Abstract
Methionine added to minimal medium overcomes the repressing effects of ammonium and cyclic AMP (cAMP) on sexual development and efficiently induces mating and sporulation in homothallic strains of Schizosaccharomyces pombe. In heterothallic strains it induces G1 arrest when cells enter stationary phase. We show that methionine reduces the intracellular cAMP pool and induces the expression of at least two cAMP-repressible genes, including fbp1 and ste11. The easiest interpretation of the results is that methionine induces sexual development via a cAMP-dependent ste11 signalling pathway.
The life cycle of Schizosaccharomyces pombe is nutritionally regulated. Under rich growth conditions cells grow, go through mitosis, and divide. Under appropriate starvation conditions cells arrest in G1 of the cell cycle and sexually differentiate, and haploid cells of opposite mating types mate and fuse to a diploid zygote which undergoes meiosis to generate four haploid spores (2). The most efficient nutritional signal for induction of sexual development is nitrogen starvation, and in most studies the effects of ammonium have been investigated. A key regulator in the control of mating and sporulation is the gene ste11. It encodes a transcription factor which activates a number of genes involved in sexual differentiation and meiosis, including mat1-P, mat1-M, mei2, rep1, ste4, ste6, and fus1 (18). Expression of ste11 is regulated by different pathways. In the nutritionally regulated pathway, ammonium starvation leads to a decrease in the level of cyclic AMP (cAMP) and as a consequence induces expression of ste11 (9). How the nitrogen status is sensed and converted to an appropriate cAMP signal is not known. An α-subunit of a heteromeric G protein which is encoded by the gene gpa2 is involved in this process (6). When this gene is deleted, the intracellular cAMP level drops and strains undergo ectopic sexual development. In addition to the nutritional pathway there is the cAMP-independent Wis1-Sty1/Phh1 stress pathway, which regulates ste11 expression positively in response to nutrient starvation (7, 16, 17). In this pathway a stress signal activates via a kinase cascade transcription factor Atf1, which in turn induces expression of other genes, including ste11, fbp1 (encoding fructose biphosphatase), and the genes encoding glycerol-3-phosphate dehydrogenase, catalase, and a tyrosine-specific phosphatase. The genes pac2 and rcd1 also control ste11 expression but are independent of the cAMP-Pka1 and Wis1-Sty1/Phh1 pathways (8, 12). rcd1 is responsible for nitrogen- but not glucose-induced ste11 expression.
To learn more about nutritional regulation of the S. pombe life cycle, we examined the effects of different nutrients on mating and sporulation. Here we report that methionine induces ste11 expression and acts as an efficient inducer of mating and sporulation.
MATERIALS AND METHODS
Strains and media.
All strains used in this study are from our collection in Bern, Switzerland. The minimal medium (MM) used has been described in detail previously (15). It is a modified version of the PM medium (1) and contains 1% glucose, 0.43 g of NH4Cl/liter, and 40 mg of Na2SO4/liter. The mutant met6-31 ura4-D18 h90 strain was selected as a methionine-auxotrophic strain from a mutagenized ura4-D18 h90 culture (13).
Molecular cloning and sequencing of the met6 gene.
The met6-31 ura4-D18 h90 strain was transformed by using the partial Sau3A genomic library ligated in the ura4+ marker containing shuttle vector pUR19, described previously (3). The methods for transformation and amplification of the plasmids have been described previously (10). The inserts of two complementing plasmids were subcloned, and about 4 kb was sequenced by the dideoxy chain termination method with modified T7 polymerase, 35S-ATP, and the Sequenase version 2.0 kit from U.S. Biochemicals.
Mating, sporulation, and fluorescence-activated cell sorter analyses.
The protocol for quantitating mating and sporulation in liquid media has been described in detail previously (15). A total of 500 to 1,000 U of vegetative cells, zygotes, and asci was counted. For fluorescence-activated cell sorter analysis cells were fixed in 70% ethanol, stained with propidium iodide, and prepared as described previously (1).
Northern analyses.
Cells were grown in MM to an optical density at 595 nm of 0.9 to 1.1. Total RNA was extracted, separated on glyoxal gels, blotted, and hybridized as described previously (14). Twenty micrograms of total RNA was loaded per slot. The blots were probed with PCR-generated fragments of ste11, fbp1, and act1. The mRNA bands were measured with a PhosphorImager, and the intensities of bands were quantified with the ImageQuant program by using the act1 signal as an internal standard.
cAMP assay.
cAMP was extracted by a metabolite extraction method described for yeast (4), and cAMP levels were determined according the protocol of Mochizuki and Yamamoto (9) by using the [3H]cAMP assay system of Amersham. All values to be compared were determined simultaneously in the same run.
