Abstract
Background
Prostate cancer in the anterior region may be missed on a transrectal systematic biopsy (SBx). Therefore, this study aimed to evaluate the performance of magnetic resonance imaging-transrectal ultrasound (MRI-TRUS) fusion targeted biopsy (TBx) in detecting anterior region cancer in patients with a history of SBxs.
Methods
Prostate biopsies were performed in 224 patients after multiparametric MRI, among whom 119 patients with prostate imaging reporting and data system (PI-RADS version 2) scores of 3 to 5 underwent MRI-TRUS fusion TBxs. Afterward, cancer detection rates (CDRs) and TBx-positive core regions were compared by categorizing patients into those with or without a history of SBxs.
Results
Total CDR was 68.8% (44/64 cases) in the initial biopsy group (Initial-Bx group) and 47.3% (26/55 cases) in the previous-negative-systematic biopsy group (Pre-Neg-SBx group) (P = 0.018). Interestingly, both TBx- and SBx-core positive cases were more common in the Initial-Bx group than in the Pre-Neg-SBx group (Initial-Bx group: 75% [33/44 cases] vs. Pre-Neg-SBx group: 42.3% [11/26 cases], P = 0.006). However, only TBx-core positive cases were more common in the Pre-Neg-SBx group than in the Initial-Bx group (Initial-Bx group: 11.4% [5/44 cases] vs. Pre-Neg-SBx group: 30.8% [8/26 cases], P = 0.043). In addition, the proportion of anterior lesions detected by TBx cores was higher in the Pre-Neg-SBx group than in the Initial-Bx group (Initial-Bx group: 26.3% [10/38 cases] vs. Pre-Neg-SBx group: 52.6% [10/19 cases], P = 0.049).
Conclusion
Using MRI-TRUS fusion TBx in the evaluation of previously negative SBx cases improved the detection rate of anterior lesions, which might have been missed in previous SBxs. Especially in patients with a history of SBxs mpMRI should be performed to screen for anterior lesions.
Keywords: Image-Guided Biopsy, Multiparametric Magnetic Resonance Imaging, Prostatic Neoplasms, Transrectal, Ultrasound
1. Introduction
Prostate cancer (PC) is one of the most common cancers in men and the sixth-largest cause of male cancer mortality worldwide.1 PC is screened based on prostate-specific antigen (PSA) levels and rectal examination findings. Generally, 10- to 12-core systematic biopsies (SBxs) are performed to diagnose PC2 and may be carried out using two biopsy approaches: transrectal and transperineal. While there is no difference in cancer detection rate (CDR) between the two approaches,3, 4, 5, 6 the transrectal biopsy is more commonly performed because it can be easily carried out without lumbar spinal anesthesia.4,7 Furthermore, since PCs are more likely to be located in the peripheral zone (PZ),8 the PZ region is primarily sampled using transrectal SBxs.9 However, some PCs occur in the anterior region, such as the transition zone (TZ), central zone (CZ), and anterior fibromuscular stroma (AFS), and transrectal SBx may miss these anterior lesions.10 For these reasons, transrectal SBx may not provide an accurate measurement in approximately 50% of PC cases.11
Recently, targeted biopsies (TBxs) based on multiparametric magnetic resonance imaging (mpMRI) have been shown to improve the detection rate of clinically significant PCs (csPCs).12, 13, 14, 15, 16 In particular, the improvement of CDR using TBx in patients with a history of previous SBx has been reported.15,17 Nevertheless, while these previous studies focused only on detection rates, only a few reports have compared PC localization with or without a history of previous SBx.
Therefore, this study aimed to verify the clinical utility of TBx in patients with a history of SBx. In addition, the CDRs and tumor localization were compared between patients with and without a previous history of SBxs.
