Abstract
Cisplatin (CDDP) stands as a highly effective chemotherapeutic agent; however, its ototoxicity remains a perplexing challenge in the field. Formononetin (FMNT), a potent flavonoid isolated from Astragalus membranaceus, displays a diverse range of promising pharmacological activities, encompassing antioxidant, anti-apoptotic, and anti-inflammatory effects. Nonetheless, the advantageous effects of FMNT on cisplatin-induced cochlear hair cell injury demand further investigation. This study aimed to assess the protective properties of FMNT against cisplatin-induced hair cell damage by conducting in vitro assays on explant-cultured cochlear hair cells. The findings revealed that FMNT exhibited a notable reduction in cisplatin-induced hair cell apoptosis. Also, FMNT effectively mitigated the accumulation of reactive oxygen species and mitochondrial damage in cochlear explants exposed to cisplatin, while also restoring the turnover of the reduced glutathione (GSH)/glutathione disulfide (GSSG) ratio. Furthermore, our study demonstrated that FMNT protects hair cells against CDDP injury through the activation of the PI3K/AKT-Nrf2 signaling pathway. Consequently, formononetin emerges as a potential therapeutic agent for the treatment of cisplatin-induced ototoxicity.
Keywords: Formononetin, Reactive oxygen species (ROS), Ototoxicity, Cisplatin, Hair cell, Phosphatidylinositol-3-kinase (PI3K)/ serine/threonine kinase 1 (AKT), Nuclear factor erythroid 2-related factor 2 (Nrf2)
1. Introduction
Cisplatin (CDDP) stands as a highly effective chemotherapeutic agent widely employed in the treatment of hematological and solid tumor malignancies [1]. However, its clinical application is often impeded by unwanted ototoxicity and nephrotoxicity [2,3]. Remarkably, over 60 % of pediatric patients reportedly experience ototoxicity following CDDP treatment [4]. Previous research has indicated that CDDP induces the death of cochlear hair cells and spiral ganglion cells [5]. The primary mechanisms underlying cisplatin-induced cochlear damage include but not limited to oxidative stress resulting from the accumulation of reactive oxygen species (ROS), activation of the apoptotic cell death pathways, and the inflammatory responses that ultimately lead to hearing loss [[6], [7], [8]]. Consequently, there is a need to explore medications capable of mitigating apoptosis and reducing oxidative stress, as they hold promise for protecting against CDDP-induced ototoxicity.
Formononetin (FMNT) is a natural flavonoid abundantly found in herbal medicines such as Angelica sinensis and Astragalus membranaceus [9,10]. Previous studies have unveiled FMNT's extensive repertoire of beneficial biological activities, including antioxidative, anti-inflammatory, anti-apoptotic, and tissue-protective properties, mediated through modulation of various signaling pathways [11,12]. Notably, FMNT has demonstrated neuroprotective effects in neurological disorders such as cerebral ischemia and Alzheimer's disease, manifesting in vitro and in vivo through the amelioration of oxidative damage and inflammation [[13], [14], [15]]. Novel research has uncovered FMNT's capacity to exert neuroprotective action against H2O2-induced cell injury in human neuroblastoma cells by impeding ROS overproduction, upregulating the expression of antioxidant genes via activation of the PI3K/Akt-Nrf2 signaling pathway, and inhibiting phosphorylation of mitogen-activated protein kinase (MAPK) [16]. Also, FMNT exhibits renal protective capabilities and the ability to restore renal function both in vitro and in vivo [[17], [18], [19], [20]]. Huang et al. reported that FMNT attenuates rhabdomyolysis-induced kidney apoptosis through the upregulation of the Nrf2-mediated signaling pathway [17]. In Hao et al.'s study, FMNT protected against cisplatin-induced kidney injury by activating the PPARα/Nrf2/HO-1/NQO1 pathway, thus mitigating oxidative stress, inflammatory response, and apoptosis of renal proximal tubular cells, ultimately improving renal function [18].
To date, the potential protective effects of FMNT against cisplatin-induced ototoxicity have not been investigated. This present study aims to explore the protective effect of FMNT against cisplatin-induced hair cell damage, offering a novel prospective therapeutic modality for addressing cisplatin-induced hearing loss.
2. Materials and methods
2.1. Ethics statement
All the animal experiments were carried out with the approval of the ethic committee of the Fudan affiliated Eye and ENT Hospital.
2.2. Reagents and antibodies
Formononetin was obtained from Selleck Chemicals Company (Houston, TX, USA; S2299). Cisplatin was purchased from Sigma-Aldrich Chemical Company (St. Louis, MO, USA, Cat. No. P4394). The following primary antibodies were used in this study: myosin-VIIa (Proteus Biosciences, Ramona, CA, USA; 25–6790), parvalbumin (Abcam, UK; ab277625), cleaved caspase-3 (Cell Signaling Technology, Beverly, MA, USA; 9661S), caspase-3 (CST, 9662S), Bax (CST, 2772S), Bcl-2 (CST, 3498S), PI3K (CST, 4249S), phospho-PI3K (CST, 17366S), AKT (CST, 4691S), phospho-AKT (CST, 4060S), and Nrf2 (CST, 12721S). MitoSOX RED (Invitrogen, Eugene, Oregon, USA, M36008) and LY294002 (Selleck, S1105) were also utilized in the experimental procedures.
2.3. Culture of cochlear explants
Neonatal cochlear explants were obtained from C57BL/6 mice at postnatal day 3 (P3). The cochlear organs were carefully dissected and placed onto coverslips coated with CellTaK (BD Biosciences, USA). Subsequently, they were cultured in DMEM/F12 medium (HyClone, Waltham, MA, USA; SH30023.01) supplemented with N2 (Invitrogen; 17502-048), B27 (Invitrogen; 17504-044), and ampicillin (Sigma-Aldrich; P0781). The cultures were maintained at 37 °C in a 5 % CO2 atmosphere.
