Abstract
Background
This study evaluated the efficiency and safety of transurethral enucleation with bipolar energy (TUEB) using a spatula loop according to prostate volume.
Methods
We retrospectively evaluated 398 patients who underwent TUEB for benign prostatic hyperplasia at a single tertiary hospital between August 2018 and December 2022. The patients were divided into three groups according to estimated prostate volume (ePV): ≤40 mL (n = 67), 40–80 mL (n = 200), and ≥80 mL (n = 131). To compare the efficiency of TUEB, perioperative parameters including TUEB and enucleation efficiencies, were calculated as enucleated tissue weight per operation time and enucleated tissue weight per enucleation time, respectively. Preoperative and postoperative functional outcomes such as the International Prostate Symptom Score (IPSS), quality-of-life (QoL) score, maximum flow rate (Qmax), and post-void residual urine volume (PVR), were also compared.
Results
The IPSS total score, voiding sub-score, Qmax, and PVR improved after TUEB in all groups (all p < 0.05). The TUEB and enucleation efficiencies increased with increasing ePVs (all P < 0.001). When comparing the three prostate volume groups, there were no significant differences in functional outcomes within 12 months after TUEB (all-Bonferroni adjusted P > 0.017). A total of 57 patients experienced adverse events after TUEB, with no significant differences between the three groups (p = 0.507)
Conclusion
As prostate volume increases, the perioperative efficiency of TUEB is enhanced. Meanwhile, small prostates did not show significant differences in the improvement of functional outcomes and complications in comparison with larger prostates.
Keywords: Benign prostate hyperplasia, Efficiency, Functional outcomes, Prostate volume, Transurethral enucleation of prostate with bipolar energy
1. Introduction
In Korea, the incidence of benign prostatic hyperplasia (BPH) was approximately 11,610 per 100,000 males, increasing with age.1 Transurethral resection of the prostate (TURP) is recognized as the gold standard surgical treatment for symptomatic BPH, especially when the prostate volume is 30-80 mL.2 However, there is a potential risk of morbidity such as TURP syndrome and hematuria, leading urologists to focus on new transurethral enucleation techniques that are equally effective but safer alternatives.3 Since 1998, when holmium laser enucleation of the prostate (HoLEP) was reported,4 it has been widely recognized as an alternative to TURP in patients with moderate-to-severe lower urinary tract symptoms (LUTS).2 Recently, transurethral enucleation with bipolar energy (TUEB) has been introduced as an alternative to TURP and HoLEP in clinical practice.2 Several studies have shown the superiority of TUEB over TURP in patients with a prostate volume >80 mL.5,6 However, few studies have compared the surgical outcomes and safety of TUEB according to various prostate volume ranges.7 Furthermore, there are few studies evaluating the surgical outcomes of TUEB in patients with small prostates, although there are several studies on HoLEP.8.9 Therefore, our study aimed to evaluate the impact of a wide range of prostate volumes on surgical outcomes after TUEB in terms of perioperative efficiency, postoperative International Prostate Symptom Score (IPSS) parameters, uroflowmetry parameters, and complication rates.
2. Materials and methods
2.1. Ethics statement
This study was approved by the Institutional Review Board of the Seoul National University Bundang Hospital (IRB no. B-2305-826-101). The requirement for written informed consent was waived because of the retrospective nature of the study. All methods were conducted in accordance with the relevant guidelines and regulations (ethical standards of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards).
2.2. Study population
Between August 2018 and December 2022, 398 patients who underwent TUEB for LUTS or benign prostatic obstruction were enrolled at our institution. Patients with a history of neurogenic bladder, prostate cancer, or BPH surgery were excluded. All patients were evaluated by medical history, physical examination, IPSS, QoL score, serum prostate-specific antigen (PSA) test, transrectal prostate ultrasonography (TRUS), and uroflowmetry before surgery.
The primary aim of the present study was to verify whether there were any significant differences in perioperative outcomes, postoperative functional outcomes, or postoperative complication rates according to the estimated prostate volume (ePV) based on preoperative TRUS. Therefore, the patients in our cohorts were subdivided into three groups: those with small-sized prostates (ePV <40 mL; group 1) those with medium-sized prostates (ePV ≥40 mL and <80 mL; group 2) and those with large-sized prostates (ePV ≥80 mL; group 3).
