Abstract
Centella asiatica (L.) Urb. has wound healing, anti-inflammatory, cognitive improvement, and neuroprotective properties which have been attributed to its centelloside content. However, the quantities of these bioactive compounds are limited and vary due to genetic and environmental factors. Light qualities are known to enhance the production of secondary metabolites in several plant species, both preharvest and postharvest. In this study, fresh leaves of C. asiatica were subjected to different light emitting diode (LED) quality including white, dark, red, blue, and green to assess centelloside content, phytochemical constituents, and transcription level expression of triterpenoid biosynthesis genes. Results showed that white and blue LEDs significantly increased centelloside content in C. asiatica leaves at 3 days postharvest (dph) by 73 % over the control group at 0 dph. Blue LEDs stimulated the expression of triterpenoid biosynthesis genes including C. asiatica squalene synthase (CaSQS), C. asiatica β-amyrin synthase (CabAS), and C. asiatica UDP gluclosyltransferase-73AH1 (CaUGT73AH1; CaUGT), while different LED conditions gave diverse results. Red LED treatment triggered higher total flavonoid content (TFC) and total triterpenoid content (TTC) while white LEDs enhanced total triterpenoid content (TTC). Taken together, our findings suggest that postharvest under blue LEDs is a great approach to increase centelloside production of C. asiatica through gene up-regulation in triterpenoid pathway. Therefore, postharvest technology by LEDs serves as an effective tool for improving raw material quality for medicinal plant industries.
Keywords: Indian pennyworth, Saponins, Bioactive compound, Centelloside pathway
Graphical abstract
1. Introduction
Centella asiatica (L.) Urb. (CA) is valued for its medicinal properties including wound healing, venous insufficiency, antibacterial, anticancer, and neuroprotective activities, and as an antidepressant [[1], [2], [3], [4], [5], [6]]. Major constituents in C. asiatica leaves are triterpenoids as the four centelloside compounds asiatic acid, madecassic acid (aglycone-type), asiaticoside, and madecassoside (glycoside-type). Pharmacological benefits of aglycones and glycosides include wound healing [1,7] and as treatment for diabetic ulcers [8], anti-inflammatory activity [8,9], cognitive improvement and neuroprotection [10], varicose veins and memory brain function improvement [4,10]. Different varieties of C. asiatica have diverse genetic backgrounds with variability in centelloside content recorded in different seasons. Highest levels of the four triterpenes were observed during January and February, which correspond to the summer season in the southern hemisphere, whereas lowest values were recorded during the winter season (June) and spring season (October) in Australia [11]. Meanwhile, in Madagascar, the period of rainfall manifested a marked enhancement in the yield of both biomass and asiaticoside of C. asiatica, whereas a significant decline was observed during the dry and cold seasons [12]. Previous studies determined seasonal inconsistency in centelloside production and biomass yield of C. asiatica.
The quality of raw materials with high amounts of bioactive compounds is pivotal in the medicinal industry for downstream processes of finalized commercial products. Fluctuation of centelloside content occurs naturally in different cultivation seasons, thus increase in triterpenoid content using postharvest technology would improve the quality of raw material for the medicinal industry. Increasing centellosides in C. asiatica using elicitors such as methyl jasmonate (MeJA) and salicylic acid (SA) was investigated by Buraphaka and Putalun (2020) [13]. Elicitors improve the bioactive compounds in C. asiatica raw material, but plant spraying is inconvenient for mass production. Therefore, instead of using chemicals, subjecting plant material to light emitting diodes (LEDs) is a viable option for greenhouse crop cultivation [14,15], and food preservation [16] to increase secondary metabolites [[17], [18], [19]]. LED treatment to enhance bioactive compound production has been widely used postharvest in a wide range of plant species such as tomato (Lycopersicon esculentum Mill.) [20], orange (Citrus sinensis Osbeck) [17], broccoli (Brassica oleracea var. Italica) [21], and lettuce (Lactuca sativa L.) [14,22]. C. asiatica was recently studied to determine the effect of blue light on growth parameters in a closed-culture system. A combination of red and blue LEDs stimulated important agronomic traits of C. asiatica such as fresh weight, dry weight, leaf width, and leaf length together with centelloside and phytochemical contents [23]. Treatment of blue LEDs inhibited the germination percentage of C. asiatica seed [24] but also enabled up-regulation of the stress-responsive genes (ABA insensitive 5 (ABI5), MYB4, and HY5-homolog (HYH) transcription factors) involved in the flavonoid synthesis pathway compared with red LEDs [25].
Numerous studies have investigated the potential of postharvest light-emitting diodes (LEDs) to enhance the secondary metabolite content of economically important plants, while blue and red LEDs were reported to affect the agronomic characteristics of C. asiatica [23]. However, few studies have addressed the effects of postharvest LEDs on C. asiatica leaves. Thus, this study postulated that postharvest LEDs could improve the centelloside content of C. asiatica leaves by modulating the expression of genes involved in triterpenoid biosynthesis, thereby augmenting the quality of the raw material for medicinal purposes.
2. Results
2.1. Leaf morphology under different LED quality
After harvesting, the leaves were exposed to six different light treatments including sunshade, white, dark, red, blue, and green LEDs for 3 days. The leaf color of C. asiatica was bleached at 2 days postharvest (dph) in all treatments compared with 0 and 1 dph. The C. asiatica leaves under sunshade and red LEDs wilted, whereas leaves under dark at 2 dph were still fresh and similar to the control at 0 dph (Fig. 1A).
Fig. 1.
Leaf morphology, pigments, phytochemical content, and antioxidation of C. asiatica leaf extracts after treatment under different LED quality for 3 days.
(A) Leaf morphology after LED treatment for 1–3 dph. (B) Determination of pigments including total chlorophyll a (Chlo_a), total chlorophyll b (Chlo_b), total chlorophyll ab (Chlo_ab), and carotene (Caro). (C) Phytochemical analysis including total phenolic content (TPC), total flavonoid content (TFC), total triterpenoid content (TTC), and free radical scavenging activity by DPPH. The C. asiatica leaves were placed under sunshade, light, dark, white, red, blue, and green LEDs for 3 days. The values of each parameter were visualized as a bar graph which standard error was used to create error bars. The asterisks represent the significant difference at p < 0.05 of each treatment when compared with C. asiatica leaves at day 0 or non-incubation (NI) by LSD test. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
2.2. Chlorophyll, phytochemical content and antioxidation of C. asiatica leaves
To evaluate the effect of different LED quality, C. asiatica leaves at 0, 1, 2, and 3 dph under sunshade, white, dark, red, blue, and green LEDs were extracted to determine total chlorophyll a, chlorophyll b, chlorophyll ab, carotenoid, total phenolic content (TPC), total flavonoid content (TFC), and total triterpenoid content (TTC) and antioxidation by DPPH (Fig. 1). C. asiatica leaves were yellowish and wilted for 3 days, while red LEDs preserved the greenish color of the leaves (Fig. 1A, Supplemental file 1). All the pigments showed increased chlorophyll with incubation time for all treatments compared with the control at 0 dph. However, at 2 dph, red and green LEDs displayed highest amounts of all pigments. In dark condition, all pigments increased the most at 1 dph and then decreased (Fig. 1B). Total phenolic content increase was time-dependent in red, blue, and green treatments, while TFC increased the highest amount in red condition, followed by blue condition. White condition gave the highest TTC value compared with the other treatments. Total triterpenoid content in red condition increased at 1 dph and then decreased, while TTC in blue condition increased in a time-dependent manner. White condition showed increased TFC and TTC contents at 1 dph which then decreased (Fig. 1C). Antioxidant activities shown by DPPH assay decreased for all treatments in a time-dependent manner, apart from white and green conditions that increased at 3 dph (Fig. 1C). Besides, nitrate content of C. asiatica leaves under different LEDs for 3 dph was measured by saliccylic acid method as results no increse amount of nitrate in any treaments when compared with C. asiatica leaves at day 0 (Supplemental file 2).
