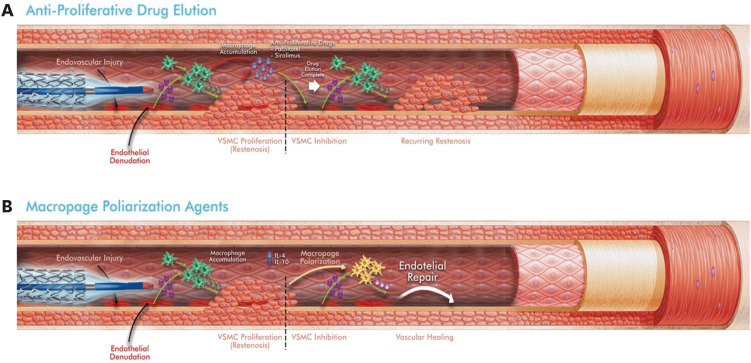
Antiproliferative drugs used to prevent vascular EC proliferation inhibit reendothelialization between the metal surface of the stent and the blood. This reduces long-term vascular healing and increases late ISR and stent thrombosis due to blood contact with the metal surface of the stent [39,40] (Fig. 2A).
Figure 2. Differences between drug eluting endovascular interventions (A) and macrophage polarization (B) in preventing restenosis. Green cells, M1 (pro-inflammatory) macrophages; Yellow cells, M2 (anti-inflammatory) macrophages. Adapted from [32], with permission from Tan et al.
VSMC, vascular smooth muscle cell.
Immunotherapy can be considered an important alternative in the prevention of ISR because the chronic vascular inflammation is known to play a central role in the progression of restenosis. However, immunotherapies are not currently recommended for the clinical management of PAD. On the other hand, several small-sized studies in human have demonstrated the therapeutic benefits of therapies that reduce inflammation, such as anti-IL-1β and anti-TNF-α agents [41,42,43]. Although they have promising anti-inflammatory effects, they may cause immune-related off-target effects in perivascular or other distant areas due to the pleiotropic nature of cytokines (affecting multiple systems or more than one phenotype), especially during systemic administration [44].
The most appropriate approach should be to identify the specific pathology in the pathways involved in restenosis and apply individual treatments. For example, rare defects in genes related to nitric oxide signaling have been observed in members of a family with early myocardial infarction. However, causal pathway pathologies remain unclear [45]. Macrophages play a dominant and central role in maintaining chronic inflammation [14]. Moreover, M1 macrophages sustain further endothelial damage, shape the immune response in the inflammatory direction, and facilitate proliferation and phenotypic changes of VSMC by secreting pro-inflammatory cytokines during restenosis development [29]. In addition, M2 macrophages shape the immune response in an anti-inflammatory manner and contribute to tissue repair [31]. In this context, macrophages can be considered an important and effective therapeutic target for preventing and resolving vascular inflammation. This approach for targeting macrophages can be achieved primarily in 2 ways:
1) By reducing the increase in the number of M1 macrophages following endovascular intervention by administration of certain agents, such as inhibitors of typical inflammatory cytokines (IL-1β, TNF-α, and IL-6), chemokines (CCL2 and CCL3), or growth factors [13], or
2) By rapidly polarizing them towards M2 phenotype by administering some polarizing molecules such as IL-4, IL-10, TGF-β1, and PGE2 [46].
