Abstract
Background
Myasthenia gravis (MG) is an autoimmune disease characterized by generalized skeletal muscle contraction weakness due to autoantibodies targeting neural-muscular junctions. Here, we investigated the relationship between key cytokines and MG type, disease course, antibodies, and comorbidities.
Method
Cytokine levels in serum samples collected from MG (n = 45) and healthy control (HC, n = 38) patients from January 2020 to June 2022 were quantified via flow cytometry.
Results
Levels of IL-6 were higher in the MG group versus healthy individuals (p = 0.026) and in patients with generalized versus ocular MG (p = 0.019). IL-6 levels were positively correlated with QMG score. In patients with MG with both AChR and Titin antibodies, serum levels of sFas and granulysin were higher than in those with AChR alone (p = 0.036, and p = 0.028, respectively). LOMG had a reduction in serum levels of IL-2 compared to EOMG (p = 0.036). LOMG patients with diabetes had lower serum levels of IL-2, IL-4, and IFN-γ (p = 0.044, p = 0.038, and p = 0.047, respectively) versus those without diabetes. sFas in the MG with Abnormal thymus were reduced compared to those in MG with Normal thymus (p = 0.008).
Conclusions
This study revealed a positive correlation between IL-6 level and MG status. Serum cytokine levels of the AChR + Titin MG group differed from those of the AChR group. LOMG had a lower IL-2 level. Comorbidities affect some cytokines in peripheral blood in MG serum.
Keywords: Myasthenia gravis, Cytokine, Titin
1. Introduction:
Myasthenia gravis (MG) is a T cell-dependent and antibody-mediated autoimmune disease characterized by generalized skeletal muscle contraction weakness [1]. The most common pathogenic antibodies are those against the acetylcholine receptor (AChR); however, antibodies against muscle-specific receptor tyrosine kinase (MuSK) and low-density lipoprotein receptor-related protein 4 (LRP4) have also been implicated in the development of MG. Furthermore, Titin antibodies are frequently detected in patients with late-onset MG (onset after the age of 50 years) and MG with thymoma [2]. Humoral immune dysregulation in MG in which multifarious cytokines play obscured roles is a hot topic in international research. Cytokines such as interferon-gamma (IFN-γ), interleukin (IL)-4, and IL-17 can stimulate naïve T cells to differentiate into different T helper (Th) cells [3,4], which participate in the pathogenesis of MG. For instance, anti-AChR antibody production depends on Th2 cell-associated cytokine IL-4, a factor that promotes B cell proliferation and differentiation [5]. The clinical condition or severity of MG is influenced by the balance between Th cells and cytokine levels [6].
Natural killer (NK) cells comprise a subset of innate lymphocytes capable of affecting both innate and adaptive immune responses. These innate immune cells act as a bridge between innate and adaptive immunity [7]. To release apoptotic proteins in a target cell after membrane fusion at immunological synapses, NK cells store lytic molecules in cytolytic granules [8]. The pore-forming glycoproteins perforin, granzymes, Fas ligand (FasL), tumor necrosis factor related apoptosis-induced ligand, and granulysin are included in these cytolytic granules [9]. NK cells play a role in the development of autoimmune diseases such as rheumatoid arthritis [10], systemic lupus erythematosus [11,12], and multiple sclerosis [13] by producing a variety of cytokines and chemokines. Prior research by our team revealed that in patients with MG versus healthy controls, changes in the distributions of subsets of NK cells in peripheral circulation [14]. Additionally, compared to HC, NK cells from the peripheral blood of new-onset MG patients produced significantly less IFN-γ [14]. However, alterations in levels of cytokines secreted by NK cells in MG have seldom been described. In this study, we examined alterations in cytokine secretion by Th cells and NK cells in the serum of patients with MG to better understand the association between the cytokines and MG subtype and disease duration.
2. Materials and methods
2.1. MG patients and controls
2.1.1. Patients and controls
Patients diagnosed with MG (n = 45) according to the Chinese Guidelines for the Diagnosis and Treatment of Myasthenia Gravis, 2020 Edition, at the department of Neurology, Shandong Provincial Qianfoshan Hospital from January 2020 to June 2022 were considered. Inclusion criteria were as follows: 1) aged >18 years and 2) meeting diagnostic criteria for MG. Exclusion criteria were as follows: (1) MG combined with malignant tumors, 2) acute infection within the previous 4 weeks, and 3) acute cerebrovascular disease. The clinical characteristics of patients with MG in this study are summarized in Table 1.
Table 1.
Demographic and clinical characteristics of MG patients and healthy controls.
| HC | MG | P-value | ||
|---|---|---|---|---|
| Number | 38 | 45 | – | |
| Age, years, Mean ± SD | 56.05 ± 14.32 | 55.87 ± 13.88 | 0.952 | |
| Sex, male, n | 14 | 21 | 0.367 | |
| MGFA, n | I | – | 19 | – |
| II | – | 24 | – | |
| III | – | 2 | – | |
| IV | – | 0 | – | |
| V | – | 0 | – | |
| Antibody, n | Negative | – | 3 | – |
| AChR | – | 24 | – | |
| MuSK | – | 3 | – | |
| AChR + Tintin | – | 11 | – | |
| AChR + MuSK | – | 1 | – | |
| AChR + LRP4 | – | 2 | – | |
| Undetected | – | 1 | – | |
| Duration (years), n | <1 | – | 23 | – |
| 1–5 | – | 13 | – | |
| >5 | – | 9 | – | |
| Comorbidity, n | Diabetes | – | 14 | – |
| Abnormal thymus (Thymic hyperplasia or thymoma) | – | 21 | – | |
| Treatment | Naïve | – | 18 | – |
| Only Symptomatic drug treatment | 5 | |||
| + Immunosuppressive drug treatment | – | 20 | – | |
| + Thymectomy | 9 | |||
Note: Symptomatic drug treatment: Pyridostigmine; Immunosuppressive drug treatment: Corticosteroid therapy or glucocorticosteroid treatment and or Immunosuppressive treatment.