2.3. Centelloside content of C. asiatica leaves under different LED treatments
C. asiatica leaves were treated under different LEDs for 1, 2, and 3 dph to assess changes in centelloside content. Total centelloside content of each treatment was determined by high performance liquid chromatography (HPLC). Centelloside content increased in a time-dependent manner under all treatments compared with the control at 0 dph, with no difference in the amount of centelloside shown at 1 dph for all treatments (Fig. 2C). Centelloside content significantly increased at 2 and 3 dph, particularly in white and blue treatments, with 3.83 ± 0.06 %DW and 3.78 ± 0.45 %DW at 3 dph, respectively (Fig. 2C). Centelloside content of C. asiatica leaves under sunshade, dark, red, and green LEDs was around 3 %DW, with increased centelloside under white and blue LEDs at approximately 20 % compared with 3 dph of sunshade (Fig. 2C).
Fig. 2.
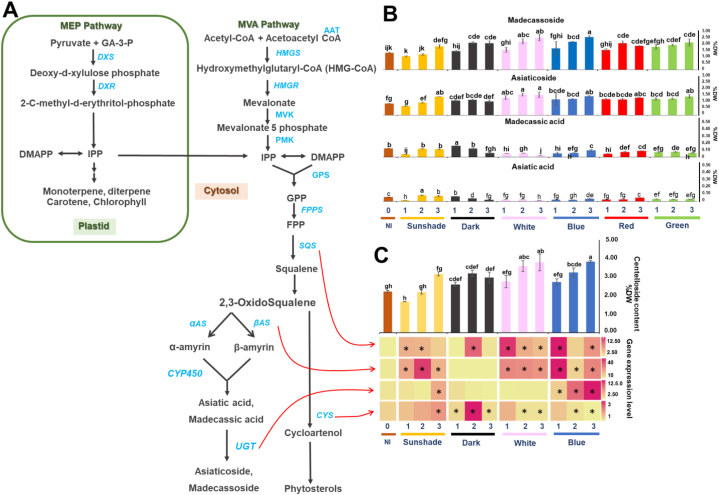
Centelloside content and expression levels of triterpenoid synthesis-relating genes on Centella asiatica leaves under different LED quality during three days.
(A) A summarized scheme of the triterpenoid biosynthesis pathway. (B) The content of madecassoside, asiaticoside, madecassic acid, and asiatic acid obtained from C. asiatica leaf extracts under different LED qualities. Each bar represents the mean ± SD of three replicates. Letters (a–g) indicate significant difference at p < 0.05 using ANOVA with Duncan's multiple range test. (C) Gene expressions of the four triterpenoid-relating genes CaSQS, CabAS, CaUGT, and CaCYS illustrated using a heatmap. The asterisks (*) represent significance difference in gene expression compared with C. asiatica leaves under non-incubation (NI) by Student's t-test at p < 0.05.
2.4. Alteration of centelloside constituents and gene expression of postharvest C. asiatica leaf treated by different LEDs
Centelloside composition including madecassoside, asiaticoside, madecassic acid and asiatic acid was investigated. Centelloside content increased at longer incubation in all treatments, with the highest amounts observed at 3 dph under white and blue LEDs at 3.78 and 3.83 %DW, respectively (Fig. 2C). At 0 dph, triterpene glycoside content including madecassoside and asiaticoside was higher than triterpene including madecassic acid, and asiatic acid (Fig. 2B). Triterpene glycosides including madecassoside and asiaticoside significantly increased in a time-dependent manner under different LED quality. White and blue conditions gave the highest amounts of madecassoside and asiaticoside (Fig. 2B), with madecassoside production under white and blue LEDs at 3 dph 2.31 ± 0.24, and 2.36 ± 0.03 %DW, respectively and twice the value of the control as 0 dph. Asiaticoside recorded higher content under white LED at 2 and 3 dph (1.47 ± 0.11 and 1.44 ± 0.21 %DW, respectively) (Fig. 2B). For triterpene aglycones, madecassic acid and asiatic acid contents were steady for all treatments, excluding sunshade which was higher at 3 dph. By contrast, dark condition decreased madecassic acid and asiatic acid contents in a time-dependent manner.
C. asiatica leaves were subjected to different light qualities including sunshade, dark, white, and blue treatments to determine the gene expression of triterpenoid biosynthesis genes including C. asiatica squalene synthase (CaSQS), C. asiatica β-amyrin synthase (CabAS), C. asiatica UDP gluclosyltransferase-73AH1 (CaUGT73AH1; CaUGT), and C. asiatica cycloartenol synthase (CaCYS) (Fig. 2A). Sunshade, white and blue treatments up-regulated the expression of four genes in a time-dependent manner in different ways for each light quality (Fig. 2C). Under sunshade treatment, the four genes were slightly up-regulated compared with the control at 0 dph, whereas white and blue treatments increased the expression level of CaSQS and CabAS, especially at 1 dph (Fig. 2C). Notably, up-regulation of CaUGT was recorded only under blue treatment in a time-dependent manner, particularly at 3 dph (Fig. 2C). The expression of CaCYS was stimulated under dark condition with the highest value at 2 dph followed by sunshade, whereas the other light quality parameters did not affect CaCYS expression (Fig. 2A).
2.5. Pairwise correlation of various parameters obtained from postharvest C. asiatica leaves
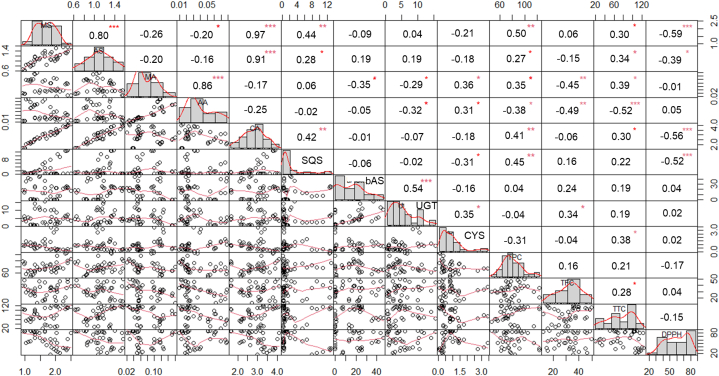
Pearson's correlation coefficient analyses of parameters including centelloside content, phytochemical and gene expressions derived from postharvest C. asiatica leaves treated with different LEDs are illustrated in Fig. 3. Results within the triterpene glycoside and triterpene aglycone groups showed significant positive correlation at r = 0.80 and 0.86, respectively whereas a negative relationship was found between triterpene glycoside and triterpene aglycone groups (r = −0.39 to −0.16). Gene expressions of CaSQS, CabAS, and CaUGT positively correlated with amounts of madecassoside and asiaticoside but negatively correlated with madecassic acid and asiatic acid, while the expression level of CaCYS exhibited negative correlation with triterpene glycosides. Expression levels of CaCYS and CaUGT showed positive correlation at r = 0.38, and findings also exhibited a significantly positive correlation between CaSQS and CabAS with r = 0.55. Phytochemical analysis indicated that gene expression of CaSQS positively correlated with TPC (r = 0.40) and negatively correlated with antioxidant activity (r = −0.52). The expression level of CaUGT had a positive correlation with TFC (r = 0.34), while the expression of CaCYS showed decreased TPC and TTC (r = −0.26 and −0.36, respectively) (Fig. 3).