2.3. Surgical technique and perioperative parameters
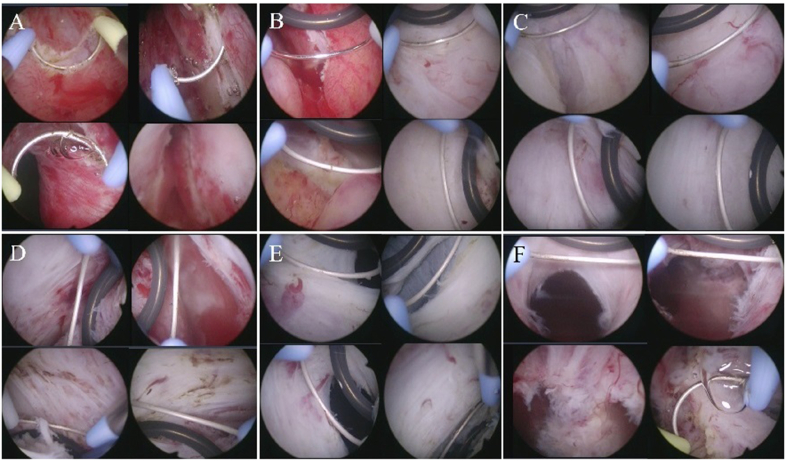
To enucleate the prostatic adenoma, an Olympus transurethral resection in saline bipolar resection system (Olympus Medical Systems Corp., Tokyo, Japan) was used, with both a standard tungsten wire loop and a PLASMA enucleation electrode (Olympus Medical Systems Corp., Tokyo, Japan) with a round spatula in front (Fig. 1A). For prostate morcellation, a DrillCut™ (Karl Storz Inc., Tuttlingen, Germany) morcellator with an oscillating tooth blade was used (Fig. 1B). Our surgical technique is similar to that reported by Bebi et al..7 TUEB was performed as described below (Fig. 2), which was applied to every TUEB case, regardless of the prostate volume or shape of the prostatic lobes. First, a groove was created at the 5 and 7 o'clock positions of the prostatic urethra at the level of the verumontanum using a standard tungsten wire loop (Fig. 2A). The initial groove was expanded circumferentially on both sides (Fig. 2B). Subsequently, the surgeon pushed the PLASMA enucleation electrode with a spatula against the prostatic adenoma from the prostate apex to the bladder neck (Fig. 2C–F). This allowed the prostatic adenoma to be gently removed from the capsule. When active bleeding occurred during enucleation, electrocauterization using a standard wire loop was performed at the discretion of the operator. After the enucleated prostatic adenoma was separated from the capsule and placed into the bladder, morcellation using the Drillcut™ morcellator was performed. When the TUEB procedure was completed, a 22-Fr 3-way Foley catheter was inserted with continuous bladder irrigation. The time of cessation of bladder irrigation and removal of the Foley catheter was based on the discretion of the operator.
Fig. 1.
Transurethral enucleation with bipolar energy (TUEB) equipment in Seoul National Bundang Hospital: (A) PLASMA enucleation electrode with spatula for prostate enucleation (https://www.olympusprofed.com/uro/plasmabph/1188/); (B) Drillcut™ morcellator system for prostate morcellation (https://www.karlstorz.com/cps/rde/xbcr/karlstorz_assets/ASSETS/3528837.pdf).
Fig. 2.
Overall procedure of transurethral enucleation with bipolar energy (TUEB) using a spatula loop: (A) circumferential marking and incision at the apical urethral mucosa surface (start at 5 and 7 o'clock and proceed upward); (B) upward enucleation of the left and right lobe from just lateral to the verumontanum (laterally and upwardly) using TUEB spatula loop; (C) enucleation of both lateral lobes using TUEB spatula loop; (D) enucleation of anterior side of the lateral lobes and anterior lobe (En-bloc procedure); (E) Bladder neck mucosa incision; (F) detachment of the most distal mucosal stalk.
The perioperative parameters included operative time, enucleated tissue weight, enucleation time, postoperative catheterization time, and length of hospital stay. To evaluate the perioperative efficiency, TUEB and enucleation efficiencies were calculated as the enucleated prostatic tissue weight per operation time and the enucleated prostatic tissue weight per enucleation time, respectively.
2.4. Postoperative follow-up and functional outcomes
Patients were followed up at the outpatient clinic at 1, 3, 6, and 12 months postoperatively to evaluate functional outcomes such as IPSS, QoL score, and uroflowmetry. Postoperative adverse events were evaluated based on electronic medical records and categorized according to the Clavien–Dindo scale.10
Functional outcomes included the IPSS total score sum, IPSS voiding subscore, IPSS storage subscore, IPSS QoL score, maximum flow rate (Qmax), and post-void residual volume (PVR). The IPSS voiding sub-score was calculated as the sum of the answers to questions 1 (incomplete emptying), 3 (intermittency), 5 (weak stream), and 6 (straining to void). The IPSS storage subscore was calculated as the sum of the answers to questions 2 (frequency), 4 (urgency), and 7 (nocturia).
2.5. Statistical analysis
All data were analyzed using IBM SPSS Statistics ver. 27.0 (IBM Corp., Armonk, New York, USA). The normality test was performed using the Shapiro–Wilk test. Continuous parameters were presented as medians (interquartile range [IQR]) and compared using the Mann–Whitney U test or Kruskal–Wallis test. In contrast, categorical parameters were presented as numbers (proportions) and evaluated using the chi-squared test or Fisher's exact test. The Wilcoxon test was used to evaluate the differences in continuous variables before and 1 month after TUEB. All statistical tests were two-sided, with p < 0.05 as the threshold for statistical significance. For post hoc analyses among the three groups according to ePV, statistical significance was considered when the Bonferroni-adjusted p value was less than 0.017 (= 0.05/3).
3. Results
Table 1 shows the preoperative characteristics of the patients according to prostate volume. There were no significant differences in age, body mass index (BMI), or comorbidities between the three groups. Serum PSA levels increased with increasing prostate volume (P < 0.001). Patients with a higher prostate volume were more likely to have a history of urinary retention before undergoing TUEB (P = 0.001). Group 2 showed the highest rate of preoperative BPH-related drug use. For the IPSS, group 1 had the highest IPSS total score, voiding subscore, and QoL score, while there were no significant differences between groups 2 and 3. Regarding uroflowmetry parameters, there was no significant difference in the preoperative Qmax, whereas the preoperative ratio of PVR to VV increased as the prostate volume increased.
Table 1.