2. Materials and methods
2.1. Patients (study population)
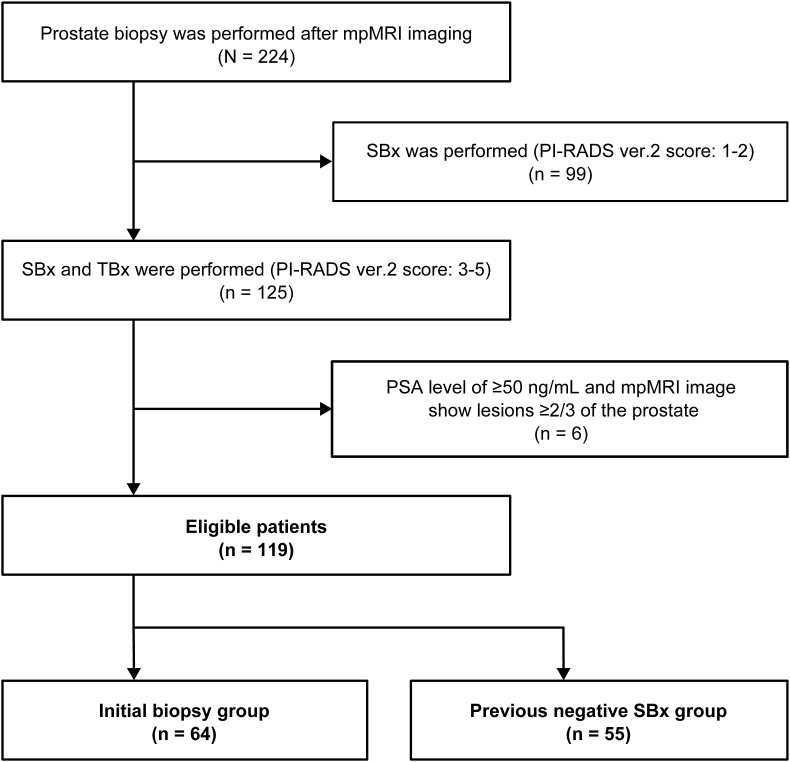
We identified 224 consecutive patients who underwent prostate biopsy after mpMRI examination with a PSA level ≥3.0 ng/mL between June 2016 and February 2018.18 Prior to this examination, no patient had undergone TBx. Among these cases, i) those with a Prostate Imaging–Reporting and Data System version 2 (PI-RADS v2) score ≤ 2 (n = 99) and ii) those with PSA levels ≥50 ng/mL and a target lesion in ≥2/3 of the prostate on mpMRI findings were excluded because only a 4- to 6-core of SBx would be sufficient to detect the cancers. Of the 119 eligible patients with a PI-RADS v2 score ≥3, 64 underwent initial biopsies (the initial-Bx group), and 55 had previously undergone SBxs (the Pre-Neg-SBx group) (Fig. 1).
Fig. 1.
Structural outline representation of the number of cases. Abbreviations: mpMRI, multiparametric magnetic resonance imaging; PSA, prostate-specific antigen; PI-RADS, Prostate Imaging Reporting and Data System; SBx, systematic biopsy; TBx, target biopsy.
This study was approved by the Institutional Review Board of our hospital and performed in accordance with the Declaration of Helsinki on human participants. Informed consent for clinical information was obtained from each participant or each participant's parent or guardian.
2.2. Multiparametric magnetic resonance imaging analysis
mpMRI was performed using a 1.5-Tesla magnetic resonance scanner (Philips Ingenia, Philips Healthcare, Best, Netherlands), and the following MRI sequences were recorded: T1-weighted imaging, T2-weighted imaging, diffusion-weighted imaging, apparent diffusion coefficient mapping, and dynamic contrast-enhanced MRI. Two radiologists with more than 10 years of clinical experience evaluated these images using PI-RADS v2,19 and one pathologist with more than 30 years of experience evaluated the specimen. Furthermore, abnormal signal regions were evaluated according to the sector map in PI-RADS v2, lesion length, and PI-RADS v2 score.
2.3. Magnetic resonance imaging-transrectal ultrasound fusion biopsy
A transrectal ultrasound (US) instrument (LOGIQ E9; GE Healthcare, Chicago, IL, USA) was used. This device can detect the magnetic field created by the transmitter using a magnetic sensor attached to a probe, synchronizing the MRI and US images based on the position and orientation of the spatial coordinates. Because this device can display MRI and US images simultaneously, it is possible to perform a puncture based on the US image while confirming the position of the lesion on the MRI scan. In addition, if there is any shift between the MRI and US images, it can be corrected using a tracker sensor.
In cases with a PI-RADS score ≥3, the TBx was performed first, followed by the SBx. The TBx was obtained by taking two or three cores per target lesion,20 and the SBx (a 10-core) was performed as follows: 6-cores were obtained from the base, middle, and apex of the prostate, and 4-cores were obtained from the far lateral middle and base on the right and left of the prostate.21 It is worth noting that the doctor who performed the previous systematic biopsies and the one who performed the current target biopsy differed, even though the protocol for systematic biopsies was consistently the same. In addition, a single urologist performed the target biopsies on all cases in this study.