Fig. 3.
Correlation among centellosides, phytochemical, and gene expressions after LED treatment of C. asiatica leaves at 3 dph.
Pearson's correlation coefficients between centellosides (CA), madecassosides (MS), asiaticosides (AS), madecassic acid (MA), asiatic acid (AA), total phenol content (TPC), total flavonoid content (TFC), and gene expression (CaSQS, CaβAS, CaUGT, and CaCYS) in C. asiatica leaves at 3 dph. Values on the upper diagonal indicate Pearson's correlation (r) between two variables. The symbols of *, **, and *** represent the p < 0.05, 0.01, and 0.001, respectively.
3. Discussion
The World Health Organization (WHO) has stated that the content of ester glycosides (asiaticoside and madecassoside) in C. asiatica raw material must not be less than 2 % [26]. According to the British Pharmacopoeia, the fermented dried aerial part of C. asiatica contains a minimum of 6 % of total triterpenoid derivatives [27] and the Thai Herbal Pharmacopoeia describes similar percentages [28]. However, significant differences have been recorded in the major active ingredients among C. asiatica samples from different countries and cultivation seasons, with amount of triterpenoids varying from 1 % to 8 % [27]. Total triterpenoid content may be lower than recorded in the pharmacopoeia text books, contributing to low quality for medicinal use (<2 %). In recent times, preharvest and postharvest LEDs have emerged as a promising approach to increase bioactive compounds in various economically important plants, in addition to chemical treatments [14,17,21,22]. Postharvest LED technology was employed to optimize the centelloside content of C. asiatica raw material and comply with pharmacopeial regulations. This study was the first to demonstrate that postharvest LED technology enhanced total triterpenoid content in the leaves of C. asiatica. Findings demonstrated that the application of white and blue LEDs on C. asiatica leaves for 3 dph significantly stimulated the accumulation of centelloside, especially asiaticoside and madecassoside content, with a 73 % increase compared with the control at 0 dph. Increased amounts of centelloside corresponded to up-regulation of the relevant genes CaUGT, CaSQS, and CabAS. Light quality impacted the phytochemistry and accumulation of pigments on C. asiatica leaves. These findings showed that LED irradiation was a possible postharvest option to augment the secondary metabolites in the raw material of C. asiatica leaves.
Apart from photosynthesis, light plays a vital role in plant morphological and developmental changes. The absorption of light by plants involves two prominent phytochromes that specifically absorb red and far-red light wavelengths, along with cryptochromes that selectively absorb blue and ultraviolet A (UV-A) light [29]. Light quality impacts the synthesis of secondary metabolites in plants. Several studies have assessed the impact of light quality on a wide range of plant species such as lettuce (Lactuca sativa L.), tea (Camellia sinensis), carrot (Daucus carota subsp. sativus) and brahmi (Bacopa monnieri (L.) Pennell), and C. asiatica [19,22,30]. Results showed that red and blue LED irradiation exhibited higher growth performance and bioactive compound accumulation than under white LEDs (normally used as the control) or other LEDs [19,[30], [31], [32], [33]], while postharvest treatment by blue LEDs on C. asiatica showed highest total centelloside content. White LED irradiation increased total centelloside content but was less effective than blue LEDs. Song et al. (2022) [23] reported optimal increase in C. asiatica centelloside content under 80 % red LED combined with 20 % blue LED treatment at 200 μmol m−2s−1. Ginsenosides, a triterpenoid ingredient, showed increase in Panax ginseng C.A.Mey. root under blue LEDs (450 nm and 470 nm) combined with dark condition. Blue LED treatment stimulated key enzymes in the isoprenoid pathway (such as squalene synthase) by inducing the generation of reactive oxygen species (ROS) which triggered the expression of several defense genes and the synthesis of ginsenosides [29,34]. Therefore, blue LEDs play an important role by triggering the triterpenoid pathway, as summarized and illustrated in Fig. 2. Relationship analysis between the triterpenoid content and gene expression suggested that the centelloside content was moderately connected with the expression level of CaSQS (r = 0.41), while the expression level of CaUGT was negatively correlated with madecassic acid and asiatic acid (r = −0.30 and −0.32, respectively). UDP-glycosyltransferases could play a role in transforming α-amyrin and β-amyrin derivatives into asiaticoside and madecassoside [5,35,36]. Kim et al. (2017) [37] performed a transcriptomic analysis of C. asiatica leaves treated by methyl jasmonate (MeJA) elicitor, with results revealing CaUGT (UGT73AH1) as a candidate gene involved in asiaticoside biosynthesis. Similarly, up-regulation of the UGT73AH1 gene in C. asiatica callus tissue enabled increase in asiaticoside content [38]. The negative relationship between the CaUGT and CaβAS genes with high correlation (r = −0.55), suggested a possible negative feedback loop that requires further investigation.
White LED irradiation showed an increase in centelloside content, similar to blue light, because white LEDs contain various wavelengths including the blue wavelength at low intensity, as an effective tool to stimulate the synthesis of bioactive compounds [39,40]. Under sunshade treatment, the expression of CaβAS was up-regulated possibly due to heat. High temperatures during the summer season impacted the expression levels of β-amyrin synthase, an oxidosqualenecyclase that plays a role in the biosynthesis of triterpenoid saponins in Glycyrrhiza glabra [11,41]. The dark condition induced the expression of CaCYS, while expressions of CaSQS, CaβAS, and CaUGT remained inactive. The CaCYS gene encoded a cycloartenol synthase to catalyze the (S)-2,3-epoxysqualene substrate with CaβAS enzyme. In this study, dark condition produced lower amounts of centelloside than the other LED treatments.
