Abstract
Objective
This study aims to assess the safety, feasibility, and potential benefits of a combined aerobic and resistance exercise intervention for patients diagnosed with advanced pancreatic or lung cancer.
Methods
A prospective, single-arm study was conducted, enrolling patients with advanced lung or pancreatic cancer. Participants engaged in a 12-week exercise intervention comprising personalized bi-weekly aerobic and resistance training tailored to individual baseline conditions. The primary study outcomes focused on safety (absence of serious adverse events) and feasibility. Secondary outcomes included assessments of functional capacity using the "Six minutes walking test", strength measured through handgrip and leg press tests, anthropometric measures including body mass index and waist–hip ratio, quality of life (QoL), and changes in blood parameters.
Results
The study involved twelve patients (mean age 57.66 ± 7.40 years), with seven having pancreatic cancer and five having lung cancer. The recruitment rate was 50%, and assessment adherence was 100%, with an 84% adherence to the exercise program and no dropouts. No exercise-related adverse events were recorded, while three non-severe, non-exercise-related adverse events were observed: treatment-related dermatitis (Grade 2), axillary lymphadenopathy (Grade 2), and migraine (Grade 1). Significant enhancements in functional capacity, emotional well-being, and social functioning within the QoL domains were observed. Anthropometric measures, specifically waist–hip ratio and body mass index, remained stable.
Conclusions
The findings suggest that a tailored 12-week exercise intervention is both feasible and safe for patients with advanced lung or pancreatic cancer. This intervention appears to enhance functional capacity, specific aspects of QoL, and contribute to maintaining body weight.
Keywords: Pancreatic cancer, Lung cancer, Cancer cachexia, Exercise intervention, Aerobic exercise, Resistance exercise
Introduction
Lung and pancreatic cancers remain two of the most aggressive disease, typically diagnosed with advanced stage.1 Although several improvements in their treatments have been made in recent years, the 5-year survival rate is still poor: 34% and 7% for advanced and metastatic lung cancer, 15% and 3% for advanced and metastatic pancreatic cancer, respectively.1
Nevertheless, both the side effects of anticancer treatments and the disease-related burden lead to a deterioration of the patient's physical and psychological condition.2,3 Patients may experience a series of symptoms, including appetite loss, nausea/vomiting, dyspnea, depression, and shortness of breath, negatively influencing their quality of life (QoL).2,3 Patients with lung or pancreatic cancers may present impairments in physical functions. For instance, patients with advanced lung cancer experience a significant decline in functional capacity, muscle strength, and anthropometric measures during anticancer treatments.4,5 Similarly, the only available data on pancreatic cancer report impairments in cardiorespiratory fitness (–24%), upper (−4.3%), and lower (−13.8%), muscle strength, compared to the healthy reference values.6 However, nearly 95% of the patients included in the just mentioned study had an early stage of the disease; it is possible to speculate that patients affected by an advanced stage may present a greater reduction in physical fitness.
Moreover, this population may suffer from weight loss and muscle wasting, which may be considered hallmarks of cancer cachexia.7,8 Cancer cachexia is associated with poor survival,8 and it is still orphaned of effective treatments. It has been suggested that exercise, especially as part of a multidisciplinary approach, may help prevent cachexia development. Beyond the possible effect on cachexia onset, exercise may help patients to manage their symptoms and treatment-related side effects. Randomized controlled studies have demonstrated that exercise may significantly enhance fatigue, anxiety, depression, nausea, sleep quality, bone health, and anemia, as well as increase patients' QoL and physical fitness, including functional capacity, muscle strength, and mass.9, 10, 11, 12
Despite the potential benefits related to exercise, patients with advanced cancer may experience a series of obstacles, such as high symptoms burden hindering them from being physically active. On the contrary, different features have been identified as potential facilitators, including social support, supervised and structured exercise programs that are easy to attend, and patients ‘preferences.13 Regarding patients’ preferences, a prior survey of our group showed that 40% of patients prefer exercising in a group-based program, 27% individually, with a program to follow at home, and 25% opt for an individual program with a personal trainer.14 Prior studies have tested the feasibility of an exercise intervention in patients affected by advanced lung or pancreatic cancer;15, 16, 17 however, all of these have proposed only a single exercise modality, e.g., individual-based or groups-based. Therefore, in this research, we have assessed the safety/feasibility and explored the preliminary efficacy of a patient-centered and patients-based exercise intervention specifically developed for patients with pancreatic and lung cancer. We hypothesized that proposing a supervised exercise intervention letting the patient choose the type of modality (e.g., home-based, individual-based, or group-based) would be safe, and feasible in patients with advanced lung or pancreatic cancer receiving systemic oncologic treatments.
Methods
Study design, participants, and procedures
A prospective, single-arm study was conducted at the University of Verona between September 2021 and November 2022 to explore the impact of a combined aerobic and resistance exercise intervention in patients with advanced lung or pancreatic cancer. The primary objective was to assess the safety and feasibility of the exercise intervention. Secondary objectives included the evaluation of functional capacity, muscle strength, flexibility, QoL, the total amount of physical activity, and blood parameters.
Patients' inclusion criteria were (1) age ≥ 18, (2) histologically/cytologically confirmed advanced (locally advanced or metastatic) non-small cell lung cancer, small cell lung cancer, or pancreatic cancer, and (3) Eastern Cooperative Oncology Group performance status, of 0–1. Patients were excluded if they had a compromised mental status, unstable bone metastases, or absolute contraindications for exercise intervention [i.e., heart insufficiency > NYHA III or uncertain arrhythmia, uncontrolled hypertension, severe renal dysfunction (glomerular filtration rate < 30%; creatine > 3 mg/dL; insufficient hematological capacities such as either hemoglobin value below 8 g/dL or thrombocytes below 30.000/μL; reduced standing or walking ability)].
Patients were recruited at the Oncology Units of the University of Verona Hospital Trust. Potential eligible patients were identified through medical records, check-up, and nutritional visits. Oncologists or dietitians proposed the study to patients, and if interested, the research staff contacted them to provide a detailed description of the study's procedures and conduction and to fix a first appointment to perform baseline assessments.