2.4. Study design and endpoints
The primary endpoint involved the comparison of anterior regional lesions between the Initial-Bx and Pre-Neg-SBx groups. The secondary endpoint included total CDRs, csPC detection rates, International Society of Urological Pathology (ISUP) grade group, total and positive cores, and number of cases per positive core compared between the Initial-Bx and Pre-Neg-SBx groups. Generally, the boundary between the anterior and posterior regions of the prostate is mainly determined by the position of the urethra.22, 23, 24 However, in this study, a line 20 mm from the rectal side, approximately the length of the biopsy needle, was set as the boundary because our objective was to verify the detection rate of anterior lesions in patients who had previously undergone SBxs.13 However, for cases with multiple target lesions on MRI, the target with the highest PI-RADS v2 score was identified as the target site. The csPC was defined as a Gleason score (GS) ≥ 3 + 4 = 7, or a maximum core length ≥4 mm.25,26
2.5. Statistical analysis
Normally distributed data are presented as mean ± standard deviation (SD), and non-normally distributed data are presented as medians and interquartile range (IQR) for continuous variables. Categorical variables are reported as frequencies and proportions. Overall, t-tests, Mann–Whitney U tests, Cochran–Armitage tests, and χ2 tests were performed to compare the significance of the statistical differences between the means and proportions, respectively. All statistical data were analyzed using JMP 13.2 software (SAS Institute Inc., Cary, NC, USA), and a P-value of <0.05 was considered statistically significant.
3. Results
3.1. Patient characteristics and biopsy outcomes
Table 1 shows the patient characteristics, mpMRI findings, and number of biopsy cores in the Initial-Bx group (n = 64) and Pre-Neg-SBx group (n = 55). The two groups had no significant differences in age, PSA levels, prostate sizes, or prostate-specific antigen density (PSADs); however, the PI-RADS v2 score was significantly higher in the Initial-Bx group than in the Pre-Neg-SBx group (P = 0.004). Notably, all patients underwent 10 cores of SBx, and the number of TBx cores did not differ between the two groups.
Table 1.
Patient characteristics, magnetic resonance imaging findings, and number of biopsy cores
| All patients (n = 119) | Initial-Bx group (n = 64) | Pre-Neg-SBx group (n = 55) | P value | |
|---|---|---|---|---|
| Patient characteristics | _ | _ | _ | _ |
| Age (years, mean ± SD) | 71.1 ± 6.7 | 70.5 ± 7.7 | 71.9 ± 5.3 | 0.432 |
| PSA level (ng/mL, mean ± SD) | 9.0 ± 8.2 | 9.2 ± 9.3 | 8.7 ± 6.6 | 0.202 |
| Estimated prostate volume (ml, mean ± SD) | 40.1 ± 18.2 | 37.4 ± 18.4 | 43.3 ± 17.9 | 0.057 |
| PSAD (ng/mL/mL, mean, SD) | 0.27 ± 0.32 | 0.30 ± 0.41 | 0.23 ± 0.16 | 0.411 |
| mpMRI findings | _ | _ | _ | _ |
| PI-RADS v2 score (n) | _ | _ | _ | _ |
| 3 | 51 | 20 | 31 | 0.018 |
| 4 | 48 | 30 | 18 | |
| 5 | 20 | 14 | 6 | |
| Target lesion size (mm, median, (IQR)) | 10 (6–14) | 10 (7–15) | 10 (5–14) | 0.402 |
| No. of biopsy cores | _ | _ | _ | _ |
| SBx (mean ± SD) | 10 ± 0 | 10 ± 0 | 10 ± 0 | 1.000 |
| TBx (mean ± SD) | 2.5 ± 0.66 | 2.4 ± 0.73 | 2.5 ± 0.60 | 0.387 |
Initial-Bx, initial biopsy; Pre-Neg-SBx, previous negative systematic biopsy; SD, standard deviation; PSA, prostate-specific antigen; PSAD, prostate-specific antigen density; mpMRI, multiparametric magnetic resonance imaging; PI-RADS, Prostate Imaging-Reporting and Data System; IQR, interquartile range; SBx, systemic biopsy; TBx, target biopsy.