Postharvest alteration of morphological features (freshness and color) of C. asiatica leaves by light quality was also determined. Freshness and color of C. asiatica leaves reduced at longer incubation time under blue and green LEDs, and especially under red LEDs because they had high intensity and energy, while amounts of chlorophyll a, chlorophyll b, chlorophyll ab, and carotene also increased, with the highest amounts in red and blue LEDs. In the review of Perera et al. (2022) [42], it summarized that intensity range at 10–35 μmol m−2s−1 of white, green, blue and red LEDs appeared to be effectively preserved the chlorophyll content in vegetable. The most efficient wavelengths for plant photosynthesis are red and blue LEDs because photosynthetic pigments mainly absorb light in the range of 600–700 nm for red LEDs and 400–500 nm for blue LEDs [43]. Red and blue LEDs also provide the maximum spectra for photosynthesis. Leaf cells may produce sugar from the remaining plant resources, contributing to high cell survival rate. The results indicated that red LEDs improved the freshness of raw materials. In brussels sprouts (B. oleracea gemmifera) and Pak-choi (B. rapa spp. chinensis), red LED light exposure at 20 °C for five days resulted in the postponement of senescence and inhibition of various enzyme activities including chlorophyllase, chlorophyll-degrading peroxidase, Mg-dechelatase, and pheophytinase, as well as suppression of the genes associated with chlorophyll degradation (BrChlase1, BrChlase2, and BrPPH). Furthermore, expression of the chlorophyll synthetase gene, BrHEMA1, increased after seven days at 20 °C [44,45]. Broccoli (B. oleracea) under red LEDs showed decreased phytochemical content with retained chlorophyll, while yellow spot was impeded for 4–5 days at 20 °C [21,45].
Alteration of the phytochemistry of C. asiatica leaves as TPC, TFC, and TTP by different LEDs stimulated the accumulation of different phytochemical compounds. Blue LEDs increased accumulation of TPC and TTC, concurring with Song et al. (2022) [23]. The expression levels of several genes (CaABI5, CaMYB4, CaMYB12, and CaHYH) in the flavonoid biosynthetic pathway under blue LEDs were higher than under red LEDs [25]. Transcription factors ABI5, MYB4, and HYH gene were active as hub nodes of the protein-protein interaction network of differential expression genes (DEGs) under blue LEDs and correlated with the expression levels of several genes [25]. Synthesis of these phytochemical ingredients also linked with the phenylalanine ammonia-lyase enzyme (PAL). A few studies revealed augmented production of plant secondary metabolites under red and blue LEDs, possibly resulting from the up-regulation of PAL [29,31].
The significant findings of postharvest white and blue LEDs at intensity of ∼30 μmol m−2s−1 enable improvement of the bioactive compounds such as triterpenoid glycosides in C. asitica leaves. However, several previous studies for postharvest LED technology have indicated that, besides selecting the appropriate wavelength, various factors need consideration. These factors encompass the type of vegetable species, cultivar, maturity level of the harvested vegetables, and the specific nutrient or desired postharvest quality attribute intended for preservation [42]. For improving quality of the raw materials of C. asiatica leaves using LED technology, the optimal intensity range of blue LEDs should be further investigated. For example, in tomatoes and sweet peppers, the lycopene content was increased under both postharvest red and blue LED at 113–118 and 100–150 μmol m− 2 s− 1, respectively [[46], [47], [48], [49]]. The higher intensities of blue or white LED might improve the triterpenoid content in C. asiatica leaves after harvesting.
4. Conclusion
This was the first study to demonstrate that postharvest blue and white LED light treatment of C. asiatica leaves stimulated the synthesis of centellosides, particularly madecassoside and asiaticoside, by increasing the expression of downstream genes in the triterpenoid pathway including CaSQS, CaβAS, and CaUGT. Different LED treatments had varying effects on the phytochemical content of the leaves. White LED treatment resulted in increased TTP content, while blue LED treatment increased both TFC and TTC. Red LED treatment, on the other hand, increased phytochemical contents, while also preserving chlorophyll, potentially prolonging the freshness of the leaves. The overview of significant findings to demonstrate the postharvest LED effect on secondary metabolite production of C. asiatica leaves was illustrated in Fig. 2. Postharvest LED treatment is an inexpensive, simple, and environmentally-friendly method that can be easily scaled up for industrial use. Results suggested that this approach has promise for enhancing the bioactive ingredients of various medicinal plants and could improve the quality of raw materials used in medicinal industries such as cosmetics and food supplements.
5. Materials and methods
5.1. Plant materials and LED treatments
Mature leaves commercially cultivated in the Nakhon Pathom accession were used for all treatments with leaf width 3.50 ± 1 cm. The C. asiatica leaves were exposed to six different light treatments comprising sunshade, white LED (27 μmol m−2s−1), dark, red LED (24.7 μmol m−2s−1 at 650 nm), blue LED (29.5 μmol m−2s−1 at 450 nm), and green LED (30.5 μmol m−2s−1 at 530 nm) for three days. In this study, white, red, blue and green LEDs at intensity range of 25–30 μmol m−3s−1 were performed due to effective preservation of chlorophyll content and some LEDs increase/preserve the nutritional value in leafy and non-leafy vegetable [42]. Leaves of C. asiatica harvested at day 0 were used as the control, with non-incubation (NI). Each treatment used ten leaves and was conducted in triplicate. All treatments were incubated for 1, 2, and 3 dph at 25 ± 2 °C, 50 % relative humidity except for sunshade which was incubated at ambient temperature. Ten leaves were collected each day and dried in a hot air oven at 60 °C. The dried leaves were stored at −20 °C for further analysis.
5.2. Methanolic extraction of C. asiatica
Leaves incubated for 1, 2, and 3 days were dried in a hot air oven at 60 °C for 48 h. The C. asiatica extract was carried out according to a modified method of Alqahtani et al. (2015) [11]. The dried leaves were then weighed at 100 mg, homogenized to powder using a BeadBug Microtube Homogenizer D1030-E (Benchmark Scientific, USA), and passed through a 2 mL sieve. The C. asiatica extract was obtained by adding 1 mL of methanol to the powder and mixing in a vortex mixer at 3500 rpm for 15 min before sonication in an ultrasonic bath for 15 min and centrifugation at 3500 rpm for 5 min. The supernatant was removed, and the rest of the pellet was repeated for extraction once. The supernatant collected from two rounds was pooled and filtrated through a 0.45 μm nylon membrane, with the volume adjusted to 2 mL by adding methanol (HPLC-grade, RCI Labscan LIMITED, Thailand) and storing at −20 °C for further chemical analysis.
5.3. Determination of centelloside content by high performance liquid chromatography (HPLC)
To determine the amount of four major triterpenoids including asiatic acid, asiaticoside, madecassic acid, and madecassoside from the different quality of light treatments, the methanolic extracts were carried out by HPLC (Agilent 1100 Series HPLC, Agilent Technologies, Germany) according to the slightly modified method of Alqahtani et al. (2015) [11]. Chromatographic separation was achieved using a reversed-phase C18 Aqua 5 μm 125 A (250 × 4.60 mm) column connected to a 1 mm Opti Guard C18 pre-column, which was kept at 27 °C. Solvent A (0.3 % phosphoric acid in HPLC-grade water) and solvent B (100 % acetonitrile, RCI Labscan LIMITED, Thailand) were used as the mobile phase. The gradient elution profile was as follows: 0 min, 0%A:6.0 % B; 15 min, 72%A:28 % B; 35 min, 40%A:60 % B; 37 min, 20%A:80 % B; 45 min, 80%A:20 % B. The solution was pumped through the system at a rate of 0.9 mL/min for a total of 55 min, followed by a re-equilibration period of 10 min. A volume of 10 μL of the sample was injected into the system. The standard solution was asiatic acid (TCI, Japan, purity >97 %), asiaticoside (Sigma-Aldrich, USA, purity 98.5 %), madecassic acid (Abcam, USA, purity >95 %), and madecassoside (Sigma-Aldrich, USA, purity ≥95 %) in methanol, with concentration ranging from 6.50 to 200 μg/mL. Chromatographic data processing software was utilized to monitor the chromatograms at a wavelength of 210 nm.