Age- and sex-matched healthy individuals were selected as healthy controls (HC; n = 38). The study included 14 males and 24 females, with a mean age of 56.05 ± 14.32 years. The Ethics Committee of Shandong Provincial Qianfoshan Hospital examined and authorized the study. All patients with MG and HCs provided written informed consent.
2.1.2. Blood serum isolation
Blood samples were obtained after overnight fasting. Serum was obtained by centrifuging the blood samples twice at 3000 rpm for 10 min and was frozen at −80 °C.
2.1.3. Cytokine measurement
Serum levels of cytokines including IL-2, IL-4, IL-10, IL-6, IL-17 A, TNF-α, sFas, IFN-γ, Granzyme A, Granzyme B, Perforin, and Granulysin were measured using a Multi-Analyte Flow Assay (740267, Biolegend) kit according to the manufacturer's instructions. Briefly, serum samples were diluted 2-fold with assay buffer. The diluted standard or sample (25 μL) was mixed with an equal volume of assay buffer and beads and incubated at 25 °C for 2 h with shaking. The beads were then centrifuged and spun down. After removing the supernatant, 25 μL of detection antibodies were added. After wells had been shaken for 1 h at 25 °C, 25 μL of SA-PE was added. Beads were spun down after shaking for 30 min. The beads were resuspended in 150 μL of wash buffer. The assay FCS files were analyzed using the LEGENDplex™ data analysis software (BioLegend).
2.1.4. Statistical analysis
All analyses were performed using SPSS (version 27.0). Normality was tested using the Shapiro-Wilk test. If a comparison failed, a nonparametric test or the Mann-Whitney test was used instead of a t-test. Differences between groups were examined using the parametric Student's t-test or appropriate nonparametric tests (e.g., the Kruskal-Wallis Test). None of the data in this study did comply with the normal test, and the data were described using quartiles (Median, P25, P75). The nonparametric Spearman correlation analysis was used to model bivariate correlations. Statistical significance was set at P < 0.05.
3. Results
-
1.
Serum levels of IL-6 were elevated in patients with MG and positively correlated with QMG score
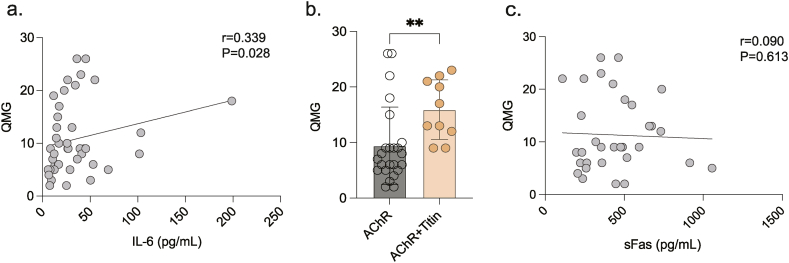
Based on the Myasthenia Gravis Foundation of America (MGFA) clinical classification [15], MG subtypes comprise the following: oculomotor (I), generalized (II-IV). Serum levels of IL-6 were higher in patients with MG than in those of the HC group (p = 0.026). Among MG subtypes, the generalized MG group (G-MG) had higher IL-6 levels than the HC group (p = 0.019), with no statistically significant difference between oculomotor MG (O-MG) and HC groups. There were no statistically significant differences observed when levels of other cytokines present in MG (O-MG and G-MG) and HC groups were compared (Table 2, Table 3). Additionally, serum levels of IL-6 were positively correlated with QMG score (Fig. 1a).
-
2.
Serum cytokine levels of the AChR + Titin MG group differ from those of the AChR MG group
Table 2.
Comparison of various cytokines in the MG and HC groups.
| Cytokine (pg/mL) | Median (P25, P75) |
Median (P25, P75) |
Mann-Whitney U |
|---|---|---|---|
| HC(n = 38) | MG(n = 45) | P | |
| IL-2 | 44.14(16.77,110.81) | 66.55(12.93,127.38) | 0.819 |
| IL-4 | 2.92(0.00,10.09) | 5.95(0.00,20.81) | 0.358 |
| IL-10 | 3.28(0.00,14.92) | 9.20(0.49,20.83) | 0.277 |
| IL-6 | 14.44(10.55,29.94) | 26.41(13.65,42.63) | 0.026 |
| IL-17 A | 0.61(0.00,5.04) | 0.49(0.00,9.46) | 0.592 |
| TNF-α | 45.77(18.19,118.84) | 89.97(29.81,278.91) | 0.12 |
| sFas | 390.10(215.11,628.01) | 429.27(258.43,503.29) | 0.884 |
| IFN-γ | 96.70(0.00,253.79) | 144.17(0.00,310.84) | 0.26 |
| Granzyme A | 579.94(371.74,1056.05) | 528.08(269.93,1244.32) | 0.688 |
| Granzyme B | 1819.99(1499.06,2516.71) | 2094.06(1673.87,2937.65) | 0.257 |
| Perforin | 3731.56(2790.35,5776.36) | 3184.83(2710.29,4784.94) | 0.231 |
| Granulysin | 5148.99(3811.19,6793.38) | 4375.73(2936.27,6481.75) | 0.249 |
Table 3.