Exercise intervention
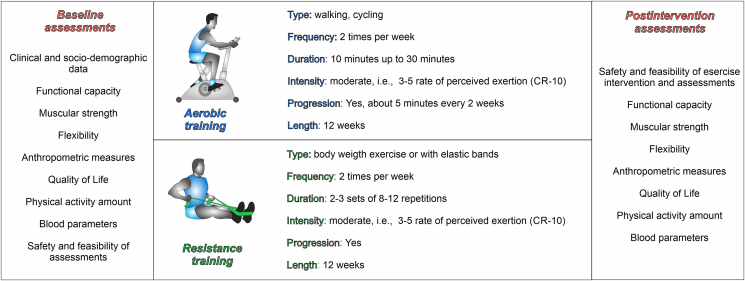
The intervention consisted of a 12-week combined aerobic and resistance program, individually tailored according to the patient's baseline evaluations and delivered at the facilities of the University of Verona (Fig. 1). Exercise sessions lasted approximately 60 min two times per week. Each exercise session was composed of warm-up, aerobic and resistance training, and cool down. Five minutes were dedicated to warm-up, which included ten dynamic stretching exercises (neck adduction, neck flexion/extension, neck rotation, shoulder rotation, elbow flexion, wrist rotation, hip flexion, hip adduction, knee flexion, and ankle rotation). The aerobic component comprised cardiovascular exercises, such as treadmill and cycle-ergometer. The duration of aerobic exercise started at 10–20 min, based on the patient's initial condition, and progressively increased over the weeks, about 5 min every two weeks, up to 25–30 min at the end of the program. The intensity of exercise was moderate and checked using the 10-point Borg Rating of the Perceived Exertion Scale (RPE) (i.e., 3–5 of RPE). Resistance training included five exercises involving major upper and lower body muscle groups, such as squats, pulleys, push presses, sit-ups, and calf raises, performed with body weight or using elastic bands (Thera-Bands, Hygienic Corp. Akron OH). Each resistance exercise was adapted to the patient's ability, e.g., changing the position, like sitting or standing, or increasing/decreasing the resistance of the elastic band, and was performed in 2–3 sets of 8–12 repetitions at moderate intensity, i.e., 3–5 RPE. The volume of the resistance training progressively increased during the training period: initially, an increase in the repetitions was preferred, followed by a gain in the series in the last six weeks of the program. Cool-down comprised five stretching exercises of the major muscle groups for 30 s each. Patients were allowed to choose to perform the training program in a fully supervised manner, at the facilities of the University of Verona and under the supervision of one specialized kinesiologist/doctoral student for each session, or in a partially supervised way. In the partially supervised training, the intervention was delivered through a personalized written exercise program in which the activities' type, frequency, duration, and intensity were specifically described, and an exercise log diary was included. Elastic bands were provided to each patient, and periodic meetings every two, four, and six weeks were scheduled in order to handle the new program, teach patients how to self-monitor exercise intensity, and try the activities with the kinesiologist. Additionally, a weekly phone call was made to monitor and support patients.
Fig. 1.
Exercise intervention and assessments.
The study was carefully followed by a dedicated post-doctoral fellow and a doctoral student, who was kinesiologists and had experience working with patients with cancer. Whereas the doctoral student was in charge, especially of the exercise sessions and the acquisition of information regarding the sessions, the post-doctoral fellow organized and conducted the baseline and post-intervention assessments and ensured that no deficits occurred during the study. Additionally, since the study was carried out during the COVID-19 outbreak, safety procedures (e.g., green pass, body temperature measurement, hand sanitizing, FFP2 mask, distancing) were adopted according to the evolving Italian legislation in that period. The research staff was careful to observe the just mentioned procedures and wear the FFP2 mask in order to provide a safe environment for the patients. All the study procedures, including exercise sessions/meetings, were conducted in a single-room-small gym, which only patients and the research staff could access in order to minimize the risk of infection.
Outcomes assessment
Safety and feasibility
Feasibility variables included recruitment rate, adherence to the exercise program, and withdrawals. The recruitment rate was evaluated by dividing the number of recruited patients by the patients considered eligible. Adherence to the intervention was determined by the number of sessions attended out of 24, whereas adherence to assessments was defined as the number of participants able to complete baseline and post-intervention evaluations. The withdrawal rate was registered, i.e., the number of patients that left the study. Safety was continuously tracked during the study period. Adverse events (AEs), defined as any undesirable medical or health-related event that occurred during study participation, were recorded. AEs were classified as either non-exercise AEs, i.e., occurred during study participation but considered unrelated to exercise, or exercise-related AEs, i.e., occurred during or as a direct result of exercise. AEs are categorized according to the Common Terminology Criteria for Adverse Events (version 5.0).18
Functional capacity
Functional capacity was assessed through the "Six minutes walking test" (6MWT), conducted according to the American Thoracic Society guidelines.19 Patients were instructed to walk in a 20-m hallway at their own pace, with the aim to walk as many meters as possible. Standardized encouragement was given each minute. Prior to initiating the test, blood pressure and saturation were screened, and patients were monitored during the test with portable pulse oximetry.
Strength
Muscular strength was evaluated through maximal voluntary isometric contraction of the upper and lower limbs. For upper limbs, the handgrip strength test was utilized. According to a standardized protocol, patients were sitting in a straight-backed chair with the feet flat on the floor, the shoulder adducted and neutrally rotated, the elbow flexed at 90°, and the forearm and wrist in a neutral position.20 For lower limbs, an isometric leg press test was proposed using a load cell mounted with a horizontal leg press. The load cell was positioned in series with the sliding axis of the leg press so that the direct line of force was registered. The dynamometer was routinely calibrated using International Organization for Standardization-certified weights. The leg-press back, on which the subjects were lying, was inclined 30° from the horizontal plane. The knee angle was set at about 90° and was controlled using a goniometer.21 For both strength assessments, five tests were performed, and each voluntary contraction was kept for 2–4 s. The highest value was collected.
Flexibility
Upper limb flexibility was recorded using the "back scratch test". This evaluation involves a combination of shoulder abduction, adduction, and internal and external rotation, measuring the distance between (or the overlap of) the middle fingers of the hands behind the back.22 The "chair sit and reach test" assessed lower limb flexibility. The patient was sitting on the edge of the chair, with one foot on the floor and the other leg extended forward with the knee straight, the heel on the floor, and the ankle flexed at 90°. The goal of the test was to reach forward as possible or past to the toes with the fingers.22 For both evaluations, two tests were performed.
Anthropometric measures
Anthropometric parameters included body weight and height and the hip and waist circumferences of the subjects, taken utilizing standardized procedures.23 Additionally, the body mass index (BMI)—obtained by the weight (in kilograms) of the subjects divided by the square of their height (in meters)—and of the waist–hip ratio—obtained by the ratio of waist and hip circumferences (in centimeters) were calculated.
