Abstract
Background
Pediatric COVID-19 patients have lower rates of hospitalization and fatal outcomes compared to adults with COVID-19; however, children represent a challenge in the detection, diagnosis, and treatment of COVID-19. Our aim was to determine the risk factors for hospital admission, invasive mechanical ventilation, and mortality in pediatric COVID-19 patients in Mexico during the COVID-19 pandemic.
Material and methods
A retrospective cohort of pediatric patients with COVID-19 from February 2020 to April 2021 was reported on the National Epidemiological Surveillance System for Viral Respiratory Disease (SISVER) platform.
Results
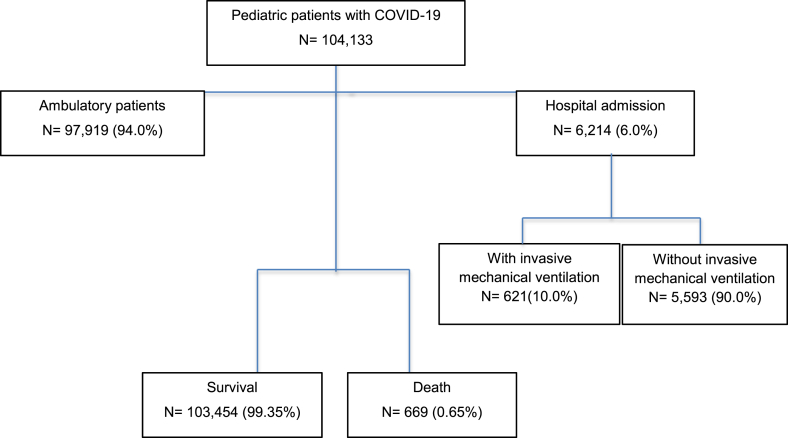
Among the 104,133 patients included in our study, 6214 were hospitalized, and 621 patients underwent invasive mechanical ventilation. A total of 0.65 % died during hospitalization. Children aged <12 months (odds ratio [OR]: 17.1; 95 % confidence interval [CI]: 15.9–19.4, p < 0.001), 1–4 years (OR: 3.69; 95 % CI: 3.2–4.1, p < 0.001), 5–9 years (OR: 1.86; 95 % CI: 1.66–2.08, p < 0.001), and 10–14 years (OR: 1.23; 95 % CI: 1.11–1.37, p < 0.001), and those diagnosed with diabetes (OR: 2.32; 95 % CI 1.68–3.20, p < 0.001) and obesity (OR: 1.24; 95 % CI 1.04–1.48, p = 0.015) were associated with hospital admission. Renal disease (OR: 3.85; 95 % CI: 2.25–6.59, p < 0.001) was associated with invasive mechanical ventilation. Pneumonia (OR: 15.9; 95 % CI: 12.6–20.1, p < 0.001) and renal disease (OR: 3.85; 95 % CI: 2.25–6.59, p value < 0.001) were associated with death.
Conclusion
Pneumonia increases the risk of death. The youngest age group has a higher risk of hospital admission. Comorbidities such as renal disease or immunosuppression increase the risk of death in all age groups.
Keywords: Risk factors, Pediatrics, Mortality, COVID-19, Hospital admission
Highlights
-
•
Age and comorbid conditions were associated with increased risk factors for fatal outcomes.
-
•
Renal disease and immunosuppression were associated with increased risk factors for mortality.
-
•
Among group age, children <1, and 1 to 4 were associated with increased risk to severe disease and died.
Data Access Statement: Research data supporting this publication are available from the SISVER platform.
Abbreviations
- Coronavirus disease 2019
(COVID-19)
- Real-time reverse transcription polymerase chain reaction
(rRT-PCR)
- National Epidemiological Surveillance System for Viral Respiratory Disease
(SISVER)
- Angiotensin-converting enzyme 2
(ACE-2)
- Body mass index
(BMI)
- Severe acute respiratory syndrome coronavirus
(SARS-CoV-2)
- Hazard ratio
(HR)
- Odds ratio
(OR)
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic was caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [1]. The disease was concentrated in middle-aged and older people, and children and adolescents showed a growing trend worldwide [1]. The latest updates make it clear that all people, including children, are susceptible to SARS-CoV-2, even though pediatric patients represent 1–5% of all cases, and the risk of developing severe or critical disease is less than that in adults [2].
SARS-CoV-2 infection can induce acute respiratory distress syndrome, pneumonia, or death, particularly in children, which represents a challenge in their detection, diagnosis, and treatment. Comorbidities such as congenital heart diseases, respiratory diseases, malnutrition, and cancer make children susceptible to COVID-19 and other severe diseases [3].
In Mexico, 274,638 cases were reported in children under the age of 18 in early January 2022. The age group with the highest confirmed cases was 15–18, with 91,304 (33 %). Approximately 96 % were treated in ambulatory care, and 4 % required inpatient treatment. The national incidence rate was 231.9, and the reported case fatality rate was 0.9 per 100,000 people [4,5]. Fever and mild coughs are the most common symptoms of disease onset in children. In mild cases, the fever was brief and rapidly resolved [3,6].