2.4. Immunofluorescence
The samples underwent three washes with PBS, followed by fixation with 4 % paraformaldehyde for 30 min. Subsequently, they were permeabilized using PBS containing 1 % Triton X-100 for 20 min and blocked with PBS containing 10 % donkey serum for 1 h at room temperature. Next, the samples were incubated overnight at 4 °C with primary antibodies, which were appropriately diluted (ranging from 1:200 to 1:500 depending on the specific antibodies) in PBST. After washing, the samples were incubated with fluorescent secondary antibodies (1:1000) in the dark for 1 h at 37 °C. The nuclei were stained with DAPI for 20 min, and the fluorescent signals were observed using a Leica SP8 confocal microscope (Leica Microsystems, Biberach, Germany).
2.5. ROS detection
To quantitatively assess the level of mitochondrial reactive oxygen species (ROS), MitoSOX™ Red reagent was employed in this study. MitoSOX™ Red was diluted with serum-free medium to achieve a final concentration of 5 μM. The diluted MitoSOX™ Red reagent was then added to the samples and incubated for 30 min at 37 °C. Fluorescent images were captured using a Leica SP8 confocal microscope (Leica Microsystems, Biberach, Germany).
2.6. Malondialdehyde (MDA) assay
The measurement of malondialdehyde (MDA) was conducted using the MDA assay kit (Biosharp, Beijing, China, BL904A) following the manufacturer's instructions. This assay is based on the reaction of MDA with thiobarbituric acid (TBA), resulting in the formation of a highly absorbent adduct that can be easily quantified using a spectrophotometer at 532 nm. To perform the assay, 25 cochlear explants were collected and homogenized with an ultrasonic homogenizer in 120 μL of PBS on ice. Subsequently, 100 μL of the supernatant was utilized for the MDA analysis using a BioTek Synergy H1 reader (Agilent, Santa Clara, CA, U.S.). The MDA concentration is expressed in nanomoles (nM) per 10 mg of tissue.
2.7. GSH/GSSG assay
The GSH/GSSG assay was conducted using the GSH/GSSG assay kit (Jiancheng, Nanjing, China, A061-1-1) following the manufacturer's instructions. This method is based on the reaction between GSH and 5,5′-di-thiobis (DTNB), which leads to the cyclic production of TNB. The constant production of TNB is then measured using spectrophotometry at a wavelength of 405 nm. To perform the assay, a total of twenty-five cochlear explants were collected and homogenized using an ultrasonic homogenizer in 120 μL of the provided homogenization reagent on ice. The concentrations of total glutathione (tGSH) and oxidized glutathione (GSSG) were determined using a BioTek Synergy H1 reader (Agilent, Santa Clara, CA, U.S.). The levels of reduced glutathione (GSH) were calculated as follows: GSH = tGSH - (GSSG * 2). The concentration of GSSG is presented as nanomoles per 10 mg of tissue.
2.8. Western blot assay
Twenty cochlear explants of each experimental group were lysed to extract proteins using radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime Biotech, Shanghai, China; P0013B) supplemented with protease inhibitor (Sigma-Aldrich; 04693132001). The lysate was placed on ice for 30 min and then centrifuged at 12,000 rpm for 10 min at 4 °C. The supernatant, containing the extracted proteins, was collected, and the protein concentration was determined using a bicinchoninic acid kit (Beyotime Biotech; P0010S). The proteins were denatured by boiling in a 5x loading buffer for 5 min. Forty microgram of the denatured proteins were loaded onto 12 % SDS gels, separated by electrophoresis, and transferred to polyvinylidene fluoride membranes through electroblotting in an ice bath. Subsequently, the membrane was blocked for 1 h and incubated overnight at 4 °C with appropriately diluted primary antibodies. Following washing, the membrane was incubated with horseradish peroxidase-conjugated secondary antibodies (1:2000 dilution) for 2 h at room temperature. Finally, the membrane was visualized using a super chemiluminescence reagent plus (Pierce, Rockford, IL, USA). GAPDH was employed as an internal reference protein.
2.9. Quantitative reverse-transcription PCR
Total RNA was extracted utilizing TRIzol reagent (Invitrogen). Subsequently, cDNA synthesis was carried out using the GoScript Reverse Transcription System (Promega, Madison, WI, USA) with 1 μg of total RNA as the starting material. Reverse transcription of mRNAs was achieved using the provided RT primer within the RT kit. Quantitative real-time PCR (qPCR) was performed on an ABI 7500 Real-Time PCR system (Applied Biosystems, Foster City, CA, USA) using the GoTaq qPCR Master Mix (Promega). A comprehensive list of primers employed for specific gene analysis is presented in Table 1. For mRNA quantification, 18S rRNA was utilized as an internal control. The relative quantities of the target genes were calculated using the 2-ΔΔCT method.
Table 1.
Primer sequences used for qRT-PCR.
| Gene | Accession No. | Forward primer | Reverse primer |
|---|---|---|---|
| Gclc | M90656 | 5′-ATGACTGTTGCCAGGTGGATGAGA | 5′- ACACGCCATCCTAAACAGCGATCA |
| Gpx2 | NM_030677 | 5′-AACCAGTTCGGACATCAGGAGAAC | 5′-GGCAAAGACAGGATGCTCGTTCTG |
| Txnrd1 | NM_182729 | 5′-CAATTGGAATCCACCCTGTC’ | 5′-CCACACTGGGGCTTAACCT |
| HO-1 | NM_002133 | 5′-GGCAGAGGGTGATAGAAGAGG | 5′-AGCTCCTGCAACTCCTCAAA |
| 18S rRNA | M10098 | 5′-GGGAGCCTGAGAAACGGC | 5′-GGGTCGGGAGTGGGTAATTT |
2.10. Statistical analysis
Statistical analyses were conducted using GraphPad Prism 8 software (San Diego, CA, USA). The data are presented as means ± standard error of the mean (SEM) and were analyzed using either a Student's t-test or a one-way analysis of variance (ANOVA). A significance level of p < 0.05 was considered indicative of a statistically significant difference. Each experiment was performed in triplicate to ensure reproducibility and reliability of the results.