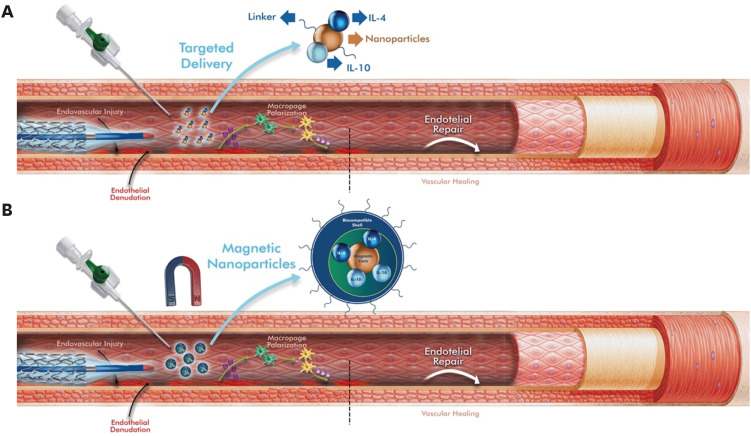
Several animal studies have shown that therapeutic M2 polarization (Table 1) [47,48,49,50,51], which is the second approach, is associated with plaque regression and has a permanent effect on the disease [52]. However, in these applications, even if the local inflammation is reduced, undesirable and uncontrollable systemic events may develop. Adopting this approach intravenously also requires further consideration of the known blood flow effects [32]. Some bioengineered materials, such as polylactic-co-glycolic acid polymer-coated scaffolds, can make a significant contribution by providing controlled and local release of some molecules that provide M2 macrophage polarization (Fig. 2B) [48]. However, it is difficult to avoid the systemic effects of these agents owing to degradable polymers or passive absorption. To localize these agents only to the areas of vascular injury, nanoparticles (NPs) decorated with target ligands may be a more reasonable solution [32] (Fig. 3A). Indeed, NPs have recently found a wide range of studies for treatment and diagnostic purposes. However, it should not be ignored that some of them tend to show toxicity at the cellular level in tissues and organs. Some NPs can even produce highly reactive forms of oxygen that can cause tissue damage, including inflammation and other toxic effects [53,54]. Modification strategies to increase the safety of NPs should be evaluated in detail, taking into account their physical and pharmacokinetic properties.
Table 1. Selected examples investigating macrophage polarization in preclinical peripheral arterial disease.
| Study | Polarizing application | Essential mechanism | Control | Experimental model | Outcomes | Ref. | |
|---|---|---|---|---|---|---|---|
| Hachim et al. (2017) | IL-4 loaded mesh | M2 polarization | Unloaded mesh | C57BL6 mice | - Decreased M1/M2 ratio | [47] | |
| - Diminished formation of fibrotic capsule surrounding implant | |||||||
| Pellegrin et al. (2014) | Ischemic condition | M1 polarization | Non-ischemic conditions | C57BL6 mice | Early stage: | [48] | |
| - Increased M1/M2 ratio | |||||||
| - Increased IFN-γ/IL-4 ratio | |||||||
| Later stages: | |||||||
| - Neutral state of the polarization | |||||||
| Ganta and Annex (2021) | Anti-VEGF165b monoclonal antibody | VEGFR1 inhibition | Placebo | C57BL6 | - Increased S100A8/S100A9 | [49] | |
| - Increased M1/M2 ratio | |||||||
| Fu et al. (2018) | Hydrogen-saturated water | M2 polarization | Dehydrogenized water | Balb/c mice | - Decreased M1/M2 ratio | [50] | |
| - Decreased ROS | |||||||
| Wolfs et al. (2014) | Helminth-derived soluble egg antigens | M2 polarization | PBS | C57BL6 mice | - Decreased M1/M2 ratio | [51] | |
| - Increased IL-10 production | |||||||
| - Decreased intraplaque TNF-a, MCP-1, ICAM-1, VCAM-1, and CD68 | |||||||
VEGFR1, downstream regulators of macrophage polarization; S100A8/S100A9, downstream mediator of M1 macrophages.
IL, interleukin; INF, interferon; PBS, phosphate buffered saline; TNF, tumor necrosis factor; MCP-1, monocyte chemotactic protein-1; ICAM-1, intercellular adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1.
Figure 3. Schematic representation of 2 proposed ways to localize cell polarizing agents at sites of vascular injury. (A) Nanoparticle loaded with targeting ligands and cell-polarizing agents. (B) Shell-coated magnetic nanoparticles loaded with targeting ligands and cell polarizing agents, the localization effect of which is enhanced by the application of an external magnetic field. Adapted from [32], with permission Tan et al.