Quality of life
Health-related QoL was measured using the European Organization for Research and Treatment of Cancer Quality of Life and Core Questionnaire-30 (EORTC QLQ C-30), Italian version. The 30-item questionnaire is composed of multi-item scales and single items that reflect the multidimensionality of the QoL construct. It incorporates five functional scales (physical, role, cognitive, emotional, and social), three symptom scales (fatigue, pain, nausea, and vomiting), and a global health and QoL scale. The remaining single item assesses additional symptoms commonly reported by cancer patients (dyspnea, appetite loss, sleep disturbance, constipation, and diarrhea).24
Exercise level
Exercise level was evaluated using Godin's Shepard Leisure Time Exercise Questionnaire. This is a 3-item questionnaire that enquires about the previous week's leisure frequency and duration of vigorous, moderate, and mild-intensity exercise.25
Blood and medical parameters and socio-demographic information
Medical data (i.e., tumor type, tumor stage, anticancer treatments, date of the diagnosis, comorbidity, and drug treatments) and blood parameters were extracted from medical records. Socio-demographic information was collected at baseline with a questionnaire investigating: age, gender (male/female), education (elementary/secondary/high school degree/undergraduate degree/postgraduate degree), marital status (unmarried/married/divorced/widow), and employment (retired/in search of employment/stay-at-home or housewife/part-time employed/full-time employed).
Data analysis
The primary study endpoint was safety and feasibility. To date, no standard criteria to evaluate the safety and feasibility of exercise intervention are available. Therefore, we have utilized the following indications based on previous literature26: a recruitment rate ≥ 25%, adherence to the assessments and exercise sessions ≥ 75%, a dropout rate < 25%, and the absence of serious AEs (i.e., Grade 3 or 4). Being an implementation study not aimed at detecting statistically significant changes, a formal sample size calculation was not performed. Considering the access rates to the facilities involved in the study, a total of 10 patients was considered sufficient to reliably estimate feasibility and preliminarily assess the effects of the intervention.
Descriptive statistic was used for patients' demographic and medical data as well as for safety and feasibility outcomes. Continuous variables are presented as mean and standard deviation (or median and interquartile range if the distribution was skewed), whereas categorical data are summarized as frequencies and percentages. To determine if a difference exists pre- and post-intervention for secondary outcomes, a paired t-test was applied, or the Wilcoxon signed-rank test if the data were not normally distributed. STATA 14.0 (Stata Corp, Texas, TX, USA) was utilized to perform the analysis. All tests were two-tailed, and P-values < 0.05 were considered significant.
Ethical considerations
The study obtained the approval of the Ethics Committee for Clinical Trials for the University of Verona (IRB No. 33320) and was conducted following the declaration of Helsinki as well as the declaration of Oviedo. The current report complies with the CONSORT Statement: extension to randomized pilot and feasibility trials (Supplementary Material). Written informed consent was signed by the study participants before the initiation of any study procedures.
Results
A total of 12 patients participated in the study. Participants' characteristics are shown in Table 1. Briefly, patients had a mean age of 57.66 years, 58% were female, 92% were married, and 41% had at least an undergraduate degree. Regarding medical variables, seven patients had pancreatic cancer, whereas five had lung cancer. The majority, 75%, had metastatic disease, and the most frequently metastatic sites were liver (33%), brain (25%), and lymph nodes (25%). All patients were currently undergoing anticancer treatment during the exercise intervention and one resulted cachectic.
Table 1.
Baseline characteristics of the participants (N = 12).
| Variables | n (%) | Mean | SD |
|---|---|---|---|
| Age (years) | 57.66 | 7.40 | |
| Male | 5 (42) | ||
| Female | 7 (58) | ||
| Education | |||
| Secondary | 2 (17) | ||
| High school degree | 5 (42) | ||
| Undergraduate degree | 4 (33) | ||
| Postgraduate degree | 1 (8) | ||
| Marital status | |||
| Married | 11 (92) | ||
| Divorced | 1 (8) | ||
| Employment | |||
| Part-time employed | 2 (17) | ||
| Full-time employed | 3 (25) | ||
| Retired | 7 (58) | ||
| Family income | |||
| Barely adequate | 1 (8) | ||
| Adequate | 7 (58) | ||
| More than adequate | 4 (33) | ||
| Tumor site | |||
| Lung—non-small cell lung cancer | 5 (42) | ||
| Pancreas—exocrine pancreatic cancer | 7 (58) | ||
| Stage | |||
| III | 3 (25) | ||
| IV | 9 (75) | ||
| Metastases sitesa | |||
| Brain | 3 (25) | ||
| Liver | 4 (33) | ||
| Lymph nodes | 3 (25) | ||
| Bone | 1 (8) | ||
| Lung | 2 (17) | ||
| Pleura | 1 (8) | ||
| Cancer cachexiab | |||
| Yes | 1 (8) | ||
| No | 11 (92) | ||
| Months since diagnosis | 17.25 | 16.36 | |
| Type of treatment | |||
| Chemotherapy | 9 (75) | ||
| Radiotherapy | 4 (33) | ||
| Surgery | 2 (17) | ||
| Immunotherapy | 1 (8) | ||
| Target therapy | 5 (42) | ||
| Current treatments status | |||
| Ongoing | 12 (100) | ||
| ECOG—performance status | |||
| 0 | 4 (33) | ||
| 1 | 8 (67) | ||
| Concomitant comorbidities | |||
| Yesc | 7 (58) | ||
| No | 5 (42) | ||
More than one answer is possible;
according to EPCRC criteria;
types of comorbidities: hypertension (17%), diabetes (8%), osteoporosis (17%), hypercholesterolemia (8%), polyradiculopathy (8%), kidney stones (8%), anxious-depressive syndrome (8%), migraine (8%), demyelinating neuropathy (8%), gastroesophageal reflux (8%), hip vasculitis (8%). SD, standard deviation; ECOG, Eastern Cooperative Oncology Group.