Comparison of cytokines in the O-MG, G-MG, and HC groups.
| Cytokine (pg/mL) | Median (P25, P75) |
Median (P25, P75) |
Median (P25, P75) |
K–W test |
|---|---|---|---|---|
| HC n = 38 | O-MG n = 19 | G-MG n = 24 | P | |
| IL-2 | 44.14(16.77,110.81) | 70.54(13.06,116.37) | 56.74(12.12,153.57) | 0.91 |
| IL-4 | 2.92(0.00,10.09) | 5.73(0.00,18.72) | 7.61(0.00,24.94) | 0.633 |
| IL-10 | 3.28(0.00,14.92) | 7.79(0.00,12.80) | 11.29(4.68,28.92) | 0.147 |
| IL-6 | 14.44(10.55,29.94) | 16.85(10.66,36.44) | 28.70(16.18,51.23)a | 0.022 |
| IL-17 A | 0.61(0.00,5.04) | 0.00(0.00,3.23) | 2.92(0.00,14.14) | 0.176 |
| TNF-α | 45.77(18.19,118.84) | 90.28(24.76,309.54) | 93.78(41.60,280.59) | 0.24 |
| sFas | 390.10(215.11,628.01) | 446.19(259.18,489.05) | 399.06(235.31,582.56) | 0.988 |
| IFN-γ | 96.70(0.00,253.79) | 156.10(0.00,294.64) | 162.20(0.00,606.88) | 0.539 |
| Granzyme A | 579.94(371.74,1056.05) | 444.32(142.30,987.76) | 631.41(381.32,1479.86) | 0.529 |
| Granzyme B | 1819.99(1499.06,2516.71) | 1866.62(1574.16,2935.77) | 2250.63(1769.08,2992.99) | 0.318 |
| Perforin | 3731.56(2790.35,5776.36) | 2964.06(2673.07,5136.98) | 3366.80(2820.74,4251.10) | 0.504 |
| Granulysin | 5148.99(3811.19,6793.38) | 3896.75(2680.39,5240.03) | 4865.37(3098.24,7508.54) | 0.247 |
HC and G-MG were statistically different.
Fig. 1.
a. IL-6 was positively correlated with QMG scores (Spearman, r = 0.357, P = 0.019). b. sFas was higher in AChR + Titin group compared with AChR group (Mann-Whitney U, P = 0.0036). c. sFas and QMG scores had no association (Spearman, r = 0.090, P = 0.613). **p < 0.01.
Concomitant titin antibodies are often detected in patients with MG who have severe symptoms [16]. Consistently, MG patients with AChR and Titin antibodies (AChR + Titin MG group) had higher QMG scores than those with only AChR antibodies (AChR MG group) (Fig. 1b). Interestingly, we found that the serum levels of sFas and granulysin were lower in the AChR + Titin MG group than in the AChR MG group (p = 0.036 and p = 0.028, respectively). No statistically significant difference in remaining cytokines was observed when the two groups were compared (Table 4).
-
3.
Serum cytokine levels of the EOMG group differ from LOMG group
Table 4.
Comparison of cytokines in the AChR-only MG and AChR + Titin MG groups.
| Cytokine (pg/mL) | Median (P25, P75)) |
Median (P25, P75) |
Mann-Whitney U |
|---|---|---|---|
| AChR MG n = 24 | AChR + Titin MG n = 11 | P | |
| IL-2 | 57.29(12.87,113.25) | 60.91(17.98,153.60) | 0.696 |
| IL-4 | 6.37(0.00,22.08) | 7.26(0.00,18.60) | 0.744 |
| IL-10 | 7.79(0.24,23.27) | 12.80(6.39,15.49) | 0.411 |
| IL-6 | 26.38(10.19,45.05) | 27.33(22.66,40.20) | 0.594 |
| IL-17 A | 0.00(0.00,1.90) | 2.51(0.00,14.45) | 0.227 |
| TNF-α | 70.22(20.75,228.50) | 275.55(43.34,477.66) | 0.177 |
| sFas | 352.74(239.04,488.73) | 548.82(429.27,673.32) | 0.036 |
| IFN-γ | 129.94(0.00,318.94) | 141.08(0.00,229.47) | 0.801 |
| Granzyme A | 434.13(152.45,1384.43) | 643.44(445.18,1369.48) | 0.166 |
| Granzyme B | 1884.96(1450.77,2928.13) | 2396.39(1932.99,3208.99) | 0.055 |
| Perforin | 3123.84(2678.56,4195.89) | 3584.49(2961.66,7061.81) | 0.127 |
| Granulysin | 3480.06(2688.92,5253.33) | 5383.10(3368.57,8148.65) | 0.028 |
Patients with MG were divided into early-onset myasthenia gravis (EOMG) and late-onset myasthenia gravis (LOMG), depending on whether the first symptoms appeared before or after the age of 50 [17]. We found that LOMG had a reduction in serum levels of IL-2 compared to EOMG (p = 0.036). No other statistically significant differences were observed with EOMG versus LOMG (Table 5).
-
4.
Comparison of cytokine levels in the MG comorbidity and MG groups
Table 5.