3. Results
3.1. FMNT reduced the cisplatin-induced cochlear hair cell death
After dissection, all the cochlear explants were cultured in culture medium for 24 h before they were treated with CDDP, FMNT or CDDP and FMNT together. In the CDDP group, the cochlear explants were treated with 20 μM concentration of cisplatin for 24 h, followed by recovery in the culture medium for 48 h. In the CDDP + FMNT group, cochlear explants were pretreated with gradient concentrations of FMNT ranging from 50 to 200 μM for 2 h. Subsequently, they were subjected to 20 μM CDDP and the corresponding concentration of FMNT for 24 h before further recovery in the culture medium supplemented with FMNT for 48 h. To assess the cytotoxicity of FMNT on cochlear hair cells, an additional group of cochlear explants was incubated with 200 μM FMNT for 72 h. Notably, no discernible hair cell loss was observed in the FMNT group (Fig. 1A and B). The three rows of outer hair cells and one row of inner hair cells exhibited normal morphology and alignment (Fig. 1A). Conversely, the CDDP group showed a significant reduction in the total number of hair cells, accompanied by the collapse of all four rows of hair cells (Fig. 1A and B). In the CDDP + FMNT group, hair cell loss was visibly attenuated, and the preservation of normal hair cell structure was evident (Fig. 1A and B). Furthermore, the protective effect of FMNT against CDDP toxicity demonstrated a concentration-dependent pattern. Collectively, these findings indicate that FMNT possesses the ability to safeguard cochlear hair cells from cisplatin-induced damage.
Fig. 1.
FMNT attenuated the damage to cochlear hair cells induced by 20 μM CDDP treatment. (A) Immunostaining with anti-Myosin-VIIa antibody (green) and DAPI (blue) revealed the preservation of hair cell integrity in the basal, middle, and apical turns of the cochlea in different experimental groups. Representative images are shown, with a scale bar of 20 μm. (B) Quantitative analysis of Myosin-VIIa-positive hair cells. The data presented are the mean ± SEM of six independent experiments. Statistical analysis revealed significant differences: ** (p < 0.01), *** (p < 0.001), and **** (p < 0.0001) compared to the control group; & (p < 0.05), &&& (p < 0.001), and &&&& (p < 0.0001) compared to the CDDP treatment group.
3.2. FMNT prevented cisplatin-induced apoptosis of cochlear hair cells
In order to investigate the impact of FMNT on cisplatin-induced apoptosis in cochlear hair cells, the researchers employed cleaved caspase-3 immunofluorescent staining to label hair cell apoptosis in cochlear organ explants. In the CDDP group, a substantial number of hair cells exhibited cleaved caspase-3 positivity, dispersed along the basal membrane, signifying severe cisplatin-induced hair cell apoptosis (Fig. 2A and B). Conversely, co-treatment with FMNT resulted in a significant reduction in the number of hair cells positive for cleaved caspase-3 in the cisplatin-injured cochlear explants. These findings provide compelling evidence that FMNT possesses the ability to inhibit cisplatin-induced hair cell apoptosis.
Fig. 2.
FMNT effectively suppressed the apoptosis of the cochlear hair cells induced by CDDP. (A) Representative images demonstrate the reduction of cleaved caspase-3 signals (green) in hair cells of CDDP + FMNT group compared to CDDP group revealed the anti-apoptotic effect of FMNT. The hair cells are labeled with anti-parvalbumin antibody (red). Representative images are shown, with a scale bar of 20 μm. (B) Quantification of the percentage of cleaved caspase-3-positive hair cells in the basal, middle, and apical turns of the cochlea in different experimental groups. The data presented are the mean ± SEM, and statistical analysis revealed significant differences: * (p < 0.05), ** (p < 0.01), and **** (p < 0.0001) compared to the control group; && (p < 0.01) and &&&& (p < 0.0001) compared to the CDDP treatment group.
3.3. FMNT reduced cisplatin-induced mitochondrial superoxide accumulation and oxidative stress in cochlear hair cells
The excessive accumulation of intracellular reactive oxygen species (ROS) is another pathway that contributes to cell death induced by CDDP. To assess the antioxidant effect of FMNT, the researchers measured ROS levels in hair cells using MitoSOX-Red staining. The fluorescence intensity of MitoSOX-Red exhibited a significant increase in the CDDP group compared to the control group (Fig. 3A and B). However, co-treatment of FMNT with CDDP resulted in a significant reduction of mitochondrial superoxide accumulation in cochlear hair cells (Fig. 3A and B). Malondialdehyde (MDA), a principal and highly toxic product of lipid peroxidation [21], was investigated in cochlear explants. Following CDDP treatment, the MDA level notably increased. Conversely, when cochlear explants were treated with FMNT alongside CDDP, a significant decrease in the MDA level was observed (Fig. 3C). The GSH/GSSG is the most abundant and crucial intracellular redox couple serving as a defender against ROS [22]. The GSH/GSSG ratio reflects the cellular redox balance [23]. In the CDDP group, the GSSG level significantly increased, accompanied by a sharp decline in the GSH/GSSG ratio, indicative of disrupted redox homeostasis. However, in the CDDP + FMNT group, the GSSG level was significantly lower compared to the CDDP group (Fig. 3D). Moreover, the GSH/GSSG ratio displayed a reversal compared to the CDDP group (Fig. 3D). These results strongly indicate that FMNT ameliorates cisplatin-induced intracellular ROS production, thereby attenuating oxidative stress in cochlear hair cells exposed to cisplatin.