IL, interleukin.
Some studies on magnetic nanoparticles (MNPs) coated with target ligands and therapeutic agents have reported that MNPs can be localized around targeted tissues such as malignant tissue by applying an external magnetic field (EMF) [55]. This type of strategy may also be envisaged for preventing luminal loss after endovascular intervention if the implant is made of nonmagnetic alloys. After systemic administration, MNPs loaded with target ligands and M2-polarizing agents can be localized at sites of vascular injury by applying an EMF (Fig. 3B). We can expect an optimal result from this application when performed from the outer surface of the body closest to the site of vascular injury. Because localized immunotherapy enhances the macrophage-polarizing effect and reduces non-specific immune responses, similar to the results of cancer treatments [56].
Various pharmacological treatments that can be applied instead of or in addition to invasive treatment to prevent restenosis have been investigated. These drugs, which can be grouped into several main groups, are used to prevent neointimal growth due to the proliferation of smooth muscle cells according to the pathophysiological mechanism that may cause restenosis (Table 2).
Table 2. Selected pharmacological drugs against in stent restenosis (modified from [57], with permission of Patel et al.).
| Group | Immunosuppressive | Antiproliferative | Migration inhibitor | Accelerator of healing | Antithrombin | |
|---|---|---|---|---|---|---|
| Selected agents | Limus group: | Taxol (paclitaxel) | Batimastat | VEGF | Heparin | |
| Sirolimus | Actinomycin | Prolylhydrosylase inhibitors | 17b-estradiol | Hirudin and iloprost | ||
| Tacrolimus | Methotrexate | C-proteinase inhibitors | EPC antibodies | Abciximab | ||
| Everolimus | Mitomycin | Metalloproteinase inhibitors | TKI | |||
| Zotarolimus | C-myc antisense | |||||
| Others: | Taxol derivative (QP-2) | |||||
| Methylprednisolone | ||||||
| Dexamethasone | ||||||
| Cyclosporine | ||||||
| Mycophenolic acid | ||||||
| Interferon-1b | ||||||
| Tranilast | ||||||
| Leflunomide | ||||||
| Main characteristics | Stopping cell cycle | Weakening neointimal growth | Preventing endothelial cell migration into the stent | Promoting healing of the vascular system | Preventing stent thrombosis and platelet aggregation | |
VEGF, vascular endothelial growth factor; EPC, endothelial progenitor cell; TKI, tyrosine kinase inhibitor.
CONCLUSION REMARK
Endovascular interventions play a life-saving role in PAD treatment. Significant advances have been made in endovascular intervention over the past 2 decades. However, restenosis after endovascular intervention remains a significant challenge. Despite the new and different platforms that release drugs, the long-term risks of morbidity and mortality remain unresolved. Current early studies show that immunotherapies aimed at modulating macrophages to the M2 subset hold strong promise. Developments that enable immunomodulatory agents to be localized only in the stented area will play a vital role in preventing side effects following PAD treatment.
LIMITATION OF THIS STUDY
This review had some limitations. Currently, there are no immunotherapeutic medications in clinical use for PAD treatment. Therefore, the review was designed in a traditional format, and only the findings of promising early study reports were highlighted. Several issues need to be addressed, such as effectiveness, safety, and standardization.
ACKNOWLEDGMENTS
The author thanks to Bilal Coban from INVAMED RD Global (Ankara, Turkey) for drawing the figures.
Footnotes
Conflict of Interest: - Authors: Nothing to declare
- Reviewers: Nothing to declare
- Editors: Nothing to declare
Ethics Approval Statement: This manuscript, which is a review article, does not include any studies with human or animal participants.
Reviewer: This article was reviewed by peer experts who are not TCP editors.