Comparison of cytokines in the EOMG and LOMG groups.
| Cytokine |
Median (P25, P75) |
Median (P25, P75) |
Mann-Whitney U |
|---|---|---|---|
| (pg/mL) | EOMG (n = 20) | LOMG (n = 25) | P |
| IL-2 | 78.07 (49.13, 138.84) | 34.42 (6.49, 106.22) | 0.036 |
| IL-4 | 8.27 (0.43, 25.02) | 1.92 (0.00, 14.44) | 0.147 |
| IL-10 | 6.94 (0.51, 18.86) | 10.70 (0.49, 24.10) | 0.407 |
| IL-6 | 17.28 (13.31, 39.15) | 28.89 (13.50, 49.28) | 0.349 |
| IL-17 A | 2.21 (0.00, 12.54) | 0.00 (0.00, 5.08) | 0.209 |
| TNF-α | 93.78 (34.77,239.07) | 76.03 (20.63, 344.04) | 0.909 |
| sFas | 351.98 (235.25, 460.70) | 453.14 (289.54, 626.61) | 0.064 |
| IFN-γ | 200.83 (13.85, 634.24) | 141.08 (0.00, 220.81) | 0.166 |
| Granzyme A | 730.64 (400.25, 1479.86) | 445.18 (216.79, 962.26) | 0.268 |
| Granzyme B | 2221.11 (1832.18, 2928.13) | 2024.84 (1494.26, 3074.25) | 0.508 |
| Perforin | 2958.9 (2691.35, 3584.51) | 3584.49 (2855.73, 5419.71) | 0.071 |
| Granulysin | 3657.61 (2692.21, 4990.96) | 5240.03 (3049.52, 6994.70) | 0.064 |
Among those with late-onset MG (LOMG), diabetes mellitus (DM) is frequently observed [18]. Therefore, patients with LOMG were divided into the following two groups: LOMG with diabetic mellitus (LOMG + DM) and LOMG without diabetes mellitus (LOMG). Table 6 showed that the LOMG + DM group had lower IL-2, IL-4, and IFN-γ levels than the LOMG group (p = 0.044, p = 0.038, and p = 0.047, respectively). There were no statistically significant differences observed when levels of the remaining cytokines were compared among MG patients with and without diabetes.
Table 6.
Comparison of cytokines in the LOMG + DM and LOMG groups.
| Cytokine (pg/mL) | Median (P25, P75) |
Median (P25, P75) |
Mann-Whitney U |
|---|---|---|---|
| LOMG + DM n = 13 | LOMG n = 12 | P | |
| IL-2 | 12.80(4.77,52.70) | 82.21(18.40,157.74) | 0.044 |
| IL-4 | 0.00(0.00,6.37) | 7.92(0.00,55.96) | 0.038 |
| IL-10 | 10.70(0.49,17.40) | 11.03(1.75,28.58) | 0.848 |
| IL-6 | 26.95(8.78,73.53) | 29.94(17.64,49.69) | 0.957 |
| IL-17 A | 0.00(0.00,0.78) | 2.47(0.00,11.69) | 0.218 |
| TNF-α | 60.02(2.82,315.10) | 137.03(41.60,361.29) | 0.253 |
| sFas | 477.66(234.73,555.73) | 444.22(326.78,669.84) | 0.624 |
| IFN-γ | 88.63(0.00,176.76) | 197.54(28.11,284.41) | 0.047 |
| Granzyme A | 354.99(162.59,631.41) | 510.08(432.29,1590.88) | 0.073 |
| Granzyme B | 1840.96(1454.08,2747.91) | 2160.00(1683.11,3397.56) | 0.328 |
| Perforin | 3184.83(2733.12,5640.27) | 4187.70(2991.66,5228.65) | 0.744 |
| Granulysin | 3104.17(2697.46,7411.70) | 5847.77(5077.38,7059.06) | 0.231 |
We defined MG patients with thymic hyperplasia or thymoma as MG with Abnormal thymus, and the remaining patients as MG with Normal thymus. The data showed (Table 7) that sFas cytokine levels in the MG with Abnormal thymus were reduced compared to those in MG with Normal thymus (p = 0.008). There were no statistically significant differences in other cytokines between groups.
-
5.
Comparison of cytokine in the MG without treatment, MG with drug treatment, and MG with Thymectomy groups
Table 7.
Comparison of cytokines in the MG with Abnormal thymus or with Normal thymus.
| Cytokine (pg/mL) | Median (P25, P75) |
Median (P25, P75) |
Mann-Whitney U |
|---|---|---|---|
| MG with Abnormal thymus (n = 21) | MG with Normal thymus (n = 24) | P | |
| IL-2 | 66.55(15.50,161.43) | 66.90(12.87,11.74) | 0.509 |
| IL-4 | 7.96(0.21,25.69) | 0.58(0.00,17.64) | 0.222 |
| IL-10 | 7.79(2.58,18.58) | 9.95(0.00,22.85) | 0.945 |
| IL-6 | 26.40(16.16,40.72) | 27.14(11.77,45.04) | 0.946 |
| IL-17 A | 2.51(0.00,11.86) | 0.02(0.00,7.11) | 0.263 |
| TNF-α | 97.58(49.50,198.17) | 63.87(23.03,372.04) | 0.946 |
| sFas | 347.43(230.01,431.57) | 481.70(332.71,601.69) | 0.008 |
| IFN-γ | 141.08(24.72,605.28) | 150.14(0.00,247.65) | 0.654 |
| Granzyme A | 643.44(295.82,1443.06) | 439.26(234.17,1090.76) | 0.363 |
| Granzyme B | 2286.84(1746.68,2951.80) | 1918.14(1599.39,2938.58) | 0.363 |
| Perforin | 3184.83(2681.60,4235.42) | 3336.85(2735.23,5051.62) | 0.733 |
| Granulysin | 4375.73(3341.98,5993.12) | 4362.52(2576.63,6739.58) | 0.509 |
In the treatment of MG, including symptomatic drug treatment, immunosuppressive drug treatment, and Thymectomy. Of the patients included in this trial, 18 were undrugged, 5 were on Pyridostigmine bromide alone, and 13 were on corticosteroid therapy or glucocorticosteroid treatment and/or immunosuppressants. At the same time, 9 of the 21 patients with thymic abnormalities have chosen thymectomy. We divided patients into MG without treatment (non-drug and symptomatic drug treatment), MG with drug treatment (immunosuppressive drug treatment without Thymectomy), and MG with Thymectomy. The results were as follows: we found no statistically significant differences between groups for any cytokines (Table 8).