Table 2 shows the comparison of biopsy outcomes between the Initial-Bx and Pre-Neg-SBx groups. Total CDRs (68.8% vs. 47.3%, P = 0.018) and csPC detection rates (63.6% vs. 45.5%, P = 0.027) were significantly higher in the Initial-Bx group than in the Pre-Neg-SBx group. Particularly for a PI-RADS v2 score of 4, the CDR was higher in the Initial-Bx group (Initial-Bx group: 83.3% vs. Pre-Neg-SBx group: 55.6%; P = 0.037), even though there was no significant difference between the two groups in PI-RADS v2 scores of 3 and 5. Furthermore, there were no significant differences between the two groups in the ISUP grades or SBx- and TBx-positive core lengths.
Table 2.
Comparison of biopsy outcomes in the initial biopsy group and the previous negative systematic biopsy group
| All (n = 119) | Initial-Bx group (n = 64) | Pre-Neg-SBx group (n = 55) | P value | |
|---|---|---|---|---|
| No. of positive cases | _ | _ | _ | _ |
| All cancers (n, %) | 70 (58.8) | 44 (68.8) | 26 (47.3) | 0.018 |
| csPCs (n, %) | 67 (56.3) | 42 (65.6) | 25 (45.5) | 0.027 |
| No. of positive cases by PI-RADS v2 score | _ | _ | _ | _ |
| 3 (n, %) | 16/51 (31.4) | 5/20 (25.0) | 11/31 (35.5) | 0.431 |
| 4 (n, %) | 35/48 (72.9) | 25/30 (83.3) | 10/18 (55.6) | 0.037 |
| 5 (n, %) | 19/20 (95.0) | 14/14 (100) | 5/6 (83.3) | 0.117 |
| ISUP grade group | _ | _ | _ | _ |
| Group 1 (n) | 9 | 4 | 5 | 0.427 |
| Group 2 (n) | 21 | 13 | 8 | |
| Group 3 (n) | 11 | 6 | 5 | |
| Group 4 (n) | 24 | 17 | 7 | |
| Group 5 (n) | 5 | 4 | 1 | |
| Biopsy cores | _ | _ | _ | _ |
| No. of total SBx cores sampled | 1190 | 640 | 550 | _ |
| No. of SBx positive cores (n) | 162 | 114 | 48 | _ |
| Positive SBx core length (mm, mean ± SD) | 5.2 ± 3.7 | 5.6 ± 4.0 | 4.2 ± 2.8 | 0.083 |
| No. of total TBx cores sampled | 295 | 155 | 140 | _ |
| No. of TBx positive cores (n) | 109 | 69 | 40 | _ |
| Positive TBx core length (mm, mean ± SD) | 8.2 ± 4.7 | 7.7 ± 4.3 | 8.9 ± 5.2 | 0.344 |
| No. of cases per positive cores | _ | _ | _ | _ |
| Both TBx and SBx (n, %) | 44 (81.4) | 33 (75.0) | 11 (42.3) | 0.006 |
| TBx only (n, %) | 13 (18.6) | 5 (11.4) | 8 (30.8) | 0.043 |
| SBx only (n, %) | 13 (18.6) | 6 (13.6) | 7 (26.9) | 0.167 |
Initial-Bx, initial biopsy; Pre-Neg-SBx, previous negative systematic biopsy; No, number; csPCs, clinically significant prostate cancers; PI-RADS, Prostate Imaging and Reporting and Data System; ISUP, International Society of Urological Pathology; SBx, systemic biopsy; TBx, Target biopsy; SD, standard deviation.
While comparing the biopsy cores, CDRs were significantly higher in the TBx cores than in the SBx cores (SBx cores: 13.6% [162/1190 cores] vs. TBx cores: 36.9% [109/295 cores], P < 0.001), which improved the CDR by 18.6% compared to that in SBx. Interestingly, both TBx and SBx core positive cases were more common in the Initial-Bx group (Initial-Bx group: 75% [33/44 cases] vs. Pre-Neg-SBx group: 42.3% [11/26 cases], P = 0.006), compared to only TBx-core positive cases found majorly in the Pre-Neg-SBx group (Initial-Bx group: 11.4% [13/44 cases] vs. Pre-Neg-SBx group: 30.8% [8/26 cases], P = 0.043).
