Abstract
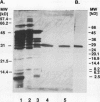
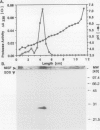
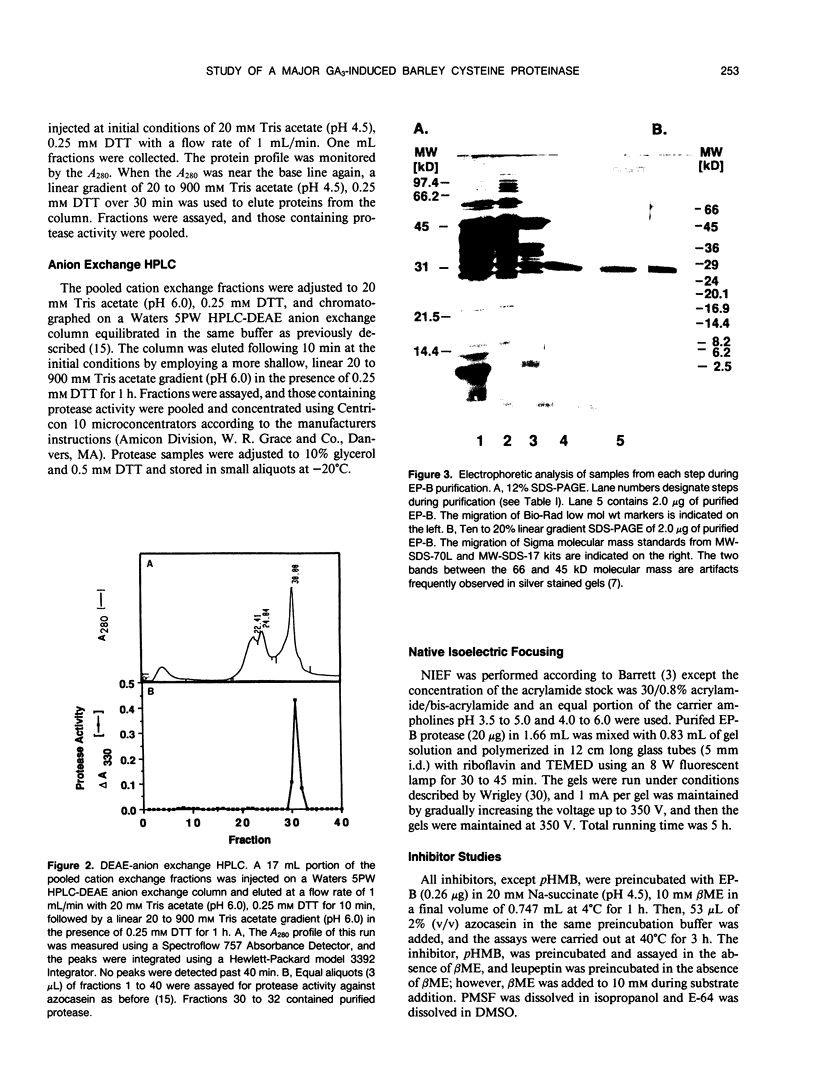
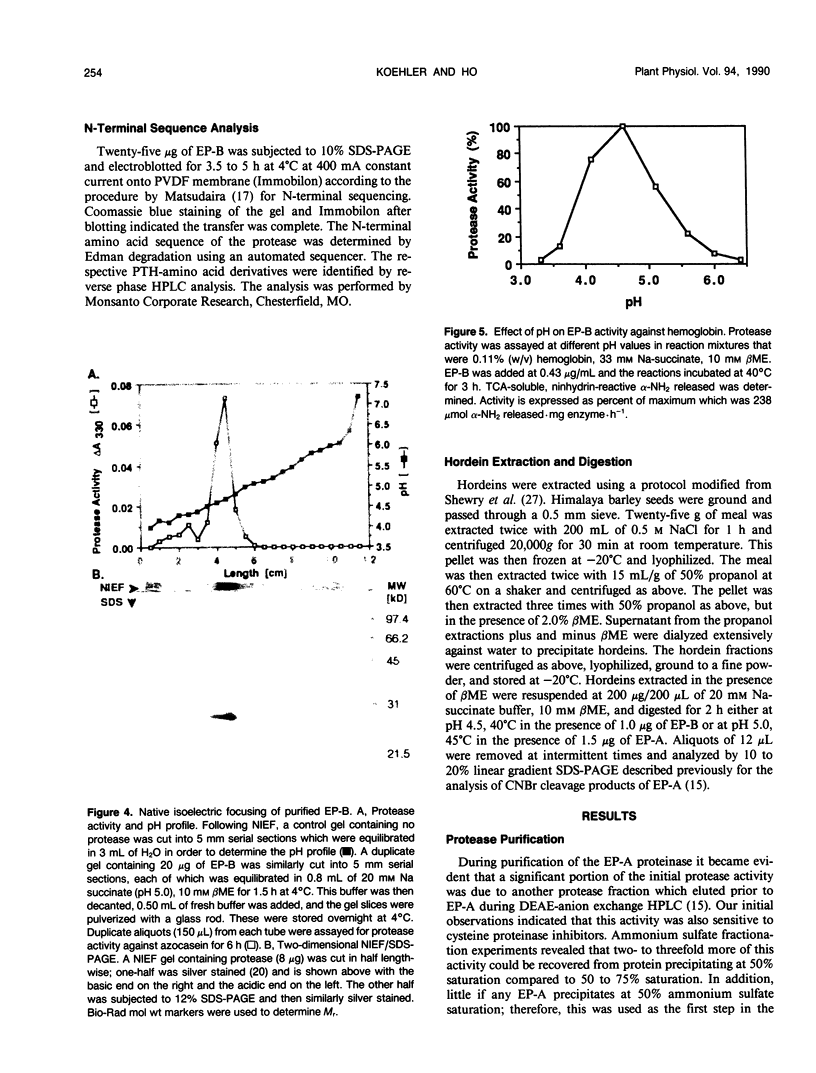
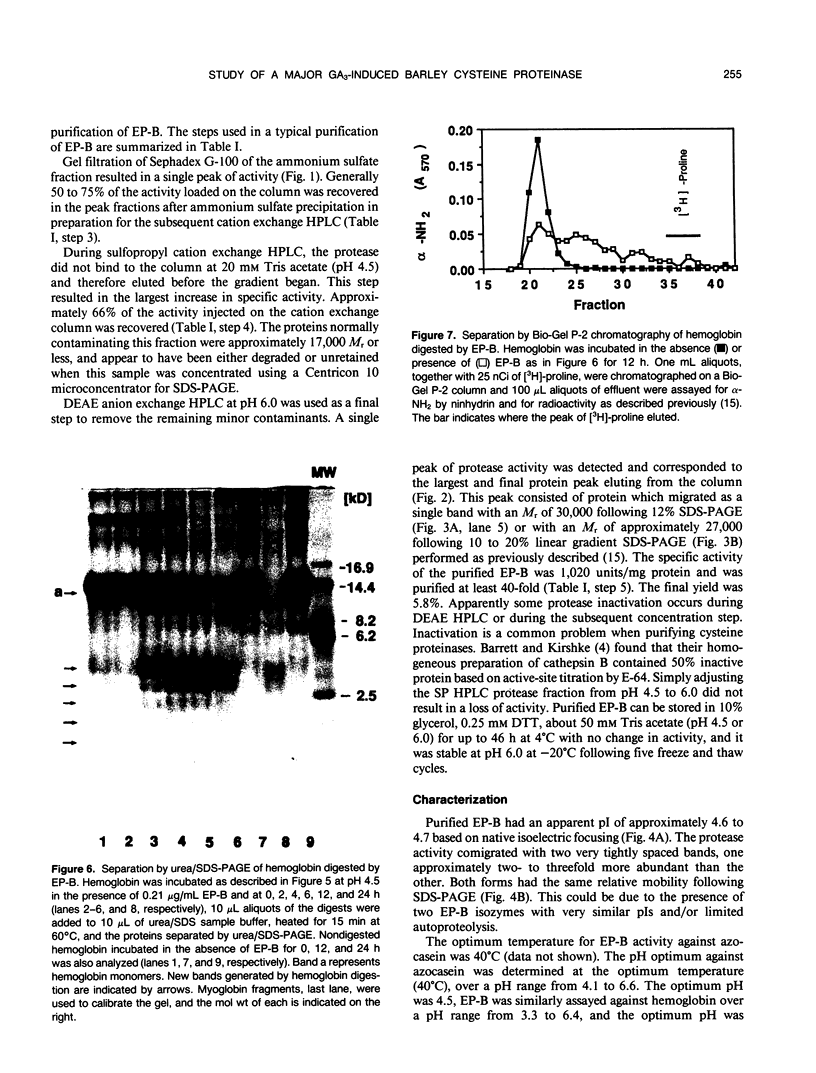
We previously described the purification and characterization of a 37,000 Mr cysteine proteinase, designated EP-A, from gibberellic acid (GA3)-induced barley (Hordeum vulgare L.) aleurone layers (S Koehler, T-HD Ho [1988] Plant Physiol 87: 95-103). A second, more abundant protease has now been purified from this tissue. This protease, designated EP-B, has an apparent Mr of 30,000 on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). It resolves into two bands during native isoelectric focusing with pl of 4.6 to 4.7. The analysis of hemoglobin digestion products by both gradient SDS-PAGE and Bio-Gel P2 chromatography, the inhibition of protease activity by E-64, leupeptin, iodoacetate, and p-hydroxymercuribenzoate, and N-terminal amino acid sequence analysis all indicate that EP-B is a cysteine proteinase. The first 22 amino acids at the N terminus of EP-B have been determined, and their sequence is 90% similar to that of EP-A. EP-B has properties similar to EP-A; however, EP-B is much more sensitive to high pH during gel electrophoresis and therefore is not detectable on native activity gels used to detect EP-A. Its pH optimum against azocasein and hemoglobin is 4.5 to 4.6. Both of these proteinases digest hordeins enriched for the B and D fractions into similar peptides of 25,000 to 2,000 Mr as determined by gradient SDS-PAGE.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akasofu H., Yamauchi D., Mitsuhashi W., Minamikawa T. Nucleotide sequence of cDNA for sulfhydryl-endopeptidase (SH-EP) from cotyledons of germinating Vigna mungo seeds. Nucleic Acids Res. 1989 Aug 25;17(16):6733–6733. doi: 10.1093/nar/17.16.