The rate of COVID-19-associated hospitalization reported in Hispanic and Latino children was 16.4 per 100,000 people, and 5.8 % required invasive mechanical ventilation [7]. Despite the low severity frequency in children, the incidence is sufficient for rapidly identifying children with COVID-19, especially those with underlying or comorbid diseases, which is essential for speeding up treatment [8]. A few publications describing the risk factors for fatal outcomes of COVID-19 involve the population of children and adolescents. Most said that the impact of COVID-19 on children was less than that on adults. Therefore, the objectives of this investigation were to identify the demographic characteristics and medical conditions associated with increased risk factors for severe COVID-19 (hospitalization/invasive mechanical ventilation) and death in children.
2. Material and methods
Epidemiological surveillance in Mexico for COVID-19 cases consists of two general strategies. Sentinel surveillance is a system of selected health units that monitor respiratory diseases (USMER in Spanish). This is the most effective way to collect high-quality and timely data on respiratory diseases in Mexico [9]. A total of 473 USMER medical units from the first, second, or third levels of care have enough resources to identify respiratory disease. These units are distributed across the country. In no USMER units, each federal entity has access codes to the National Epidemiological Surveillance System for Viral Respiratory Disease (SISVER) platform to upload their data; there are 6253 no USMER units. Both epidemiological surveillance strategies use the SISVER online platform.
2.1. Study design and setting
A retrospective cohort study was performed. The information collected from the SISVER platform was collected from February 2020 to April 2021. In total, 104,133 pediatric patients were included.
2.2. Study population
We included all cases reported in the database of patients who were ≤18 years of age, who had a positive SARS-CoV-2 test to real-time reverse transcription polymerase chain reaction (rRT-PCR), who were antigen-positive, and whose status was confirmed by clinical epidemiological association. Confirmation of COVID-19 was based on an operational definition: a person of any age who had at least one of the following signs and symptoms in the past 10 days: respiratory distress, cough, fever, or headache, accompanied by at least one of the following signs or symptoms: myalgia, arthralgia, odynophagia, shaking chills, chest pain, rhinorrhea, polypnea, anosmia, dysgeusia, and conjunctivitis plus confirmation by rRT-PCR, rapid antigenic test, or clinical epidemiological association [10]. Clinical epidemiological association refers to the person's case meeting the operational definition of a suspected case and the person being in close contact (living less than 1 m away for 15 min or more—continuously or accumulated) with a patient with laboratory-confirmed case by PCR-RT or rapid antigenic test for SARS-CoV-2 from 2 to 14 days before the onset of symptoms that are confirmed.
Demographic data, comorbidities, clinical signs, and symptoms were recorded. The included population was divided into three outcomes: hospitalization, invasive mechanical ventilation, and death (Fig. 1). Nonsevere symptoms included cough, expectoration, and other upper respiratory tract symptoms. On admission, severe types of respiratory disease were defined by pneumonia and other organ failures that required intensive care monitoring or treatment.
Fig. 1.
Summary of patients.
For covariate evaluation, the attending physician collected epidemiological, comorbidity, laboratory, and other treatment information, as well as demographic characteristics, using a standardized questionnaire. Pneumonia was evaluated as present or absent; however, it was not possible to determine whether it was ventilator-associated, bacterial, or community-acquired [11].
2.3. Statistical analysis
Categorical variables were expressed as frequencies and percentages. The Shapiro–Wilk test was used to test the normality of continuous variables; normal continuous variables were expressed as the mean and the standard deviation, while nonnormal variables were expressed as medians and 25–75th percentiles. Comparisons among study groups were analyzed using the χ2 test or Fisher's test for categorical variables and the unpaired Student's t-test or Mann–Whitney test for continuous variables.
The risk of hospital admission, need for invasive mechanical ventilation, and death were analyzed using a simple logistic regression performed with an odds ratio (OR) with 95 % confidence interval (95 % CI). A multivariable regression was used to group variables to identify comorbidities and symptom predictors of the outcomes. The multivariate logistic regression model was adjusted using univariate analysis variables at p < 0.05, and interactions between the variables were tested. Statistical significance was set at p < 0.05. All statistical tests were two-sided and considered statistically significant when the p value was <0.05. The analysis was performed using STATA 16 software (Stata Corp. LP, College Station, TX, USA). Graphs were created using GraphPad Prism software (version 8.0; GraphPad Software, La Jolla, CA, USA).
3. Results
A total of 104,133 pediatric patients were analyzed. A total of 50.1 % were female, and the groups aged 10–14 and 14–18 were the most frequent. Obesity (3.7 %) and asthma (2.9 %) were the most prevalent comorbidities. For hospitalized patients, the most prevalent comorbidities were immunosuppression (8.3 %) and obesity (5.4 %); for invasive mechanical ventilation patients, immunosuppression (10.2 %) and cardiac disease (6.4 %) were the most frequent comorbidities.
The most frequent symptoms in the general population were fever (46.2 %), cough (52.3 %), and headache (52.7 %). For hospitalized patients, fever (69.9 %), cough (48.0 %), and general condition attack (46.5 %) were the most frequent symptoms. However, for patients that required invasive mechanical ventilation the most frequent symptoms were fever (73.0 %), dyspnea (71.9), and general condition attack (60.1 %) (see Table 1).
Table 1.
