Abstract
Background
Liposarcoma originating from peripancreatic fat tissue is extremely rare. This case report presents a surgical case of a giant liposarcoma originating from peripancreatic fat tissue with origin identification using 3-Dimensional Computed Tomography Angiography (3D-CTA).
Case presentation
A 59-year-old female was referred to our hospital with a giant abdominal tumor. Computed tomography revealed a 34 cm tumor composed of fatty tissue, exerting pressure on the posterior aspect of the pancreas. Suspecting liposarcoma, we planned for surgery. At first, the tumor appeared to be intra-abdominal tumor, based on the identification of the tumor’s feeding artery as a branch of the dorsal pancreatic artery using 3D-CTA, we concluded that the liposarcoma originated from the peripancreatic fat tissue and situated in the retroperitoneum. During surgery, we observed a well-capsulated, elastic, yellowish mass without infiltration into surrounding tissues. We carefully dissected the tumor from the greater omentum and transverse mesocolon while preserving the tumor capsule. We ligated the feeding artery at the border with the pancreatic parenchyma and successfully completed the excision of the tumor. The resected specimen weighted 2620 g and was pathologically diagnosed as a well-differentiated liposarcoma. There was no injury to the tumor’s capsule, and the surgical margins were negative.
Conclusions
In this report, we present an extremely rare case of a liposarcoma originating in the peripancreatic fat tissue. The use of 3D-CTA was instrumental in identifying the primary site of this giant tumor, enabling us to guide the surgery and achieve complete resection successfully.
Keywords: Liposarcoma, Peripancreatic fat tissue, 3D-CT angiography, Dorsal pancreatic artery, Giant tumor
Background
The first-line treatment for liposarcoma is surgery; performing en bloc resection with the capsule is of utmost importance. We experienced a rare case of a giant liposarcoma originating from the peripancreatic fat tissue. In this instance, the preoperative identification of the tumor’s feeding artery using 3-Dimensional Computed Tomography Angiography (3D-CTA) played a crucial role in determining its origin and contributed to the successful complete resection. We present this significant in this report.
Case presentation
A 59-year-old female with a history of chest X-ray examination revealing bilateral lung ground-glass appearance 4 years ago was undergoing regular chest computed tomography (CT) for follow-up observation. On the recent CT, a giant abdominal tumor was identified, and she referred to our hospital. Her height was 158 cm, and body weight was 66 kg. The abdominal examination revealed a flat and soft abdomen, with no palpable mass detected on the surface. Laboratory findings showed no significant abnormalities. An abdominal contrast-enhanced CT revealed a 34 cm tumor with a similar density to subcutaneous fat (Fig. 1). A high density area was observed on the ventral side of the tumor. Abdominal plain magnetic response imaging (MRI) revealed that the tumor was suppressed on the fat-suppressed images, indicating a predominance of fatty components. The area that showed high density on CT exhibited equivalent-signal intensity area to renal parenchyma on T2-weighted images, and not suppressed on fat-suppressed images. The upper and lower gastrointestinal endoscopy revealed no abnormalities without hiatal hernia.
Fig. 1.
Abdominal CT and MRI. A, B Abdominal contrast-enhanced CT. C Abdominal plain MRI T2-weighted image. D Abdominal plain MRI fat-suppressed T2-weighted image. A giant tumor measuring 34 cm predominantly composed of fat tissue was observed in the abdomen (white triangles). A high density area of the ventral side of the tumor was detected on CT (white arrows), and the region showed equivalent-signal intensity area to renal parenchyma on MRI T2-weighted image and not suppressed on fat-suppressed images (white arrows)
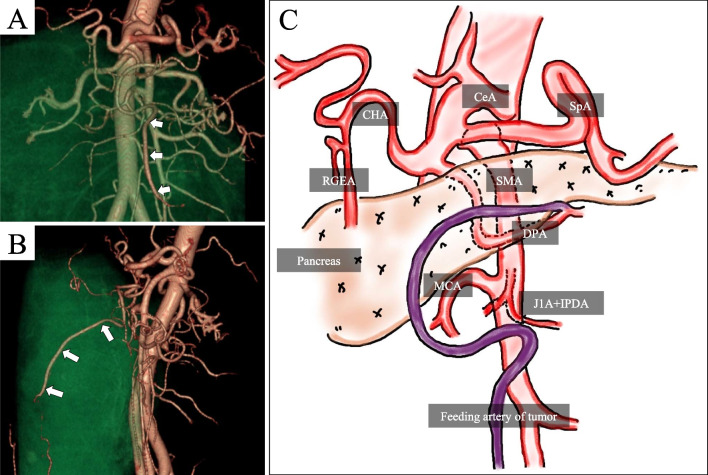
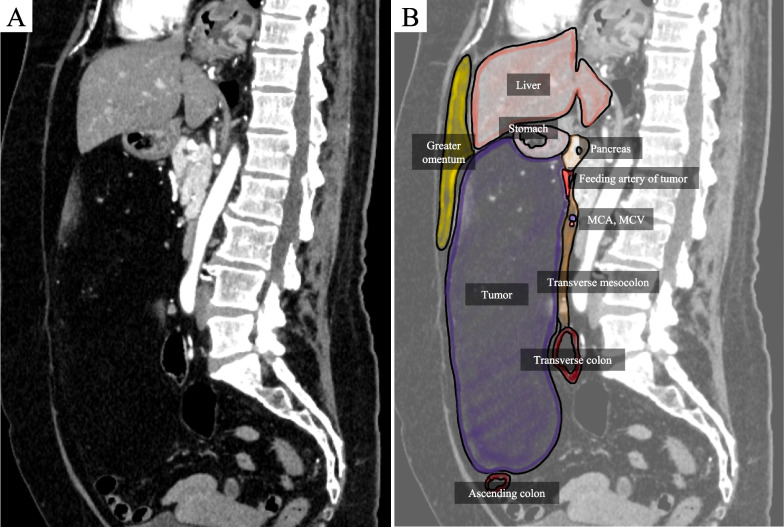