References
- 1.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 2.Kohn CG, Alberts MJ, Peacock WF, Bunz TJ, Coleman CI. Cost and inpatient burden of peripheral artery disease: findings from the National Inpatient Sample. Atherosclerosis. 2019;286:142–146. doi: 10.1016/j.atherosclerosis.2019.05.026. [DOI] [PubMed] [Google Scholar]
- 3.Criqui MH, Matsushita K, Aboyans V, Hess CN, Hicks CW, Kwan TW, et al. Lower extremity peripheral artery disease: contemporary epidemiology, management gaps, and future directions: a scientific statement from the American Heart Association. Circulation. 2021;144:e171–e191. doi: 10.1161/CIR.0000000000001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Writing Committee Members. Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary. Vasc Med. 2017;22:NP1–NPNP43. doi: 10.1177/1358863X17701592. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Carrio J, Bangueses R, Rodriguez I, Pevida M, Llames S, Meana A, et al. Distinct profiles of immune cell populations underlie in-stent restenosis: a cluster analysis approach. Eur Heart J. 2021;42(Suppl 1):ehab724.3398 [Google Scholar]
- 6.Schillinger M, Sabeti S, Loewe C, Dick P, Amighi J, Mlekusch W, et al. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med. 2006;354:1879–1888. doi: 10.1056/NEJMoa051303. [DOI] [PubMed] [Google Scholar]
- 7.Shammas NW, Shammas GA, Halupnik G, Fedele N, Comp K, Taleb EM, et al. Auryon laser in peripheral arterial interventions: a single-center experience (Auryon-SCE) J Invasive Cardiol. 2022;34:E428–E432. doi: 10.25270/jic/21.00375. [DOI] [PubMed] [Google Scholar]
- 8.Paramasivam G, Devasia T, Ubaid S, Shetty A, Nayak K, Pai U, et al. In-stent restenosis of drug-eluting stents: clinical presentation and outcomes in a real-world scenario. Egypt Heart J. 2019;71:28. doi: 10.1186/s43044-019-0025-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinc R, Yerebakan H. Atlas drug-eluting coronary stents inhibits neointimal hyperplasia in sheep modeling. bioRxiv. doi: 10.1101/2023.04.19.537461. April 20, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baerlocher MO, Kennedy SA, Rajebi MR, Baerlocher FJ, Misra S, Liu D, et al. Meta-analysis of drug-eluting balloon angioplasty and drug-eluting stent placement for infrainguinal peripheral arterial disease. J Vasc Interv Radiol. 2015;26:459–473.e4. doi: 10.1016/j.jvir.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Liistro F, Reccia MR, Angioli P, Ducci K, Ventoruzzo G, Falsini G, et al. Drug-eluting balloon for below the knee angioplasty: five-year outcome of the DEBATE-BTK randomized clinical trial. Cardiovasc Intervent Radiol. 2022;45:761–769. doi: 10.1007/s00270-022-03104-3. [DOI] [PubMed] [Google Scholar]
- 12.Hiatt WR, Armstrong EJ, Larson CJ, Brass EP. Pathogenesis of the limb manifestations and exercise limitations in peripheral artery disease. Circ Res. 2015;116:1527–1539. doi: 10.1161/CIRCRESAHA.116.303566. [DOI] [PubMed] [Google Scholar]
- 13.Kiguchi N, Kobayashi D, Saika F, Matsuzaki S, Kishioka S. Pharmacological regulation of neuropathic pain driven by inflammatory macrophages. Int J Mol Sci. 2017;18:2296. doi: 10.3390/ijms18112296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu YC, Zou XB, Chai YF, Yao YM. Macrophage polarization in inflammatory diseases. Int J Biol Sci. 2014;10:520–529. doi: 10.7150/ijbs.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang H, Huang W. Regulation of endothelial progenitor cell functions in ischemic heart disease: new therapeutic targets for cardiac remodeling and repair. Front Cardiovasc Med. 2022;9:896782. doi: 10.3389/fcvm.2022.896782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaabane C, Otsuka F, Virmani R, Bochaton-Piallat ML. Biological responses in stented arteries. Cardiovasc Res. 2013;99:353–363. doi: 10.1093/cvr/cvt115. [DOI] [PubMed] [Google Scholar]