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J. Cathepsin D. Purification of isoenzymes from human and chicken liver. Biochem J. 1970 Apr;117(3):601–607. doi: 10.1042/bj1170601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 1981;80(Pt 100):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- Baumgartner B., Chrispeels M. J. Purification and characterization of vicilin peptidohydrolase, the major endopeptidase in the cotyledons of mung-bean seedlings. Eur J Biochem. 1977 Jul 15;77(2):223–233. doi: 10.1111/j.1432-1033.1977.tb11661.x. [DOI] [PubMed] [Google Scholar]
- Brown P. H., Ho T. H. Barley aleurone layers secrete a nuclease in response to gibberellic Acid : purification and partial characterization of the associated ribonuclease, deoxyribonuclease, and 3'-nucleotidase activities. Plant Physiol. 1986 Nov;82(3):801–806. doi: 10.1104/pp.82.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerton R. W., Ho T. H. Hormonal regulation of the development of protease and carboxypeptidase activities in barley aleurone layers. Plant Physiol. 1986 Mar;80(3):692–697. doi: 10.1104/pp.80.3.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen J. V., Higgins T. J. Characterization of the alpha-Amylases Synthesized by Aleurone Layers of Himalaya Barley in Response to Gibberellic Acid. Plant Physiol. 1982 Dec;70(6):1647–1653. doi: 10.1104/pp.70.6.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler S. M., Ho T. H. Hormonal regulation, processing, and secretion of cysteine proteinases in barley aleurone layers. Plant Cell. 1990 Aug;2(8):769–783. doi: 10.1105/tpc.2.8.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler S., Ho T. H. Purification and characterization of gibberellic Acid-induced cysteine endoproteases in barley aleurone layers. Plant Physiol. 1988 May;87(1):95–103. doi: 10.1104/pp.87.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Mitsuhashi W., Koshiba T., Minamikawa T. Separation and Characterization of Two Endopeptidases from Cotyledons of Germinating Vigna mungo Seeds. Plant Physiol. 