Demographic and clinic characteristics according the admission status in pediatric patients with COVID-19.
| Total n = 104,133 |
Hospital admission n = 6214 |
Invasive mechanical ventilation n = 621 |
|||
|---|---|---|---|---|---|
| Yes | No | Yes | No | ||
| Sex | |||||
| Female, n (%) | 52,170 (50.1) | 2.856 (45.9) | 49,314 (50.3) | 285 (45.8) | 2569 (45.9) |
| Male, n (%) | 51,963 (49.9) | 3358 (54.0) | 48,605 (49.6) | 336 (54.1) | 3017 (54.0) |
| Age, years | 13 (7–16) | 6 (1–14) | 14 (10–17) | 2 (0–12) | 6 (1–14) |
| Age | |||||
| <1 | 3389 (3.2) | 1471 (23.6) | 1918 (1.9) | 194 (31.2) | 1277 (22.8) |
| 1–4 | 8363 (8.0) | 1325 (21.3) | 7038 (7.1) | 157 (25.2) | 1169 (20.9) |
| 5 - 9 | 14,591 (14.0) | 911 (14.6) | 13,680 (13.9) | 71 (11.4) | 840 (15.0) |
| 10–14 | 29,624 (28.4) | 1104 (17.7) | 28,520 (29.1) | 94 (15.1) | 1004 (17.9) |
| 14 - 18 | 29,624 (28.4) | 1403 (22.5) | 46,763 (47.6) | 105 (16.9) | 1296 (23.2) |
| Pregnant | 640 (1.0) | 120 (3.0) | 520 (0.9) | 2 (0.5) | 119 (3.0) |
| Comorbidities | |||||
| Diabetes | 643 (0.6) | 167 (2.6) | 476 (0.4) | 26 (4.1) | 138 (2.4) |
| Hypertension | 553 (0.5) | 139 (2.2) | 414 (0.4) | 15 (2.4) | 122 (2.1) |
| Obesity | 3910 (3.7) | 338 (5.4) | 3572 (3.6) | 30 (4.8) | 308 (5.5) |
| Asthma | 3065 (2.9) | 179 (2.8) | 2886 (2.9) | 8 (1.2) | 171 (3.0) |
| Immunosuppression | 826 (0.7) | 518 (8.3) | 308 (0.3) | 63 (10.2) | 456 (8.1) |
| HIV/AIDS | 112 (0.11) | 20 (0.3) | 92 (0.09) | 1 (0.1) | 19 (0.3) |
| Cardiac disease | 573 (0.5) | 191 (3.0) | 382 (0.3) | 40 (6.4) | 151 (2.7) |
| Kidney disease | 323 (0.3) | 129 (2.0) | 194 (0.2) | 27 (4.3) | 101 (1.8) |
| Symptoms | |||||
| Fever | 48,151 (46.2) | 4343 (69.9) | 43,808 (44.7) | 452 (73.0) | 3884 (69.6) |
| Cough | 54,429 (52.3) | 2982 (48.0) | 51,447 (52.5) | 286 (46.2) | 2688 (48.1) |
| Odynophagia | 34,358 (33.0) | 1260 (20.6) | 33,098 (33.8) | 98 (16.7) | 1158 (21.0) |
| Dyspnea | 10,462 (10.6) | 2831 (45.6) | 7631 (7.8) | 446 (71.9) | 2376 (42.6) |
| Diarrhea | 12,316 (11.8) | 1153 (18.6) | 11,163 (11.4) | 103 (16.7) | 1049 (18.8) |
| Thoracic pain | 10,272 (9.8) | 889 (14.6) | 9383 (9.6) | 92 (15.8) | 794 (14.4) |
| Headache | 54,752 (52.7) | 2384 (39.1) | 52,368 (53.5) | 192 (32.3) | 2182 (39.7) |
| Myalgia | 29,564 (28.4) | 1602 (26.3) | 27,962 (28.6) | 122 (20.9) | 1477 (26.9) |
| Arthralgia | 23,938 (23.0) | 1467 (24.1) | 22,471 (22.9) | 109 (18.7) | 1355 (24.7) |
| General condition attack | 23,808 (22.9) | 2870 (46.5) | 20,938 (21.4) | 367 (60.1) | 2498 (45.0) |
| Rhinorrhea | 32,104 (30.8) | 1305 (21.1) | 30,800 (31.5) | 108 (17.6) | 1197 (21.5) |
| Polypnea | 4320 (4.1) | 1470 (23.7) | 2850 (2.9) | 280 (45.5) | 1189 (21.3) |
| Vomit | 5881 (5.6) | 1119 (18.0) | 4762 (4.8) | 117 (19.0) | 1004 (18.0) |
| Conjunctivitis | 8124 (7.8) | 452 (7.3) | 7672 (7.8) | 46 (7.4) | 407 (7.3) |
| Cyanosis | 1764 (1.7) | 546 (8.8) | 1218 (1.2) | 130 (21.1) | 414 (7.4) |
| Anosmia | 14,614 (15.1) | 261 (5.0) | 14,353 (15.6) | 16 (3.8) | 241 (5.0) |
| Disgeusya | 13,026 (13.5) | 230 (4.4) | 12,796 (14.0) | 11 (2.6) | 219 (4.6) |
| Irritability | 15,072 (14.4) | 2304 (37.1) | 12,768 (13.0) | 308 (50.0) | 1993 (35.7) |
| Calophrios | 19,811 (19.0) | 1378 (22.5) | 18,433 (18.8) | 114 (19.2) | 1263 (22.9) |
Qualitative variables are presented in n (%), variables with free distribution are presented in median and 25th −75th percentile.