5.4. Determination of total triterpenoid content (TTC)
The total triterpenoid content (TTC) was determined using a modified method of Wei et al. (2015) [50] and reported as mg ursolic acid (Sigma-Aldrich, USA, purity >90 %) equivalent per gram of dry weight. Initially, the extracts were diluted ten times, and 30 μL of the resulting solution was placed into a 96-well microplate. The extract was evaporated using a hot air oven, followed by the addition of 10 μL of fresh mixed 5 % (w/v) vanillin-acetic solution (vanillin purchased from Sigma-Aldrich, USA, purity 99 %) and 38 μL of 98 % (v/v) sulfuric acid. After mixing, the solution was incubated at 70 °C for 30 min and cooled before being diluted to 200 μL with acetic acid. The absorbance of the resulting solution was measured at 573 nm using a microplate reader (BioTek Synergy H1 Multimode Reader, Agilent Technologies, USA) against a blank that contained all reagents and solvents except for the sample solution. To create the standard curve, ursolic acid (Sigma-Aldrich, USA, purity ≥90 %) was used in concentrations ranging from 0 to 100 μg/mL. The results were expressed as mg ursolic acid equivalent per gram of dry weight (mg UAE/g), and sterile distilled water was used as the negative control.
5.5. Determination of total flavonoid content (TFC)
To determine the amount of total flavonoid content (TFC) in a 96-well microplate [51], an aliquot (30 μL) of the methanolic extract solution was mixed with 120 μL of sterile distilled water and 10 μL of 5 % (w/v) NaNO2 solution. The mixture was incubated for 5 min at 25 °C before the addition of 10 % (w/v) AlCl3 solution (10 μL). The mixture was allowed to stand for 6 min at ambient temperature before the addition of 1 N NaOH solution (60 μL). The reaction mixture was then diluted to a volume of 300 μL with sterile distilled water, thoroughly mixed, and incubated at 25 °C for 15 min. The intensity of the pink color was measured at 510 nm using a microplate reader (BioTek Synergy H1 Multimode Reader, Agilent Technologies, USA). To create the standard curve, quercetin (Fisher Scientific, USA, purity >95 %) was used in concentrations ranging from 0 to 100 μg/mL. The results were expressed as mg quercetin equivalent per gram of dry weight (mg QCE/g), and sterile distilled water was used as the negative control.
5.6. Determination of total phenolic content (TPC)
Phenolic content of the plant extract was estimated using Folin-Ciocalteu's (FC) phenol reagent following Govarthanan et al. (2015) [51] with minor modifications. Briefly, 10 μL of a standard solution of gallic acid (Bio Basic INC, Canada, purity >98 %) at different concentrations or diluted C. asiatica extracted (1:5) were added to 96-well microplates containing 125 μL of sterile distilled water. Then, Folin-Ciocalteu's phenol reagent (Carlo Erba Reagents, Italy) was added for 10 μL to the 96-well microplates, thoroughly mixed and incubated for another 5 min at room temperature. Lastly, Na2CO3 solution (7.5 % w/v) was added for 100 μL to the above mixture with constant stirring, and the volume was immediately made up to 300 μL with sterile distilled water. The reaction mixture was incubated at ambient temperature for 1 h and the absorbance was measured at 750 nm using a microplate reader (BioTek Synergy H1 Multimode Reader, Agilent Technologies, USA). Total phenolic content in C. asiatica plants was expressed as mg gallic acid equivalent per gram dry weight (0–100 μg/mL) (mg GAE/g), with sterile distilled water used as the negative control. All experiments were conducted in duplicate and mean values were recorded.
5.7. Determination of free radical scavenging activity by 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay
To evaluate the scavenging activity of DPPH free radicals, Govarthanan et al. (2015) [51] method was used with a slight modification. Initially, a methanolic extract of the sample (diluted 1:10) or gallic acid at various concentrations (ranging from 6.25 to 200 μg/mL) was mixed with 100 μL of 0.1 mM methanolic DPPH solution (Sigma-Aldrich, USA), followed by incubation for 20 min at room temperature in the dark. The resulting mixture was then measured at a wavelength of 517 nm using a microplate reader (BioTek Synergy H1 Multimode Reader, Agilent Technologies, USA). The negative control was sterile distilled water. The scavenging activity of the extracts was determined using the following formula: inhibition (%) = [(absorbance of control – absorbance of sample) x 100]/absorbance of control, where absorbance control is the absorbance of DPPH solution without extract and Abs sample is the absorbance of the sample with DPPH solution.
5.8. Chlorophyll analysis
The amount of chlorophyll in five field-grown leaves was estimated according to Ramírez-Mosqueda et al. (2015) [52]. The process involved homogenizing 0.05 g of fresh leaves for each treatment in a mortar that was pre-chilled, using a 1 mL mixture of 80 % cold acetone and absolute ethanol. After homogenization, the resulting mixture was transferred to a 1.5-mL test tube and centrifuged at 10,000 rpm for 5 min. The supernatant was then collected, and the absorbance was measured at 663 nm (for chlorophyll a), 645 nm (for chlorophyll b), and 441 nm (for carotenes) using a microplate reader (BioTek Synergy H1 Multimode Reader, Agilent Technologies, USA). The amounts of chlorophyll and carotenes were determined using following formulas,
| Chl a = [(12.25 x A663− 2.25 x A645)] x [1/(100 x W)] |
| Chl b = [(20.30 x A645− 4.91 x A663)] x [1/(100 x W)] |
| Chl a+b = [(7.34 x A663 + 17.76 x A645)] x [1/(100 x W)] |
| Car = [(4.46 x A441 – Chl a) + Chl b)] x [1/(100 x W)] |
where V is the total volume of acetone extract (mL), and W is the fresh weight (g) of the sample.
5.9. Gene expression relating to centelloside synthesis pathway by realtime quantitative PCR (RT-qPCR)
Results of centelloside content by high performance liquid chromatography (HPLC) and phytochemical analysis indicated that white and blue LED treatments exhibited potential to increase centelloside content, total flavonoid content, and total triterpenoid content. Therefore, a few leaves were selected from non-incubation, dark, white LED, and blue LED samples at 1, 2, and 3 dph to investigate the level of gene expression by RT-qPCR. The incubated leaves of each treatment were isolated for RNA using the RNeasy Plant Mini Kit (Qiagen, Germany), according to the manufacturer's instructions. RNA integrity was assessed using 1 % agarose gel electrophoresis and the NanoDrop Lite (Thermo Fisher Scientific, Inc., MA, USA) at 260 and 280 nm. RevertAid First strand cDNA Synthesis Kit (Thermo Fisher Scientific, USA) was used for synthesis of first-strand cDNA. The cDNA obtained from each treatment was used to determine the gene expression of CaSQS, CabAS, CaUGT, and CaCYS using a reaction of 25 μL consisting of 12.5 μL of 2X SYBR Green qPCR Master Mix (Thermo Fisher Scientific, USA), 0.2 μM forward primer and 0.2 μM reverse primer (Table 1), and 1 μL of 1:3 cDNA, with sterile water added up to 25 μL. The DNA amplification was performed using a RT-qPCR realtime PCR machine (Bioer Line-Gene 9600 Plus Real Time Thermal Cycler PCR Systems, USA) with initial denaturation at 95 °C for 5 min followed by amplification at 95 °C for 30 s, 58 °C for 15 s, and 72 °C for 30 s for 40 cycles and a final extension at 72 °C for 5 min. After completing the reactions, the threshold cycle (Ct) value for each reaction was obtained. C. asiatica actin (CaActin) was used for the normalization of gene expression. The relative gene expression to non-incubation treatment was derived using the 2−ΔΔCt method [53].