Fig. 3.
(A) FMNT attenuated CDDP-induced mitochondrial ROS overload in cochlear hair cells. MitoSOX™ Red (red) stained the mitochondrial superoxide in cochlear hair cells. Hair cells were stained with anti-parvalbumin antibody (green). Representative images are shown, with a scale bar of 20 μm. (B) Relative level of MitoSOX™ Red intensity in different experimental groups. The data presented are the mean ± SEM, and statistical analysis revealed significant differences: * (p < 0.05), *** (p < 0.001), and **** (p < 0.0001) compared to the control group; &&&& (p < 0.0001) compared to the CDDP treatment group. (C) FMNT inhibited CDDP-induced MDA overproduction in cochlear explants. MDA level is presented as nmol per 10 mg of tissue. (D) FMNT mitigated CDDP-induced GSSG overproduction and the turnover of GSH/GSSG ratio in cochlear explants. The concentration of GSSG is presented as nmol per 10 mg of tissue. The data presented are the mean ± SEM, and statistical analysis revealed significant differences: ** (p < 0.01), *** (p < 0.001).
3.4. FMNT inhibited cochlear hair cell apoptosis and oxidative damage by activating PI3K/AKT-Nrf2 signaling pathway
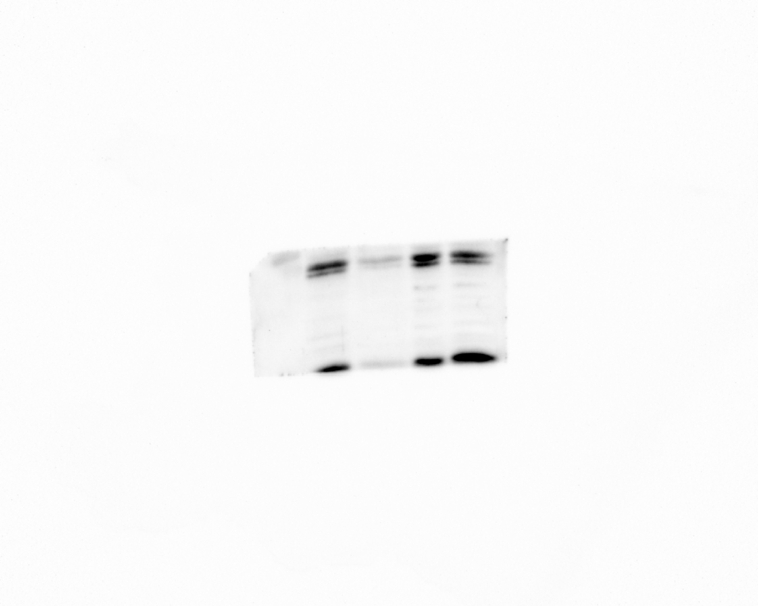
To further elucidate the underlying mechanisms by which FMNT protects against cisplatin-induced hair cell apoptosis and enhances antioxidant defense, the researchers conducted a Western blot assay to assess the levels of apoptosis-related proteins in cochlear explants under different treatment conditions. Upon CDDP treatment, the expression of the anti-apoptotic protein B-cell lymphoma 2 (Bcl-2) in cochlear explants significantly decreased compared to the control group, while the expression of the pro-apoptotic protein apoptosis regulator (Bax) increased (Fig. 4A and B). However, co-treatment with FMNT markedly upregulated the expression of Bcl-2 and downregulated the expression of Bax compared to the CDDP group (Fig. 4A and B). The caspase-3 serves as a key executioner in the apoptosis cascade [24]. The researchers measured the c-caspase-3/caspase-3 ratio in cochlear explants treated with CDDP and CDDP + FMNT. The CDDP treatment led to a noticeable increase in the c-caspase-3/caspase-3 ratio. In contrast, FMNT treatment significantly lowered the c-caspase-3/caspase-3 ratio, indicating the inhibition of apoptosis by FMNT (Fig. 4A and B).
Fig. 4.
(A–B) FMNT inhibits the expression of BAX while promoting Bcl-2 expression. Protein bands and relative protein expression of Bax, Bcl-2, Cleaved caspase-3 (C-Casp-3), and Caspase-3 as determined by Western blot. The original images of Western blot are enclosed in supplementary material. (C) FMNT enhanced the phosphorylation of PI3K and AKT while promoting the expression of Nrf2. Western blot analysis was conducted to assess the protein expression levels of p-PI3K, PI3K, p-AKT, AKT, and Nrf2. The original images of Western blot are enclosed in supplementary material. (D) Quantitative analysis of the p-PI3K/PI3K ratio, p-AKT/AKT ratio and Nrf2/GAPDH ratio in different experimental groups. All data represent mean ± SEM; and statistical analysis revealed significant differences: ** (p < 0.01), **** (p < 0.0001) compared to the control group; &&&& (p < 0.0001) compared to the CDDP treatment group. (E) Hair cell counts treated with CDDP (20 μM), CDDP (20 μM) + FMNT (200 μM), CDDP (20 μM) + FMNT (200 μM) + LY294002 (100 μM) and sham, *** indicates p < 0.001. (F) The relative mRNA levels of Gclc, Gpx2, Txnrd1, and HO-1 in cochlear explants were measured at 3h and 12h after treatment with CDDP, CDDP + FMNT, and sham. Data are presented as the mean ± S.D. from three independent experiments. Statistical analysis revealed significant differences: * (p < 0.05); ** (p < 0.01); and *** (p < 0.001).