“In the nonsurvival group, <1 year (27.8 %), and in the 1 to 4 age group, it was 22.4 % was the most frequent. Among comorbidities, non-survival subjects had more frequent of diabetes, hypertension, obesity, immunosuppression, HIV/AIDS, cardiac disease and kidney disease than survival subjects. Respect to sing and symptoms, non-survival subjects had more frequent of fever, cough, odynophagia, dyspnea, diarrhea, thoracic pain, headache, general condition attack, rhinorrhea, polypnea, vomit, cyanosis, anosmia, dysgeusia, irritability and, calophrios than survival subjects. (see Table 2).”
Table 2.
Demographic and clinic characteristics according the survival or not survival in pediatric patients with COVID-19.
| Survival n = 103,454 | Non-survival n = 669 | p-value | |
|---|---|---|---|
| Sex | 0.019 | ||
| Female | 51,865 (50.1) | 305 (45.5) | |
| Male | 51,599 (49.8) | 365 (54.4) | |
| Age, years | 14 (9–17) | 4 (0–15) | <0.001 |
| Age | |||
| <1 | 3203 (3.1) | 186 (27.8) | |
| 1-4 | 8213 (7.9) | 150 (22.4) | <0.001 |
| 5-9 | 14,523 (14.0) | 68 (10.1) | |
| 10-14 | 29,527 (28.5) | 97 (14.5) | |
| 15-18 | 47,998 (46.3) | 168 (25.1) | |
| Pneumonia | 2615 (2.5) | 443 (66.2) | <0.001 |
| Comorbidities | |||
| Diabetes | 601 (0.5) | 42 (6.3) | <0.001 |
| Hypertension | 510 (0.4) | 43 (6.4) | <0.001 |
| Obesity | 3858 (3.7) | 52 (7.7) | <0.001 |
| Asthma | 3054 (2.9) | 11 (1.6) | 0.047 |
| Immunosuppression | 762 (0.7) | 64 (9.6) | <0.001 |
| HIV/AIDS | 109 (0.1) | 3 (0.4) | 0.007 |
| Cardiac disease | 536 (0.5) | 37 (5.5) | <0.001 |
| Kidney disease | 281 (0.2) | 42 (6.3) | <0.001 |
| Symptoms | |||
| Fever | 47,644 (46.1) | 507 (75.9) | <0.001 |
| Cough | 54,050 (52.2) | 379 (56.7) | 0.022 |
| Odynophagia | 34,198 (33.1) | 160 (24.5) | <0.001 |
| Dyspnea | 9978 (9.6) | 484 (72.3) | <0.001 |
| Diarrhea | 12,204 (11.8) | 112 (16.8) | <0.001 |
| Thoracic pain | 10,147 (9.8) | 125 (19.3) | <0.001 |
| Headache | 54,482 (52.7) | 270 (41.4) | <0.001 |
| Myalgia | 29,379 (28.4) | 185 (28.4) | 0.971 |
| Arthralgia | 23,766 (23.0) | 172 (26.5) | 0.035 |
| General condition attack | 14,092 (21.6) | 204 (60.7) | <0.001 |
| Rhinorrhea | 31,984 (30.9) | 120 (18.0) | <0.001 |
| Polypnea | 4077 (3.9) | 243 (36.4) | <0.001 |
| Vomit | 5779 (5.6) | 102 (15.3) | <0.001 |
| Conjunctivitis | 8037 (7.8) | 37 (5.5) | 0.029 |
| Cyanosis | 1637 (1.5) | 127 (19.0) | <0.001 |
| Anosmia | 14,590 (15.1) | 24 (4.6) | <0.001 |
| Dysgeusia | 13,006 (13.5) | 20 (3.9) | <0.001 |
| Irritability | 14,780 (14.3) | 292 (43.9) | <0.001 |
| Calophrios | 19,663 (19.0) | 148 (22.6) | 0.021 |
Chi-square test was performed for categorical variables and Student t-test or Mann-Whitney U test for continuous variables. Qualitative variables are presented in n (%).