The tumor was suspected of liposarcoma and decided to proceed with surgery. The tumor appeared to be composed of homogeneous fatty tissue, and there was no clear evidence of infiltration into the surrounding organs on imaging. Therefore, a well-differentiated liposarcoma was the most likely diagnosis, but the areas showing high density on CT and not suppressed on MRI fat-suppression images were considered to potentially indicate dedifferentiation. Artery running through the tumor was recognized, and 3D-CTA showed that the tumor was supplied by a branch of the dorsal pancreatic artery (DPA) (Fig. 2). The branches of the right gastroepiploic artery (RGEA) and superior mesenteric artery (SMA), such as the middle colic artery (MCA), were observed to be compressed and displaced by the tumor, and they were not supplying blood to the tumor. Regarding the origin of the tumor, at first, given the appearance of the tumor occupying the intraperitoneal space, we thought it originated from an intra-abdominal organ. However, considering that the tumor’s feeding artery was branches of the DPA, we concluded that the tumor originated retroperitoneally. Upon reevaluating the CT images, we suspected that the tumor arose from the peripancreatic fat tissue, exerting pressure on the stomach and greater omentum ventrally and displacing the transverse colon and transverse mesocolon dorsally (Fig. 3). In addition, the drainage vein was flowing into the middle colic vein (MCV).
Fig. 2.
3D-CT angiography. A Coronal image, B Sagittal image, C Schema. The tumor’s feeding artery was identified as a branch of the DPA. CeA celiac artery, SpA splenic artery, CHA common hepatic artery, RGEA right gastroepiploic artery, SMA superior mesenteric artery, DPA dorsal pancreatic artery, MCA middle colic artery, J1A first jejunal artery, IPDA inferior pancreaticoduodenal artery
Fig. 3.
Preoperative CT and schema. A Abdominal contrast-enhanced CT sagittal image, B schema. The tumor was located on the ventral side of pancreas. Transverse colon and transverse mesocolon were displaced dorsally and stomach and greater omentum were compressed ventrally by the tumor. Based on its localization and vascular supply, the tumor was considered to originate from the peripancreatic fat tissue and situated in the retroperitoneum
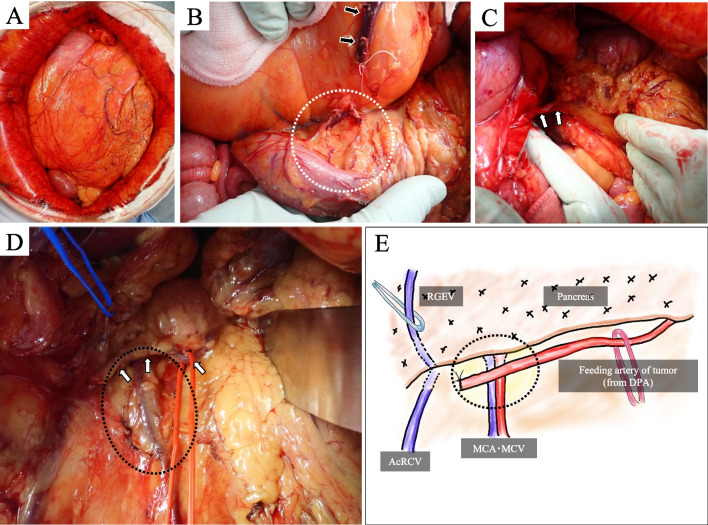
She underwent midline laparotomy from the xiphoid process to the lower abdomen, and the tumor was found to be a soft, yellowish mass, approximately the size of an adult’s head, with an extremely thin but distinct capsule (Fig. 4). It did not invade the abdominal wall or other organs. The greater omentum and the tumor capsule could be easily separated without causing injury, as their color and texture differed from the surrounding tissue. Similarly, during the dissection of the transverse colon and transverse mesocolon, separation at a layer that did not injure the visceral peritoneum or tumor capsule was mostly possible. The vein on the back of the tumor was found to be flowing into the transverse mesocolon, confirming the preoperative assessment of the venous drainage into the MCV. To avoid injuring the tumor capsule, the transverse mesocolon was partially attached to the tumor and dissected. Once the dissection between the greater omentum, transverse colon, and its mesentery, and the tumor was completed, only an artery remained at the lower border of the pancreas. Based on the preoperative 3D-CTA, it was determined that this artery was branch of the DPA suppling as the feeding artery. The point of origin of the feeding artery was the fat tissue at the lower border of the pancreas, which was initially suspected as the primary site of the tumor. Therefore, a portion of the pancreatic capsule and the transverse mesocolon were attached to the tumor, and the feeding artery was dissected without injuring the tumor capsule, leading to successful tumor resection. After the tumor was resected, the right gastroepiploic artery and vein (RGEV), MCA, MCV, accessary right colic vein (AcRCV) were clearly visualized, confirming that the feeding arteries of the tumor were distinct from these vessels. The operation time was 1 h and 47 min, with a blood loss of 30 g.