The PI3K/AKT signaling pathway plays an essential role in cell metabolism, growth, proliferation, and survival [25]. Nrf2, a pivotal transcription factor, occupies a central position in multiple signaling pathways that regulate cytoprotective responses against cellular stress [26]. Furthermore, Nrf2 lies downstream of the PI3K/AKT signaling pathway [27]. Based on this knowledge, the researchers hypothesized that FMNT protects hair cells against CDDP-induced ototoxicity by activating the PI3K/AKT-Nrf2 signaling pathway. To investigate this hypothesis, the expression of p-PI3K, p-AKT, and Nrf2 was examined in cochlear explants treated with CDDP alone or in combination with FMNT. The protein level of Nrf2, as well as the p-PI3K/PI3K and p-AKT/AKT ratios, significantly decreased following CDDP treatment (Fig. 4C and D). However, co-treatment with FMNT led to a marked increase in Nrf2 expression, as well as the p-PI3K/PI3K and p-AKT/AKT ratios (Fig. 4C and D). To further confirm that FMNT improves hair cell survival by activating PI3K/AKT, the researchers utilized the PI3K inhibitor LY294002 to downregulate the PI3K signaling. When cochlear explants were co-treated with CDDP, FMNT, and LY294002, hair cell loss was noticeably exacerbated compared to the CDDP + FMNT group (Fig. 4E).
Nrf2 transcriptionally regulates downstream genes to counteract oxidative stress and facilitate detoxification. The expression of several target genes of Nrf2, known for their antioxidant activities, was explored under CDDP and CDDP + FMNT treatment. Notably, the transcriptional levels of glutamate-cysteine ligase catalytic subunit (Gclc), glutathione peroxidase 2 (Gpx2), thioredoxin reductase 1 (Txnrd1), and heme oxygenase (HO-1) were significantly higher in the CDDP + FMNT group compared to the CDDP group (Fig. 4F). These results strongly suggest that the protective effects of FMNT against CDDP-induced cellular toxicity are mediated through the activation of the PI3K/AKT-Nrf2 signaling pathway.
4. Discussion
The ototoxicity induced by cisplatin is a common complication in clinical practice, particularly in patients undergoing multiple rounds of chemotherapy. Currently, there are no effective strategies to rescue cisplatin-induced hair cell damage. Developing novel medications that provide sufficient protection against cisplatin ototoxicity represents a potential approach to address this issue. Formononetin, a naturally occurring flavonoid derived from plants, possesses various properties, including anti-apoptotic, antioxidant, and anti-inflammatory effects. In this study, the researchers demonstrated that formononetin has the ability to protect the cochlea from cisplatin-induced hair cell death. Formononetin exhibited anti-apoptotic activities by upregulating the expression of anti-apoptotic proteins and downregulating the expression of pro-apoptotic proteins. Additionally, formononetin alleviated cisplatin-induced oxidative stress by reducing mitochondrial reactive oxygen species (ROS) accumulation, lipid peroxidation, and the imbalance of glutathione (GSH) and oxidized glutathione (GSSG). Furthermore, the study provided evidence that formononetin exerts its effects through the PI3K/AKT-Nrf2 signaling pathway (Fig. 5).
Fig. 5.
Proposed mechanism of FMNT protects cochlear hair cells against CDDP-induced cell damage.
Oxidative stress-induced cellular damage plays a significant role in the pathophysiology of various diseases [[28], [29], [30]]. Previous studies have demonstrated that cisplatin enters hair cells and triggers intracellular ROS and reactive nitrogen species (RNS) production, resulting in an imbalance between oxidants and antioxidants and dysfunction of organelles such as mitochondria and endoplasmic reticulum, ultimately leading to programmed cell death. Conversely, reducing excessive production of ROS and RNS attenuates hair cell death [31,32]. Formononetin has been reported to exhibit antioxidant and anti-inflammatory actions in multiple disease models by suppressing oxidative stress [[33], [34], [35]]. For instance, formononetin ameliorated methotrexate-induced acute kidney injury by inhibiting oxidative damage and inflammation through the suppression of ROS and nitric oxide (NO) generation [11]. Moreover, formononetin protected pancreatic beta cells against IL-1β-mediated apoptosis by blocking NF-κB pathway activation and reducing excess NO production in the rat beta cell line INS-1 [35]. Based on these findings, it was hypothesized that formononetin enhances hair cell survival after cisplatin exposure by inhibiting ROS overproduction and maintaining oxidant balance. In the present study, cisplatin significantly increased ROS levels in hair cells, whereas formononetin attenuated ROS levels. ROS can induce irreversible oxidative modifications of biomolecules such as proteins, lipids, and glycans, impairing their function and inducing cell death. Malondialdehyde (MDA), the principal and highly toxic product of lipid peroxidation, can form DNA-protein cross-links, potentially leading to gene mutations [36]. Furthermore, MDA has been reported to react with amines, which may interfere with the interaction of modified lipoproteins and macrophages [37]. In this study, formononetin significantly decreased the MDA level in cisplatin-treated cochlear explants, indicating a reduction in intracellular lipid peroxidation. GSH is a crucial component of the antioxidant defense system in the human body, scavenging increased ROS production. In response to oxidative stress, two GSH molecules are oxidized to GSSG. GSH and GSH-related enzymes act to prevent oxidative damage and detoxify electrophiles. Maintaining the balance of GSH and GSSG is crucial for normal cellular physiology [23]. Previous studies have shown that cisplatin induces oxidative damage in the liver and kidney of mice, leading to GSH/GSSG imbalance, liver cell apoptosis, and renal dysfunction [38,39]. Interestingly, intraperitoneal injection of the antioxidant L-N-acetylcysteine (LNAC) restored both the GSH/GSSG ratio in guinea pig cochlea and their hearing [40]. In this study, formononetin successfully inhibited the rise of GSSG under the toxic effects of cisplatin and restored the GSH/GSSG balance in cochlear explants. These results suggest that formononetin has antioxidant potential and may protect hair cells from cisplatin-induced ototoxicity.