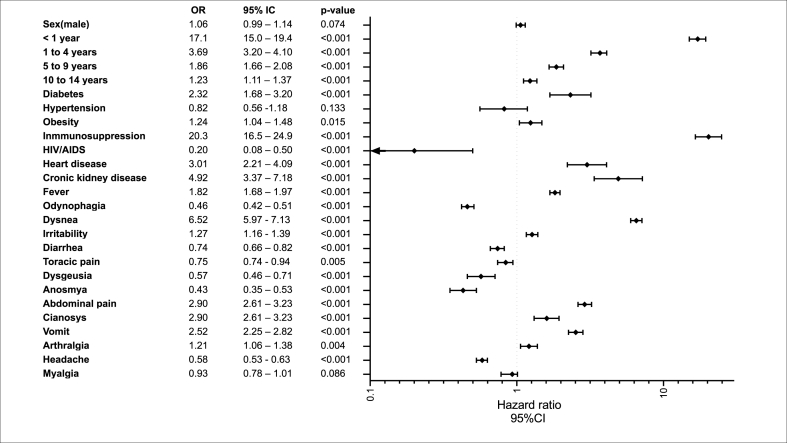
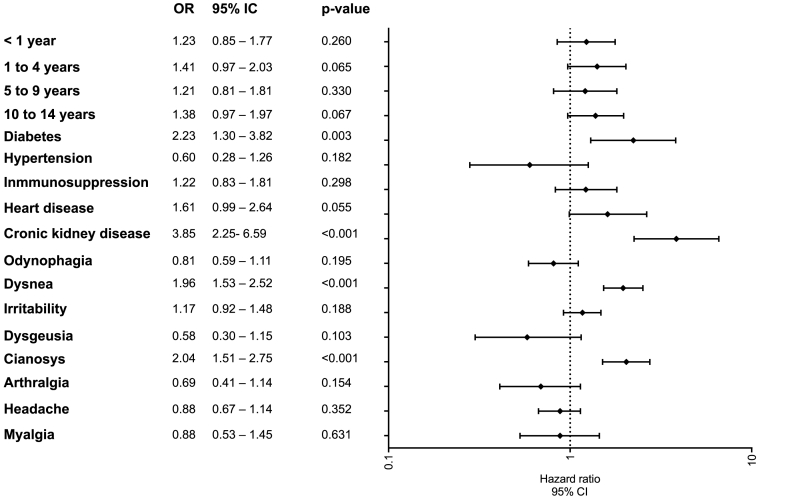
Fig. 2 shows the risk factors associated with hospital admissions. In the multivariate model, showed that subjects aged less than one year (OR 17.1, 95 % CI: 15.0–19.4), and 1 to 4 age (OR 3.69, 95 % CI:3.20–4.10), 5 to 9 age (OR:1.89, 95 % CI; 1.66–2.08), and 10 to 14 age group (OR 1.23, 95 % CI: 1.11–1.371) had the highest risk of hospital admission 15 to 18 age, who were the reference age group. However, the comorbidities with high risk were immunosuppression, kidney disease, cardiac disease, diabetes, and obesity (see Fig. 2). The risk factors associated with invasive mechanical ventilation were kidney disease (OR 3.85, 95 % CI: 2.25–6.59, p < 0.001), diabetes (OR 2.23, 95 % CI: 1.30–3.82, p = 0.003), dyspnea (OR 1.96, 95 % CI: 1.53–2.52, p < 0.001), and cyanosis (OR 2.04, 95 % CI: 1.51–2.75, p < 0.001) (see Fig. 3).
Fig. 2.
Risk factors for hospital admission in pediatric patients with COVID-19.
Fig. 3.
Risk factors for invasive mechanical ventilation in pediatric patients with COVID-19.
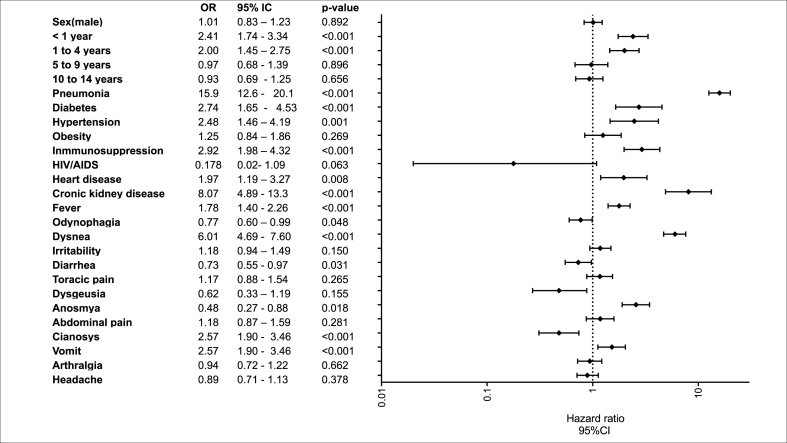
The risk factors associated with COVID-19 deaths were age <1 year (OR 2.41, 95 % CI: 1.74–3.34, p < 0.001), age 1–4 years (OR 2.0, 95 % CI: 1.4–2.7, p < 0.001), pneumonia (OR 15.9, 95 % CI: 12.6–20.1, p < 0.001), kidney disease (OR 8.07, 95 % CI:4.89–13.3, p < 0.001), immunosuppression (OR 2.92, 95 % CI:1.98–4.32, p < 0.001), diabetes (OR 2.74, 95 % CI:1.65–4.53, p < 0.001), hypertension (OR 2.48, 95 % CI:1.46–4.19, p = 0.001), cardiac disease (OR 1.97, 95 % CI:1.19–3.27, p < 0.001), and dyspnea (OR 6.01, 95 % CI:4.69–7.60, p < 0.001) (see Fig. 4).
Fig. 4.
Risk factors for COVID-19 deaths in the pediatric population.
4. Discussion
The main aim of the present study was to determine the risk factors for COVID-19 hospital admissions, invasive mechanical ventilation, and death in pediatric patients in Mexico. The strength of this study was its large sample size. The diversity of children required medical attention, which allowed for the identification of medical characteristics associated with hospital admission, invasive mechanical ventilation, and death associated with COVID-19 among pediatric subgroups.
Various studies have reported that the risk factors associated with severe COVID-19 and higher mortality were age, sex, ethnicity, lower socioeconomic status, duration of diabetes, body mass index, poorer glycemic control, and preexisting cardiovascular disease [12,13]. Children with COVID-19, and especially younger ones, present milder symptoms and a lower risk of hospitalization, severe illness, and life-threatening conditions; nevertheless, there are records of infant mortality associated with COVID-19 worldwide [3,14].