- 17.Cimmino G, Di Serafino L, Cirillo P. Pathophysiology and mechanisms of acute coronary syndromes: atherothrombosis, immune-inflammation, and beyond. Expert Rev Cardiovasc Ther. 2022;20:351–362. doi: 10.1080/14779072.2022.2074836. [DOI] [PubMed] [Google Scholar]
- 18.Bui TB, Burgering BM, Goga A, Rugo HS, van ’t Veer LJ. Biomarkers for cyclin-dependent kinase 4/6 inhibitors in the treatment of hormone receptor-positive/human epidermal growth factor receptor 2-negative advanced/metastatic breast cancer: translation to clinical practice. JCO Precis Oncol. 2022;6:e2100473. doi: 10.1200/PO.21.00473. [DOI] [PubMed] [Google Scholar]
- 19.Rocha LA, Piccinato CE, Ribiero MS, Becari C, Joviliano RD, Joviliano EE. The role of the kallikrein-kinin system, matrix metalloproteinases, and tissue inhibitors of metalloproteinases in the early restenosis of covered stents in the femoropopliteal arterial segment. J Vasc Surg. 2017;65:119–127. doi: 10.1016/j.jvs.2016.06.106. [DOI] [PubMed] [Google Scholar]
- 20.Abu-Saleh N, Yaseen H, Kinaneh S, Khamaisi M, Abassi Z. Combination of hyperglycaemia and hyperlipidaemia induces endothelial dysfunction: role of the endothelin and nitric oxide systems. J Cell Mol Med. 2021;25:1884–1895. doi: 10.1111/jcmm.15787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng J, Hu W, Zheng F, Wu Y, Li M. hsa_circ_0058092 protects against hyperglycemia-induced endothelial progenitor cell damage via miR-217/FOXO3. Int J Mol Med. 2020;46:1146–1154. doi: 10.3892/ijmm.2020.4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiao J, Qi K, Chu P, Mi H, Yang N, Yao H, et al. Infusion of endothelial progenitor cells ameliorates liver injury in mice after haematopoietic stem cell transplantation. Liver Int. 2015;35:2611–2620. doi: 10.1111/liv.12849. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Q, Liu Z, Wang Z, Yang C, Liu J, Lu J. Effect of prepro-calcitonin gene-related peptide-expressing endothelial progenitor cells on pulmonary hypertension. Ann Thorac Surg. 2007;84:544–552. doi: 10.1016/j.athoracsur.2007.03.067. [DOI] [PubMed] [Google Scholar]
- 24.Ke X, Shu XR, Wu F, Hu QS, Deng BQ, Wang JF, et al. Overexpression of the β2AR gene improves function and re-endothelialization capacity of EPCs after arterial injury in nude mice. Stem Cell Res Ther. 2016;7:73. doi: 10.1186/s13287-016-0335-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kastora SL, Eley J, Gannon M, Melvin R, Munro E, Makris SA. What went wrong with VEGF-A in peripheral arterial disease? A systematic review and biological insights on future therapeutics. J Vasc Res. 2022;59:381–393. doi: 10.1159/000527079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol. 2014;5:514. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willecke F, Rupprecht B, Gissler MC, Pfeiffer K, Anto-Michel N, Stachon P, et al. Tumor necrosis factor receptor-associated factor 5 promotes arterial neointima formation through smooth muscle cell proliferation. J Vasc Res. 2019;56:308–319. doi: 10.1159/000501615. [DOI] [PubMed] [Google Scholar]