Safety and feasibility
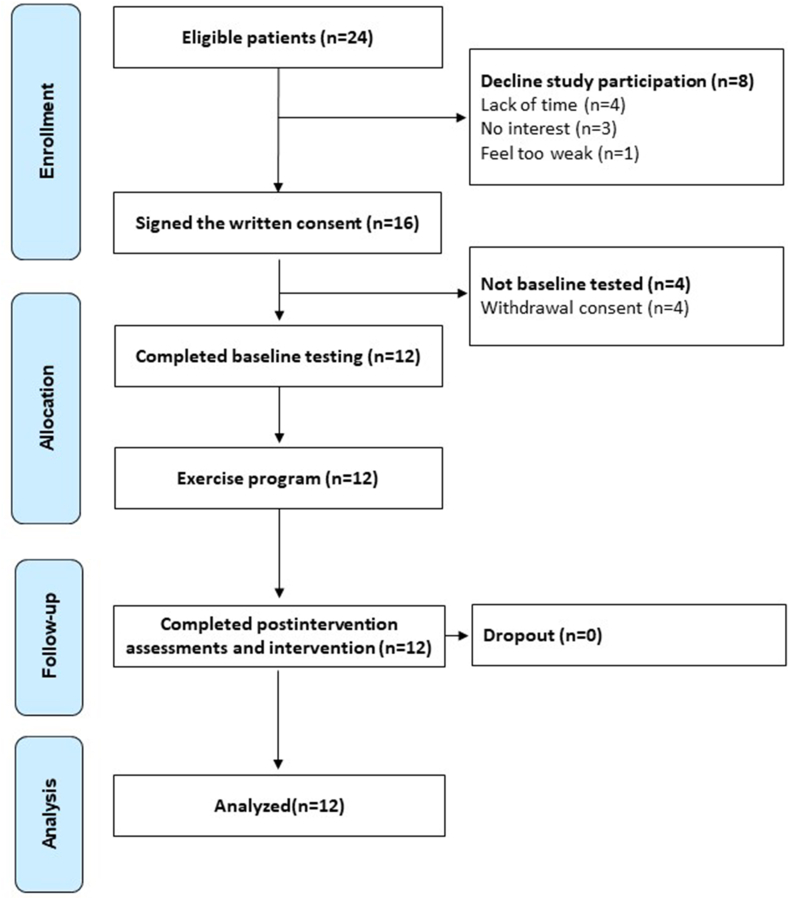
Among the 24 eligible patients, 8 declined study participation due to lack of time (n = 4), no interest in the study (n = 3), and one patient felt too weak to participate. A total of 16 patients signed the informed consent, but four withdrew their consent to participate before the baseline assessments and did not initiate the program (50% recruitment rate) (Fig. 2). Adherence to baseline and post-intervention assessments was excellent (100%), and no patients were lost at follow-up. Overall, the adherence to the exercise sessions was 84%. In detail, the adherence to aerobic exercise was 85%, whereas the resistance component was 82%. Seven patients performed the partially supervised exercise program, and five patients performed the fully supervised one. Overall, the adherence to exercise was slightly lower in patients who chose the partially supervised program, 79% (83% for aerobic and 75% for strength), compared to patients performing the fully supervised training, 87% (87% for aerobic and 87% for strength). During the trial, no exercise-related AEs occurred, whereas three non-exercise-related AEs were registered, in the partially supervised exercise group 11: treatment-related dermatitis at the hands (Grade 2),2 axillary lymphadenopathy (Grade 2), and migraine (Grade 1).3 Although these AEs did not occur as a direct consequence of exercise, they required an adaptation to the exercise program.
Fig. 2.
Study flowchart.
Preliminary efficacy outcomes
The preliminary efficacy of functional measures is exposed in Table 2. No significant improvements were detected for the anthropometric measures, muscle strength, and flexibility. Functional capacity was significantly increased from baseline to post-intervention (528.33 ± 82.06 m vs. 564.83 ± 69.84 m, P = 0.021), as well as the total amount of physical activity (250.83 ± 195.79 min vs. 404.50 ± 229.63 min, P = 0.008) and the physical activity at moderate intensity (0.00 [0.00; 120.0] min vs. 140.00 [67.50; 330.00] min, P = 0.002). QoL outcomes are reported in Table 3. Significant enhancements were observed for emotional functioning (75.00 [68.75; 89.85] vs. 91.67 [83.33; 100.00], P = 0.004) and social functioning (61.11 ± 25.95 vs. 83.33 ± 15.89, P = 0.003), whereas no improvements were detected for the other domains. Blood parameters (Table 4) did not significantly change, except urea, which increased in the post-evaluation (10.40 ± 12.46 mg/dL vs. 31.26 ± 12.10, P < 0.001). Non-significant differences between the exercise modalities for outcomes emerged (Supplementary Material).
Table 2.
Functional assessments before and after the intervention (N = 12).
| Variable | Baseline | Post-intervention | Significant |
|---|---|---|---|
| Anthropometric measures | |||
| Body weight (kg)a | 68.08 (13.68) | 69.04 (13.02) | 0.436 |
| Body mass index (kg/m2)a | 24.65 (3.98) | 25.00 (3.73) | 0.406 |
| Waist (cm)a | 87.20 (13.83) | 85.16 (14.11) | 0.153 |
| Hip (cm)a | 100.50 (7.33) | 99.68 (7.22) | 0.593 |
| Waist–hip ratioa | 0.86 (0.09) | 0.85 (0.12) | 0.498 |
| Chair sit and reach (cm) | |||
| Right lega | −3.45 (10.25) | −2.08 (16.34) | 0.611 |
| Left lega | −4.54 (11.06) | −4.12 (18.15) | 0.886 |
| Back scratch (cm) | |||
| Right armb | 2.0 (−7.0; 4.5) | 4.5 (−12.0; 7.0) | 0.233 |
| Left arma | −6.45 (12.66) | −5.29 (13.99) | 0.408 |
| Handgrip (kg) | |||
| Right arma | 32.83 (11.51) | 33.87 (10.59) | 0.387 |
| Left arma | 30.75 (9.24) | 31.41 (2.71) | 0.494 |
| Leg press (kg)a | 104.24 (48.72) | 124.79 (79.61) | 0.098 |
| 6-min walking test (m)a | 528.33 (82.06) | 564.83 (69.84) | 0.021∗∗∗ |
| Physical activity level (min/week) | |||
| Vigorousb | 0.00 (0.00; 0.00) | 0.00 (0.00; 67.50) | 0.250 |
| Moderateb | 0.00 (0.00; 120.00) | 140.00 (67.50; 330.00) | 0.002∗∗∗∗ |
| Lighta | 183.33 (174.68) | 137.50 (148.88) | 0.461 |
| Totala | 250.83 (195.79) | 404.50 (229.63) | 0.008∗∗∗∗ |
∗∗∗P < 0.05, ∗∗∗∗P < 0.01.
Data presented as mean and standard deviation.
Data presented as median and interquartile range.
Table 3.
Quality of life, evaluated with the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (score 0–100), before and after the intervention (N = 12).
| Variables | Baseline | Postintervention | Significant |
|---|---|---|---|
| Physical functioning | 84.40 (13.43) | 89.44 (9.62) | 0.108 |
| Role functioning | 75.00 (66.67; 100.00) | 100.00 (54.17; 100.00) | 0.625 |
| Emotional functioningb | 75.00 (68.75; 89.58) | 91.67 (83.33; 100.00) | 0.004∗∗∗ |
| Cognitive functioninga | 84.72 (13.21) | 86.11 (15.62) | 0.723 |
| Social functioninga | 61.11 (25.95) | 83.33 (15.89) | 0.003∗∗∗ |
| Global health statusa | 66.66 (23.30) | 68.05 (20.66) | 0.782 |
| Fatiguea | 30.55 (26.00) | 26.85 (23.90) | 0.339 |
| Nausea/vomitingb | 0.00 (0.00; 16.67) | 8.33 (0.00; 16.67) | 0.375 |
| Painb | 8.33 (0.00; 33.33) | 0.00 (0.00; 3.33) | 1.000 |
| Dyspneab | 33.33 (0.00; 33.33) | 16.67 (0.00; 58.33) | 0.750 |
| Insomniab | 33.33 (0.00; 33.33) | 16.67 (0.00; 58.33) | 0.750 |
| Appetite lossb | 16.67 (0.00; 58.33) | 0.00 (0.00; 33.33) | 0.156 |
| Constipationb | 0.00 (0.00; 0.00) | 0.00 (0.00; 0.00) | 1.000 |
| Diarrheab | 0.00 (0.00; 25.00) | 0.00 (0.00; 33.33) | 1.000 |
| Financial problemsb | 0.00 (0.00; 25.00) | 0.00 (0.00; 25.00) | 1.000 |
∗∗∗P < 0.01.