The BCL-2 family consists of anti-apoptotic proteins such as Bcl-2, Bcl-xL, and A1, as well as pro-apoptotic members like Bax and Bak, which regulate the balance between apoptosis and survival and maintain mitochondrial function [41]. Upon exposure to stress or apoptotic stimuli, mitochondria become fragile, leading to increased permeability of the outer mitochondrial membrane and release of cytochrome c, subsequently activating the caspase cascade [42]. In this study, formononetin effectively reversed the cisplatin-induced imbalance between Bax and Bcl-2, as well as reduced the expression of the apoptosis executor cleaved caspase-3. Thus, anti-apoptosis may be one of the critical mechanisms by which formononetin protects hair cells against cisplatin-induced ototoxicity.
The PI3K/AKT signaling pathway has been recognized as a prominent regulator of sensory hair cell survival against oxidative stress by regulating the transcription of several antioxidant genes [43,44]. Activation of the PI3K/AKT pathway represents a promising strategy for hair cell protection [45,46]. Recent studies have shown that formononetin can activate the PI3K/AKT signaling pathway in different cell types [47]. For instance, it has been reported that formononetin protects against cell death and prevents oxidative stress by enhancing PI3K/AKT signaling in H2O2-treated SH-SY5Y cells [16]. Additionally, formononetin induces vasorelaxation in rat thoracic aorta by regulating the PI3K/PTEN/Akt signaling pathway [48]. Notably, the PI3K/AKT pathway has been identified as an upstream regulator of Nrf2, and activation of the PI3K/AKT pathway triggers the activation of Nrf2 [49,50]. Formononetin has been shown to improve the survival of random skin flaps, prevent oxidative stress and inflammation by activating PI3K/AKT signal-mediated Nrf2 antioxidant defense system [47]. In this study, it was observed that formononetin enhanced the phosphorylation of PI3K and AKT, promoting hair cell survival under the assault from cisplatin. Conversely, treatment with the PI3K inhibitor LY294002 abolished the protective effect of formononetin, further confirming that formononetin protects hair cells by activating the PI3K/AKT pathway.
Nrf2 is a core transcription factor that plays important roles in stress response and promoting cell survival. It is regulated by various signaling pathways, including Nf-KB, Notch, PI3K-AKT, and Brca-1. Nrf2 modulates the transcription of key elements involved in the glutathione and thioredoxin antioxidant systems, enzymes responsible for NADPH regeneration, ROS and xenobiotic detoxification, and heme metabolism. Upon intracellular ROS accumulation, Nrf2 translocates into the cell nucleus and activates antioxidant proteins such as Gclc, Gpx2, Txnrd1, and HO-1 [51]. Gclc is a component of the glutamate-cysteine ligase complex, which is the rate-limiting enzyme in GSH synthesis, maintaining intracellular GSH content and preventing stress-induced cytotoxicity [52]. Gpx2 and Txnrd1 are primary antioxidant enzymes responsible for scavenging hydrogen peroxide and organic hydroperoxides, transferring electrons from NADPH to detoxify ROS and RNS [53]. HO-1 exerts beneficial effects through modulation of inflammation, protection against oxidative injury, regulation of apoptosis, and the removal of toxic heme, producing biliverdin, iron ions, and carbon monoxide [54]. The results of this study indicate that formononetin ameliorates cisplatin-induced hair cell death through the activation of the PI3K/AKT-Nrf2 signaling pathway and its downstream antioxidant genes (Fig. 5).
In conclusion, the findings of this study suggest that formononetin has a protective effect against cisplatin-induced hair cell death by preventing apoptosis. These effects are associated with the reduction of ROS overload and the inhibition of apoptosis. The activation of the PI3K/AKT-Nrf2 signaling pathway in cisplatin-treated hair cells mediates the protective effects of formononetin. Formononetin holds promise as a natural medication for preventing cisplatin-induced hair cell damage. Translation of these findings into clinical practice necessitates further in vivo investigations in future studies.
Data availability statement
Data will be made available on request.
CRediT authorship contribution statement
Yimeng Li: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Writing - original draft, Writing - review & editing. Jingfang Wu: Data curation, Formal analysis, Investigation, Methodology. Huiqian Yu: Formal analysis, Resources, Writing - review & editing. Xiaoling Lu: Data curation, Investigation, Methodology, Writing - original draft. Yusu Ni: Funding acquisition, Resources, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 82271169).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e23750.
Contributor Information
Yimeng Li, Email: eent06214@qq.com.
Yusu Ni, Email: niyusu@aliyun.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
figs1.
figs2.
figs3.
figs4.
figs5.
figs6.
figs7.
figs8.
figs9.
figs10.
figs11.