Baseline characteristics of patients according to prostate volume groups; <40 mL (n = 67), 40–80 mL (n = 200), and ≥80 mL (n = 131)
| Variable | Group 1 ePV <40 mL (n = 67) | Group 2 ePV 40–80 mL (n = 200) | Group 3 ePV ≥80 mL (n = 131) | P |
|---|---|---|---|---|
| Age (year) | 72 (66–78) | 71 (65–77) | 73 (66–78) | 0.637 |
| BMI (kg/m2) | 23.8 (21.8–25.7) | 24.1 (22.6–26.1) | 24.6 (22.6–26.5) | 0.174 |
| HTN | 35 (52.2%) | 100 (50.0%) | 67 (51.1%) | 0.945 |
| DM | 15 (22.4%) | 43 (21.5%) | 33 (25.2%) | 0.733 |
| Neurologic disease | 12 (17.9%) | 29 (14.5%) | 26 (19.8%) | 0.431 |
| Cardiovascular disease | 16 (23.9%) | 34 (17.0%) | 19 (14.5%) | 0.253 |
| CKD | 4 (6.0%) | 5 (2.5%) | 9 (6.9%) | 0.143 |
| History of AUR | 11 (16.4%) | 54 (27.0%) | 54 (41.2%) | 0.001 |
| Prior AP/AC therapy | 21 (31.3%) | 52 (26.0%) | 43 (32.8%) | 0.373 |
| PSA (ng/mL) | 1.28 (0.63–2.50) | 2.62 (1.57–6.14) ∗ | 7.68 (4.62–12.11) ∗† | <0.001 |
| BPH-related drugs | 58 (86.6%) | 189 (94.5%) | 110 (84.0%) | 0.006 |
| Alpha-blockers | 37 (55.2%) | 87 (43.5%) | 62 (47.3%) | 0.008 |
| 5-ARIs | 1 (1.5%) | 8 (4.0%) | 4 (3.1%) | |
| Combination | 20 (29.9%) | 94 (47.0%) | 44 (33.6%) | |
| ePV (mL) | 34.0 (28.0–37.8) | 57.0 (48.9–68.0) ∗ | 102.7 (91.1–128.0) ∗† | <0.001 |
| IPSS total score | 23.0 (19.0–28.0) | 19.0 (13.0–26.0) ∗ | 17.0 (12.0–25.0) ∗ | 0.010 |
| IPSS voiding subscore | 14.0 (11.5–19.0) | 12.0 (7.0–16.0) ∗ | 11.0 (6.5–15.0) ∗ | 0.001 |
| IPSS storage subscore | 8.0 (5.5–12.0) | 8.0 (5.0–11.0) | 8.0 (4.5–11.0) | 0.553 |
| IPSS QoL score | 5.0 (4.0–6.0) | 4.0 (3.3–5.0) ∗ | 4.0 (3.0–5.0) ∗ | 0.001 |
| Qmax (mL/s) | 9.0 (6.5–12.0) | 9.0 (6.0–12.0) | 9.0 (6.0–11.0) | 0.712 |
| PVR (mL) | 45.0 (13.8–95.0) | 64.0 (30.0–115.0) | 112.0 (58.0–166.5) ∗† | <0.001 |
ePV, estimated prostate volume; BMI, body mass index; HTN, hypertension; DM, diabetes mellitus; CKD, chronic kidney disorder; AUR, acute urinary retention; AP/AC, antiplatelet/anticoagulant; PSA, prostate-specific antigen; BPH, benign prostate hyperplasia; 5-ARI, 5-alpha reductase inhibitor; IPSS, International Prostate Symptom Score; QoL, quality of life; Qmax, maximum flow rate; PVR, post-void residual volume; VV, voided volume. Data presented are median (interquartile range) or number (%). In post hoc analysis, the statistical significance was considered when the Bonferroni adjusted p value was less than 0.017. ∗p < 0.017 vs. Group 1, †p < 0.017 vs. Group 2.
3.1. Perioperative outcomes of TUEB according to prostate volume
The weight of the enucleated prostate tissue, operation time, enucleation time, and enucleation efficiency increased with increasing prostate volume (all P < 0.001) (Table 2). Median enucleated prostate tissue weight was significantly lower for the smaller prostates in group 1 (9.0 g [IQR 6.0–12.0]) than in groups 2 and 3 (20.0 g [IQR 14.0-26.0] and 42.0 g [32.0-61.0], respectively, P < 0.001). Patients with smaller prostates in group 1 had significantly shorter operation and enucleation times (50 min [IQR 45–55] and 25 min [IQR 20–25], respectively) than group 2 (65 min [IQR 55–75] and 45 min [IQR 40–50]) and group 3 (90 min [IQR 80–110] and 60 min [IQR 50–70]) (all P < 0.001). Furthermore, median TUEB efficiency and enucleation efficiency were 0.18 g/min (IQR 0.12–0.24) and 0.36 g/min (IQR 0.28–044), respectively, in group 1 versus 0.30 g/min (IQR 0.23–0.39) and 0.44 g/min (IQR 0.34–0.60), respectively, in group 2 versus 0.45 g/min (IQR 0.35–0.60) and 0.73 g/min (IQR 0.53–0.96), respectively, in groups 3, showing a tendency to increase as prostate volume increased (all P < 0.001). In contrast, the duration of hospital stay and catheterization were the longest in group 3 (all P < 0.001), without significant differences between groups 1 and 2 (P = 0.383 and P = 0.596, respectively).
Table 2.