Data presented as mean and standard deviation.
Data presented as median and interquartile range.
Table 4.
Circulatory parameters before and after the intervention (N = 12).
| Variables | Baseline | Postintervention | Normality range | Significant |
|---|---|---|---|---|
| Erythrocytes (1012/L) | 4.05 (0.45) | 4.12 (0.49) | 4.00–5.20 | 0.529 |
| Hemoglobin (g/L)a | 117.66 (13.16) | 120.33 (16.77) | 135–175 | 0.480 |
| MCV (fL) | 89.75 (86.40; 94.73) | 90.20 (87.78; 92.78) | 80.0–99.0 | 0.622 |
| MCH (pg)a | 29.47 (1.51) | 29.22 (1.91) | 26–34 | 0.570 |
| MCHC (g/L)a | 326.41 (14.23) | 324.25 (14.77) | 310–360 | 0.559 |
| RDW (%)b | 13.65 (13.00; 16.8) | 14.600 (13.40; 17.10) | 11.5–15.0 | 0.846 |
| Platelets (109/L)a | 219.16 (86.32) | 239.66 (82.49) | 150–400 | 0.248 |
| Erythroblasts (109/L)a | Absent | Absent | Absent | N/A |
| MPV (fL)a | 10.23 (0.94) | 10.41 (1.00) | 9.6–12.9 | 0.228 |
| Leukocytes (109/L)a | 5.18 (2.26) | 5.49 (3.67) | 4.50–11.0 | 0.661 |
| Neutrophils (109/L)a | 3.16 (1.76) | 3.43 (3.45) | 1.80–8.00 | 0.679 |
| Lymphocytes (109/L)a | 1.54 (0.74) | 1.47 (0.74) | 1.20–4.00 | 0.620 |
| Neutrophils- lymphocytes ratio (109/L)a | 2.52 (1.56) | 2.56 (2.02) | N/A | 0.919 |
| Monocytes (109/L)a | 0.51 (0.26) | 0.54 (0.16) | 0.20–1.00 | 0.835 |
| Eosinophils (109/L)a | 0.07 (0.04) | 0.11 (0.07) | < 0.45 | 0.112 |
| Basophils (109/L)a | 0.02 (0.01) | 0.02 (0.01) | < 0.20 | 0.719 |
| Urea (mg/dL)a | 10.40 (12.46) | 31.26 (12.10) | 17.1–47.1 | < 0.001∗∗∗ |
| Creatinine (μmol/L)a | 71.16 (11.98) | 73.25 (15.66) | 53.0–115.0 | 0.378 |
| Bilirubin (μmol/L)a | 7.06 (5.33) | 7.11 (2.70) | Less than 18.0 | 0.972 |
| Calcium (mmol/L)a | 2.33 (0.10) | 2.34 (0.10) | 2.10–2.60 | 0.692 |
| Sodium (mmol/L)a | 139.75 (1.60) | 139.66 (2.64) | 135–145 | 0.901 |
| Potassium (mmol/L)a | 4.05 (0.35) | 3.90 (0.40) | 3.40–4.80 | 0.072 |
| Glucose (mg/dL)a | 97.41 (18.20) | 99.33 (23.94) | 63–99 | 0.575 |
| P-ast (U/L)a | 37.32 (0.317) | 32.0 (10.55) | 5–50 | 0.317 |
| P-alt (U/L)a | 48.16 (32.67) | 49.33 (24.51) | 6–50 | 0.855 |
| P-alp (U/L)a | 96.75 (45.71) | 102.60 (62.72) | 50–130 | 0.601 |
| Cholesterol (mg/dL)b | 159.00 (132.75; 188.50) | 165.50 (158.25; 184.00) | Less than 200 | 0.563 |
| Triglycerides (mg/dL)a | 107.0 (74.27) | 135.28 (81.19) | Less than 150 | 0.521 |
∗∗∗P < 0.001. MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RDW, red cell distribution width; MPV, mean platelet volume; P-ast, aspartate aminotransferase; P-alt, alanine aminotransferase; P-alp, alkaline phosphatase; N/A, not available.
Data presented as mean and standard deviation.
Data presented as median and interquartile range.
Discussion
This study aimed to investigate safety, feasibility, and explore the preliminary efficacy of a tailored exercise program specifically designed for patients with high cachectic potential, such as those affected by advanced pancreatic or lung cancer.