Fig. 4.
Intraoperative photographs. A The tumor was yellow and approximately size of an adult’s head. B We dissected of the transverse mesocolon (dotted white circle) and ligated drainage vein (black arrows) at the site where it flows into the transverse mesocolon. C We identified the feeding artery at the lower border of the pancreas (white arrows). D, E (Schema): after tumor resection, we clarified the relationship between the feeding artery (white arrows) and other vessels. We also removed the portion of the pancreatic capsule and the transverse mesocolon attached to the tumor, which were considered to be the origin of the tumor (dotted black circle). RGEV right gastroepiploic vein, DPA dorsal pancreatic artery, AcRCV accessary right colic vein, MCA middle colic artery, MCV middle colic vein
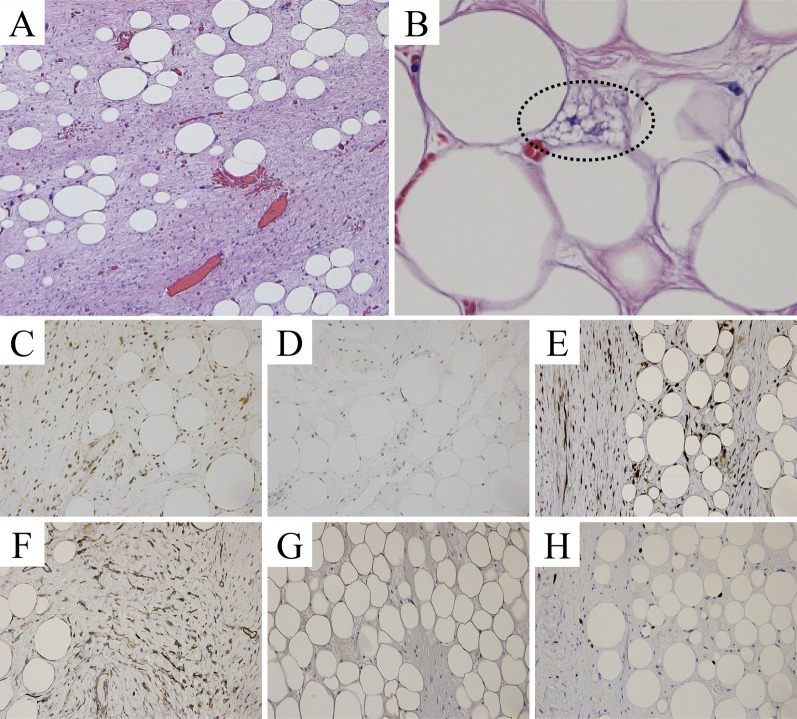
The resected specimen measured 34 × 23 × 7 cm, 2620 g (Fig. 5). The tumor had a thin fibrous capsule, and its cut surface was predominantly composed of yellowish fatty tissue, with some areas showing white nodules. Histopathological examination revealed that the tumor was mostly composed of slightly enlarged adipocytes of varying sizes, along with atypical stromal cells and lipoblast (Fig. 6). The white nodules corresponded to the high density areas observed in the CT and showed an increase in fibrous tissue, but no signs of necrosis, nuclear atypia, or mitotic figures were observed, and no dedifferentiation was evident. Immunohistochemical examination revealed positive results for CDK4, MDM2, and p16, weakly positive results for CD34 and S-100, with a Ki-67 index of 2%. Based on these findings, the tumor was diagnosed as a well-differentiated liposarcoma. The tumor was resected without injuring the capsule, and the surgical margins were negative, indicating complete resection.
Fig. 5.
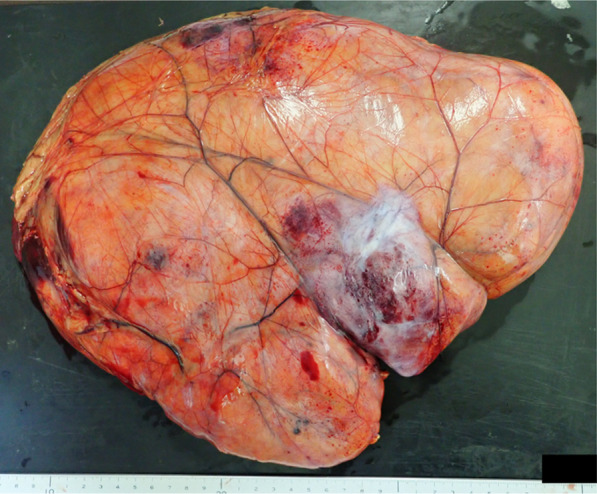
Resected specimen. The size of tumor was 34 × 23 × 7 cm, and its weight was 2590 g. Although the tumor had a thin fibrous capsule, we were able to resect it without injuring the capsule
Fig. 6.
Histopathological and immunohistochemical examinations. A, B: Hematoxylin and eosin stain (×20). The tumor was mostly composed of slightly enlarged adipocytes of varying sizes, along with atypical stromal cells and lipoblast (dotted black circle). C CDK4 positive, D MDM2 positive, E p16 positive, F CD34 weak positive, G S-100 weak positive, H Ki-67 index of 2%. The tumor was diagnosed as a well-differentiated liposarcoma
The postoperative course was uneventful, and she was discharged on the third day after surgery. She did not receive any postoperative therapy. Follow-up evaluations were performed with CT or MRI every 3 months. Two years postoperatively, she remains alive with no evidence of disease recurrence.