1986 Mar;80(3):628–634. doi: 10.1104/pp.80.3.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi W., Minamikawa T. Synthesis and Posttranslational Activation of Sulfhydryl-Endopeptidase in Cotyledons of Germinating Vigna mungo Seeds. Plant Physiol. 1989 Jan;89(1):274–279. doi: 10.1104/pp.89.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Poulle M., Jones B. L. A Proteinase from Germinating Barley : I. Purification and Some Physical Properties of a 30 kD Cysteine Endoproteinase from Green Malt. Plant Physiol. 1988 Dec;88(4):1454–1460. doi: 10.1104/pp.88.4.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi V., Oaks A. Hydrolysis of storage proteins in barley endosperms : analysis of soluble products. Plant Physiol. 1986 Jul;81(3):901–906. doi: 10.1104/pp.81.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J. C., Dean D., Heck G. R. Aleurain: a barley thiol protease closely related to mammalian cathepsin H. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6512–6516. doi: 10.1073/pnas.82.19.6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J. C. Two barley alpha-amylase gene families are regulated differently in aleurone cells. J Biol Chem. 1985 Mar 25;260(6):3731–3738. [PubMed] [Google Scholar]
- Sopanen T., Laurière C. Release and Activity of Bound beta-Amylase in a Germinating Barley Grain. Plant Physiol. 1989 Jan;89(1):244–249. doi: 10.1104/pp.89.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart I. M., Loi L., Fincher G. B. Development of (1-->3,1-->4)-beta-d-Glucan Endohydrolase Isoenzymes in Isolated Scutella and Aleurone Layers of Barley (Hordeum vulgare). Plant Physiol. 1986 Feb;80(2):310–314. doi: 10.1104/pp.80.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrigley C. W. Analytical fractionation of plant and animal proteins by gel electrofocusing. J Chromatogr. 1968 Aug 27;36(3):362–365. doi: 10.1016/s0021-9673(01)92959-0. [DOI] [PubMed] [Google Scholar]