References
- 1.Wang D., Lippard S.J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 2005;4(4):307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 2.Gentilin E., et al. Cisplatin-induced ototoxicity: updates on molecular targets. Trends Mol. Med. 2019;25(12):1123–1132. doi: 10.1016/j.molmed.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Duan Z., et al. Cisplatin-induced renal toxicity in elderly people. Ther Adv Med Oncol. 2020;12 doi: 10.1177/1758835920923430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chadha S., Kamenov K., Cieza A. The world report on hearing, 2021. Bull. World Health Organ. 2021;99(4):242. doi: 10.2471/BLT.21.285643. 242A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kros C.J., Steyger P.S. Aminoglycoside- and cisplatin-induced ototoxicity: mechanisms and otoprotective strategies. Cold Spring Harb Perspect Med. 2019;9(11) doi: 10.1101/cshperspect.a033548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheth S., et al. Mechanisms of cisplatin-induced ototoxicity and otoprotection. Front. Cell. Neurosci. 2017;11:338. doi: 10.3389/fncel.2017.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.So H., et al. Evidence that cisplatin-induced auditory damage is attenuated by downregulation of pro-inflammatory cytokines via Nrf2/HO-1. JARO J. Assoc. Res. Otolaryngol. 2008;9(3):290–306. doi: 10.1007/s10162-008-0126-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rybak L.P. Mechanisms of cisplatin ototoxicity and progress in otoprotection. Curr. Opin. Otolaryngol. Head Neck Surg. 2007;15(5):364–369. doi: 10.1097/MOO.0b013e3282eee452. [DOI] [PubMed] [Google Scholar]
- 9.Machado Dutra J., Espitia P.J.P., Andrade Batista R., Formononetin Biological effects and uses - a review. Food Chem. 2021;359 doi: 10.1016/j.foodchem.2021.129975. [DOI] [PubMed] [Google Scholar]
- 10.Tay K.C., et al. Formononetin: a review of its anticancer potentials and mechanisms. Front. Pharmacol. 2019;10:820. doi: 10.3389/fphar.2019.00820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aladaileh S.H., et al. Formononetin upregulates Nrf2/HO-1 signaling and prevents oxidative stress, inflammation, and kidney injury in methotrexate-induced rats. Antioxidants. 2019;8(10) doi: 10.3390/antiox8100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yi L., et al. Formononetin attenuates airway in fl ammation and oxidative stress in murine allergic asthma. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.533841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma X., Wang J. Formononetin: a pathway to protect neurons. Front. Integr. Neurosci. 2022;16 doi: 10.3389/fnint.2022.908378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian J., Wang X.Q., Tian Z. Focusing on formononetin: recent perspectives for its neuroprotective potentials. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.905898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fei H.X., et al. Neuroprotective effect of formononetin in ameliorating learning and memory impairment in mouse model of Alzheimer's disease. Biosci. Biotechnol. Biochem. 2018;82(1):57–64. doi: 10.1080/09168451.2017.1399788. [DOI] [PubMed] [Google Scholar]
- 16.Sugimoto M., et al. Formononetin attenuates H(2)O(2)-induced cell death through decreasing ROS level by PI3K/Akt-Nrf2-activated antioxidant gene expression and suppressing MAPK-regulated apoptosis in neuronal SH-SY5Y cells. Neurotoxicology. 2021;85:186–200. doi: 10.1016/j.neuro.2021.05.014. [DOI] [PubMed] [Google Scholar]
- 17.Huang D., et al. Protective effects of formononetin against rhabdomyolysis-induced acute kidney injury by upregulating Nrf2 in vivo and in vitro. Rsc Advances. 2016;6(112):110874–110883. [Google Scholar]
- 18.Hao Y., et al. Formononetin protects against cisplatin-induced acute kidney injury through activation of the PPAR alpha/Nrf2/HO-1/NQO1 pathway. Int. J. Mol. Med. 2021;47(2):511–522. doi: 10.3892/ijmm.2020.4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Q., et al. Formononetin attenuates renal tubular injury and mitochondrial damage in diabetic nephropathy partly via regulating sirt1/PGC-1alpha pathway. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.901234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Althunibat O.Y., et al. Formononetin ameliorates renal dysfunction, oxidative stress, inflammation, and apoptosis and upregulates Nrf2/HO-1 signaling in a rat model of gentamicin-induced nephrotoxicity. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.916732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Del Rio D., Stewart A.J., Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metabol. Cardiovasc. Dis. 2005;15(4):316–328. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Circu M.L., Aw T.Y. Glutathione and apoptosis. Free Radic. Res. 2008;42(8):689–706. doi: 10.1080/10715760802317663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giustarini D., et al. Assessment of glutathione/glutathione disulphide ratio and S-glutathionylated proteins in human blood, solid tissues, and cultured cells. Free Radic. Biol. Med. 2017;112:360–375. doi: 10.1016/j.freeradbiomed.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Cohen G.M. Caspases: the executioners of apoptosis. Biochem. J. 1997;326(Pt 1):1–16. doi: 10.1042/bj3260001. Pt 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hemmings B.A., Restuccia D.F. PI3K-PKB/Akt pathway. Cold Spring Harb Perspect Biol. 2012;4(9):a011189. doi: 10.1101/cshperspect.a011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torrente L., DeNicola G.M. Targeting NRF2 and its downstream processes: opportunities and challenges. Annu. Rev. Pharmacol. Toxicol. 2022;62:279–300. doi: 10.1146/annurev-pharmtox-052220-104025. [DOI] [PubMed] [Google Scholar]
- 27.Nakaso K., et al. Novel cytoprotective mechanism of anti-parkinsonian drug deprenyl: PI3K and Nrf2-derived induction of antioxidative proteins. Biochem. Biophys. Res. Commun. 2006;339(3):915–922. doi: 10.1016/j.bbrc.2005.11.095. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y.K., et al. GALNT3 protects against vascular calcification by reducing oxidative stress and apoptosis of smooth muscle cells. Eur. J. Pharmacol. 2022 doi: 10.1016/j.ejphar.2022.175447. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y., et al. Ginsenoside Rk1 prevents UVB irradiation-mediated oxidative stress, inflammatory response, and collagen degradation via the PI3K/AKT/NF-kappaB pathway in vitro and in vivo. J. Agric. Food Chem. 2022;70(50):15804–15817. doi: 10.1021/acs.jafc.2c06377. [DOI] [PubMed] [Google Scholar]