Comparisons of perioperative characteristics after surgery according to prostate volume groups; <40 mL (n = 67), 40-80 mL (n = 200), and ≥80 mL (n = 131)
| Variable | Group 1 ePV <40 mL (n = 67) | Group 2 ePV 40–80 mL (n = 200) | Group 3 ePV ≥80 mL (n = 131) | P |
|---|---|---|---|---|
| Enucleated tissue weight (g) | 9.0 (6.0–12.0) | 20.0 (14.0–26.0) | 42.0 (32.0–61.0) | G1 vs. G2 <0.001 G2 vs. G3 <0.001 G1 vs. G3 <0.001 |
| Operation time (min) | 70 (55–85) | 100 (80–120) | 145 (125–175) | G1 vs. G2 <0.001 G2 vs. G3 <0.001 G1 vs. G3 <0.001 |
| Enucleation time (min) | 25 (20–35) | 45 (40–50) | 60 (50–70) | G1 vs. G2 <0.001 G2 vs. G3 <0.001 G1 vs. G3 <0.001 |
| TUEB efficiency (g/min) | 0.13 (0.09–0.18) | 0.20 (0.15–0.26) | 0.30 (0.23–0.38) | G1 vs. G2 <0.001 G2 vs. G3 <0.001 G1 vs. G3 <0.001 |
| Enucleation efficiency (g/min) | 0.36 (0.28–0.44) | 0.44 (0.34–0.60) | 0.73 (0.53–0.96) | G1 vs. G2 <0.001 G2 vs. G3 <0.001 G1 vs. G3 <0.001 |
| Catheterization duration (day) | 3 (2–4) | 3 (2–4) | 4 (3–5) | G1 vs. G2 0.383 G2 vs. G3 <0.001 G1 vs. G3 <0.001 |
| Hospitalization duration (day) | 5 (4–5) | 5 (4–5) | 5 (5–7) | G1 vs. G2 0.596 G2 vs. G3 <0.001 G1 vs. G3 <0.001 |
ePV, estimated prostate volume; IPSS, International Prostate Symptom Score; QoL, quality of life; Qmax, maximum flow rate; PVR, post-void residual volume; VV, voided volume. Data presented are median (interquartile range). In post hoc analysis, the statistical significance was considered when the Bonferroni adjusted p value was less than 0.017.
3.2. Functional outcomes after TUEB according to prostate volume
Table 3 shows the comparisons between the baseline functional outcomes before and within 1 month after TUEB. In every group, the patients showed substantial improvements in the IPSS total score, voiding subscore, Qmax, PVR, and ratio of PVR to VV after TUEB (all P < 0.05). The postoperative QoL of patients significantly improved in groups 1 (P = 0.002) and 2 (P = 0.019), but group 3 failed to show a statistically significant improvement in QoL (P = 0.059).
Table 3.
Comparisons of preoperative and postoperative functional outcomes for each prostate volume group; <40 mL (n = 67), 40–80 mL (n = 200), and ≥80 mL (n = 131)
| Variable | Before TUEB | After TUEB | P |
|---|---|---|---|
| Group 1: PV < 40 mL (n = 67) | |||
| IPSS total score | 23.0 (19.0–28.0) | 16.5 (10.5–23.0) | 0.003 |
| IPSS voiding subscore | 14.0 (11.5–19.0) | 9.0 (3.5–15.0) | 0.001 |
| IPSS storage subscore | 8.0 (5.0–12.0) | 7.0 (5.0–10.0) | 0.580 |
| IPSS QoL score | 5.0 (4.0–6.0) | 3.5 (3.0–5.0) | 0.002 |
| Qmax (mL/s) | 9.0 (6.5–12.0) | 14.0 (9.0–20.5) | <0.001 |
| PVR (mL) | 45.0 (13.8–94.5) | 20.0 (1.5–40.0) | 0.001 |
| Group 2: PV 40–80 mL (n = 200) | |||
| IPSS total score | 19.0 (13.0–16.0) | 11.0 (6.0–17.0) | <0.001 |
| IPSS voiding subscore | 12.0 (7.0–16.0) | 4.0 (1.0–9.0) | <0.001 |
| IPSS storage subscore | 8.0 (5.0–11.0) | 6.0 (4.0–9.0) | 0.202 |
| IPSS QoL score | 4.0 (3.3–5.0) | 3.0 (2.0–4.0) | 0.019 |
| Qmax (mL/s) | 9.0 (6.0–12.0) | 17.0 (11.0–24.0) | <0.001 |
| PVR (mL) | 63.5 (30.8–114.8) | 30.0 (10.0–50.0) | <0.001 |
| Group 3: PV ≥ 80 mL (n = 131) | |||
| IPSS total score | 17.0 (12.0–25.0) | 9.5 (5.0–16.8) | 0.002 |
| IPSS voiding subscore | 11.0 (6.5–15.0) | 3.5 (0.0–6.0) | 0.001 |
| IPSS storage subscore | 8.0 (4.5–11.0) | 6.5 (3.3–9.0) | 0.026 |
| IPSS QoL score | 4.0 (3.0–5.0) | 3.0 (1.0–4.0) | 0.059 |
| Qmax (mL/s) | 9.0 (6.0–11.0) | 17.5 (13.0-24.3) | <0.001 |
| PVR (mL) | 111.5 (57.0–163.0) | 40.0 (20.0–60.0) | <0.001 |
TUEB, transurethral enucleation of bipolar energy; IPSS, International Prostate Symptom Score; QoL, quality of life; Qmax, maximum flow rate; PVR, post-void residual volume; VV, voided volume. Data presented are median (interquartile range).