Discussion
Liposarcoma is one of the most common soft tissue sarcomas, accounting for approximately 10% of soft tissue sarcomas [1]. 15% of primary sarcoma arise from retroperitoneum, and 25–35% of liposarcoma consist of soft tissue sarcomas located in retroperitoneal cavity [2]. The early symptoms of retroperitoneal liposarcomas are nonspecific, and the complaints of patients often involve a significant increase in tumor size, leading to direct invasion or compression of adjacent organs [3, 4]. Therefore, retroperitoneal liposarcomas are often diagnosed at advanced stages when tumors develop to large size [5]. In our case, despite the tumor’s enormous size (34 cm), the patient remained asymptomatic, and the diagnosis was incidentally made during CT performed for an unrelated issue. The tumor, occupying the abdominal cavity extensively, initially suggested an origin from intra-abdominal organs. However, it was actually originated from peripancreatic fat tissue and arose in the retroperitoneum. Histologically, liposarcomas are classified into well-differentiated, myxoid, pleomorphic, and round cell types, each with varying prognoses [6]. The 5-year survival rates are closely related to the histological subtypes, with well-differentiated type showing a favorable outcome (85%), compared to myxoid (77%), pleomorphic (18%), and round cell types (21%) [6]. CT is the most common used imaging modalities for the diagnosis and preoperative evaluation [7]. Previous studies have identified certain CT findings suggestive of malignancy, including internal heterogeneity, irregular margins, higher density than normal fat tissue, and enhancement on contrast-enhanced CT [8]. In our case, there were regions with high density on CT and non-suppressed on fat-suppressed MRI, raising suspicion of dedifferentiated liposarcoma. However, the resected specimen confirmed a well-differentiated liposarcoma. The main treatment for non-metastatic retroperitoneum liposarcoma is surgery [9]. It is important to completely resect the tumor with its capsule, and often adjacent organ resection is necessary [7]. The recurrence rate after curative resection is approximately 70%, and this high recurrence rate can be attributed to several reasons, such as the pseudo-capsule formed by tumor cells, the difficulty in ensuring sufficient margins due to the presence of important organs, and the unclear boundary with normal fat tissue [6, 10]. Despite performing extensive resections, there have been reports of local recurrences, suggesting that the recurrence may also be influenced by the malignant potential of the tumor [11]. In our case, with the aid of accurate preoperative localization and well-circumscribed capsule, an appropriate dissection plane was identified. Consequently, there was no need for combined resection of adjacent organs to ensure surgical margin. The histological diagnosis of liposarcoma is considered useful through the detection of protein overexpression due to CDK4 and MDM2 gene amplification [12]. The positivity rate for liposarcoma is approximately 90%, while benign lipomas show a positivity rate of 2–4% [12].
The significance of our report is the identification of the origin of giant abdominal liposarcoma using preoperative 3D-CTA and its application in surgery. Therefore, we conducted a literature review on giant abdominal liposarcomas. 55 cases of primary liposarcoma reported were obtained in PubMed between 2002 and 2023 using the keywords “giant/huge”, “liposarcoma” and “abdomen”, including our case (Table 1) [1–3, 7, 9, 13–59]. The median age was 55 years, with no gender difference observed. The most common origin of liposarcoma was found to be the retroperitoneum in 39 cases (70.6%). The patient’s complaint most frequently reported was abdominal distention, and there were 5 cases (9.1%) where the diagnosis was incidental during unrelated examinations as our case. Regarding surgical procedures, 23 cases (41.8%) underwent tumor resection, while 29 cases (52.7%) required resection involving adjacent organs. Preoperative diagnosis was conducted in 34 cases (61.8%), and the identification of feeding artery was observed in only 3 cases (5.5%). While preoperative site diagnosis was established for cases clearly originating from retroperitoneal fat tissue, such as around the kidney, a considerable number of cases involving retroperitoneal liposarcomas protruding into the abdominal cavity, as our case, were commonly subjected to laparotomy for “abdominal tumor” excision. Intraoperatively, these cases were diagnosed as originating from the retroperitoneum. The median tumor diameter was 32 cm, and the median tumor weight was 9.9 kg. Among the histological subtypes, well-differentiated liposarcoma was the most common, accounting for 24 cases (43.6%), followed by dedifferentiated liposarcoma with 18 cases (32.7%).
Table 1.