Nucleotide sequence accession number.
The sequence for the met6 gene is available under EMBL/GenBank accession no. AJ223985.
RESULTS AND DISCUSSION
Methionine induces G1 arrest, mating, and sporulation in S. pombe.
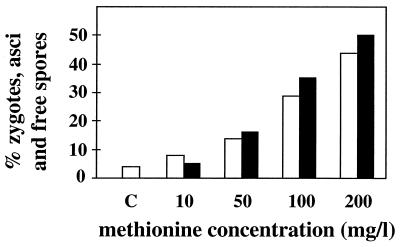
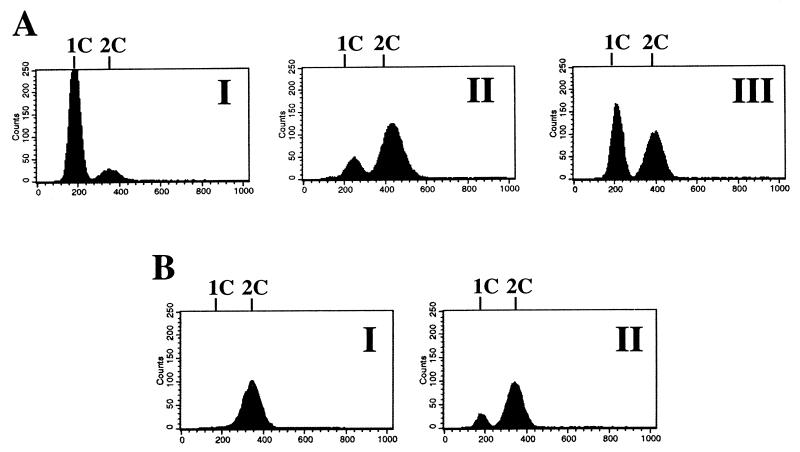
In our standard MM, cultures of the homothallic strain 968 (h90) or a mixture of the heterothallic strains 975 (h+) and 972 (h−) mated and sporulated. By lowering the sulfate concentration of the medium by a factor of 2 and more we observed a drastic decrease of mating and sporulation. The effect could be reversed by lowering the ammonium concentration in addition to the sulfate concentration (data not shown). We took this as an indication that in addition to nitrogen metabolism, sulfur metabolism is a parameter in the nutritional control of mating and sporulation in S. pombe; we therefore started to examine various sulfur metabolites for their effects on the life cycle. Cysteine at 100 mg/liter had a weak inhibitory effect on mating and sporulation in liquid cultures and glutathione, cystathione, and homocysteine had very weak or no effects, but methionine clearly induced mating and meiosis, both in the h90 strain and in a mixture of the two heterothallic strains. The results are shown for MM containing large amounts of ammonium (Fig. 1). In media containing small amounts of ammonium, exogenously added methionine did not drastically increase the frequency of but very significantly accelerated (by 2 to 4 h) the rate of mating and sporulation (results not shown). In yeast extract medium methionine had no effect. To test the effect of methionine in a strain defective in methionine biosynthesis, we isolated a methionine-auxotrophic (met6-31) mutant. We isolated and sequenced the met6 gene and demonstrated that it codes for homoserine acyltransferase, the enzyme catalyzing the first reaction in the methionine-specific biosynthetic pathway. (Its amino acid sequence shows 55% homology to the corresponding sequences of Saccharomyces cerevisiae and Ascobolus immersus.) As shown in Fig. 1 for the met6-31 strain, mating and sporulation were affected the same way as in the wild-type strain, implying that methionine can induce sexual development without a functional methionine biosynthetic pathway. We also examined the effect of methionine in diploids heterozygous at the mating type locus. In medium containing 5 g of NH4Cl/liter, sporulation frequency was stimulated by a factor of roughly 10 upon addition of 100 mg of methionine/liter (results not shown). We observed that in homothallic strains methionine induces G1 arrest in the stationary phase. The same effect was also apparent in heterothallic strains (Fig. 2), indicating that methionine, rather than the mating factors, is responsible for the G1 arrest.
FIG. 1.