Overall, no significant differences in the postoperative functional outcomes were identified from baseline according to the prostate volume (all Bonferroni-adjusted P > 0.017) (Table 4). Only PVR 1 month after TUEB, in group 3, showed significant improvement compared with that in the other groups (all P < 0.001). However, this difference disappeared 12 months after TUEB.
Table 4.
Comparison of postoperative functional outcomes according to prostate volume groups; <40 mL (n = 67), 40–80 mL (n = 200), and ≥80 mL (n = 131)
| Variable | Group 1 ePV <40 mL (n = 67) | Group 2 ePV 40–80 mL (n = 200) | Group 3 ePV ≥80 mL (n = 131) | P |
|---|---|---|---|---|
| IPSS total score improvement | ||||
| 1M | 4.5 (1.8-13.3) | 6.0 (−1.0–11.0) | 8.5 (6.0–14.5) | G1 vs. G2 0.685, G2 vs. G3 0.091. G1 vs. G3 0.282 |
| 3M | 8.0 (1.0–13.0) | 8.0 (3.0–14.0) | 9.0 (3.0–15.0) | G1 vs. G2 0.777, G2 vs. G3 0.701 G1 vs. G3 0.579 |
| 6M | 8.0 (1.3–12.5) | 9.0 (4.0–18.0) | 9.0 (3.0–15.0) | G1 vs. G2 0.243, G2 vs. G3 0.919 G1 vs. G3 0.301 |
| 12M | 4.0 (−2.0–11.5) | 10.0 (3.0–16.0) | 8.0 (4.0–14.5) | G1 vs. G2 0.039, G2 vs. G3 0.597 G1 vs. G3 0.115 |
| IPSS voiding subscore improvement | ||||
| 1M | 6.5 (2.5–11.3) | 5.0 (1.0–9.0) | 5.0 (3.0–12.5) | G1 vs. G2 0.359, G2 vs. G3 0.397 G1 vs. G3 0.929 |
| 3M | 6.0 (1.0–12.0) | 7.0 (2.0–10.0) | 5.5 (2.0–10.0) | G1 vs. G2 0.881, G2 vs. G3 0.933 G1 vs. G3 0.659 |
| 6M | 6.5 (2.3–9.0) | 7.0 (3.0–12.0) | 7.0 (3.0–11.0) | G1 vs. G2 0.481, G2 vs. G3 0.843 G1 vs. G3 0.508 |
| 12M | 2.0 (−1.0 – 8.5) | 6.5 (2.0–11.3) | 5.0 (2.5–10.5) | G1 vs. G2 0.060, G2 vs. G3 0.670 G1 vs. G3 0.129 |
| IPSS storage subscore improvement | ||||
| 1M | 0.5 (–3 –3.5) | 1.0 (−2.0–3.0) | 3.0 (0.3–4.0) | G1 vs. G2 0.822, G2 vs. G3 0.061 G1 vs. G3 0.171 |
| 3M | 1.0 (−2.0–4.0) | 2.0 (0.0–5.0) | 2.0 (0.0–5.0) | G1 vs. G2 0.168, G2 vs. G3 0.685 G1 vs. G3 0.092 |
| 6M | 1.0 (−1.0–6.5) | 3.0 (1.0–6.0) | 3.0 (0.0–5.0) | G1 vs. G2 0.107, G2 vs. G3 0.716 G1 vs. G3 0.369 |
| 12M | 1.0 (−1.0–4.0) | 3.0 (1.0–5.0) | 2.0 (1.0–5.0) | G1 vs. G2 0.049, G2 vs. G3 0.424 G1 vs. G3 0.188 |
| IPSS QoL score improvement | ||||
| 1M | 1.0 (0.0–2.3) | 0.0 (0.0–1.3) | 1.0 (−0.8–2.8) | G1 vs. G2 0.083, G2 vs. G3 0.440 G1 vs. G3 0.614 |
| 3M | 1.0 (0.0–3.0) | 2.0 (0.0–3.0) | 2.0 (1.0–3.0) | G1 vs. G2 0.307, G2 vs. G3 0.716 G1 vs. G3 0.146 |
| 6M | 1.0 (0.3–2.0) | 2.0 (0.5–3.0) | 2.0 (0.0–3.0) | G1 vs. G2 0.205, G2 vs. G3 0.765 G1 vs. G3 0.549 |
| 12M | 1.0 (0.0–2.0) | 2.0 (0.0–3.0) | 1.0 (0.0–3.0) | G1 vs. G2 0.066, G2 vs. G3 0.335 G1 vs. G3 0.331 |
| Qmax improvement (mL/s) | ||||
| 1M | 2.0 (−6.5–10.0) | 6.0 (−1.0–13.0) | 7.0 (1.0–16.0) | G1 vs. G2 0.050, G2 vs. G3 0.248 G1 vs. G3 0.015 |
| 3M | 8.0 (2.0–11.0) | 8.0 (1.5–17.0) | 11.0 (3.0–18.0) | G1 vs. G2 0.461, G2 vs. G3 0.232, G1 vs. G3 0.076 |
| 6M | 3.4 (−0.8–7.8) | 5.0 (−1.0–12.0) | 9.0 (6.0–13.0) | G1 vs. G2 0.343, G2 vs. G3 0.025 G1 vs. G3 0.003 |
| 12M | 3.6 (1.0–10.0) | 8.0 (1.0–14.0) | 11.0 (5.0–18.0) | G1 vs. G2 0.116, G2 vs. G3 0.125 G1 vs. G3 0.004 |
| PVR decrease (mL) | ||||
| 1M | 20.0 (0.0–75.0) | 42.5 (10.0–85.0) | 73.5 (21.3–132.5) | G1 vs. G2 0.165, G2 vs. G3 <0.001 G1 vs. G3 <0.001 |
| 3M | 31.0 (6.0–116.5) | 40.0 (10.0–102.5) | 90.0 (43.0–159.0) | G1 vs. G2 0.572, G2 vs. G3 0.005 G1 vs. G3 0.019 |
| 6M | 30.0 (−20.0–114.5) | 46.0 (9.5–104.5) | 95.0 (36.5–135.5) | G1 vs. G2 0.294, G2 vs. G3 0.048 G1 vs. G3 0.029 |
| 12M | 50.0 (5.5–99.0) | 54.0 (8.0–110.0) | 85.0 (45.0–143.0) | G1 vs. G2 0.906, G2 vs. G3 0.017 G1 vs. G3 0.066 |
ePV, estimated prostate volume; IPSS, International Prostate Symptom Score; QoL, quality of life; Qmax, maximum flow rate; PVR, post-void residual volume; VV, voided volume. Data presented are median (interquartile range). In post hoc analysis, the statistical significance was considered when the Bonferroni adjusted p value was less than 0.017.