3.2. Comparison of target regions classified by previous systematic biopsies
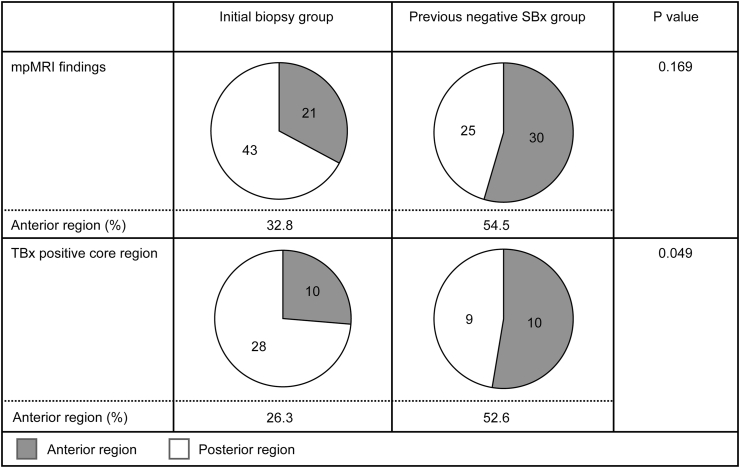
Fig. 2 shows a comparison of target regions on mpMRI findings and the TBx-positive cores between the Initial-Bx and Pre-Neg-SBx groups. The proportion of anterior regions of the TBx-positive core was significantly higher in the Pre-Neg-SBx group (52.6%) than in the Initial-Bx group (26.3%, P = 0.049). Furthermore, as the number of previous SBxs increases, the proportion of TBx-core-only positive cases (P = 0.024) and anterior lesion cases (P = 0.004) significantly increases (Table 3).
Fig. 2.
Comparison of anterior regions between the initial biopsy group and the previously negative systematic biopsy group. The proportions of anterior regions on mpMRI and TBx-positive cores were compared between the two groups. Abbreviations: mpMRI, multiparametric magnetic resonance imaging; Initial-Bx, initial biopsy; Pre-Neg-SBx, previous negative systematic biopsy; TBx, targeted biopsy.
Table 3.
Proportions of target biopsy only positive cases and anterior lesions classified by the number of previous systematic biopsies
| Initial-Bx group |
Pre-Neg-SBx group |
P value | |||
|---|---|---|---|---|---|
| Number of previous SBxs | 0 | 1 | 2 | 3≤ | |
| Number of patients (n) | 44 | 14 | 6 | 6 | _ |
| TBx-core only positive cases (n, %) | 5 (11.4) | 1 (6.6) | 3 (50) | 4 (66.7) | 0.024 |
| Anterior lesion cases (n, %) | 10 (22.7) | 2 (14.3) | 3 (50) | 5 (83.3) | 0.004 |
SBx, Systematic biopsy; TBx, Target biopsy.
4. Discussion
In this study, we found that MRI-TRUS fusion TBx improved CDRs and detected anterior lesions, and we observed that these benefits were greater in patients with previous histories of SBxs than in those undergoing an initial biopsy. In particular, our findings suggest that cancers missed by previous transrectal SBxs are often anterior regional lesions and that MRI-TRUS fusion TBx may be beneficial for detecting these missed anterior lesions. Although several reports have shown that TBx improves CDR in patients with a history of SBx,15,17 this study is the first to compare tumor localization between the Initial Bx and Pre-Neg-SBx groups.
Generally, CDRs in patients with histories of negative SBxs are lower than those in patients undergoing an initial biopsy,27 which may be because they excluded cases whose cancer was detected by a previous SBx. Consistent with previous studies, PI-RADS v2 scores, total CDRs, and csPC detection rates were lower in the Pre-Neg-SBx group than in the Initial-Bx group in this study. However, during subanalyses, CDRs were higher in the Initial-Bx group than in the Pre-Neg-SBx group in cases with a PI-RADS v2 score of 4, and it remains unclear why only the CDRs differed significantly between the two groups in cases with PI-RADS v2 scores of 4. Thus, these results should be further examined in future studies.