- 28.Rai V, Agrawal DK. Immunomodulation of IL-33 and IL-37 with vitamin D in the neointima of coronary artery: a comparative study between balloon angioplasty and stent in hyperlipidemic microswine. Int J Mol Sci. 2021;22:8824. doi: 10.3390/ijms22168824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang F, King MW. Immunomodulation strategies for the successful regeneration of a tissue-engineered vascular graft. Adv Healthc Mater. 2022;11:e2200045. doi: 10.1002/adhm.202200045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poudel B, Ekperikpe US, Mandal S, Wilson GE, Shields CA, Cornelius DC, et al. Chronic treatment with IL-25 increases renal M2 macrophages and reduces renal injury in obese Dahl salt-sensitive rats during the prepubescent stage. Am J Physiol Renal Physiol. 2023;325:F87–F98. doi: 10.1152/ajprenal.00209.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chistiakov DA, Myasoedova VA, Revin VV, Orekhov AN, Bobryshev YV. The impact of interferon-regulatory factors to macrophage differentiation and polarization into M1 and M2. Immunobiology. 2018;223:101–111. doi: 10.1016/j.imbio.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Tan RP, Ryder I, Yang N, Lam YT, Santos M, Michael PL, et al. Macrophage polarization as a novel therapeutic target for endovascular intervention in peripheral artery disease. JACC Basic Transl Sci. 2021;6:693–704. doi: 10.1016/j.jacbts.2021.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scafa Udriste A, Niculescu AG, Grumezescu AM, Bădilă E. Cardiovascular stents: a review of past, current, and emerging devices. Materials (Basel) 2021;14:2498. doi: 10.3390/ma14102498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katsanos K, Spiliopoulos S, Karunanithy N, Krokidis M, Sabharwal T, Taylor P. Bayesian network meta-analysis of nitinol stents, covered stents, drug-eluting stents, and drug-coated balloons in the femoropopliteal artery. J Vasc Surg. 2014;59:1123–1133.e8. doi: 10.1016/j.jvs.2014.01.041. [DOI] [PubMed] [Google Scholar]
- 35.Mori M, Sakamoto A, Kawakam R, et al. Paclitaxel-and sirolimus-coated balloons in peripheral artery disease treatment: current perspectives and concerns. Vasc Endovascular Rev. 2021;4:e03 [Google Scholar]
- 36.Katsanos K, Spiliopoulos S, Kitrou P, Krokidis M, Karnabatidis D. Response to letter by Bonassi on article, “Risk of Death Following Application of Paclitaxel-Coated Balloons and Stents in the Femoropopliteal Artery of the Leg: A Systematic Review and Meta-Analysis of Randomized Controlled Trials”. J Am Heart Assoc. 2019;8:e012172. doi: 10.1161/JAHA.119.012172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vahabli E, Mann J, Heidari BS, Lawrence-Brown M, Norman P, Jansen S, et al. The technological advancement to engineer next-generation stent-grafts: design, material, and fabrication techniques. Adv Healthc Mater. 2022;11:e2200271. doi: 10.1002/adhm.202200271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Canfield J, Totary-Jain H. 40 Years of percutaneous coronary intervention: history and future directions. J Pers Med. 2018;8:33. doi: 10.3390/jpm8040033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palmerini T, Biondi-Zoccai G, Stone GW. Stent selection to minimize the risk of stent thrombosis. Curr Opin Cardiol. 2014;29:578–585. doi: 10.1097/HCO.0000000000000102. [DOI] [PubMed] [Google Scholar]
- 40.Nakano M, Otsuka F, Yahagi K, Sakakura K, Kutys R, Ladich ER, et al. Human autopsy study of drug-eluting stents restenosis: histomorphological predictors and neointimal characteristics. Eur Heart J. 2013;34:3304–3313. doi: 10.1093/eurheartj/eht241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bissonnette R, Tardif JC, Harel F, Pressacco J, Bolduc C, Guertin MC. Effects of the tumor necrosis factor-α antagonist adalimumab on arterial inflammation assessed by positron emission tomography in patients with psoriasis: results of a randomized controlled trial. Circ Cardiovasc Imaging. 2013;6:83–90. doi: 10.1161/CIRCIMAGING.112.975730. [DOI] [PubMed] [Google Scholar]