3.3. Postoperative adverse events
Among the 398 patients, 57 (14.3%) experienced postoperative adverse events requiring emergency center visits. Table 5 presents the details of the postoperative adverse events according to prostate volume. In groups 1, 2, and 3, 10 (14.9%), 32 (16.0%), and 15 (11.5%) patients experienced adverse events after TUEB, respectively. There were no significant differences in the prevalence (P = 0.507) or severity (P = 0.199) of postoperative adverse events between the three groups based on the Clavien–Dindo scale.
Table 5.
Comparison of postoperative adverse events among the prostate volume groups according to the Clavien-Dindo classification. The following adverse events and consecutive managements is demonstrated in the table
| Adverse events | Group 1 ePV <40 mL (n = 67) | Group 2 ePV 40–80 mL (n = 200) | Group 3 ePV ≥80 mL (n = 131) | P |
|---|---|---|---|---|
| Overall AEs, n (%) | 10 (14.9%) | 32 (16.0%) | 15 (11.5%) | 0.507 |
| Clavien-Dindo I | 7 (70.0%) | 16 (50.0%) | 4 (26.7%) | |
| Clavien-Dindo II | 0 (0.0%) | 5 (15.6%) | 2 (13.3%) | 0.199 |
| Clavien-Dindo III | 3 (30.0%) | 11 (34.4%) | 9 (60.0%) |
| Clavien-Dindo grade | Adverse events | Management |
|---|---|---|
| I | Gross hematuria ± urinary retention due to blood clot (n = 21) | Foley catheter insertion and bladder irrigation |
| Acute urinary retention without hematuria (n = 6) | Foley catheter insertion | |
| II | Epididymitis (n = 2) | PO medication |
| Prostatitis (n = 1) | PO medication | |
| Acute pyelonephritis (n = 1) | IV antibiotics | |
| Other UTI (n = 1) | IV antibiotics | |
| Urethral stricture (n = 1) | sounding | |
| Gross hematuria (n = 1) | PO Tranexamic acid | |
| III | Gross hematuria ± urinary retention due to blood clot (n = 11) | Transurethral fulguration under anesthesia |
| Urethral stricture (n = 2) | Urethral dilation under anesthesia | |
| Bladder neck contraction (n = 7) | Transurethral incision of bladder neck | |
| Bladder stone (n = 3) | Cystolitholapaxy under anesthesia |
ePV, estimated prostate volume; AE, adverse event.
4. Discussion
Both HoLEP and TUEB have been accepted in clinical practice as effective and safe alternatives to TURP in BPH patients with moderate-to-severe LUTS.2 HoLEP has been reported as a ‘size-independent’ surgical management option for BPH in several studies.9,11,12 Although HoLEP is recommended for moderate BPH with a prostate volume of 30–80 mL, even BPH with a small prostate size can be treated using HoLEP.8,13 Therefore, it may be reasonable to consider TUEB as a size-independent surgical treatment option for patients with BPH. Indeed, a prospective study showed that TUEB could be a more favorable alternative to TURP, especially in patients with prostate volumes >80 g.14 Endo et al.15 also concluded that TUEB could be a safe and effective surgical option for BPH regardless of PV after comparing the functional outcomes and rates of perioperative adverse events between the standard group (PV < 80 mL) and the large group (PV ≥ 80 mL). However, there are few studies on surgical outcomes after TUEB for small prostates.16,17
Moreover, to the best of our knowledge, only one previous study has compared surgical efficiency, postoperative functional outcomes, and complication rates according to various prostate volume ranges.7 Furthermore, few studies have evaluated the association between a wide range of prostate volumes and the surgical outcomes of TUEB, especially with long-term follow-up. Therefore, we aimed to evaluate the perioperative efficiency and postoperative functional outcomes according to a wide range of prostate volumes within a 1-year postoperative follow-up period.