The main finding of this feasibility trial is that a combined aerobic and resistance training program performed at moderate intensity was shown to be safe, feasible, and tolerated by patients with advanced pancreatic or lung cancer undergoing anticancer treatments. Prior studies found considerable heterogeneity in terms of the recruitment rate, adherence, and retention rate. A systematic review found that in patients with pancreatic cancer, the recruitment rate varied between 21% and 93%, attendance to exercise programs between 64% and 100%, and retention rate ranged from 68% to 90% across the included studies,27 and a similar variability is observed in the lung cancer context.28, 29, 30 Whereas the recruitment rate was sufficient, 50%, and the reasons for the decline suggest that additional efforts in terms of patients’ education and information should be made, the adherence rate in our study was high, overall 84%. This result is particularly important since adherence is one of the major determinants for expecting to obtain benefits from an exercise intervention.31 However, adherence to exercise behavior is often challenging in this population, with several factors that may potentially influence the adoption of an active lifestyle in patients with advanced cancer. Disease-correlated barriers, such as fatigue, insomnia, and weakness due to anticancer therapies, are commonly reported by patients as impediments to exercise32; intriguingly, such variables have been demonstrated to improve after an exercise intervention.10 In this sense, informing and educating patients about the benefits of exercise, oncologist's advice33 as well as the availability of skilled specialists, such as exercise physiologists, kinesiologists, and physiotherapists, to adapt the program considering these features, might be the first steps to try overcoming the potential difficulties related to participation.34 Nevertheless, investigations also reported the distance from the gym facility and transportation problems as obstacles that may interfere with patients' participation and adherence.35 To overcome this well-recognized impediment, we proposed a partially supervised program in which patients could exercise at home without overshadowing the personalization of the exercise prescription. At the same time, this exercise modality may not always be the preferred one because the fear of getting injured, and the thought that exercise is harmful or may trigger symptoms35,36 may make patients more afraid of exercising at home. This is the reason why our intervention included the possibility of performing exercise in a fully-supervised way, with kinesiologists supervision each session, and was the patient to choose the modality that he/she thought was most suitable for him/her. Both exercise modalities had demonstrated to be feasible, with good adherence and a safety profile; thus, proposing different ways to exercise might be an optimal strategy to meet and answer different patients' needs. Also, safety is an important outcome: no exercise-related AEs have been observed. This may be crucial, especially for the consequences that injury may generate on patients' health. For instance, in patients with bone metastases, skeletal-related events may affect patients' QoL and increase mortality risk with a consistent economic burden.37, 38, 39
Regarding QoL, we detected a significant increase in emotional and social functioning. Feelings of fear, hopelessness, anxiety, frustration, and a sense of isolation are frequently reported by patients with advanced cancer and often remain an unmet care need.40 The beneficial effect of training from psychological and social points of view has already been reported,10,28,41 and thus exercise may be considered one of the suitable interventions to address these issues. However, the other domains, including symptoms, despite showing improvements, were not significant. Although the literature describes exercise as a method for managing different treatment-related side effects, such as fatigue,42 anemia,9 and nausea/vomiting,10 it is worth remembering that patients with advanced stages of the disease may have different symptoms burden that may progressively and rapidly worsen. Prior meta-analysis43 and randomized controlled trials41 have found the inability of exercise to produce effective improvements on these parameters. On the other hand, we recognize that the small sample size and the lack of a control group suggest that these results should be taken with caution. In this sense, it cannot exclude that without the exercise intervention, symptoms may deteriorate instead of remaining stable. Similar speculations may be carried out for strength and flexibility outcomes.
An important finding regards the impact of exercise on functional capacity. We found that 12 weeks of aerobic and strength training was significantly able to increment about 36.5 m the distance walking in the 6MWT. A prior study on patients with pancreatic cancer testing resistance training did not detect any effect on functional capacity,44 whereas the investigations on advanced lung cancer show conflicting results.43,45 This result may have important clinical implications. Indeed, functional capacity was found to be a prognostic factor in patients with advanced lung cancer.46,47 Kasymjanova et al. found that patients walking a distance < 400 m have more frequent progression of the disease and a median survival significantly shorter than those who performed more than 400 m.47 In addition, Jones et al., besides finding 6MWT, a strong independent predictor of survival, estimated that every 50 m of improvement correspond to a 13% of mortality risk reduction.46 Therefore, trying to increase functional capacity in this population is fundamental and may be considered a crucial aim.
The last consideration concerns the anthropometric assessments: no significant changes in BMI and waist–hip ratio were observed. This finding may be particularly interesting in the context of cachexia, where the preservation or the increase of body weight is an important step. To date, just one study has tested the impact of resistance exercise alone in cachectic patients with head and neck cancer and found that exercise was not able to counteract the progressive loss of weight, lean body, and mass, as well as to improve functional parameters and QoL.48 One possible explanation could be related to the different populations included in the studies: our trial included patients with a high risk of developing cachexia, whereas, in the study of Grote, patients were already cachectic. This difference could highlight the importance of prevention. Indeed, it could be easier to manage and revert the pre-cachexia phase rather than the already established syndrome. In this sense, some cancer types have a greater risk of developing cachexia than others; consequently, screening at regular intervals to identify those patients with an elevated risk early and referring them to an appropriate intervention could be a helpful strategy to reduce the development of this syndrome.
Limitations
The present study has some limitations that should be noted. First, the study population was heterogeneous in terms of medical characteristics and treatments, and additionally, we cannot exclude a potential patient selection bias, i.e., patients more motivated could be more likely to participate in the exercise intervention, thus limiting the generalizability of this investigation. Second, the non-randomized controlled design and the small sample size, underpowered to detect the intervention's efficacy, suggest carefully interpreting those outcome changes. Another potential source of bias could be related to wearing of protective masks during the exercise sessions and evaluations. Indeed, especially for aerobic exercise, wearing a mask could alter the response to exercise, potentially decreasing cardiorespiratory fitness. Nevertheless, on one side, the assessments could not be performed without wearing the mask for safety issues; on the other, we have utilized a submaximal test to assess cardiorespiratory. Different studies have demonstrated that the use or not of a mask does in such test, not produce significant differences in its result, suggesting that the COVID-19 safety procedures adopted should not have affected the data. Moreover, we have evaluated adherence only by the number of sessions attended by the patients. Investigating the tolerance to the program through the ratio of the total volume of exercise compared to that prescribed could have provided additional information regarding the program's feasibility. A follow-up period could have provided important information regarding the long-term effect of exercise in particular regarding the preventive role of exercise on cachexia onset since the EPCRC criteria for cachexia diagnosis is based on the changes in body weight, BMI, and/or skeletal muscle mass over the past six months. Finally, body composition changes and circulatory inflammatory parameters were not evaluated. However, the study has the strength of proposing a patient-centered intervention, which considered their preferences and needs and which left to have a proactive role of the patients in deciding the delivery of exercise modality. Moreover, a core set of validated and reliable measures to assess safety, feasibility, and efficacy was applied.
Conclusions
The current study reports that 12 weeks of combined aerobic and resistance training may be feasible in patients with advanced lung or pancreatic cancer. Additionally, the investigation also shows that different proposed modalities in delivering exercise are feasible and do not significantly affect adherence. This study provides the basis for future large trials, using an intervention that has been proven to be feasible to establish the real impact on outcomes. Moreover, other future perspectives could be related to exploring this kind of intervention in patients with an established diagnosis of cancer-cachexia, as well as investigating its feasibility within a multimodal approach.