Table 8.
Comparison of cytokines in the MG without treatment, MG with drug treatment, and MG with Thymectomy groups.
| Cytokine |
Median (P25, P75) |
Median (P25, P75) |
Median (P25, P75) |
K–W test |
|---|---|---|---|---|
| (pg/mL) | MG without treatmenta (n = 23) | MG with drug treatmentb (n = 13) | MG with thymectomy (n = 9) | P |
| IL-2 | 70.54 (47.58, 116.37) | 17.98 (3.99, 146.00) | 34.42 (8.08, 138.13) | 0.159 |
| IL-4 | 7.81 (0.42, 22.91) | 0.00 (0.00, 16.67) | 5.95 (0.00, 25.69) | 0.214 |
| IL-10 | 9.92 (6.39, 13.00) | 6.80 (0.00, 30.38) | 6.14 (0.49, 21.68) | 0.552 |
| IL-6 | 26.95 (16.85, 41.25) | 14.92 (9.94, 49.28) | 26.40 (12.55, 40.71) | 0.467 |
| IL-17 A | 1.07 (0.00, 10.52) | 0.00 (0.00, 16.19) | 0.00 (0.00, 4.76) | 0.419 |
| TNF-α | 89.97 (42.83, 275.55) | 114.89 (17.00, 456.93) | 67.96 (32.27, 128.71) | 0.716 |
| sFas | 453.14 (259.18, 517.65) | 446.19 (350.86, 695.41) | 237.21 (216.69, 392.53) | 0.454 |
| IFN-γ | 180.23 (0.00, 294.64) | 156.10 (4.25, 446.38) | 95.27 (0.00, 605.28) | 0.748 |
| Granzyme A | 643.44 (434.19, 1119.16) | 431.65 (257.18, 1300.79) | 354.99 (111.21, 1443.06) | 0.188 |
| Granzyme B | 2094.06 (1840.96, 2905.19) | 2111.61 (1373.22, 3543.03) | 1980.63 (1426.66, 2943.07) | 0.427 |
| Perforin | 2961.66 (2695.02, 4774.36) | 4204.08 (3325.05, 6408.89) | 3146.36 (2681.60, 3557.20) | 0.286 |
| Granulysin | 3896.75 (2714.49, 5335.16) | 6603.13 (2490.31, 8217.21) | 3818.80 (3080.99, 4865.37) | 0.388 |
Without treatment: non-drug and symptomatic drug treatment.
Drug treatment: immunosuppressive drug treatment without thymectomy.
4. Discussion
MG is an autoimmune disease characterized by muscle weakness and fatigue that is mediated by the formation of autoantibodies against AChR, MuSK, or LRP4 in the postsynaptic membrane of neuromuscular junctions [17]. The relationship between cytokines or chemokines secreted by CD4+ T cells and serum MG levels has been frequently reported. However, the relationship between cytokines secreted by NK cells and MG has rarely been investigated. It has been documented that there is a redistribution of NK cell subsets in MG and that these NK cells have a reduced killing effect on CD4+ T cells and follicular helper T cells in MG, and that NK also promotes Tfh differentiation, suggesting that NK cells are also involved in the pathogenesis of MG [14].
IL-6 is produced by various immune cells in MG, such as B cells, T cells, macrophages, and DCs [19]. Moreover, IL-6 is produced by muscle cells in an anti-AChR antibody-dependent manner [20]. IL-6 can promote the proliferation of activated B cells and the secretion of antibodies. It can also stimulate T cell proliferation, promote the activation of cytotoxic T lymphocytes (CTL, CD8+ T cells), and induce the differentiation of Th17 and Tfh cells, which have been implicated in the pathogenesis of MG [21]. Serum IL-6 levels were elevated in MG, correlated with the severity of MG, and decreased after immunosuppressive treatment, indicating that IL-6 contributes to the exacerbation of MG pathogenesis [21]. Similar to most studies [19,22,23], our study showed that serum IL-6 levels were higher in patients with MG particularly those of the G-MG group than those in the HC group. We also found that IL-6 levels were positively correlated with QMG score. However, since the increase of IL-6 was a comprehensive effect of multiple immune cells, the pathogenesis of various immune cells in MG cannot be specified.
IFN-γ is primarily generated by CD8+ T cells and NK cells [3], which have broad antiviral effects and immunomodulatory activities that function in innate and adaptive immunity, autoimmune disorders, and tumor immunity [24]. Our research revealed that NK cells in peripheral circulation in patients with new-onset MG release considerably less IFN-γ than HC [14]. However, serum levels of IFN-γ of patients with MG and HC were comparable, probably due to the fact that IFN-γ may be generated by both T and NK cells. Further analysis indicated that serum IFN-γ levels were associated with concomitant disease and disease course. Results showed that serum levels of IFN-γ in the LOMG + DM group were significantly lower than those of the LOMG alone group. This is consistent with previous studies that have shown that intracellular IFN-γ production by CD4+ and CD8+ lymphocytes in patients with type 2 diabetes is lower than that in HC [25,26].