In our study, TUEB using a specialized spatula loop resulted in significant improvements in functional outcomes after surgery. In terms of functional outcomes, the IPSS total score, voiding subscore, Qmax, PVR, and ratio of PVR to VV improved significantly in all prostate volume groups at 1 month postoperatively from baseline values. When evaluating the improvements in functional outcomes within 12 months of follow-up after TUEB, the differences between postoperative functional outcomes and baseline values according to prostate volume were indefinite, suggesting that TUEB can be considered a size-independent surgical procedure, which is consistent with the results of a previous study.7
Meanwhile, in comparison with the results of previous studies,7 postoperative functional outcomes such as Qmax are relatively poor. As our study was based on a single surgeon's experience, the proficiency or learning curve of the operator might affect the surgical outcomes, considering TUEB at our institution was first introduced in 2018. A further large-scale study based on multiple surgeons experiences should be performed.
In contrast to the perioperative outcomes, there was a tendency for enucleation efficiency to increase as prostate volume increased, which is consistent with previous studies. Bebi et al.7 showed that the enucleation efficiency of TUEB increased significantly as the prostate volume increased. This association was also observed in other enucleation techniques, such as HoLEP.11,18 This would be partially because of surgeons’ reluctance to recommend surgical treatment for patients with small prostates, which retards the learning curve. Moreover, Xiong et al. mentioned technical difficulties in recognizing the appropriate surgical enucleation plane when performing TUEB for small prostates.19
In our cohort, there were a total of 57 patients (14.3%) who experienced postoperative adverse events, which is comparable with the results of previous studies reporting the rate of overall complications after TUEB as 6.9%–38.7%.7,15,20, 21, 22, 23, 24 There were no significant differences in the rates and severities of the overall complications according to prostate volume, suggesting the size-independent safety of TUEB. These results were consistent with those of previous studies.7,15
Our study had some limitations. Primarily owing to its retrospective design, our study missed several pieces of information including postoperative medication usage, such as anticholinergics. We also could not include an assessment of sexual function before and after TUEB because of the retrospective nature of the present study. In addition, when comparing prostate volumes, BPH with an extremely large prostate volume over 150 cc was not available for evaluation because of the small sample size. For similar reasons, we used 40 mL as the cutoff value for small-sized prostates instead of 30 mL, which is used in current clinical guidelines. Furthermore, our study was based on the experience of a single surgeon at a single institution. The initial learning curve effect of the operator on surgical outcome could not be excluded. Moreover, comparison of the surgical outcomes of TUEB with those of other surgical procedures such as TURP or HoLEP, was not available in our study. Therefore, further multicenter studies are required to generalize these findings. Despite these limitations, our study is valuable as the largest population-based study with the longest follow-up period to the best of our knowledge, especially considering the lack of available studies evaluating the efficiency and safety of TUEB according to a wide range of prostate volumes.
5. Conclusion
Our single surgeon-based experience showed that although the perioperative efficiency of TUEB using a specialized spatula loop increases in larger prostates, there are no significant differences in postoperative functional improvements or overall complication rates according to prostate volume. A further large-scale study should be performed to evaluate if TUEB could be considered a prostate volume-independent procedure.
Funding
No funding was received for our work.
Author contributions
BS and SJJ contributed to protocol and project development. BS and SJJ contributed to manuscript writing and editing. BS and SHS were involved in data collection, analysis, and management. SJJ supervised the study.
Conflicts of interest
This study was conducted in the absence of commercial or financial relationships that could be interpreted as potential conflicts of interest. The authors declare no conflicts of interest.
References
- 1.Kim D.K., Park J.J., Yang W.J., Doo S.W., Kim J.H., Song Y.S. Changes in diagnosis rate and treatment trends of benign prostatic hyperplasia in Korea: A nationwide population-based cohort study. Prostate Int. 2021;9:215–220. doi: 10.1016/j.prnil.2021.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Office EG . 2023. EAU guidelines on management of nonneurogenic male lower urinary tract symptoms (LUTS), incl. benign prostatic obstruction (BPO): 2023 update.https://uroweb.org/guideline/treatment-of-non-neurogenic-male-luts/ Available from: [Google Scholar]
- 3.Reddy S.K., Utley V., Gilling P.J. The evolution of endoscopic prostate enucleation: a historical perspective. Andrologia. 2020;52 doi: 10.1111/and.13673. [DOI] [PubMed] [Google Scholar]
- 4.Gilling P.J., Kennett K., Das A.K., Thompson D., Fraundorfer M.R. Holmium laser enucleation of the prostate (HoLEP) combined with transurethral tissue morcellation: an update on the early clinical experience. J Endourol. 1998;12:457–459. doi: 10.1089/end.1998.12.457. [DOI] [PubMed] [Google Scholar]