Our data showed that children aged <1 year had the highest risk associated with hospital admission. Consistent with other studies, children aged between 0 and 3 months had a higher risk of hospital admission (OR 7.86, 95 % CI: 3.0–20.4, p < 0.001) [13]. In addition, in pediatric patients, age under 30 days and presence of comorbidities were strongly associated with unfavorable outcomes [15]. In addition, children aged less than 1 year and aged 1–4 years had higher risk of death, and the group aged 10–14 years showed a higher risk of requiring mechanical ventilation. A systematic review described that 108 out of 5686 (1.9 %) patients aged 0–18 years required ventilation, increasing the risk associated with death; it also stated that 35 % of all mechanically ventilated children were younger than one year, suggesting that infants of age less than 1 year may be significantly affected by severe COVID-19 [16]. In addition, Woodruff et al. described that among hospitalized children aged less than 2 years, chronic lung disease, neurological disorders, cardiovascular disease, prematurity, and airway abnormality were associated with severe COVID-19; among those aged 2–17 years, diabetes mellitus and obesity were associated with adverse events [17].
Previous investigations documented several comorbidities as risk factors for severe COVID-19 (e.g., hospital admission, respiratory support, and critical care) in adults and children [13]. Diabetes is one of the most prevalent comorbidities in Mexico, affecting approximately 15.6 % of adults, according to ENSANUT 2020 [18]. We found that diabetes was associated with hospital admission, invasive mechanical ventilation, and death. Similar results have been recorded in pediatric populations younger than 12 months, and those with comorbidities have a worse prognosis [19,20]. Consistent with other studies, adult patients with diabetes had higher hospital admission rates and a higher risk of dying [12].
Obesity is a public health problem in Mexico. The prevalence of obesity in children aged 5–11 years, according to ENSANUT 2020, was 18.6 %, and in adolescents, it was 17 % [18]. Obesity in children can be considered a risk factor for the severity of COVID-19, because it is associated with respiratory, renal, and immunological alterations, which increase the risk of complications [21]. Kompaniyets showed that obesity is a risk factor for hospitalization in patients aged 18 years or younger [22]. Our study observed a significant risk of hospital admission in obese patients. Studies have reported that children with obesity have an inadequate response to infections and high mortality rates due to COVID-19 (10). Similar results were shown by Pranata et al. who demonstrated that obesity was associated with severe COVID-19 and mortality in adults [23,24].
Additionally, studies in rats reported a higher expression of angiotensin-converting enzyme 2 with a high-fat diet [25], which triggers a systemic inflammatory response. They may partly explain the association between obesity and the severity of clinical symptoms. Obesity is also related to insulin resistance, endothelial dysfunction, and chronic inflammation, which increase the susceptibility and risk of severe COVID-19. Obesity causes structural, metabolic, and hemodynamic changes in the kidneys, leading to reduced functional reserves in these organs [26].
In addition, studies on adults with kidney disease reported risk factors for severe COVID-19. Kidney disease is an important comorbidity associated with poor prognosis in adults with COVID-19 and with an increased risk of pneumonia in inpatients and outpatients [27]. Children with kidney disease may have the greatest risk due to immunosuppressive therapy and may require medical care. In our study, kidney disease was associated with a significant risk of hospitalization, invasive mechanical ventilation, and death.
We observed a higher risk factor for death due to pneumonia when compared to the rest of the characteristics studied for this outcome. Hospitalized patients with COVID-19 and pneumonia often receive invasive mechanical ventilation; this risk factor is higher especially in those over 65 years of age.
Our data indicated that fever was a significant risk factor for hospital admission and death.
Unfortunately, based on our data, we needed to be more accurate about those children who presented with multisystemic inflammatory syndrome versus those who did not. In terms of symptoms, we observed a higher incidence of headache (53 %), cough (52 %), fever (46 %), and odynophagia (33 %), consistent with other investigators. Children with COVID-19 presented with fever (51 %), cough (41 %), and sore throat (16 %) [1]. The symptoms with the highest risk were dyspnea and cyanosis, both for hospital admission and death. In a cohort study, adults with COVID-19 and dyspnea had a higher risk of hospitalization and mechanical ventilation and a higher risk of mortality [28]. These results may help with health education, prevention measures, and recommendations for vaccination among younger children [29,30].
This study's limitations include the fact that the information is based on electronic data collected to monitor the epidemic and not more specific clinical data or strict follow-up of patients. Second, most cases were defined by self-reporting; in the case of children, some of them could have been misclassified if the underlying conditions were not noted in their electronic medical records or specified by parents or guardians. Third, the etiological origin of pneumonia is unknown, as we do not have specific information in the database.
Despite the limitations, one of the strengths of this study was the sample size, which provided an overview of this population, focusing on characteristics that would provide a better understanding of the clinical and sociodemographic features of COVID-19 in children. In adults, we know that the COVID-19 risk factors for fatal outcomes have been established; however, in children, this has not yet been accurately determined, as these results are derived from studies in small percentages. Therefore, a large sample size is needed, and our study allows for the assessment of risk factors for fatal outcomes.
Statistics on children may be underestimated by the fact that children are not an economically active population that is routinely screened. In addition, this population was confined to home through a large part of the pandemic because of the suspension of school activities, which could have prevented many infections at the beginning of the pandemic.
Some studies have indicated that many children have asymptomatic symptoms, which contributes partly to the low detection rate in this age group. Our results could guide preventive and alerting actions for clinicians, vaccination strategies, and future treatments that could influence the disease burden in this age group.