IL-2 is mainly synthesized and secreted by CD4+ T cells, and can also be produced by B cells, NK cells, and monocytes-macrophages [27]. IL-2, as a T cell growth factor, enhances the cytolytic activity of NK cells and promotes the production of immunoglobulins by B cells [27]. It has been determined that IL-2 is a T-cell-stimulating cytokine that enhances the differentiation and proliferation of effector T cells and encourages Th1 immunological polarization [28]. IL-2 plays a role in the immune system to enhance immunity, only elevated serum IL-2 has been observed in MG patients [29], but little attention has been paid to changes in IL-2 in early-onset and late-onset MG. This study found that IL-2 in EOMG was elevated compared to LOMG. Comorbidities such as LOMG with hypertension and diabetes are more common and increase with age [30], and patients with LOMG in combination with diabetes have reduced IL-2 levels versus those without comorbidity. In non-obese diabetic mice with diabetes, reduced levels of IL-2 expression have been associated with an increased risk of developing the disease [31]. Further, IL-2 controls inflammation by preventing IL-6-dependent signaling events and Th17 differentiation [32]. Levels of IL-2 decreased in both type 1 and type 2 diabetes compared with HC [33,34]. Low-dose IL-2 is also used as a treatment for type 1 diabetes [35]. We hypothesized that with age, the immune capacity decreases. The decrease of IL-2 has some significance in the pathogenesis of LOMG, and also, it may also be a risk factor for LOMG combined with DM.
Antibodies to AChR, MuSK, LRP4, and Titin are involved in MG pathogenesis, in which cytokines regulate the antibody response, and previous studies have shown that cytokines such as IL-21, CD40L, IL-13, lymphotoxin, and IL-5 are altered in serum of patients with MG, and have an impact on the antibody response [[36], [37], [38]]. Research has shown that clinical symptoms of anti-AChR positive MG combined with Titin antibody were more severe and progressed faster than those in the AChR + LRP4 and AChR groups [39]; AChR + Titin-MG showed a shorter transition time from ocular to systemic MG, a higher incidence of thymoma, and was more severe than AChR + LRP4-MG [39]. Our findings showed that QMG scores of the AChR + Titin MG group were significantly higher than those of the AChR MG group, while levels of cytokines such as sFas, and granulysin were higher than the AChR MG group than the AChR + Titin MG group, possibly suggesting that sFas, and granulysin cytokines are associated with the development of clinical symptoms of MG.
Granulysin is a cytolytic and pro-inflammatory peptide expressed in NK and CTL cytotoxic granules. It is the first alarmin to leave lymphocytes, as opposed to the traditional alarmin produced by early leukocytes [40]. It acts as a chemoattractant for T lymphocytes, monocytes, and other inflammatory cells and stimulates the expression of several cytokines including IL-1, IL-6, IL-10, and IFN-α [41]. Granulysin operates as an alarmin when galvanized antigen-presenting cells trigger innate and adaptive immune responses [42].
sFas levels are increased in autoimmune diseases such as SLE [[43], [44], [45]], Graves' disease, and autoimmune hypothyroidism [46] and can be utilized as a measure of disease activity. This study revealed that sFas levels are increased in patients with MG with AChR and titin antibodies; however, no association between sFas and QMG was established (Fig. 1c). Interestingly, we found a decrease in sFas in the MG combined with abnormal thymus group, so there must be some kind of connection. Future research should examine the correlation between sFas, and granulysin levels in MG patients with AChR combined with Titin antibodies.
Some limitations in this study need to be addressed. The first and most important point is that this study only measured the level of cytokines in the serum, which only showed the total level in the serum, and did not explain what cells secrete the altered cytokines. Second, the inclusion of MG patients in this paper has limitations, because it is a single-center sample, MG patients have relatively low disease severity, mainly concentrated in MGFA I and II. At the same time, due to insufficient sample size, many group comparisons, such as AChR and AChR combined with LRP4, cannot be carried out.
Ethics approval and consent to participate
All experiments were performed in accordance with the Declaration of Helsinki guidelines and regulations. The research received approval from the Research Ethics Committee of the First Affiliated Hospital of Shandong First Medical University (No. S219) and all patients with MG and healthy controls provided written informed consent.
Funding
This study was funded by the National Natural Science Foundation of China (grant number 82071345); the Academic Promotion Programme of Shandong First Medical University (grant number 2019QL013); and the Cultivation Fund for the First Affiliated Hospital of Shandong First Medical University (grant number QYPY2020NSFC0610).
Data availability statement
The raw data used to support the findings of this study are available from the corresponding author upon request.
Additional information
No additional information is available for this paper.
CRediT authorship contribution statement
Shu-Li Wei: Writing – review & editing, Writing – original draft, Visualization, Investigation, Formal analysis. Chun-Lin Yang: Writing – review & editing. Wei-Yue Si: Investigation. Jing Dong: Investigation. Xue-Lu Zhao: Investigation. Peng Zhang: Resources. Heng Li: Resources. Cong-Cong Wang: Resources. Min Zhang: Resources. Xiao-Li Li: Conceptualization. Rui-Sheng Duan: Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Not applicable.
Contributor Information
Xiao-Li Li, Email: li2006xl@163.com.
Rui-Sheng Duan, Email: ruisheng_duan@163.com.