Reported cases of Giant Abdominal Liposarcoma (2002–2023, including our case)
| Age, median, year-old | 55 | (30–55) |
| Gender | ||
| Male (%) | 28 | (50.9%) |
| Female (%) | 27 | (49.1%) |
| Origin | ||
| Retroperitoneum | 39 | (70.9%) |
| Mesentery | 12 | (21.8%) |
| Greater omentum | 4 | (7.3%) |
| Patient's complaint *overlapping | ||
| Abdominal distention | 26 | (47.3%) |
| Abdominal mass palpation | 16 | (29.1%) |
| Abdominal pain | 13 | (23.6%) |
| Loss of body weight | 10 | (18.2%) |
| Anorexia | 6 | (10.9%) |
| None (Incidentally detected during unrelated examination) | 5 | (9.1%) |
| Gain of body weight | 4 | (7.3%) |
| Others | 12 | (21.8%) |
| Operative procedure | ||
| Tumor resection | 23 | (41.8%) |
| Tumor resection with other organs | 29 | (52.7%) |
| Not mentioned | 3 | (5.5%) |
| Preoperative diagnosis of tumor's origin | 34 | (61.8%) |
| Preoperative identification of feeding artery | 3 | (5.5%) |
| By contrast-enhanced CT | 1 | (1.8%) |
| By angiography | 1 | (1.8%) |
| By 3D-CT Angiography | 1 | (1.8%) |
| Tumor size, median, centimeter | 32 | (10–66) |
| Tumor weight, median, kilogram | 9.9 | (2.6–46.0) |
| Histology | ||
| Well-differentiated Liposarcoma | 24 | (43.6%) |
| Dedifferentiated Liposarcoma | 18 | (32.7%) |
| Myxoid liposarcoma | 4 | (7.3%) |
| Mixed liposarcoma | 7 | (12.7%) |
| Not mentioned | 2 | (3.6%) |
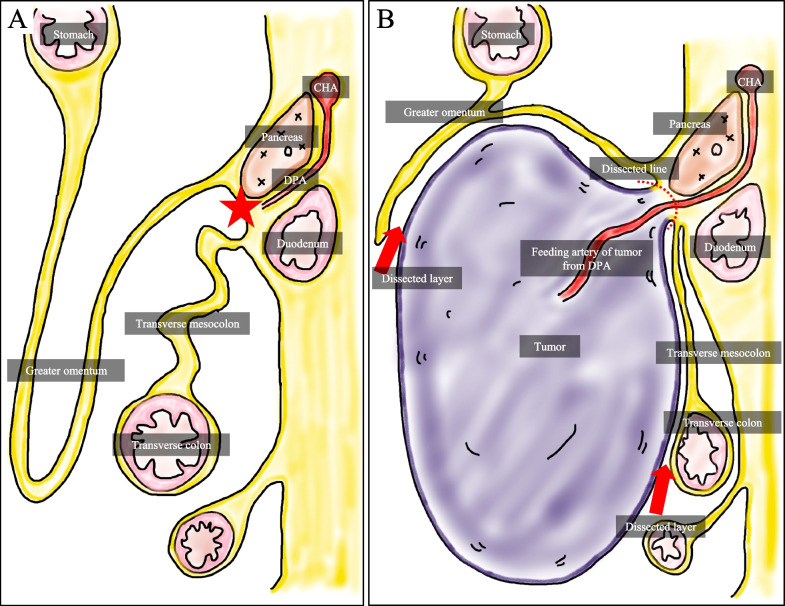
Our case represents a rare origin of retroperitoneum liposarcoma from the peripancreatic fat tissue, and there have been no previous reports of liposarcoma from a similar location. During embryonic development, the greater omentum and transverse mesocolon fuse. We consider that the tumor originated from the peripancreatic fat tissue and extended into the space to abdominal cavity between them, as indicated in the schema (Fig. 7). By utilizing preoperative 3D-CTA, we were able to identify the feeding artery and accurately diagnose the tumor’s origin, leading to a safe and complete resection without injuring the capsule. Without detailed preoperative origin identification, it would have been difficult to determine whether the greater omentum or transverse mesocolon could be dissected, potentially resulting in capsule injury or unnecessary resection of adjacent organs. We were able to treat the patient with a minimally invasive surgery in terms of surgical time, blood loss, and postoperative hospital stay. As mentioned above, among the previously reported cases, feeding artery was identified preoperatively in only three cases. Excluding our case, there are only two cases, one diagnosed with renal artery by angiography in 2010 and another diagnosed with omental artery by contrast-enhanced CT in 2019 [1, 13]. Although angiography is useful for the diagnosis of tumor’s origin, but it is a highly invasive examination and not performed for all patients [60]. In contrast, the 3D-CTA we utilized in our case could be easily created from contrast-enhanced CT and significantly contributed not only to tumor’s origin identification but also to understanding the tumor’s relationship with vessels and facilitating surgical planning. Therefore, we believe that preoperative assessment using 3D-CTA is highly valuable, even for giant tumors, to prevent resorting to a “take potluck” surgery.
Fig. 7.
Schema. A Embryonic anatomy, B Our case. The tumor was supplied by a branch of the DPA, suggesting its origin in the peripancreatic fat tissue (star). Therefore, we considered that the tumor extended from the peripancreatic fat tissue toward the space between the greater omentum and transverse mesocolon. CHA common hepatic artery, DPA dorsal pancreatic artery
Conclusions
We experienced an extremely rare case of a liposarcoma originating in the peripancreatic fat tissue. We reported the rarity of the tumor’s origin and the utility of preoperative identification and localization of the tumor’s feeding artery using 3D-CTA for achieving complete resection.
Acknowledgements
We would like to thank American Journal Experts for English language editing.
Abbreviations
- 3D-CTA
3-Dimensional Computed Tomography Angiography
- CT
Computed tomography
- MRI
Magnetic response imaging
- DPA
Dorsal pancreatic artery
- RGEA
Right gastroepiploic artery
- SMA
Superior mesenteric artery
- MCA
Middle colic artery
- MCV
Middle colic vein
- RGEV
Right gastroepiploic vein
- AcRCV
Accessary right colic vein
Author contributions
Conception and design: KN. Manuscript writing: KN. Final approval of manuscript: all authors.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Availability of data and materials
Data sharing is not applicable since no datasets were generated or analyzed during the present study.