5. Conclusion
We found that age, especially in very young children, was associated with a higher risk of severe COVID-19 outcomes. Comorbid conditions, mainly chronic kidney disease, immunosuppression, and diabetes increase mortality risk factors. Identifying these factors may help prevent or modify the conditions for survival in children with COVID-19.
Funding/support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics approval and consent to participate
Does not apply.
Authors’ contributions
Laura Flores-Cisneros: conceived and designed the experiments; analyzed and interpreted the data; contributed reagents, materials, analysis tools or data; wrote the paper Rosaura Gutiérrez-Vargas: conceived and designed the experiments; performed the experiments; contributed reagents, materials, analysis tools or data; wrote the paper Carlos Escondrillas-Maya: analyzed and interpreted the data; contributed reagents, materials, analysis tools or data; wrote the paper Dr. Zaragoza-Jiménez: conceived and designed the experiments; contributed reagents, materials, analysis tools or data; wrote the paper. Gabriel García-Rodríguez: conceived and designed the experiments; contributed reagents, materials, analysis tools or data; wrote the paper. Hugo López-Gatell: conceived and designed the experiments; contributed reagents, materials, analysis tools or data; wrote the paper. González-Islas Dulce: performed the experiments; analyzed and interpreted the data; wrote the paper. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Christian Zaragoza-Jiménez, Email: tian19808@gmail.com.
Dulce González- Islas, Email: gzz.dulce@gmail.com.
References
- 1.Cui X., Zhao Z., Zhang T., Guo W., Guo W., Zheng J., Zhang J., Dong C., Na R., Zheng L., et al. A systematic review and meta-analysis of children with coronavirus disease 2019 (COVID-19) J. Med. Virol. 2021;93:1057–1069. doi: 10.1002/jmv.26398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carlotti A.P.C.P., de Carvalho W.B., Johnston C., Gilio A.E., de Sousa Marques H.H., Ferranti J.F., Rodriguez I.S., Delgado A.F. Update on the diagnosis and management of COVID-19 in pediatric patients. Clinics. 2020;75:e2353. doi: 10.6061/clinics/2020/e2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.She J., Liu L., Liu W. COVID-19 epidemic: disease characteristics in children. J. Med. Virol. 2020;92:747–754. doi: 10.1002/jmv.25807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Government of Mexico, S.o.H Base de datos abiertos. https://www.gob.mx/salud/documentos/datos-abiertos-152127 Available online: on August 15.
- 5.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiehao C., Jin X., Daojiong L., Zhi Y., Lei X., Zhenghai Q., Yuehua Z., Hua Z., Ran J., Pengcheng L., Xiangshi W., Yanling G., Aimei X., He T., Hailing C., Chuning W., Jingjing L., Jianshe W., Mei Z. A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin. Infect. Dis. 2020;71:1547–1551. doi: 10.1093/cid/ciaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim L., Whitaker M., O'Halloran A., Kambhampati A., Chai S.J., Reingold A., Armistead I., Kawasaki B., Meek J., Yousey-Hindes K., Anderson E.J., Openo K.P., Weigel A., Ryan P., Monroe M.L., Fox K., Kim S., Lynfield R., Bye E., Shrum Davis S., Smelser C., Barney G., Spina N.L., Bennett N.M., Felsen C.B., Billing L.M., Shiltz J., Sutton M., West N., Talbot H.K., Schaffner W., Risk I., Price A., Brammer L., Fry A.M., Hall A.J., Langley G.E., Garg S., COVID-NET Surveillance Team Hospitalization rates and characteristics of children aged <18 Years hospitalized with laboratory-confirmed COVID-19 - COVID-NET, 14 states, march 1-july 25, 2020. Morb. Mortal. Wkly. Rep. 2020;69:1081–1088. doi: 10.15585/mmwr.mm6932e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moreira A., Chorath K., Rajasekaran K., Burmeister F., Ahmed M., Moreira A. Demographic predictors of hospitalization and mortality in US children with COVID-19. Eur. J. Pediatr. 2021;180:1659–1663. doi: 10.1007/s00431-021-03955-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Organization, P.A.H.O.W.H. Operational Guidelines for Sentinel Severe Acute Respiratory Infection (SARI) Surveillance.Available online: (accessed on December 10)..