Table 1.
Primer sequences used to amplify triterpenoid biosynthesis gene expression.
| Gene | Sequence | Amplicon size (bp) | References |
|---|---|---|---|
| CaCYS | Forward primer 5′-GAATCCACGCCATGAAGTCT–3′ | 421 | [54] |
| Reverse primer 5′-ACCACCATGATCCAGAATCC-3′ | |||
| CabAS | Forward primer 5′-TGGTTGGGGAGAAAGTCTTG-3′ | 302 | [54] |
| Reverse primer 5′-ACAAGCGTTTGCGGTACTCT-3′ | |||
| CaSQS | Forward primer 5′-TGGGTTAGGGTTGTCAAAGC-3′ | 324 | [54] |
| Reverse primer 5′-CGGAAGATAGCAGGATCTCG-3′ | |||
| CaUGT (UGT73AH1) | Forward primer 5′-GCATCATGTCTGAAGATGAGG-3′ | 100–200 | [38] |
| Reverse primer 5′- GCTTAATGCTAACCTATCCTT-3′ | |||
| CaActin | Forward primer 5′-GATGACATGGAAAAGATTTGGCATC-3′ | 325 | [37] |
| Reverse primer 5′-TGTTGTACGACCACTGCATACAGG-3′ |
5.10. Statistical analysis
The mean and standard deviation (SD) were used to express the centelloside content, phytochemical analysis, chlorophyll, and relative gene expression. To determine if there were any statistically significant differences among treatments, analysis of variance (ANOVA) was performed, and Duncan’ s test was conducted to compare multiple treatments. Significant differences were considered when p < 0.01 or p < 0.05. To examine the relationship among all parameters, including triterpene glycosides, antioxidant activities, TTC, TPC, TFC, and relative gene expression in C. asiatica leaves, pairwise correlation using Pearson's correlation coefficients was calculated in R.
Data availability
Data associated with our study has not been deposited into a publicly available repository. Data included in article. The data that support the results presented in this paper are available from the corresponding author, upon reasonable request. The raw figures of C. asiatica leaves irradiated under different light qualities for three days were showed in Supplemental File 1 and the nitrate determination of C. asiatica leaves was showed in Supplemental File 2. The average and standard deviation (S.D.) of major ingredients, phytochemicals, pigments, antioxidants and gene expression were deposited in Supplemental File 3.
CRediT authorship contribution statement
Puntitra Kamol: Writing – review & editing, Writing – original draft, Visualization, Methodology, Investigation, Formal analysis, Data curation. Wanrachon Nukool: Investigation, Data curation. Sakuntala Pumjaroen: Investigation, Data curation. Phithak Inthima: Methodology, Investigation, Conceptualization. Anupan Kongbangkerd: Supervision, Methodology, Investigation. Nungruthai Suphrom: Supervision, Resources, Methodology, Investigation, Formal analysis. Kittisak Buddhachat: Writing – review & editing, Writing – original draft, Project administration, Methodology, Funding acquisition, Formal analysis, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was supported by Naresuan University (NU), Thailand and the National Science, Research and Innovation Fund (NSRF), Thailand under grant number R2565B076, with partial support from the Global and Frontier Research University Fund, Naresuan University, Thailand; Grant number R2566C051.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e23639.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Mathavaraj S., Sabu K.K. Genetic status of Centella asiatica (L.) Urb. (Indian pennywort): a review. Curr. Bot. 2021;12:150–160. doi: 10.25081/cb.2021.v12.6971. [DOI] [Google Scholar]
- 2.Tan S.C., Bhattamisra S.K., Chellappan D.K., Candasamy M. Actions and therapeutic potential of madecassoside and other major constituents of Centella asiatica: a review. Appl. Sci. 2021;11:8475. doi: 10.3390/app11188475. [DOI] [Google Scholar]
- 3.Bandopadhyay S., Mandal S., Ghorai M., Jha N.K., Kumar M., Radha, Ghosh A., Proćków J., Perez de la Lastra J.M., Dey A. Therapeutic properties and pharmacological activities of asiaticoside and madecassoside: a review. J. Cell Mol. Med. 2023;27(5):593–608. doi: 10.1111/jcmm.17635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tripathy S., Verma D.K., Thakur M., Chakravorty N., Singh S., Srivastav P.P. Recent trends in extraction, identification and quantification methods of Centella asiatica phytochemicals with potential applications in food industry and therapeutic relevance: a review. Food Biosci. 2022;49:1–25. doi: 10.1016/j.fbio.2022.101864. [DOI] [Google Scholar]
- 5.Prakash V., Jaiswal N., Srivastava M. A review on medicinal properties of Centella asiatica. Asian J. Pharmaceut. Clin. Res. 2017;10(10):69–74. doi: 10.22159/ajpcr.2017.v10i10.20760. [DOI] [Google Scholar]
- 6.Wang P., Wei G., Feng L. Research advances in oxidosqualene cyclase in plants. Forests. 2022;13:1382. doi: 10.3390/f13091382. [DOI] [Google Scholar]
- 7.Park K.S. Pharmacological Effects of Centella asiatica on skin diseases: evidence and possible mechanisms. Evid. Based Complementary Altern. Med. 2021:1–8. doi: 10.1155/2021/5462633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J., Zhang H., Tan Q. Asiaticoside expedites recovery of diabetic ulcers through activation of Wnt1/β-catenin signaling cascade. Res. Sq. 2022:1–27. doi: 10.21203/rs.3.rs-1396209/v1. [DOI] [Google Scholar]
- 9.Wong J.H., Barron A.M., Abdullah J.M. Mitoprotective effects of Centella asiatica (L.) Urb.: anti-inflammatory and neuroprotective opportunities in neurodegenerative disease. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.687935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biswas D., Mandal S., Saha S.C., Tudu C.K., Nandy S., Batiha G.E.S., Shekhawat S.M., Pandey D.K. Ethnobotany, phytochemistry, pharmacology, and toxicity of Centella asiatica (L.) Urban: a comprehensive review. Phytother Res. 2021;5:6624–6654. doi: 10.1002/ptr.7248. [DOI] [PubMed] [Google Scholar]
- 11.Alqahtani A., Tongkao-on W., Li K.M., Razmovski-Naumovski V., Chan K., Li G.Q. Seasonal variation of triterpenes and phenolic compounds in Australian Centella asiatica (L.) Urb, Phytochem. Anal. 2015;26:436–443. doi: 10.1002/pca.2578. [DOI] [PubMed] [Google Scholar]