Declarations
Ethics approval and consent to participate
This study was carried out in accordance with the principles of the Declaration of Helsinki.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and her anonymity has been protected.
Competing interests
None of the authors have competing interests to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hashimoto S, Arai J, Nishimuta M, Matsumoto H, Fukuoka H, Muraoka M, et al. Resection of liposarcoma of the greater omentum: a case report and literature review. Int J Surg Case Rep. 2019;61:20–25. doi: 10.1016/j.ijscr.2019.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yildirim O, Namdaroglu OB, Menekse E, Albayrak AL. Giant well-differentiated liposarcoma of retroperitoneum. Bratisl Lek Listy. 2008;109:418–420. [PubMed] [Google Scholar]
- 3.Yang J, Zhao Y, Zheng CH, Wang Q, Pang XY, Wang T, et al. Huge retroperitoneal liposarcoma with renal involvement requires nephrectomy: a case report and literature review. Mol Clin Oncol. 2016;5:607–609. doi: 10.3892/mco.2016.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winn B, Gao J, Akbari H, Bhattacharya B. Dedifferentiated liposarcoma arising from the sigmoid mesocolon: a case report. World J Gastroenterol. 2007;13:4147–4148. doi: 10.3748/wjg.v13.i30.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Francis IR, Cohan RH, Varma DGK, Sondak VK. Retroperitoneal sarcomas. Cancer Imaging. 2005;5:89–94. doi: 10.1102/1470-7330.2005.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enzinger FM, Winslow DJ. Liposarcoma. A study of 103 cases. Virchows Arch Pathol Anat Physiol Klin Med. 1962;335:367–388. [PubMed] [Google Scholar]
- 7.Xu C, Ma Z, Zhang H, Yu J, Chen S. Giant retroperitoneal liposarcoma with a maximum diameter of 37 cm: a case report and review of literature. Ann Transl Med. 2020;8:1248. doi: 10.21037/atm-20-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frideman AC, Hartman DS, Sherman J, Lautin EM, Goldman M. Computed tomography of abdominal fatty masses. Radiology. 1981;139:415–429. doi: 10.1148/radiology.139.2.7220888. [DOI] [PubMed] [Google Scholar]
- 9.Nureta TH, Shale WT, Belete TD. Giant retroperitoneal well differentiated liposarcoma: a case report and literature review. Int J Surg Case Rep. 2023;110:108679. doi: 10.1016/j.ijscr.2023.108679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.deSantos LA, Ginaldi S, Wallace S. Computed tomography in liposarcoma. Cancer. 1981;47:46–54. doi: 10.1002/1097-0142(19810101)47:1<46::aid-cncr2820470110>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 11.Rino Y, Jojima T, Abe M, Tsuchida M, Take H, Morohoshi T, et al. A case of liposarcoma of the mesenterium. J Jpn Surg Assoc. 1996;57:1707–1712. [Google Scholar]
- 12.S Sugita, T Hasegawa. Liposarcoma. In: Hasegawa T, Oda Y, editors. Differential diagnostic atlas of tumor pathology: malignant soft tissue tumor. 2nd ed. Tokyo: Bunkodo; 2021.
- 13.Izumi H, Dowaki S, Matsuyama M, Yazawa N, Tobita K, Imaizumi T, et al. Retroperitoneal liposarcoma: a case report. J Jpn S Gastroenterol. 2010;107:1505–1512. [PubMed] [Google Scholar]
- 14.Mansour S, Azzam N, Kluger Y, Khuri S. Retroperitoneal liposarcoma: the giant type. J Med Cases. 2022;13:517–520. doi: 10.14740/jmc4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei X, Qin Y, Ouyang S, Qian J, Tu S, Yao J. Challenging surgical treatment of giant retroperitoneal liposarcoma: a case report. Oncol Lett. 2022;24:314. doi: 10.3892/ol.2022.13434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inoue K, Higaki Y, Yoshida H. Giant retroperitoneal liposarcoma. Int J Urol. 2005;12:220–222. doi: 10.1111/j.1442-2042.2005.01019.x. [DOI] [PubMed] [Google Scholar]
- 17.Qiao Z, Zhan R, Pang Y, Wu J. Giant retroperitoneal dedifferentiated liposarcoma in an old man. Asian J Surg. 2021;44:1076–1078. doi: 10.1016/j.asjsur.2021.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Antinori A, Antonacci V, Magistrelli P. Giant retroperitoneal liposarcoma. Am J Surg. 2002;184:56–57. doi: 10.1016/s0002-9610(02)00880-2. [DOI] [PubMed] [Google Scholar]