Several reports have shown the improvement of CDR by TBx in patients with histories of previous SBx.15,17 From our results, the proportion of TBx-only positive cases was higher in the Pre-Neg-SBx group than in the Initial-Bx group. Likewise, the proportion of anterior lesions was higher in the Pre-Neg-SBx group than in the Initial-Bx group. Therefore, these findings suggest that anterior lesions that may have been missed in previous transrectal SBxs procedures were detected using MRI-TRUS fusion TBxs. Moreover, it is worth noting that the higher the number of previous SBx procedures, the stronger the tendency to detect these lesions. Thus, we hypothesized that this might reflect the fact that the Pre-Neg-SBx group had a relatively high proportion of anterior lesions because cases of posterior region cancers were excluded by previous SBxs.
Furthermore, the differences between transrectal and transperineal approaches should be noted. Although the transrectal approach is more commonly used because it can be performed easily at the bedside without lumbar anesthesia, it has been demonstrated that the transperineal approach is more effective in detecting anterior lesions and has fewer complications of infection.4,7,14,24,28 Thus, since biopsy approaches vary among institutions, pre-biopsy MRI should be performed actively to screen for anterior lesions before a transrectal biopsy is performed.
Regardless of the abovementioned demerit, it has been noted that SBx should not be left out because there are some cases in which cancer is missed by TBx and detected by SBx alone.15,29 For instance, in this study, some cases were detected by SBx alone (n = 13). Of the 13 cases, in half of the cases, the lesion site on mpMRI findings matched the site of the SBx-positive core, whereas the other half did not match. Owing to these observations, we hypothesized that there are two patterns of SBx-only positive cases: (i) cases in which lesions were identified by mpMRI but could not be detected by TBx cores, and (ii) cases in which lesions could not be identified by mpMRI.
This study has some limitations. First, 3T MRIs are recommended for MRI-TRUS fusion TBx; however, a 1.5 T MRI was used in this study. Notwithstanding, we consider this appropriate because, even though the accuracy of mpMRI findings was possibly inferior to that of 3T MRI, several reports have shown that 1.5 T was sufficient for PC screening.25,26 Second, there may be confounding factors among cases that have previously undergone three or more SBxs, leading to variations among the included participants. Third, it was not possible to draw comparisons with saturation biopsies or total excision specimens, which may have led to pathological underestimation in this study, as research has shown that total excision specimens produce approximately 36% more pathological upgrades compared with biopsy results.30 Fourth, the MRI-TRUS fusion system used in this study was not adapted to the deformation of the prostate by the pressure of the echo probe; thus, it is necessary to consider distortion by the echo probe. Fifth, since the boundary between the anterior and posterior regions of the prostate was defined as a line 20 mm from the rectal side, the proportion of the anterior region may differ depending on the prostate volume. Moreover, the prostate volume was relatively higher in the Pre-Neg-SBx group, which had a higher proportion of anterior lesions, compared to the Initial-Bx group (37.4 ml vs. 43.3 ml, P = 0.057, Table 1). Therefore, these findings suggest that anterior lesions may be missed if only transrectal SBx is performed in cases of high prostate volume.
In conclusion, our findings suggest that mpMRI should be performed to screen for anterior lesions that may be missed on transrectal SBx, especially in patients with a history of SBx. Additionally, we have demonstrated that the MRI-TRUS fusion of TBx is useful for the detection of anterior lesions in PCs.
Conflicts of interest
All authors have no conflict of interest to declare.