- 42.Klein AL, Imazio M, Brucato A, Cremer P, LeWinter M, Abbate A, et al. RHAPSODY: rationale for and design of a pivotal phase 3 trial to assess efficacy and safety of rilonacept, an interleukin-1α and interleukin-1β trap, in patients with recurrent pericarditis. Am Heart J. 2020;228:81–90. doi: 10.1016/j.ahj.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 43.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 44.Rider P, Carmi Y, Cohen I. Biologics for targeting inflammatory cytokines, clinical uses, and limitations. Int J Cell Biol. 2016;2016:9259646. doi: 10.1155/2016/9259646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erdmann J, Stark K, Esslinger UB, Rumpf PM, Koesling D, de Wit C, et al. Dysfunctional nitric oxide signalling increases risk of myocardial infarction. Nature. 2013;504:432–436. doi: 10.1038/nature12722. [DOI] [PubMed] [Google Scholar]
- 46.Liu J, Geng X, Hou J, Wu G. New insights into M1/M2 macrophages: key modulators in cancer progression. Cancer Cell Int. 2021;21:389. doi: 10.1186/s12935-021-02089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hachim D, LoPresti ST, Yates CC, Brown BN. Shifts in macrophage phenotype at the biomaterial interface via IL-4 eluting coatings are associated with improved implant integration. Biomaterials. 2017;112:95–107. doi: 10.1016/j.biomaterials.2016.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pellegrin M, Bouzourène K, Poitry-Yamate C, Mlynarik V, Feihl F, Aubert JF, et al. Experimental peripheral arterial disease: new insights into muscle glucose uptake, macrophage, and T-cell polarization during early and late stages. Physiol Rep. 2014;2:e00234. doi: 10.1002/phy2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ganta VC, Annex BH. Peripheral vascular disease: preclinical models and emerging therapeutic targeting of the vascular endothelial growth factor ligand-receptor system. Expert Opin Ther Targets. 2021;25:381–391. doi: 10.1080/14728222.2021.1940139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fu J, Zou J, Chen C, Li H, Wang L, Zhou Y. Hydrogen molecules (H2) improve perfusion recovery via antioxidant effects in experimental peripheral arterial disease. Mol Med Rep. 2018;18:5009–5015. doi: 10.3892/mmr.2018.9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wolfs IM, Stöger JL, Goossens P, Pöttgens C, Gijbels MJ, Wijnands E, et al. Reprogramming macrophages to an anti-inflammatory phenotype by helminth antigens reduces murine atherosclerosis. FASEB J. 2014;28:288–299. doi: 10.1096/fj.13-235911. [DOI] [PubMed] [Google Scholar]
- 52.Barrett TJ. Macrophages in atherosclerosis regression. Arterioscler Thromb Vasc Biol. 2020;40:20–33. doi: 10.1161/ATVBAHA.119.312802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolfram J, Zhu M, Yang Y, Shen J, Gentile E, Paolino D, et al. Safety of nanoparticles in medicine. Curr Drug Targets. 2015;16:1671–1681. doi: 10.2174/1389450115666140804124808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vishwakarma V, Samal SS, Manoharan N. Safety and risk associated with nanoparticles-a review. J Miner Mater Charact Eng. 2010;9:455–459. [Google Scholar]
- 55.Furlani EP. Magnetic biotransport: analysis and applications. Materials (Basel) 2010;3:2412–2446. [Google Scholar]
- 56.Dinc R. The role of immune mechanisms in abdominal aortic aneurysm: could it be a promising therapeutic strategy? Acta Cardiol Sin. 2023;39:675–686. doi: 10.6515/ACS.202309_39(5).20230531A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patel MJ, Patel SS, Patel NS, Patel NM. Current status and future prospects of drug eluting stents for restenosis. Acta Pharm. 2012;62:473–496. doi: 10.2478/v10007-012-0036-8. [DOI] [PubMed] [Google Scholar]