- 19.Ioannidis A, Koutserimpas C, Konstantinidis M, Drikos I, Voulgaris P, Economou N. Dyspnea caused by a giant retroperitoneal liposarcoma: a case report. Oncol Lett. 2018;16:1539–1542. doi: 10.3892/ol.2018.8791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lam-Chung CE, Rodríguez-Orihuela DL, Arízaga-Ramírez R, Almeda-Valdés P, Castillo-Valdez AK, Magaña-Pérez K, et al. Acromegaly and a giant retroperitoneal liposarcoma producing IGF-1. AACE Clin Case Rep. 2020;6:e165–e169. doi: 10.4158/ACCR-2020-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meher S, Mishra TS, Rath S, Sasmal PK, Mishra P, Patra S. Giant dedifferentiated liposarcoma of small bowel mesentery: a case report. World J Surg Oncol. 2016;14:250. doi: 10.1186/s12957-016-1007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tani A, Tarumi Y, Kakibuchi A, Aoyama K, Kokabu T, Kataoka H, et al. Giant retroperitoneal dedifferentiated liposarcoma mimicking ovarian cancer: a case report. Gynecol Oncol Rep. 2022;44:101088. doi: 10.1016/j.gore.2022.101088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park N, Kuk JC, Shin EJ, Lim DR. Surgery of intraabdominal giant dedifferentiated liposarcoma of ascending colon mesentery: a rare case report. Int J Surg Case Rep. 2022;98:107482. doi: 10.1016/j.ijscr.2022.107482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kassi ABF, Yenon KS, Kassi FMH, Adjeme AJ, Diarra KM, Bombet-Kouame C, et al. Giant dedifferentiated liposarcoma of the gastrocolic ligament: a case report. World J Gastrointest Surg. 2023;15:2376–2381. doi: 10.4240/wjgs.v15.i10.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fodor M, Maglione M, Kogler P, Kafka-Ritsch R, Ofner D, Perathoner A. Challenges in the treatment of a giant retroperitoneal liposarcoma. Ann Ital Chir. 2020;9:S2239253X20033162. [PubMed] [Google Scholar]
- 26.Herzberg J, Niehaus K, Holl-Ulrich K, Honarpisheh H, Guraya SY, Strate T. Giant retroperitoneal liposarcoma: a case report and literature review. J Taibah Univ Med Sci. 2019;14:466–471. doi: 10.1016/j.jtumed.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moshref LH. A successful en bloc excision of a giant retroperitoneal liposarcoma with distal splenopancreatectomy. Cureus. 2021;13:e18903. doi: 10.7759/cureus.18903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu BE, Wu CL, Liu JP, Zhang WJ. Surgical approach for complete resection of giant retroperitoneal liposarcoma with diaphragmatic hernia via ninth rib thoracotomy. Front Oncol. 2023;13:1239962. doi: 10.3389/fonc.2023.1239962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu T, Zhang J, Xu Z, Zhou H. Abdominal viscera and gone? A rare case of giant retroperitoneal liposarcoma. Asian J Surg. 2022;45:2963–2964. doi: 10.1016/j.asjsur.2022.06.124. [DOI] [PubMed] [Google Scholar]
- 30.Smrkolj S, Rakar S, Stolfa A, Kobal B. Giant pelvic retroperitoneal liposarcoma: case report. Eur J Gynaecol Oncol. 2010;31:705–708. [PubMed] [Google Scholar]
- 31.Almas T, Ullah M, Ehtesham M, Akbar A, Khan NK. En bloc resection of a giant retroperitoneal liposarcoma: a surgical challenge. Cureus. 2020;12:e8730. doi: 10.7759/cureus.8730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khanduri A, Bansal N, Singh A, Gupta J, Gupta R. Multifocal dedifferentiated liposarcoma of the jejunal mesentery. Cureus. 2021;13:e19780. doi: 10.7759/cureus.19780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duman K, Girgin M, Artas G. A case report: giant intra-abdominal liposarcoma presenting acute renal failure. Ann Med Surg. 2016;12:90–93. doi: 10.1016/j.amsu.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakajima Y, Fukunari H, Kato T, Yoshino J, Okajima C, Shitara K, et al. Two cases of giant retroperitoneal liposarcoma. Jpn J Cancer Chemother. 2014;41:2468–2471. [PubMed] [Google Scholar]
- 35.Amir M, Akhtar S, Pervaiz M, Asad-ur-Rahman, Khawaja A, Ahmad I. Giant dedifferentiated retroperitoneal liposarcoma. J Coll Physicians Surg Pak. 2011;21:569–571. [PubMed] [Google Scholar]
- 36.Ishiguro T, Muta Y, Ito T, Chika N, Hatano S, Amano K, et al. A case of giant retroperitoneal liposarcoma resected after trabectedin chemotherapy. Jpn J Cancer Chemother. 2018;45:2132–2134. [PubMed] [Google Scholar]
- 37.Farese S, Palasciano N. A case of giant retroperitoneal liposarcoma. Chir Ital. 2002;54:95–98. [PubMed] [Google Scholar]
- 38.Tripathi M, Pavithira GJ, Dubey S, Verma R, Garg V. Surgical excision of a giant retroperitoneal liposarcoma with renal cell carcinoma: a case report of the largest retroperitoneal sarcoma. Int J Surg Case Rep. 2023;109:108515. doi: 10.1016/j.ijscr.2023.108515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maàmouri N, Cheikh I, Ouerghi H, Oukaà A, Belkahla N, Mnif E, et al. Giant retroperitoneal liposarcoma. One case report. Rev Med Interne. 2005;26:145–148. doi: 10.1016/j.revmed.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 40.Jukić Z, Brcić I, Zovak M, Vucić M, Mijić A, Kruslin B. Giant mixed-type liposarcoma of the mesentery: case report. Acta Clin Croat. 2012;51:97–101. [PubMed] [Google Scholar]