Induction of mating and sporulation in S. pombe by methionine. Wild-type strains 972 (h−) and 975 (h+) mixed in a 1:1 ratio (white columns) and the homothallic mutant met6-31 ura4-D18 h90 strain (black columns) were cultured in liquid MM containing 5 g of NH4Cl/liter and increasing concentrations of methionine as indicated. The percentage of zygotes, asci, and free spores was determined after 24 h. C, mating and sporulation in MM containing no methionine.
FIG. 2.
Flow cytometric analysis of S. pombe cultivated under different growth conditions. (A) Media I (MM containing 0.026 g of NH4Cl/liter), II (MM containing 5 g of NH4Cl/liter), and III (MM containing 5 g of NH4Cl/liter plus 100 mg of methionine/liter) were inoculated with a preculture of strain 972 (h−) to an optical density at 530 nm of 0.1 and incubated at 30°C. After 47 h, when cells had reached stationary phase, the cells were analyzed. Peaks of 1C and 2C DNA contents are shown. (B) Strain 972 (h−) was cultivated in medium I (MM containing 2 mM cAMP) and medium II (MM containing 2 mM cAMP and 200 mg of methionine/liter). Inoculation, incubation, and preparation of cells were done as described for panel A.
Methionine induces ste11 and fbp1 expression and lowers the cAMP level.
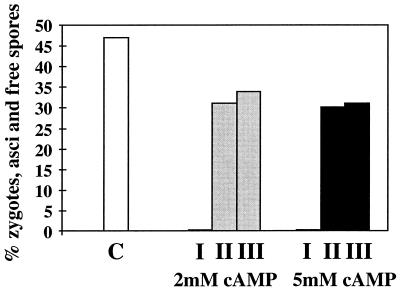
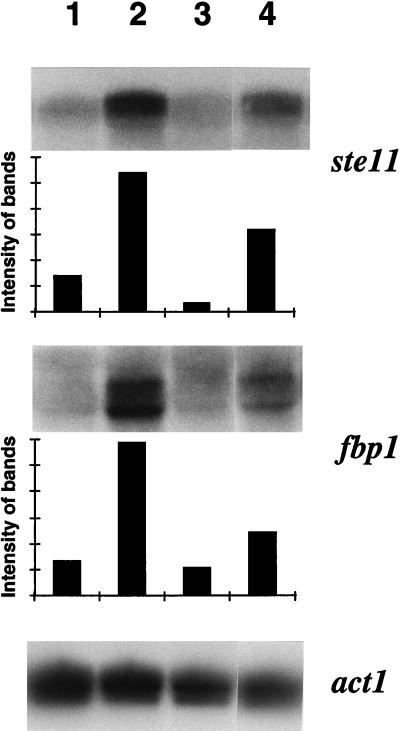
In heterothallic strains methionine partially suppressed cAMP-induced G2 arrest (Fig. 2), and in a mixture of heterothallic strains of opposite mating types and the homothallic strain it suppressed the effect of cAMP on mating and sporulation (Fig. 3). This indicates that methionine interferes with the cAMP-dependent nutritional signalling pathway. The gene ste11 is a central regulator of sexual development and is under the control of nitrogen and cAMP. We thus tested its expression and found that methionine reverses the repressing effect of ammonium and cAMP and induces ste11 expression (Fig. 4). Another gene also known to be repressed by cAMP but not involved in the control of mating and sporulation is fbp1, which encodes fructose-1,6-biphosphatase (5). Mutants defective in activation of adenylate cyclase are derepressed for both ste11 and fbp1 (11). As shown in Fig. 4, expression of fbp1 is also induced by methionine.
FIG. 3.
Methionine suppresses effects of cAMP on mating and sporulation. Wild-type strains 972 (h−) and 975 (h+) were cultured in a 1:1 ratio in liquid MM containing either 2 or 5 mM cAMP and increasing amounts of methionine (I, without methionine; II, with 100 mg of methionine/liter; III, with 250 mg of methionine/liter) and examined for mating and sporulation as described in the legend to Fig. 1. C, standard MM containing no methionine or cAMP.
FIG. 4.
Quantification of ste11 and fbp1 mRNA from cells grown in the presence or absence of cAMP and methionine. The homothallic wild-type strain 968 (h90) was precultured in MM containing 5 g of NH4Cl/liter, and at time zero cells were shifted to the same medium containing either no additional ingredients (lane 1), 200 mg of methionine/liter (lane 2), 2 mM cAMP (lane 3), or 2 mM cAMP and 200 mg of methionine/liter (lane 4). After 4 h, samples were taken for preparation of total RNA and Northern blotting. The percentages of zygotes and asci in the different media after 16 h were as follows: lane 1, 2%; lane 2, 47%; lane 3, 0%; and lane 4, 35%.