- 5.Jiang Y., Bai X., Zhang X., Wang M., Tian J., Mu L., et al. Comparative study of the effectiveness and safety of transurethral bipolar plasmakinetic enucleation of the prostate and transurethral bipolar plasmakinetic resection of the prostate for massive benign prostate hyperplasia (>80 ml) Med Sci Mon Int Med J Exp Clin Res. 2020;26 doi: 10.12659/MSM.921272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samir M., Tawfick A., Mahmoud M.A., Elawady H., Abuelnaga M., Shabayek M., et al. Two-year follow-up in bipolar transurethral enucleation and resection of the prostate in comparison with bipolar transurethral resection of the prostate in treatment of large prostates. Randomized controlled trial. Urology. 2019;133:192–198. doi: 10.1016/j.urology.2019.07.029. [DOI] [PubMed] [Google Scholar]
- 7.Bebi C., Turetti M., Lievore E., Ripa F., Rocchini L., Spinelli M.G., et al. Bipolar transurethral enucleation of the prostate: Is it a size-independent endoscopic treatment option for symptomatic benign prostatic hyperplasia? PLoS One. 2021;16 doi: 10.1371/journal.pone.0253083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsuboi I., Maruyama Y., Sadahira T., Ando N., Nishiyama Y., Araki M., et al. Efficacy of holmium laser enucleation in patients with a small (less than 30 mL) prostate volume. Investig Clin Urol. 2021;62:298–304. doi: 10.4111/icu.20200450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yılmaz S., Yalçın S., Yılmaz M., Açıkgöz O., Aybal H.Ç., Gazel E., et al. Comparison of outcomes of holmium enucleation of the prostate for small- and moderate-sized prostates. Andrologia. 2021;53 doi: 10.1111/and.13970. [DOI] [PubMed] [Google Scholar]
- 10.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamalunas A., Westhofen T., Schott M., Keller P., Atzler M., Stief C.G., et al. Holmium laser enucleation of the prostate: A truly size-independent method? Low Urin Tract Symptoms. 2022;14:17–26. doi: 10.1111/luts.12404. [DOI] [PubMed] [Google Scholar]
- 12.Das A.K., Han T.M., Hardacker T.J. Holmium laser enucleation of the prostate (HoLEP): size-independent gold standard for surgical management of benign prostatic hyperplasia. Can J Urol. 2020;27:44–50. [PubMed] [Google Scholar]
- 13.Park S., Kwon T., Park S., Moon K.H. Efficacy of holmium laser enucleation of the prostate in patients with a small prostate (≤30 mL) World J Mens Health. 2017;35:163–169. doi: 10.5534/wjmh.17011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdulwahab Al-Radhi M., Lun L.K., Safi M., Al-Danakh A., M Al-Kohlany K., Al-Najar A., et al. Can bipolar transurethral enucleation of the prostate be a better alternative to the bipolar transurethral resection of the prostate?: A prospective comparative study. Medicine (Baltim) 2021;100 doi: 10.1097/MD.0000000000025745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Endo Y., Shimizu H., Akatsuka J., Minaguchi S., Hasegawa H., Toyama Y., et al. Efficacy and safety of transurethral enucleation with bipolar energy for treatment of benign prostatic hyperplasia: Does prostate volume matter? J Nippon Med Sch. 2022;89:436–442. doi: 10.1272/jnms.JNMS.2022_89-411. [DOI] [PubMed] [Google Scholar]
- 16.Giulianelli R., Gentile B.C., Mirabile G., Tema G., Nacchia A., Albanesi L., et al. Bipolar plasma enucleation of the prostate: 5 years outcomes. J Endourol. 2019;33:396–399. doi: 10.1089/end.2019.0050. [DOI] [PubMed] [Google Scholar]
- 17.Hirasawa Y., Kato Y., Fujita K. Transurethral enucleation with bipolar for benign prostatic hyperplasia: 2-year outcomes and the learning curve of a single surgeon's experience of 603 consecutive patients. J Endourol. 2017;31:679–685. doi: 10.1089/end.2017.0092. [DOI] [PubMed] [Google Scholar]
- 18.Lin C.H., Wu W.J., Li C.C., Wen S.C. Preoperative predictors of enucleation time during en bloc ‘no-touch’ holmium laser enucleation of the prostate. BMC Urol. 2020;20:185. doi: 10.1186/s12894-020-00758-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong W., Sun M., Ran Q., Chen F., Du Y., Dou K. Learning curve for bipolar transurethral enucleation and resection of the prostate in saline for symptomatic benign prostatic hyperplasia: experience in the first 100 consecutive patients. Urol Int. 2013;90:68–74. doi: 10.1159/000343235. [DOI] [PubMed] [Google Scholar]
- 20.Boeri L., Capogrosso P., Ventimiglia E., Fontana M., Sampogna G., Zanetti S.P., et al. Clinical comparison of holmium laser enucleation of the prostate and bipolar transurethral enucleation of the prostate in patients under either anticoagulation or antiplatelet therapy. Eur Urol Focus. 2020;6:720–728. doi: 10.1016/j.euf.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Patard P.M., Roumiguie M., Sanson S., Beauval J.B., Huyghe E., Soulié M., et al. Endoscopic enucleation for prostate larger than 60 mL: comparison between holmium laser enucleation and plasmakinetic enucleation. World J Urol. 2021;39:2011–2018. doi: 10.1007/s00345-020-03382-x. [DOI] [PubMed] [Google Scholar]
- 22.Habib E., Ayman L.M., ElSheemy M.S., El-Feel A.S., Elkhouly A., Nour H.H., et al. Holmium laser enucleation vs bipolar plasmakinetic enucleation of a large volume benign prostatic hyperplasia: a randomized controlled trial. J Endourol. 2020;34:330–338. doi: 10.1089/end.2019.0707. [DOI] [PubMed] [Google Scholar]
- 23.Higazy A., Tawfeek A.M., Abdalla H.M., Shorbagy A.A., Mousa W., Radwan A.I. Holmium laser enucleation of the prostate versus bipolar transurethral enucleation of the prostate in management of benign prostatic hyperplasia: a randomized controlled trial. Int J Urol. 2021;28:333–338. doi: 10.1111/iju.14462. [DOI] [PubMed] [Google Scholar]
- 24.Magistro G., Schott M., Keller P., Tamalunas A., Atzler M., Stief C.G., et al. Enucleation vs. resection: A matched-pair analysis of TURP, HoLEP and bipolar TUEP in medium-sized prostates. Urology. 2021;154:221–226. doi: 10.1016/j.urology.2021.04.004. [DOI] [PubMed] [Google Scholar]