CRediT author statement
Alice Avancini: Conceptualization, Study design, Software, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Writing – review and editing, Visualization, Supervision, Project administration. Anita Borsati: Investigation, Resources, Data curation, Writing – review and editing, Visualization. Ilaria Trestini: Resources, Writing – review and editing, Visualization. Daniela Tregnago: Resources, Writing – review and editing, Visualization. Lorenzo Belluomini: Writing – review and editing, Visualization. Marco Sposito: Writing – review and editing, Visualization. Michele Rota: Writing – review and editing, Visualization. Jessica Insolda: Writing – review and editing, Visualization. Federico Schena: Writing – review and editing, Visualization. Michele Milella: Conceptualization, Data curation, Writing – original draft, Writing – review and editing, Visualization, Supervision, Project administration. Sara Pilotto: Conceptualization, Data curation, Writing – original draft, Writing – review and editing, Visualization, Supervision, Project administration. All authors had full access to all the data in the study, and the corresponding author had final responsibility for the decision to submit for publication. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Declaration of competing interest
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. M.M. reports personal fees from Pfizer, EUSA Pharma and Astra Zeneca, outside the submitted manuscript. S.P. received honoraria or speakers’ fee from Astra-Zeneca, Eli-Lilly, BMS, Boehringer Ingelheim, MSD and Roche, outside the submitted manuscript. All authors had full access to all the data in the study, and the corresponding author had final responsibility for the decision to submit for publication. The corresponding author attests that all listed authorsmeet authorship criteria and that no others meeting the criteria have been omitted.
Disclosure
Publication of this paper is supported by Helsinn Health care SA. Helsinn does not have any influence on the content and all items are subject to independent peer and editorial review.
Funding
This study received no external funding.
Ethics statement
The study obtained the approval of the Ethics Committee for Clinical Trials for the University of Verona (IRB No. 33320). All participants provided written informed consent.
Data availability statement
The data that support the findings of this study are available from the corresponding author, SP, upon reasonable request.
Declaration of Generative AI and AI-assisted technologies in the writing process
No AI tools/services were used during the preparation of this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.apjon.2023.100298.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Society A.C. 2023. Cancer Facts & Figures. [Google Scholar]
- 2.Iyer S., Taylor-Stokes G., Roughley A. Symptom burden and quality of life in advanced non-small cell lung cancer patients in France and Germany. Lung Cancer. 2013;81(2):288–293. doi: 10.1016/j.lungcan.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Janda M., Neale R.E., Klein K., et al. Anxiety, depression and quality of life in people with pancreatic cancer and their carers. Pancreatology. 2017;17(2):321–327. doi: 10.1016/j.pan.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Naito T., Okayama T., Aoyama T., et al. Skeletal muscle depletion during chemotherapy has a large impact on physical function in elderly Japanese patients with advanced non-small-cell lung cancer. BMC Cancer. 2017;17(1):571. doi: 10.1186/s12885-017-3562-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Granger C.L., McDonald C.F., Irving L., et al. Low physical activity levels and functional decline in individuals with lung cancer. Lung Cancer. 2014;83(2):292–299. doi: 10.1016/j.lungcan.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Clauss D., Tjaden C., Hackert T., et al. Cardiorespiratory fitness and muscle strength in pancreatic cancer patients. Support Care Cancer. 2017;25(9):2797–2807. doi: 10.1007/s00520-017-3694-8. [DOI] [PubMed] [Google Scholar]
- 7.Fearon K., Strasser F., Anker S.D., et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 8.Avancini A., Trestini I., Tregnago D., et al. A multimodal approach to cancer-related cachexia: from theory to practice. Expert Rev Anticancer Ther. 2021;21(8):819–826. doi: 10.1080/14737140.2021.1927720. [DOI] [PubMed] [Google Scholar]
- 9.Avancini A., Belluomini L., Tregnago D., et al. Exercise and anemia in cancer patients: could it make the difference? Expert Rev Hematol. 2021;14(11):979–985. doi: 10.1080/17474086.2021.2007764. [DOI] [PubMed] [Google Scholar]
- 10.Campbell K.L., Winters-Stone K.M., Wiskemann J., et al. Exercise guidelines for cancer Survivors: consensus statement from international multidisciplinary Roundtable. Med Sci Sports Exerc. 2019;51(11):2375–2390. doi: 10.1249/MSS.0000000000002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avancini A., Sperduti I., Borsati A., et al. Effect of exercise on functional capacity in patients with advanced cancer: a meta-analysis of randomized controlled trials. Crit Rev Oncol Hematol. 2022;175 doi: 10.1016/j.critrevonc.2022.103726. [DOI] [PubMed] [Google Scholar]
- 12.Avancini A., Benato G., Borsati A., et al. Exercise and bone health in cancer: Enemy or ally? Cancers. 2022;14(24) doi: 10.3390/cancers14246078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mikkelsen M.K., Nielsen D.L., Vinther A., Lund C.M., Jarden M. Attitudes towards physical activity and exercise in older patients with advanced cancer during oncological treatment - a qualitative interview study. Eur J Oncol Nurs. 2019;41:16–23. doi: 10.1016/j.ejon.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Avancini A., Pala V., Trestini I., et al. Exercise levels and preferences in cancer patients: a cross-sectional study. Int J Environ Res Public Health. 2020;17(15) doi: 10.3390/ijerph17155351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mikkelsen M.K., Lund C.M., Vinther A., et al. Effects of a 12-week multimodal exercise intervention among older patients with advanced cancer: results from a randomized controlled trial. Oncol. 2022;27(1):67–78. doi: 10.1002/onco.13970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naito T., Mitsunaga S., Miura S., et al. Feasibility of early multimodal interventions for elderly patients with advanced pancreatic and non-small-cell lung cancer. J Cachexia Sarcopenia Muscle. 2019;10(1):73–83. doi: 10.1002/jcsm.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo H., Galvão D.A., Newton R.U., Tang C.I., Spry N., Taaffe D.R. Multimodal exercise in older patients with advanced pancreatic cancer undergoing first-line chemotherapy: a Case series Examining feasibility and preliminary efficacy. Eur J Cancer Care. 2023;2023 [Google Scholar]
- 18.Institute N.C. 2022. Common Terminology Criteria for Adverse Events (CTCAE) [Available from: CTEP (cancer.gov) [Google Scholar]
- 19.Laboratories ACoPSfCPF ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 20.Innes E. Handgrip strength testing: a review of the literature. Aust Occup Ther J. 1999:120–140. [Google Scholar]
- 21.Impellizzeri F.M., Rampinini E., Maffiuletti N., Marcora S.M. A vertical jump force test for assessing bilateral strength asymmetry in athletes. Med Sci Sports Exerc. 2007;39(11):2044–2050. doi: 10.1249/mss.0b013e31814fb55c. [DOI] [PubMed] [Google Scholar]