Acknowledgments
The authors thank all patients who participated in this study and the staff at Iwate Prefectural Ofunato Hospital and Editage (www.editage.com) for English language editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.prnil.2023.08.002.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Lee A., Chia S.J. Contemporary outcomes in the detection of prostate cancer using transrectal ultrasound-guided 12-core biopsy in Singaporean men with elevated prostate specific antigen and/or abnormal digital rectal examination. Asian J Urol. 2015;2:187–193. doi: 10.1016/j.ajur.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hara R., Jo Y., Fujii T., Kondo N., Yokoyoma T., Miyaji Y., et al. Optimal approach for prostate cancer detection as initial biopsy: prospective randomized study comparing transperineal versus transrectal systematic 12-core biopsy. Urology. 2008;71:191–195. doi: 10.1016/j.urology.2007.09.029. [DOI] [PubMed] [Google Scholar]
- 4.Takenaka A., Hara R., Ishimura T., Fujii T., Jo Y., Nagai A., et al. A prospective randomized comparison of diagnostic efficacy between transperineal and transrectal 12-core prostate biopsy. Prostate Cancer Prostat Dis. 2008;11:134–138. doi: 10.1038/sj.pcan.4500985. [DOI] [PubMed] [Google Scholar]
- 5.Xue J., Qin Z., Cai H., Zhang C., Li X., Xu W., et al. Comparison between transrectal and transperineal prostate biopsy for detection of prostate cancer: a meta-analysis and trial sequential analysis. Oncotarget. 2017;8:23322–23336. doi: 10.18632/oncotarget.15056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiang J., Yan H., Li J., Wang X., Chen H., Zheng X. Transperineal versus transrectal prostate biopsy in the diagnosis of prostate cancer: a systematic review and meta-analysis. World J Surg Oncol. 2019;17:31. doi: 10.1186/s12957-019-1573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marenco Jimenez J.L., Claps F., Ramón-Borja J.C., Mascarós Martinez J.M., Gutierrez A.W., Lozano Á.G.F., et al. Rebiopsy rate after transperineal or transrectal prostate biopsy. Prostate Int. 2021;9:78–81. doi: 10.1016/j.prnil.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McNeal J.E., Redwine E.A., Freiha F.S., Stamey T.A. Zonal distribution of prostatic adenocarcinoma. Correlation with histologic pattern and direction of spread. Am J Surg Pathol. 1988;12:897–906. doi: 10.1097/00000478-198812000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Eichler K., Hempel S., Wilby J., Myers L., Bachmann L.M., Kleijnen J. Diagnostic value of systematic biopsy methods in the investigation of prostate cancer: a systematic review. J Urol. 2006;175:1605–1612. doi: 10.1016/S0022-5347(05)00957-2. [DOI] [PubMed] [Google Scholar]
- 10.Pepe P., Garufi A., Priolo G., Pennisi M. Transperineal versus transrectal MRI/TRUS fusion targeted biopsy: detection rate of clinically significant prostate cancer. Clin Genitourin Cancer. 2017;15:e33–e36. doi: 10.1016/j.clgc.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 11.El-Shater Bosaily A., Parker C., Brown L.C., Gabe R., Hindley R.G., Kaplan R., et al. PROMIS--Prostate MR imaging study: a paired validating cohort study evaluating the role of multi-parametric MRI in men with clinical suspicion of prostate cancer. Contemp Clin Trials. 2015;42:26–40. doi: 10.1016/j.cct.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore C.M., Robertson N.L., Arsanious N., Middleton T., Villers A., Klotz L., et al. Image-guided prostate biopsy using magnetic resonance imaging-derived targets: a systematic review. Eur Urol. 2013;63:125–140. doi: 10.1016/j.eururo.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Tay K.J., Villers A., Polascik T.J. Targeted anterior gland focal therapy-a novel treatment option for a better defined disease. Curr Urol Rep. 2016;17:69. doi: 10.1007/s11934-016-0628-9. [DOI] [PubMed] [Google Scholar]
- 14.Komai Y., Numao N., Yoshida S., Matsuoka Y., Nakanishi Y., Ishii C., et al. High diagnostic ability of multiparametric magnetic resonance imaging to detect anterior prostate cancer missed by transrectal 12-core biopsy. J Urol. 2013;190:867–873. doi: 10.1016/j.juro.2013.03.078. [DOI] [PubMed] [Google Scholar]