References
- 1.Gilhus N.E. Myasthenia gravis. N. Engl. J. Med. 2016;375:2570–2581. doi: 10.1056/NEJMra1602678. [DOI] [PubMed] [Google Scholar]
- 2.Romi F., Gilhus N.E., Aarli J.A. Myasthenia gravis: clinical, immunological, and therapeutic advances. Acta Neurol. Scand. 2005;111:134–141. doi: 10.1111/j.1600-0404.2005.00374.x. [DOI] [PubMed] [Google Scholar]
- 3.Billiau A., Matthys P. vol. 20. Cytokine Growth Factor Rev.; 2009. (Interferon-gamma: a Historical Perspective). [DOI] [PubMed] [Google Scholar]
- 4.Conti-Fine B.M., Milani M., Wang W. CD4+ T cells and cytokines in the pathogenesis of acquired myasthenia gravis. Ann. N. Y. Acad. Sci. 2008;1132:193–209. doi: 10.1196/annals.1405.042. [DOI] [PubMed] [Google Scholar]
- 5.Zhang G.X., Navikas V., Link H. Cytokines and the pathogenesis of myasthenia gravis. Muscle Nerve. 1997;20:543–551. doi: 10.1002/(sici)1097-4598(199705)20:5<543::aid-mus2>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 6.Wang L., Zhang Y., Zhu M., et al. Effects of follicular helper T cells and inflammatory cytokines on myasthenia gravis. Curr. Mol. Med. 2019;19:739–745. doi: 10.2174/1566524019666190827162615. [DOI] [PubMed] [Google Scholar]
- 7.Kucuksezer U.C., Aktas Cetin E., Esen F., et al. The role of natural killer cells in autoimmune diseases. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.622306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krzewski K., Strominger J.L. The killer's kiss: the many functions of NK cell immunological synapses. Curr. Opin. Cell Biol. 2008;20:597–605. doi: 10.1016/j.ceb.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gwalani L.A., Orange J.S. Single degranulations in NK cells can mediate target cell killing. J. Immunol. 2018;200:3231–3243. doi: 10.4049/jimmunol.1701500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuwabara T., Ishikawa F., Kondo M., Kakiuchi T. The role of IL-17 and related cytokines in inflammatory autoimmune diseases. Mediat. Inflamm. 2017;2017 doi: 10.1155/2017/3908061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin S.-J., Kuo M.-L., Hsiao H.-S., et al. Cytotoxic function and cytokine production of natural killer cells and natural killer T-like cells in systemic lupus erythematosis regulation with interleukin-15. Mediat. Inflamm. 2019;2019 doi: 10.1155/2019/4236562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hervier B., Beziat V., Haroche J., et al. Phenotype and function of natural killer cells in systemic lupus erythematosus: excess interferon-γ production in patients with active disease. Arthritis Rheum. 2011;63:1698–1706. doi: 10.1002/art.30313. [DOI] [PubMed] [Google Scholar]
- 13.Lünemann A., Tackenberg B., DeAngelis T., et al. Impaired IFN-γ production and proliferation of NK cells in multiple sclerosis. Int. Immunol. 2011;23:139–148. doi: 10.1093/intimm/dxq463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu R.-T., Li W., Guo D., et al. Natural killer cells promote the differentiation of follicular helper T cells instead of inducing apoptosis in myasthenia gravis. Int. Immunopharm. 2021;98 doi: 10.1016/j.intimp.2021.107880. [DOI] [PubMed] [Google Scholar]
- 15.Jaretzki A., Barohn R.J., Ernstoff R.M., et al. Myasthenia gravis: recommendations for clinical research standards. Task force of the medical scientific advisory board of the myasthenia gravis foundation of America. Neurology. 2000;55:16–23. doi: 10.1212/wnl.55.1.16. [DOI] [PubMed] [Google Scholar]
- 16.Lazaridis K., Tzartos S.J. Autoantibody specificities in myasthenia gravis; implications for improved diagnostics and therapeutics. Front. Immunol. 2020;11:212. doi: 10.3389/fimmu.2020.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilhus N.E., Verschuuren J.J. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol. 2015;14:1023–1036. doi: 10.1016/S1474-4422(15)00145-3. [DOI] [PubMed] [Google Scholar]
- 18.Zhang P., Yang C.-L., Du T., et al. Diabetes mellitus exacerbates experimental autoimmune myasthenia gravis via modulating both adaptive and innate immunity. J. Neuroinflammation. 2021;18:244. doi: 10.1186/s12974-021-02298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uzawa A., Akamine H., Kojima Y., et al. High levels of serum interleukin-6 are associated with disease activity in myasthenia gravis. J. Neuroimmunol. 2021;358 doi: 10.1016/j.jneuroim.2021.577634. [DOI] [PubMed] [Google Scholar]
- 20.Maurer M., Bougoin S., Feferman T., et al. IL-6 and Akt are involved in muscular pathogenesis in myasthenia gravis. Acta Neuropathol Commun. 2015;3:1. doi: 10.1186/s40478-014-0179-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z., Wang W., Chen Y., Wei D. T helper type 17 cells expand in patients with myasthenia-associated thymoma. Scand. J. Immunol. 2012;76:54–61. doi: 10.1111/j.1365-3083.2012.02703.x. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y., Zhang Y., Li H., et al. Increased expression of P2X7 receptor in peripheral blood mononuclear cells correlates with clinical severity and serum levels of Th17-related cytokines in patients with myasthenia gravis. Clin. Neurol. Neurosurg. 2017;157:88–94. doi: 10.1016/j.clineuro.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 23.Uzawa A., Kawaguchi N., Himuro K., Kanai T., Kuwabara S. Serum cytokine and chemokine profiles in patients with myasthenia gravis. Clin. Exp. Immunol. 2014;176:232–237. doi: 10.1111/cei.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schroder K., Hertzog P.J., Ravasi T., Hume D.A. Interferon-gamma: an overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 25.Tsiavou A., Hatziagelaki E., Chaidaroglou A., Koniavitou K., Degiannis D., Raptis S.A. Correlation between intracellular interferon-gamma (IFN-gamma) production by CD4+ and CD8+ lymphocytes and IFN-gamma gene polymorphism in patients with type 2 diabetes mellitus and latent autoimmune diabetes of adults (LADA) Cytokine. 2005;31:135–141. doi: 10.1016/j.cyto.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 26.Tsiavou A., Degiannis D., Hatziagelaki E., Koniavitou K., Raptis S.A. Intracellular IFN-gamma production and IL-12 serum levels in latent autoimmune diabetes of adults (LADA) and in type 2 diabetes. J. Interferon Cytokine Res. 2004;24:381–387. doi: 10.1089/1079990041535665. [DOI] [PubMed] [Google Scholar]