- 22.Rikli R.E., Jones C.J. Development and validation of criterion-referenced clinically relevant fitness standards for maintaining physical independence in later years. Gerontol. 2013;53(2):255–267. doi: 10.1093/geront/gns071. [DOI] [PubMed] [Google Scholar]
- 23.Organization W.H. The use and interpretation of anthropometry. Report of a World Health Organization Expert Comitee. World Health Organization technical report series. 1995;854:1–452. [PubMed] [Google Scholar]
- 24.Aaronson N.K., Ahmedzai S., Bergman B., et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 25.Amireault S., Godin G., Lacombe J., Sabiston C.M. The use of the Godin-Shephard Leisure-Time Physical Activity Questionnaire in oncology research: a systematic review. BMC Med Res Methodol. 2015;15:60. doi: 10.1186/s12874-015-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh B., Spence R.R., Steele M.L., Sandler C.X., Peake J.M., Hayes S.C. A systematic review and meta-analysis of the safety, feasibility, and effect of exercise in women with stage II+ Breast cancer. Arch Phys Med Rehabil. 2018;99(12):2621–2636. doi: 10.1016/j.apmr.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 27.Luo H., Galvão D.A., Newton R.U., et al. Exercise medicine in the Management of pancreatic cancer: a systematic review. Pancreas. 2021;50(3):280–292. doi: 10.1097/MPA.0000000000001753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quist M., Adamsen L., Rorth M., Laursen J.H., Christensen K.B., Langer S.W. The impact of a multidimensional exercise intervention on physical and functional capacity, anxiety, and depression in patients with advanced-stage lung cancer undergoing chemotherapy. Integr Cancer Ther. 2015;14(4):341–349. doi: 10.1177/1534735415572887. [DOI] [PubMed] [Google Scholar]
- 29.Quist M., Rorth M., Langer S., et al. Safety and feasibility of a combined exercise intervention for inoperable lung cancer patients undergoing chemotherapy: a pilot study. Lung Cancer. 2012;75(2):203–208. doi: 10.1016/j.lungcan.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Quist M., Langer S.W., Lillelund C., et al. Effects of an exercise intervention for patients with advanced inoperable lung cancer undergoing chemotherapy: a randomized clinical trial. Lung Cancer. 2020;145:76–82. doi: 10.1016/j.lungcan.2020.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Mouri T., Naito T., Morikawa A., et al. Promotion of behavioral change and the impact on quality of life in elderly patients with advanced cancer: a physical activity intervention of the multimodal nutrition and exercise treatment for advanced cancer program. Asia Pac J Oncol Nurs. 2018;5(4):383–390. doi: 10.4103/apjon.apjon_21_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frikkel J., Götte M., Beckmann M., et al. Fatigue, barriers to physical activity and predictors for motivation to exercise in advanced Cancer patients. BMC Palliat Care. 2020;19(1):43. doi: 10.1186/s12904-020-00542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avancini A., Belluomini L., Milella M., Schena F., Novello S., Pilotto S. Drive the oncologists into exercise promotion in lung cancer. Lung Cancer. 2022;176:1–3. doi: 10.1016/j.lungcan.2022.12.009. [DOI] [PubMed] [Google Scholar]
- 34.Avancini A., Belluomini L., Tregnago D., et al. Exercise oncology: it is time to make a change. Patient Educ Counsel. 2022;105(7):2629–2631. doi: 10.1016/j.pec.2022.01.019. [DOI] [PubMed] [Google Scholar]
- 35.Granger C.L., Connolly B., Denehy L., et al. Understanding factors influencing physical activity and exercise in lung cancer: a systematic review. Support Care Cancer. 2017;25(3):983–999. doi: 10.1007/s00520-016-3484-8. [DOI] [PubMed] [Google Scholar]
- 36.Avancini A., Tregnago D., Rigatti L., et al. Factors influencing physical activity in cancer patients during oncological treatments: a qualitative study. Integr Cancer Ther. 2020;19 doi: 10.1177/1534735420971365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McDougall J.A., Bansal A., Goulart B.H., et al. The clinical and economic impacts of skeletal-related events Among Medicare Enrollees with prostate cancer metastatic to bone. Oncol. 2016;21(3):320–326. doi: 10.1634/theoncologist.2015-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Howard L.E., De Hoedt A.M., Aronson W.J., et al. Do skeletal-related events predict overall survival in men with metastatic castration-resistant prostate cancer? Prostate Cancer Prostatic Dis. 2016;19(4):380–384. doi: 10.1038/pcan.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morin S., Lix L.M., Azimaee M., Metge C., Caetano P., Leslie W.D. Mortality rates after incident non-traumatic fractures in older men and women. Osteoporos Int. 2011;22(9):2439–2448. doi: 10.1007/s00198-010-1480-2. [DOI] [PubMed] [Google Scholar]
- 40.Wang T., Molassiotis A., Chung B.P.M., Tan J.Y. Unmet care needs of advanced cancer patients and their informal caregivers: a systematic review. BMC Palliat Care. 2018;17(1):96. doi: 10.1186/s12904-018-0346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steindorf K., Clauss D., Tjaden C., et al. Quality of life, fatigue, and sleep problems in pancreatic cancer patients—a randomized trial on the effects of exercise. Dtsch Arztebl Int. 2019;116(27-28):471–478. doi: 10.3238/arztebl.2019.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fabi A., Bhargava R., Fatigoni S., et al. Cancer-related fatigue: ESMO clinical practice guidelines for diagnosis and treatment. Ann Oncol. 2020;31(6):713–723. doi: 10.1016/j.annonc.2020.02.016. [DOI] [PubMed] [Google Scholar]
- 43.Peddle-McIntyre C.J., Singh F., Thomas R., Newton R.U., Galvão D.A., Cavalheri V. Exercise training for advanced lung cancer. Cochrane Database Syst Rev. 2019;2(2):CD012685. doi: 10.1002/14651858.CD012685.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiskemann J., Clauss D., Tjaden C., et al. Progressive resistance training to impact physical fitness and body weight in pancreatic cancer patients: a randomized controlled trial. Pancreas. 2019;48(2):257–266. doi: 10.1097/MPA.0000000000001221. [DOI] [PubMed] [Google Scholar]
- 45.Temel J.S., Greer J.A., Goldberg S., et al. A structured exercise program for patients with advanced non-small cell lung cancer. J Thorac Oncol. 2009;4(5):595–601. doi: 10.1097/JTO.0b013e31819d18e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones L.W., Hornsby W.E., Goetzinger A., et al. Prognostic significance of functional capacity and exercise behavior in patients with metastatic non-small cell lung cancer. Lung Cancer. 2012;76(2):248–252. doi: 10.1016/j.lungcan.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kasymjanova G., Correa J.A., Kreisman H., et al. Prognostic value of the six-minute walk in advanced non-small cell lung cancer. J Thorac Oncol. 2009;4(5):602–607. doi: 10.1097/JTO.0b013e31819e77e8. [DOI] [PubMed] [Google Scholar]
- 48.Grote M., Maihöfer C., Weigl M., Davies-Knorr P., Belka C. Progressive resistance training in cachectic head and neck cancer patients undergoing radiotherapy: a randomized controlled pilot feasibility trial. Radiat Oncol. 2018;13(1):215. doi: 10.1186/s13014-018-1157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, SP, upon reasonable request.