- 12.Rahajanirina V., Rakotondralambo Raoseta S.N.O., Roger E., Razafindrazaka H., Pirotais S., Boucher M., Danthu P. The influence of certain taxonomic and environmental parameters on biomass production and triterpenoid content in the leaves of Centella asiatica (L.) Urb. from Madagascar. Chem. Biodivers. 2012;9(2):298–308. doi: 10.1002/cbdv.201100073. [DOI] [PubMed] [Google Scholar]
- 13.Buraphaka H., Putalun W. Stimulation of health-promoting triterpenoids accumulation in Centella asiatica (L.) Urban leaves triggered by postharvest application of methyl jasmonate and salicylic acid elicitors. Ind. Crops Prod. 2020;146:1–12. doi: 10.1016/j.indcrop.2020.112171. [DOI] [Google Scholar]
- 14.Nicole C.C.S., Mooren J., Pereira Terra A.T., Larsen D.H., Woltering E.J., Marcelis L.F.M., Verdonk J., Schouten R., Troost F. Effects of LED lighting recipes on postharvest quality of leafy vegetables grown in a vertical farm. Acta Hortic. 2019;1259:481–488. doi: 10.1111/j.1744-7348.1989.tb06577. [DOI] [Google Scholar]
- 15.Bantis F., Ouzounis T., Radoglou K. Artificial LED lighting enhances growth characteristics and total phenolic content of Ocimum basilicum, but variably affects transplant success. Sci. Hortic. 2016;198:277–283. doi: 10.1016/j.scienta.2015.11.014. [DOI] [Google Scholar]
- 16.Poonia A., Pandey S. Application of light emitting diodes (LEDs) for food preservation, post-harvest losses and production of bioactive compounds: a review. Food Prod. Process. Nutr. 2022;4(1):8. doi: 10.1186/s43014-022-00086-0. [DOI] [Google Scholar]
- 17.Liu S., Hu L., Jiang D., Xi W. Effect of post-harvest LED and UV light irradiation on the accumulation of flavonoids and limonoids in the segments of newhall navel oranges (Citrus sinensis Osbeck) Molecules. 2019;24:1–20. doi: 10.3390/molecules24091755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baenas N., Iniesta C., González-Barrio R., Nuñez-Gómez V., Periago M.J., García-Alonso F.J. Post-harvest use of ultraviolet light (UV) and light emitting diode (LED) to enhance bioactive compounds in refrigerated tomatoes. Molecules. 2021;26:1847. doi: 10.3390/molecules26071847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martínez-Zamora L., Castillejo N., Gómez P.A., Artés-Hernández F. Amelioration effect of LED lighting in the bioactive compounds synthesis during carrot sprouting. Agronomy. 2021;11(2):304. doi: 10.3390/agronomy11020304. [DOI] [Google Scholar]
- 20.Nájera C., Guil-Guerrero J.L., Enríquez L.J., Álvaro J.E., Urrestarazu M. LED-enhanced dietary and organoleptic qualities in postharvest tomato fruit. Postharvest Biol. Technol. 2018;145:151–156. doi: 10.1016/j.postharvbio.2018.07.008. [DOI] [Google Scholar]
- 21.Jiang A.L., Zuo J.H., Zheng Q.L., Guo L., Gao L.P., Zhao S.G., Wang Q., Hu W.Z. Red LED irradiation maintains the postharvest quality of broccoli by elevating antioxidant enzyme activity and reducing the expression of senescence-related genes. Sci. Hortic. 2019;251:73–79. doi: 10.1016/j.scienta.2019.03.016. [DOI] [Google Scholar]
- 22.Shao M., Liu W., Zha L., Zhou C., Zhang Y., Li B. Differential effects of high light duration on growth, nutritional quality, and oxidative stress of hydroponic lettuce under red and blue LED irradiation. Sci. Hortic. 2020;268:109–366. doi: 10.1016/j.scienta.2020.109366. [DOI] [Google Scholar]
- 23.Song J.W., Bhandari S.R., Shin Y.K., Lee J.G. The influence of red and blue light ratios on growth performance, secondary metabolites, and antioxidant activities of Centella asiatica (L.) Urban. Horticulturae. 2022;8(601):1–15. doi: 10.3390/horticulturae807060. [DOI] [Google Scholar]
- 24.Devkota A., Kumar J.P. Effect of different light levels on the growth traits and yield of Centella asiatica, Middle East. J. Sci. Res. 2010;5(4):226–230. http://doi:10.1016/j.bse.2009.12.019 [Google Scholar]
- 25.Nawae W., Yoocha T., Narong N., Paemanee A., Ketngamkum Y., Romyanon K., Toojinda T., Tangphatsornruang S., Pootakham W. Transcriptome sequencing revealed the influence of blue light on the expression levels of light-stress response genes in Centella asiatica. PLoS One. 2021:1–19. doi: 10.1371/journal.pone.0260468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization, Geneva. WHO Monographs on Selected Medicinal Plants Volume I : Herba Centellae. 1999. pp. 59–85. [Google Scholar]
- 27.Delbo M., Calapai G. European Medicines Agency; London, UK: 2010. Assessment Report on Centella Asiatica (L.) Urban, Herba. European Medicines Agency Science Medicines Health. [Google Scholar]
- 28.Supplement to Thai Herbal Pharmacopoeia. Nonthaburi: Department of Medical Sciences, Ministry of Public Health; 2016. pp. 36–44. [Google Scholar]
- 29.Hasan M., Bashir T., Ghosh R., Lee S.K., Bae H. An overview of LEDs' effects on the production of bioactive compounds and crop quality. Molecules. 2017;22:1–12. doi: 10.3390/molecules2209142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang P., Chen S., Gu M., Chen X., Chen X., Yang J., Zhao F., Ye N. Exploration of the effects of different blue LED light Intensities on flavonoid and lipid metabolism in tea plants via transcriptomics and metabolomics. Int. J. Mol. Sci. 2020;21:1–17. doi: 10.3390/ijms21134606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y., Shi R., Jiang H., Wu L., Zhang Y., Song S., Su W., Liu H. End-of-day LED lightings Influence the leaf color, growth and phytochemicals in two cultivars of lettuce. Agronomy. 2020;10(10):1–19. doi: 10.3390/agronomy10101475. [DOI] [Google Scholar]
- 32.Watcharatanon K., Ingkaninan K., Putalun W. Improved triterpenoid saponin glycosides accumulation in in vitro culture of Bacopa monnieri (L.) Wettst with precursor feeding and LED light exposure. Ind. Crops Prod. 2019;134:303–308. doi: 10.1016/j.indcrop.2019.04.011. [DOI] [Google Scholar]
- 33.Solouki A., Mehrjerdi M.Z., Aliniaeifard S., Azimi R. Postharvest light and temperature elicitors improve chemical composition and level of essential oils in basil (Ocimum basilicum L.) through boosting antioxidant machinery. Postharvest Biol. Technol. 2023;19:1–14. doi: 10.1016/j.postharvbio.2023.112279. [DOI] [Google Scholar]
- 34.Di P., Sun Z., Cheng L., Han M., Yang L., Yang L. LED light irradiations differentially affect the physiological characteristics, ginsenoside content, and expressions of ginsenoside biosynthetic pathway genes in panax ginseng. Agriculture. 2023;13:807. doi: 10.3390/agriculture13040807. [DOI] [Google Scholar]