- 27.Liao W., Lin J.-X., Leonard W.J. IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr. Opin. Immunol. 2011;23:598–604. doi: 10.1016/j.coi.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwakura Y., Ishigame H., Saijo S., Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Hu Y., Wang J., Rao J., et al. Comparison of peripheral blood B cell subset ratios and B cell-related cytokine levels between ocular and generalized myasthenia gravis. Int. Immunopharm. 2020;80 doi: 10.1016/j.intimp.2019.106130. [DOI] [PubMed] [Google Scholar]
- 30.Klimiec-Moskal E., Quirke M., Leite M.I. Comorbidities in older patients with myasthenia gravis - comparison between early- and late-onset disease. Acta Neurol. Scand. 2022;145:371–374. doi: 10.1111/ane.13549. [DOI] [PubMed] [Google Scholar]
- 31.Hulme M.A., Wasserfall C.H., Atkinson M.A., Brusko T.M. Central role for interleukin-2 in type 1 diabetes. Diabetes. 2012;61:14–22. doi: 10.2337/db11-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laurence A., Tato C.M., Davidson T.S., et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Suri S., Mitra P., Abhilasha A., et al. Role of interleukin-2 and interleukin-18 in newly diagnosed type 2 diabetes mellitus. J. Basic Clin. Physiol. Pharmacol. 2021;33:185–190. doi: 10.1515/jbcpp-2020-0272. [DOI] [PubMed] [Google Scholar]
- 34.Khalil R.G., Abdel-Moneim A., Yousef A.I., Abdel-Rahman H., Zanaty M.I., El-Sayed A. Association of interleukin-2, interleukin-21 and interleukin-23 with hyperlipidemia in pediatric type 1 diabetes. Mol. Biol. Rep. 2021;48:5421–5433. doi: 10.1007/s11033-021-06545-0. [DOI] [PubMed] [Google Scholar]
- 35.Rosenzwajg M., Churlaud G., Hartemann A., Klatzmann D. Interleukin 2 in the pathogenesis and therapy of type 1 diabetes. Curr. Diabetes Rep. 2014;14:553. doi: 10.1007/s11892-014-0553-6. [DOI] [PubMed] [Google Scholar]
- 36.Li Y., Rauniyar V.K., Yin W.F., et al. Serum IL-21 levels decrease with glucocorticoid treatment in myasthenia gravis. Neurol. Sci. 2014;35:29–34. doi: 10.1007/s10072-013-1460-3. [DOI] [PubMed] [Google Scholar]
- 37.Yilmaz V., Oflazer P., Aysal F., et al. Differential cytokine changes in patients with myasthenia gravis with antibodies against AChR and MuSK. PLoS One. 2015;10 doi: 10.1371/journal.pone.0123546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huan X., Zhao R., Song J., et al. Increased serum IL-2, IL-4, IL-5 and IL-12p70 levels in AChR subtype generalized myasthenia gravis. BMC Immunol. 2022;23:26. doi: 10.1186/s12865-022-00501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y., Tao X., Wang Y., et al. Clinical characteristics and prognosis of anti-AChR positive myasthenia gravis combined with anti-LRP4 or anti-titin antibody. Front. Neurol. 2022;13 doi: 10.3389/fneur.2022.873599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zitvogel L., Kroemer G. The multifaceted granulysin. Blood. 2010;116:3379–3380. doi: 10.1182/blood-2010-08-299214. [DOI] [PubMed] [Google Scholar]
- 41.Krensky A.M., Clayberger C. Biology and clinical relevance of granulysin. Tissue Antigens. 2009;73:193–198. doi: 10.1111/j.1399-0039.2008.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tewary P., Yang D., de la Rosa G., et al. Granulysin activates antigen-presenting cells through TLR4 and acts as an immune alarmin. Blood. 2010;116:3465–3474. doi: 10.1182/blood-2010-03-273953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng J., Zhou T., Liu C., et al. Protection from Fas-mediated apoptosis by a soluble form of the Fas molecule. Science. 1994;263:1759–1762. doi: 10.1126/science.7510905. [DOI] [PubMed] [Google Scholar]
- 44.Jodo S., Kobayashi S., Kayagaki N., et al. Serum levels of soluble Fas/APO-1 (CD95) and its molecular structure in patients with systemic lupus erythematosus (SLE) and other autoimmune diseases. Clin. Exp. Immunol. 1997;107:89–95. doi: 10.1046/j.1365-2249.1997.d01-901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vincent F.B., Kandane-Rathnayake R., Koelmeyer R., et al. Associations of serum soluble Fas and Fas ligand (FasL) with outcomes in systemic lupus erythematosus. Lupus Sci Med. 2020;7 doi: 10.1136/lupus-2019-000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mysliwiec J., Oklota M., Nikolajuk A., Waligorski D., Gorska M. Serum CD40/CD40L system in Graves' disease and Hashimoto's thyroiditis related to soluble Fas, FasL and humoral markers of autoimmune response. Immunol. Invest. 2007;36:247–257. doi: 10.1080/08820130601069715. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data used to support the findings of this study are available from the corresponding author upon request.