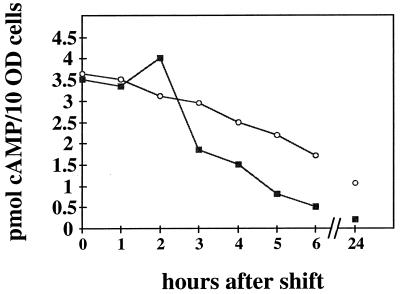
We shifted homothallic h90 cells from a methionine-free medium into either methionine-containing or methionine-free medium and measured the intracellular cAMP levels. Depletion of the cAMP pool in cells grown in the presence of methionine is indeed more efficient than in cells cultivated in the absence of exogenously added methionine (Fig. 5). The observed difference was reproducible in a second independent experiment. The reduction of the cAMP level by methionine is in a range similar to that observed when cells are shifted from a nitrogen-rich to a nitrogen-free medium (9). The most straightforward explanation of the data is that methionine lowers the intracellular cAMP level and thereby induces expression of cAMP-repressed genes. We cannot exclude the possibility that methionine interferes with another cascade activating ste11 (for example, the Wis1-Sty1/Phh1 stress pathway) and that the reduced cAMP level is only a consequence of G1 arrest.
FIG. 5.
Intracellular level of cAMP in wild-type S. pombe cells cultivated in the presence and absence of methionine. The homothallic wild-type strain 968 (h90) was precultured in MM containing 5 g of NH4Cl/liter and shifted to the same medium without (○) or with (■) 200 mg of methionine/liter. At 0, 1, 2, 3, 4, 5, 6, and 24 h after the shift, aliquots were taken and the intracellular cAMP level was measured as described previously (5). The percentages of zygotes and asci were measured after 24 h and were as follows: 4% for the culture incubated in medium without methionine and 26% for the culture incubated with methionine. cAMP values are the averages of two measurements from one experiment. Two independent experiments were performed; they gave essentially the same results. 10 OD cells, 108 cells.
To summarize, our results suggest that methionine induces G1 arrest, mating, and sporulation via an ste11-dependent signalling pathway, and we favor the idea that signalling occurs via cAMP. The data imply that S. pombe development can at least partially be controlled via regulation of methionine metabolism. Ammonium starvation is also signalled via a cAMP-dependent ste11 pathway, and it is possible that the two pathways controlling cAMP levels overlap. Methionine could simply act by inhibiting ammonium transport. We measured uptake of the ammonium analog methylamine but obtained no evidence favoring this idea (data not shown). Another possibility is that ammonium lowers the methionine level or vice versa. The possibility that methionine and ammonium regulate the cAMP pool via separate pathways remains. Further insight into the way methionine lowers the cAMP level may come from the analyses of mutants that are defective in methionine metabolism as well as in the life cycle.
ACKNOWLEDGMENTS
We thank M. Stalder for technical assistance and Thomas Seebeck for critical comments on the manuscript.
This study was supported by the Swiss National Foundation.