- 35.Alcalde M.A., Cusido R.M., Moyano E., Palazon J., Bonfill M. Metabolic gene expression and centelloside production in elicited Centella asiatica hairy root cultures. Ind. Crops Prod. 2022;184 doi: 10.1016/j.indcrop.2022.114988. [DOI] [Google Scholar]
- 36.Kim O.T., Um Y., Jin M.L., Kim J.U., Hegebarth D., Busta L., Racovita R.C., Jetter R. A novel multifunctional C-23 oxidase, CYP714E19, is involved in asiaticoside biosynthesis. Plant Cell Physiol. 2018;59(6):1200–1213. doi: 10.1093/pcp/pcy055. [DOI] [PubMed] [Google Scholar]
- 37.Kim O.T., Jin M.L., Lee D.Y., Jetter R. Characterization of the asiatic acid glucosyltransferase, UGT73AH1, involved in asiaticoside biosynthesis in Centella asiatica (L.) Urban. Int. J. Mol. Sci. 2017;18(12):2630. doi: 10.3390/ijms18122630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muhsinin S., Ahmad F.S.T., Lia M. Study of UGT73AH1 gene expression in Centella asiatica L. from tissue culture using semi-quantitative PCR method. Int. J. Pharm. Phytopharmacol. Res. 2021;11(1):167–172. doi: 10.51847/v8wcD0FKCN. [DOI] [Google Scholar]
- 39.Sharakshane A. Whole high-quality light environment for humans and plants. Life Sci. Space Res. 2017:18–22. doi: 10.1016/j.lssr.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Ma Y., Xu A., Cheng Z.M. Effects of light emitting diode lights on plant growth, development and traits a meta-analysis. Hortic. Plant J. 2021;7(6):552–564. doi: 10.1016/j.hpj.2020.05.007. [DOI] [Google Scholar]
- 41.Hayashi H., Huang P., Takada S., Obinata M., Inoue K., Shibuya M., Ebizuka Y. Differential expression of three oxidosqualene cyclase mRNAs in Glycyrrhiza glabra. Biol. Pharm. Bull. 2004;27(7):1086–1092. doi: 10.1248/bpb.27.1086. [DOI] [PubMed] [Google Scholar]
- 42.Perera W.P.T.D., Navaratne S., Wickramasinghe I. Impact of spectral composition of light from light-emitting diodes (LEDs) on postharvest quality of vegetables: a review. Postharvest Biol. Technol. 2022;191:1–11. doi: 10.1016/j.postharvbio.2022.111955. [DOI] [Google Scholar]
- 43.Xu Y., Yang M., Fei Cheng F., Liu S., Liang Y. Effects of LED photoperiods and light qualities on in vitro growth and chlorophyll fluorescence of Cunninghamia lanceolate. BMC Plant Biol. 2020;20:1–30. doi: 10.1186/s12870-020-02480-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shezi S., Magwaza L.S., Mditshwa A., Tesfay S.Z. Changes in biochemistry of fresh produce in response to ozone postharvest treatment. Sci. Hortic. (Canterb.) 2020;269 doi: 10.1016/j.scienta.2020.109397. [DOI] [Google Scholar]
- 45.Zhou F., Zuo J., Gao L., Sui Y., Wang Q., Jiang A., Shi J. An untargeted metabolomic approach reveals significant postharvest alterations in vitamin metabolism in response to LED irradiation in pak-choi (Brassica campestris L. ssp. chinensis (L.) Makino var. communis Tsen et Lee) Metabolomics. 2019;15(12):1–11. doi: 10.1007/s11306-019-1617-z. [DOI] [PubMed] [Google Scholar]
- 46.Ngcobo B.L., Bertling I., Clulow A.D. Post-harvest alterations in quality and health-related parameters of cherry tomatoes at different maturity stages following irradiation with red and blue LED lights. J. Hortic. Sci. Biotechnol. 2021;96(3):383–391. doi: 10.1080/14620316.2020.1847696. [DOI] [Google Scholar]
- 47.Panjai L., Noga G., Fiebig A., Hunsche M. Effects of continuous red light and short daily UV exposure during postharvest on carotenoid concentration and antioxidant capacity in stored tomatoes. Sci. Hortic. 2017;226:97–103. doi: 10.1016/j.scienta.2017.08.035. [DOI] [Google Scholar]
- 48.Panjai L., Noga G., Hunsche M., Fiebig A. Optimal red light irradiation time to increase health-promoting compounds in tomato fruit postharvest. Sci. Hortic. 2019;251:189–196. doi: 10.1016/j.scienta.2019.03.019. [DOI] [Google Scholar]
- 49.Favre N., Barcena A., Bahima J.V., Martinez G., Costa L. Pulses of low intensity light as promising technology to delay postharvest senescence of broccoli. Postharvest Biol. Technol. 2018;142:107–114. doi: 10.1016/j.postharvbio.2017.11.006. [DOI] [Google Scholar]
- 50.Wei L., Zhang W., Yin L., Yan F., Xu Y., Chen F. Extraction optimization of total triterpenoids from Jatropha curcas leaves using response surface methodology and evaluations of their antimicrobial and antioxidant capacities. Electron. J. Biotechnol. 2015;18:88–95. doi: 10.1016/j.ejbt.2014.12.005. [DOI] [Google Scholar]
- 51.Govarthanan M., Rajinikanth R., Kamala-Kannan S., Selvankumar T. A comparative study on bioactive constituents between wild and in vitro propagated Centella asiatica. J. Genet. Eng. Biotechnol. 2015;13(1):25–29. doi: 10.1016/j.jgeb.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramírez-Mosqueda M.A., Iglesias-Andreu L.G. Evaluation of different temporary immersion systems (BIT®, BIG, and RITA®) in the micropropagation of Vanilla planifolia Jacks, in Vitro Cell. Dev. Biol. Plant. 2016;52:154–160. doi: 10.1007/s11627-015-9735-4. [DOI] [Google Scholar]
- 53.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using realtime quantitative PCR and the 2ΔΔCT. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 54.Mangas S., Moyano E., Osuna L., Cusido R.M., Bonfill M., Palazón J. Triterpenoid saponin content and the expression level of some related genes in calli of Centella asiatica. Biotechnol. Lett. 2008;30:1853–1859. doi: 10.1007/s10529-008-9766-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data associated with our study has not been deposited into a publicly available repository. Data included in article. The data that support the results presented in this paper are available from the corresponding author, upon reasonable request. The raw figures of C. asiatica leaves irradiated under different light qualities for three days were showed in Supplemental File 1 and the nitrate determination of C. asiatica leaves was showed in Supplemental File 2. The average and standard deviation (S.D.) of major ingredients, phytochemicals, pigments, antioxidants and gene expression were deposited in Supplemental File 3.