- 15.Washino S., Kobayashi S., Okochi T., Kameda T., Konoshi T., Miyagawa T., et al. Cancer detection rate of prebiopsy MRI with subsequent systematic and targeted biopsy are superior to non-targeting systematic biopsy without MRI in biopsy naïve patients: a retrospective cohort study. BMC Urol. 2018;18:51. doi: 10.1186/s12894-018-0361-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drost F.H., Osses D.F., Nieboer D., Steyerberg E.W., Bangma C.H., Roobol M.J., et al. Prostate MRI, with or without MRI-targeted biopsy, and systematic biopsy for detecting prostate cancer. Cochrane Database Syst Rev. 2019;4:CD012663. doi: 10.1002/14651858.CD012663.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valerio M., Donaldson I., Emberton M., Ehdaie B., Hadaschik B.A., Marks L.S., et al. Detection of clinically significant prostate cancer using magnetic resonance imaging-ultrasound fusion targeted biopsy: a systematic review. Eur Urol. 2015;68:8–19. doi: 10.1016/j.eururo.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Schröder F.H., Carter H.B., Wolters T., van den Bergh R.C., Gosselaar C., Bangma C.H., et al. Early detection of prostate cancer in 2007. Part 1: PSA and PSA kinetics. Eur Urol. 2008;53:468–477. doi: 10.1016/j.eururo.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 19.Weinreb J.C., Barentsz J.O., Choyke P.L., Cornud F., Haider M.A., Macura K.J., et al. PI-RADS prostate imaging – reporting and data system: 2015, version 2. Eur Urol. 2016;69:16–40. doi: 10.1016/j.eururo.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cetin S., Huseyinli A., Koparal M.Y., Bulut E.C., Ucar M., Gonul, et al. How many cores should be taken from each region of interest when performing a targeted transrectal prostate biopsy. Prostate Int. 2023;11:122–126. doi: 10.1016/j.prnil.2023.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eskicorapci S.Y., Tuncay L., Eichler K., Hempel S., Wilby J., Myers L., et al. R e: Diagnostic value of systematic biopsy methods in the investigation of prostate cancer: a systematic review. J Urol, 175: 1605–1612. J Urol. 2006;176:2745. doi: 10.1016/S0022-5347(05)00957-2. author reply 2745-6. [DOI] [PubMed] [Google Scholar]
- 22.Shinmoto H., Tamura C., Soga S., Okamura T., Horiguchi A., Asano T., et al. Anterior prostate cancer: diagnostic performance of T2-weighted MRI and an apparent diffusion coefficient map. AJR Am J Roentgenol. 2015;205:W185–W192. doi: 10.2214/AJR.14.13392. [DOI] [PubMed] [Google Scholar]
- 23.Dason S., Allard C.B., Wright I., Shayegan B. Transurethral resection of the prostate biopsy of suspected anterior prostate cancers identified by multiparametric magnetic resonance imaging: a pilot study of a novel technique. Urology. 2016;91:129–135. doi: 10.1016/j.urology.2015.12.063. [DOI] [PubMed] [Google Scholar]
- 24.Sakamoto Y., Fukaya K., Haraoka M., Kitamura K., Toyonaga Y., Tanaka M., et al. Analysis of prostate cancer localization toward improved diagnostic accuracy of transperineal prostate biopsy. Prostate Int. 2014;2:114–120. doi: 10.12954/PI.14052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasivisvanathan V., Dufour R., Moore C.M., Ahmed H.U., Abd-Alazeez M., Charman S.C., et al. Transperineal magnetic resonance image targeted prostate biopsy versus transperineal template prostate biopsy in the detection of clinically significant prostate cancer. J Urol. 2013;189:860–866. doi: 10.1016/j.juro.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed H.U., Hu Y., Carter T., Arumainayagam N., Lecornet E., Freeman A., et al. Characterizing clinically significant prostate cancer using template prostate mapping biopsy. J Urol. 2011;186:458–464. doi: 10.1016/j.juro.2011.03.147. [DOI] [PubMed] [Google Scholar]
- 27.Djavan B., Zlotta A., Remzi M., Ghawidel K., Basharkhah A., Schulman C.C., et al. Optimal predictors of prostate cancer on repeat prostate biopsy: a prospective study of 1051 men. J Urol. 2000;163:1144–1148. discussion 1148-9. [PubMed] [Google Scholar]
- 28.Pirola G.M., Gubbiotti M., Rubilotta E., Castellani D., Trabacchin N., Tafuri A., et al. Is antibiotic prophylaxis still mandatory for transperineal prostate biopsy? Results of a comparative study. Prostate Int. 2022;10:34–37. doi: 10.1016/j.prnil.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahdoot M., Wilbur A.R., Reese S.E., Lebastchi A.H., Mehralivand S., Gomella P.T., et al. MRI-targeted, systematic, and combined biopsy for prostate cancer diagnosis. N Engl J Med. 2020;382:917–928. doi: 10.1056/NEJMoa1910038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Epstein J.I., Feng Z., Trock B.J., Pierorazio P.M. Upgrading and downgrading of prostate cancer from biopsy to radical prostatectomy: incidence and predictive factors using the modified Gleason grading system and factoring in tertiary grades. Eur Urol. 2012;61:1019–1024. doi: 10.1016/j.eururo.2012.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.