REFERENCES
- 1.Beach J, Rodgers L, Gould J. RAN1+ controls the transition from mitotic division to meiosis in fission yeast. Curr Genet. 1985;10:297–311. doi: 10.1007/BF00365626. [DOI] [PubMed] [Google Scholar]
- 2.Egel R. The Mycota. Berlin, Germany: Springer Verlag; 1994. Regulation of meiosis and sporulation in Schizosaccharomyces pombe; pp. 251–265. [Google Scholar]
- 3.Fankhauser H, Zurlinden A, Schweingruber A M, Edenharter E, Schweingruber M E. Schizosaccharomyces pombe thiamine pyrophosphokinase is encoded by gene tnr3 and is a regulator of thiamine metabolism, phosphate metabolism, mating, and growth. J Biol Chem. 1995;270:28457–28462. doi: 10.1074/jbc.270.47.28457. [DOI] [PubMed] [Google Scholar]
- 4.Gonzales B, Francois J, Renaud M. A rapid and reliable method for metabolite extraction in yeast using boiling buffered ethanol. Yeast. 1997;13:1347–1356. doi: 10.1002/(SICI)1097-0061(199711)13:14<1347::AID-YEA176>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman C S, Winston F. Glucose repression of transcription of the Schizosaccharomyces pombe fpb1 gene occurs by a cAMP signaling pathway. Genes Dev. 1991;5:561–571. doi: 10.1101/gad.5.4.561. [DOI] [PubMed] [Google Scholar]
- 6.Isshiki T, Mochizuki N, Maeda T, Yamamoto M. Characterization of a fission yeast gene, gpa2, that encodes a Gα subunit involved in the monitoring of nutrition. Genes Dev. 1992;6:2455–2462. doi: 10.1101/gad.6.12b.2455. [DOI] [PubMed] [Google Scholar]
- 7.Kato T, Okazaki K, Murakami H, Stettler S, Fantes P A, Okayama H. Stress signal, mediated by a Hog1-like MAP kinase, controls sexual development in fission yeast. FEBS Lett. 1996;378:207–212. doi: 10.1016/0014-5793(95)01442-x. [DOI] [PubMed] [Google Scholar]
- 8.Kunitomo H, Sugimoto A, Wilkinson C R, Yamamoto M. Schizosaccharomyces pombe pac2+ controls the onset of sexual development via a pathway independent of the cAMP cascade. Curr Genet. 1995;28:32–38. doi: 10.1007/BF00311879. [DOI] [PubMed] [Google Scholar]
- 9.Mochizuki N, Yamamoto M. Reduction in the intracellular cAMP level triggers initiation of sexual development in fission yeast. Mol Gen Genet. 1992;233:17–24. doi: 10.1007/BF00587556. [DOI] [PubMed] [Google Scholar]
- 10.Niederberger C, Gräub R, Schweingruber A M, Fankhauser H, Rusu M, Poitelea M, Edenharter L, Schweingruber M E. Exogenous inositol and genes responsible for inositol transport are required for mating and sporulation in Schizosaccharomyces pombe. Curr Genet. 1998;33:255–261. doi: 10.1007/s002940050334. [DOI] [PubMed] [Google Scholar]
- 11.Nocero M, Isshiki T, Yamamoto M, Hoffman C S. Glucose repression of fbp1 transcription of Schizosaccharomyces pombe is partially regulated by adenylate cyclase activation by a G protein alpha subunit encoded by gpa2 (git8) Genetics. 1994;138:39–45. doi: 10.1093/genetics/138.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okazaki N, Okazaki K, Watanabe Y, Kato-Hayashi M, Yamamoto M, Okayama H. Novel factor highly conserved among eukaryotes controls sexual development in fission yeast. Mol Cell Biol. 1998;18:887–895. doi: 10.1128/mcb.18.2.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schweingruber A M, Dlugonski J, Edenharter E, Schweingruber M E. Thiamine in Schizosaccharomyces pombe: dephosphorylation, intracellular pool, biosynthesis and transport. Curr Genet. 1991;19:249–254. doi: 10.1007/BF00355050. [DOI] [PubMed] [Google Scholar]
- 14.Schweingruber A-M, Fankhauser H, Dlugonski J, Steinmann-Loss C, Schweingruber M E. Isolation and characterization of regulatory mutants from Schizosaccharomyces pombe involved in thiamine-regulated gene expression. Genetics. 1992;130:445–449. doi: 10.1093/genetics/130.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schweingruber M E, Edenharter E. Thiamin regulates agglutination and zygote formation in Schizosaccharomyces pombe. Curr Genet. 1990;17:191–194. doi: 10.1007/BF00312609. [DOI] [PubMed] [Google Scholar]
- 16.Shiozaki K, Russell P. Conjugation, meiosis, and the osmotic stress response are regulated by Spc1 kinase through Atf1 transcription factor in fission yeast. Genes Dev. 1996;10:2276–2288. doi: 10.1101/gad.10.18.2276. [DOI] [PubMed] [Google Scholar]
- 17.Wilkinson M G, Samuels M, Takeda T, Toone W M, Shieh J C, Toda T, Millar J B A, Jones N. The Atf1 transcription factor is a target for the Sty1 stress-activated MAP kinase pathway in fission yeast. Genes Dev. 1996;10:2289–2301. doi: 10.1101/gad.10.18.2289. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto M, Imai Y, Watanabe Y. Mating and sporulation in Schizosaccharomyces pombe. In: Pringle J R, Broach J R, Jones E W, editors. Yeast III. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 1037–1106. [Google Scholar]