- 41.Atram MA, Deshmukh A, Shivkumar VB, Gangane NM. Intraperitoneal dissemination of primary dedifferentiated liposarcoma of omentum simulating an ovarian cancer – a case report. Indian J Cancer. 2022;59:422–425. doi: 10.4103/ijc.IJC_1128_20. [DOI] [PubMed] [Google Scholar]
- 42.Mori R, Ogino T, Fujino S, Takahashi H, Miyoshi N, Uemura M. An oncologic emergency case of massive dedifferentiated liposarcoma of the small bowel mesentery. Clin J Gastroenterol. 2021;14:759–764. doi: 10.1007/s12328-021-01350-5. [DOI] [PubMed] [Google Scholar]
- 43.Korukluoglu B, Ergul E, Sisman IC, Yalcin S, Kusdemir A. Giant primary synchronously bilateral mesenteric dedifferentiated liposarcoma with hyperparathyroidism, hyperthyroidism, type-2 diabetes mellitus and hypertension. J Pak Med Assoc. 2009;59:563–565. [PubMed] [Google Scholar]
- 44.Wen SC, Lin C. Kidney displaced by giant retroperitoneal liposarcoma in HIV patient. Int Braz J Urol. 2020;46:673–675. doi: 10.1590/S1677-5538.IBJU.2019.0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu H, Hu T, Li Y, Yue Z, Zhang F, Fu J. Successful intraoperative management in patients with abdominal compartment syndrome induced by giant liposarcomas: two case reports. Medicine. 2020;99:e22575. doi: 10.1097/MD.0000000000022575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sato K, Ashitomi Y, Takahashi R, Ashino K, Sugawara S, Futakuchi M, et al. A case of dedifferentiated liposarcoma with reducing surgery followed by chemotherapy and additional reducing surgery. Jpn J Cancer Chemother. 2022;49:1411–1413. [PubMed] [Google Scholar]
- 47.Cerullo G, Marrelli D, Rampone B, Perrotta E, Caruso S, Roviello F. Giant liposarcoma of the mesentery. Report of a case. Ann Ital Chir. 2007;78:443–445. [PubMed] [Google Scholar]
- 48.Khan N, Afroz N, Fatima U, Raza MH, Rab AZ. Giant primary mesenteric liposarcoma: a rare case report. Indian J Pathol Microbiol. 2007;50:787–789. [PubMed] [Google Scholar]
- 49.Khan MI, Zafar A, Younas M, Malik I. Huge mesenteric liposarcoma. J Pak Med Assoc. 2013;63:775–777. [PubMed] [Google Scholar]
- 50.Ki EY, Park ST, Park JS, Hur SY. A huge retroperitoneal liposarcoma: case report. Eur J Gynaecol Oncol. 2012;33:318–320. [PubMed] [Google Scholar]
- 51.Esaki M, Moriya Y. A case of huge liposarcoma in the abdomen. Jpn J Clin Oncol. 2006;36:532. doi: 10.1093/jjco/hyl090. [DOI] [PubMed] [Google Scholar]
- 52.Oh SE, Kim HJ, Choi SJ, Oh SY, Roh CR, Kim JH. A case of huge retroperitoneal liposarcoma in pregnancy. Obstet Gynecol Sci. 2014;57:236–239. doi: 10.5468/ogs.2014.57.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang S, Han X, Liu S, Xu G, Li J. Primary retroperitoneal liposarcoma: a rare case report. J Int Med Res. 2021;49:3000605211063085. doi: 10.1177/03000605211063085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Teng JF, Zeng X, Shi J, Xie WF, Lin Y. A huge retroperitoneal liposarcoma presenting as a hepatic space-occupying lesion: a case report. J Dig Dis. 2012;13:120–122. doi: 10.1111/j.1751-2980.2011.00553.x. [DOI] [PubMed] [Google Scholar]
- 55.Ishikawa H, Ishiguro T, Kumakura M, Ito T, Yamamoto A, Chika N, et al. A case of curative resection of a retroperitoneal liposarcoma. Cancer Chemother. 2022;49:1956–1958. [PubMed] [Google Scholar]
- 56.Paloyo SR, Ramirez AD, David-Paloyo FP, Dofitas RB. Wide excision of a retroperitoneal liposarcoma with en bloc ureterectomy and renal salvage by autotransplantation. Case Rep Transplant. 2019;2019:9725169. doi: 10.1155/2019/9725169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miura Y, Sakata J, Ando T, Soma D, Yuza K, Hirose Y, et al. Mixed type liposarcoma with intra-abdominal bleeding - report of a case. Jpn J Cancer Chemother. 2017;44:1155–1157. [PubMed] [Google Scholar]
- 58.Ishiguro S, Yamamoto S, Chuman H, Moriya Y. A case of resected huge ileocolonic mesenteric liposarcoma which responded to pre-operative chemotherapy using doxorubicin, cisplatin and ifosfamide. Jpn J Clin Oncol. 2006;36:735–738. doi: 10.1093/jjco/hyl087. [DOI] [PubMed] [Google Scholar]
- 59.Noguchi T, Kawamura M, Kawamura T, Sasaki K, Hosono T, Sato R, et al. A case of huge omental-origin liposarcoma controlled by a combination of ifosfamide and Adriamycin. Jpn J Cancer Chemother. 2011;38:1357–1359. [PubMed] [Google Scholar]
- 60.Weiss SW. Histologic Classification of soft tissue tumors. World Health Organization Histological Classification of Tumors. Berlin: Springer. 1994.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable since no datasets were generated or analyzed during the present study.