- 10.México, S.d.S.d . 2021. Lineamiento Estandarizado de Vigilancia Epidemiologica y por Laboratorio de la Enfermedad Respiratoria Viral.pdf>. [Google Scholar]
- 11.Fernandes D.M., Oliveira C.R., Guerguis S., Eisenberg R., Choi J., Kim M., Abdelhemid A., Agha R., Agarwal S., Aschner J.L., et al. Severe acute respiratory syndrome coronavirus 2 clinical syndromes and predictors of disease severity in hospitalized children and youth. J. Pediatr. 2021;230:23–31.e10. doi: 10.1016/j.jpeds.2020.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dave J.A., Tamuhla T., Tiffin N., Levitt N.S., Ross I.L., Toet W., Davies M.A., Boulle A., Coetzee A., Raubenheimer P.J. Risk factors for COVID-19 hospitalisation and death in people living with diabetes: a virtual cohort study from the Western Cape Province, South Africa. Diabetes Res. Clin. Pract. 2021;177 doi: 10.1016/j.diabres.2021.108925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graff K., Smith C., Silveira L., Jung S., Curran-Hays S., Jarjour J., Carpenter L., Pickard K., Mattiucci M., Fresia J., et al. Risk factors for severe COVID-19 in children. Pediatr. Infect. Dis. J. 2021;40:e137–e145. doi: 10.1097/inf.0000000000003043. [DOI] [PubMed] [Google Scholar]
- 14.Dong Y., Mo X., Hu Y., Qi X., Jiang F., Jiang Z., Tong S. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145 doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 15.Hendler J.V., Miranda do Lago P., Müller G.C., Santana J.C., Piva J.P., Daudt L.E. Risk factors for severe COVID-19 infection in Brazilian children. Braz. J. Infect. Dis. 2021;25 doi: 10.1016/j.bjid.2021.101650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams N., Radia T., Harman K., Agrawal P., Cook J., Gupta A. COVID-19 Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review of critically unwell children and the association with underlying comorbidities. Eur. J. Pediatr. 2021;180:689–697. doi: 10.1007/s00431-020-03801-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woodruff R.C., Campbell A.P., Taylor C.A., Chai S.J., Kawasaki B., Meek J., Anderson E.J., Weigel A., Monroe M.L., Reeg L., et al. Risk factors for severe COVID-19 in children. Pediatrics. 2022:149. doi: 10.1542/peds.2021-053418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shamah-Levy T., Romero-Martínez M., Barrientos-Gutiérrez T., Cuevas-Nasu L., Bautista-Arredondo S., Colchero M., GaonaPineda E., Lazcano-Ponce E., Martínez-Barnetche J., Alpuche-Arana C. Instituto Nacional de Salud Pública; Cuernavaca, México: 2021. Encuesta nacional de salud y nutrición 2020 sobre Covid-19. Resultados nacionales; p. 2021. [Google Scholar]
- 19.Tsankov B.K., Allaire J.M., Irvine M.A., Lopez A.A., Sauvé L.J., Vallance B.A., Jacobson K. Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int. J. Infect. Dis. 2021;103:246–256. doi: 10.1016/j.ijid.2020.11.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solórzano-Santos F., Miranda-Lora A.L., Márquez-González H., Klünder-Klünder M. Survival analysis and mortality predictors of COVID-19 in a pediatric cohort in Mexico. Front. Public Health. 2022;10 doi: 10.3389/fpubh.2022.969251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nogueira-de-Almeida C.A., Del Ciampo L.A., Ferraz I.S., Del Ciampo I.R.L., Contini A.A., Ued F.D.V. COVID-19 and obesity in childhood and adolescence: a clinical review. J. Pediatr. 2020;96:546–558. doi: 10.1016/j.jped.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kompaniyets L., Agathis N.T., Nelson J.M., Preston L.E., Ko J.Y., Belay B., Pennington A.F., Danielson M.L., DeSisto C.L., Chevinsky J.R., et al. Underlying medical conditions associated with severe COVID-19 illness among children. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pranata R., Lim M.A., Yonas E., Vania R., Lukito A.A., Siswanto B.B., Meyer M. Body mass index and outcome in patients with COVID-19: a dose-response meta-analysis. Diabetes & Metabolism. 2021;47 doi: 10.1016/j.diabet.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang F., Xiong Y., Wei Y., Hu Y., Wang F., Li G., Liu K., Du R., Wang C.Y., Zhu W. Obesity predisposes to the risk of higher mortality in young COVID-19 patients. J. Med. Virol. 2020;92:2536–2542. doi: 10.1002/jmv.26039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al Heialy S., Hachim M.Y., Senok A., Gaudet M., Abou Tayoun A., Hamoudi R., Alsheikh-Ali A., Hamid Q. Regulation of angiotensin-converting enzyme 2 in obesity: implications for COVID-19. Front. Physiol. 2020;11 doi: 10.3389/fphys.2020.555039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavie C.J., Sanchis-Gomar F., Henry B.M., Lippi G. COVID-19 and obesity: links and risks. Expet Rev. Endocrinol. Metabol. 2020;15:215–216. doi: 10.1080/17446651.2020.1767589. [DOI] [PubMed] [Google Scholar]
- 27.Chou C.Y., Wang S.M., Liang C.C., Chang C.T., Liu J.H., Wang I.K., Hsiao L.C., Muo C.H., Huang C.C., Wang R.Y. Risk of pneumonia among patients with chronic kidney disease in outpatient and inpatient settings: a nationwide population-based study. Medicine. 2014;93:e174. doi: 10.1097/md.0000000000000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guan W.J., Liang W.H., Zhao Y., Liang H.R., Chen Z.S., Li Y.M., Liu X.Q., Chen R.C., Tang C.L., Wang T., et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur. Respir. J. 2020:55. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallace M., Woodworth K.R., Gargano J.W., Scobie H.M., Blain A.E., Moulia D., Chamberland M., Reisman N., Hadler S.C., MacNeil J.R., et al. The advisory committee on immunization practices' interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine in adolescents aged 12-15 years - United States, May 2021. MMWR (Morb. Mortal. Wkly. Rep.) 2021;70:749–752. doi: 10.15585/mmwr.mm7020e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woodworth K.R., Moulia D., Collins J.P., Hadler S.C., Jones J.M., Reddy S.C., Chamberland M., Campos-Outcalt D., Morgan R.L., Brooks O., et al. The advisory committee on immunization practices' interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine in children aged 5-11 years - United States, November 2021. MMWR (Morb. Mortal. Wkly. Rep.) 2021;70:1579–1583. doi: 10.15585/mmwr.mm7045e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.