- 30.Dresen E., et al. JPEN J Parenter Enteral Nutr; 2022. Overview of Oxidative Stress and the Role of Micronutrients in Critical Illness. [DOI] [PubMed] [Google Scholar]
- 31.Wang X., et al. Cisplatin-induced ototoxicity: from signaling network to therapeutic targets. Biomed. Pharmacother. 2022;157 doi: 10.1016/j.biopha.2022.114045. [DOI] [PubMed] [Google Scholar]
- 32.Tang Q., et al. Cisplatin-induced ototoxicity: updates on molecular mechanisms and otoprotective strategies. Eur. J. Pharm. Biopharm. 2021;163:60–71. doi: 10.1016/j.ejpb.2021.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Marzocchella L., et al. Dietary flavonoids: molecular mechanisms of action as anti- inflammatory agents. Recent Pat. Inflamm. Allergy Drug Discov. 2011;5(3):200–220. doi: 10.2174/187221311797264937. [DOI] [PubMed] [Google Scholar]
- 34.Mu H., et al. Research on antioxidant effects and estrogenic effect of formononetin from Trifolium pratense (red clover) Phytomedicine. 2009;16(4):314–319. doi: 10.1016/j.phymed.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y., et al. Formononetin attenuates IL-1beta-induced apoptosis and NF-kappaB activation in INS-1 cells. Molecules. 2012;17(9):10052–10064. doi: 10.3390/molecules170910052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voitkun V., Zhitkovich A. Analysis of DNA-protein crosslinking activity of malondialdehyde in vitro. Mutat. Res. 1999;424(1–2):97–106. doi: 10.1016/s0027-5107(99)00011-1. [DOI] [PubMed] [Google Scholar]
- 37.Palinski W., et al. ApoE-deficient mice are a model of lipoprotein oxidation in atherogenesis. Demonstration of oxidation-specific epitopes in lesions and high titers of autoantibodies to malondialdehyde-lysine in serum. Arterioscler. Thromb. 1994;14(4):605–616. doi: 10.1161/01.atv.14.4.605. [DOI] [PubMed] [Google Scholar]
- 38.Martins N.M., et al. Cisplatin induces mitochondrial oxidative stress with resultant energetic metabolism impairment, membrane rigidification and apoptosis in rat liver. J. Appl. Toxicol. 2008;28(3):337–344. doi: 10.1002/jat.1284. [DOI] [PubMed] [Google Scholar]
- 39.Chen M.F., et al. Vitamin C protects against cisplatin-induced nephrotoxicity and damage without reducing its effectiveness in C57BL/6 mice xenografted with Lewis lung carcinoma. Nutr. Cancer. 2014;66(7):1085–1091. doi: 10.1080/01635581.2014.948211. [DOI] [PubMed] [Google Scholar]
- 40.Mohan S., et al. Targeted amelioration of cisplatin-induced ototoxicity in Guinea pigs. Otolaryngol. Head Neck Surg. 2014;151(5):836–839. doi: 10.1177/0194599814544877. [DOI] [PubMed] [Google Scholar]
- 41.Siddiqui W.A., Ahad A., Ahsan H. The mystery of BCL2 family: Bcl-2 proteins and apoptosis: an update. Arch. Toxicol. 2015;89(3):289–317. doi: 10.1007/s00204-014-1448-7. [DOI] [PubMed] [Google Scholar]
- 42.Van Opdenbosch N., Lamkanfi M. Caspases in cell death, inflammation, and disease. Immunity. 2019;50(6):1352–1364. doi: 10.1016/j.immuni.2019.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kucharava K., et al. vol. 10. Cell Death & Disease; 2019. (Pasireotide Protects Mammalian Cochlear Hair Cells from Gentamicin Ototoxicity by Activating the PI3K-Akt Pathway). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y., et al. Key signaling pathways regulate the development and survival of auditory hair cells. Neural Plast. 2021:2021. doi: 10.1155/2021/5522717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chung W.H., et al. A PI3K pathway mediates hair cell survival and opposes gentamicin toxicity in neonatal rat organ of Corti. JARO J. Assoc. Res. Otolaryngol. 2006;7(4):373–382. doi: 10.1007/s10162-006-0050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma W.J., et al. Ginkgolide B protects against cisplatin-induced ototoxicity: enhancement of Akt-Nrf2-HO-1 signaling and reduction of NADPH oxidase. Cancer Chemother. Pharmacol. 2015;75(5):949–959. doi: 10.1007/s00280-015-2716-9. [DOI] [PubMed] [Google Scholar]
- 47.Li H.L., et al. Formononetin improves the survival of random skin flaps through PI3K/Akt-Mediated Nrf2 antioxidant defense system. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.901498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li T., et al. Formononetin induces vasorelaxation in rat thoracic aorta via regulation of the PI3K/PTEN/Akt signaling pathway. Drug Des Devel Ther. 2018;12:3675–3684. doi: 10.2147/DDDT.S180837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun X., Chen L., He Z. PI3K/Akt-Nrf2 and anti-inflammation effect of macrolides in chronic obstructive pulmonary disease. Curr Drug Metab. 2019;20(4):301–304. doi: 10.2174/1389200220666190227224748. [DOI] [PubMed] [Google Scholar]
- 50.Lai T.T., Yang C.M., Yang C.H. Astaxanthin protects retinal photoreceptor cells against high glucose-induced oxidative stress by induction of antioxidant enzymes via the PI3K/Akt/Nrf2 pathway. Antioxidants. 2020;9(8) doi: 10.3390/antiox9080729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tonelli C., Chio I.I.C., Tuveson D.A. Transcriptional regulation by Nrf2. Antioxid Redox Signal. 2018;29(17):1727–1745. doi: 10.1089/ars.2017.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peng Z., et al. Inhibitor of kappaB kinase beta regulates redox homeostasis by controlling the constitutive levels of glutathione. Mol. Pharmacol. 2010;77(5):784–792. doi: 10.1124/mol.109.061424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benhar M. Roles of mammalian glutathione peroxidase and thioredoxin reductase enzymes in the cellular response to nitrosative stress. Free Radic. Biol. Med. 2018;127:160–164. doi: 10.1016/j.freeradbiomed.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 54.Loboda A., et al. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016;73(17):3221–3247. doi: 10.1007/s00018-016-2223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.