Abstract
Background
Maternal anticoagulation use may increase indeterminate result rates on cell-free DNA (cfDNA) based screening, but existing studies are confounded by inclusion of individuals with autoimmune disease, which alone is associated with indeterminate results. Changes in chromosome level Z-scores are proposed by others as a reason for indeterminate results, but the etiology of this is uncertain.
Objective(s)
We evaluated differences in fetal fraction, indeterminate result rate, and total cfDNA concentration in individuals on anticoagulation without autoimmune disease compared to controls undergoing non-invasive prenatal screening (NIPS). Secondly, using a nested case-control design, we evaluated differences in fragment size, GC content, and Z-scores to evaluate laboratory-level test characteristics.
Study Design
Retrospective single institution study of pregnant individuals undergoing cfDNA-based NIPS using low-pass whole genome sequencing between 2017 and 2021. Individuals with autoimmune disease, suspected aneuploidy, and cases where FF was not reported were excluded. Anticoagulation included heparin-derived products (unfractionated heparin, low molecular weight heparin), clopidogrel, and fondaparinux with a separate group for those on aspirin alone. An indeterminate result was defined as FF<4%. We evaluated the association between maternal anticoagulation or aspirin use, and FF, indeterminate results, and total cfDNA concentration using univariate and multivariate analyses, controlling for body mass index (BMI), gestational age at sample collection, and fetal sex. For the anticoagulation cohort, we compared laboratory-level test characteristics among cases (on anticoagulation) and a subset of controls. Lastly, we evaluated for differences in chromosome level Z-scores among those on anticoagulation with and without indeterminate results.
Results
A total of 1,707 pregnant individuals met inclusion criteria. Of those, 29 were on anticoagulation and 81 were on aspirin alone. For those on anticoagulation, the FF was significantly lower (9.3% vs. 11.7%, p<0.01), the indeterminate result rate was significantly higher (17.2% vs. 2.7%; p<0.001), and the total cfDNA concentration was significantly higher (218 pg/uL vs. 83.7 pg/uL, p<0.001). Among those on aspirin alone, the FF was lower (10.6% vs 11.8%, p=0.04); however, there were no differences in the rate of indeterminate results (3.7% vs 2.7%, p=0.57) or total cfDNA concentration (90.1 pg/uL vs 83.8 pg/uL p=0.31). After controlling for maternal BMI, gestational age at sample collection, and fetal sex, anticoagulation was associated with an over 8-fold increase in the likelihood of an indeterminate result (aOR 8.7, 95% CI 3.1–24.9, p<0.001), but not aspirin (aOR 1.2, 95% CI 0.3–4.1, p=0.8). Anticoagulation was not associated with appreciable differences in cfDNA fragment size or GC content. Although differences in chromosome 13 Z-scores were observed, none were observed for chromosomes 18 or 21, and this difference did not contribute to the indeterminate result call.
Conclusion(s)
In the absence of autoimmune disease, anticoagulation use, but not aspirin, is associated with lower FF, higher total cfDNA concentration, and higher rates of indeterminate results. Anticoagulation use was not accompanied by differences in cfDNA fragment size or GC content. Statistical differences in chromosome level Z-scores did not clinically affect aneuploidy detection. This suggests a likely dilutional effect by anticoagulation on cfDNA-based NIPS assays contributing to low FF and indeterminate results, and not laboratory or sequencing level changes.
Keywords: Anticoagulation, aspirin, cell-free DNA, fetal fraction, indeterminate results, non-invasive prenatal screening, Z-scores
INTRODUCTION
Noninvasive prenatal screening (NIPS) using cell-free DNA (cfDNA) is a reliable aneuploidy screening tool with failure rates of up to 8%.1 Placental-derived cfDNA (referred to as the fetal fraction, FF) is detectable early in pregnancy2 and increases with gestational age.3–7 Maternal and fetal factors contribute to indeterminate results due to low FF either due to a relatively higher maternal contribution or lower placental contribution. Such factors include aneuploidy, obesity, autoimmune disease, and possibly anticoagulation use.5,8–13
The impact of maternal anticoagulation on NIPS test characteristics is poorly understood. Studies to date have included small numbers, were confounded by autoimmune disease, or did not evaluate other analytic parameters.9,10,14 We have previously demonstrated that autoimmune disease is associated with lower FF even when anticoagulation use is excluded.13 Most in vivo studies suggest that heparin is associated with a greater chance of test failure due to low FF;15–17 however, other in vivo and in vitro studies suggest no effect on FF.16,18 One small study (n=5), that evaluated NIPS characteristics before and after administration of low molecular weight heparin (LWMH), suggested that LMWH was associated with an increase in the proportion of shorter (7 bp on average) cfDNA fragments and an increase in the proportion of GC content in those shorter fragments. The authors propose that these changes may influence FF determination (when using size discrimination) and increase false positive results, particularly for Trisomy 13 and 18, due to elevated Z-scores.9 Another study found no difference in FF with anticoagulation use, but noted a possible increase in shorter cfDNA fragments with higher GC content and hypothesized that this may influence aneuploidy detection.17 Mechanisms explaining these findings, however, are lacking.
Studies to date have largely focused on heparin-derived products and the effect of aspirin, an antithrombotic agent,19 are not well described. In one analysis combined with other medications, it did not influence the FF.18 As aspirin is frequently prescribed in pregnancy,20 it is important to understand its influence on NIPS test characteristics, particularly as pregnant individuals prescribed heparin products are often also prescribed aspirin.21
The aims of this study were threefold: 1) to evaluate if anticoagulation alone or aspirin alone, in the absence of maternal autoimmune disease and with control of relevant confounders, influences NIPS test characteristics; 2) to evaluate differences in cfDNA fragment size and GC content between those on anticoagulation and matched controls (nested case-control); and 3) to evaluate chromosome level Z-scores among those on anticoagulation with and without an indeterminate result. We hypothesized that maternal anticoagulation use, independent of autoimmune disease, will be associated with lower FF due to increases in the total cfDNA concentration; that this would be associated with more indeterminate results; and that aspirin alone would not affect NIPS test metrics. We also hypothesized that anticoagulation administration would not be associated with differences in cfDNA fragment size, GC content, and chromosome level Z-scores.
MATERIALS AND METHODS
This is a retrospective study of patients undergoing NIPS at a tertiary academic institution where we offer an internally developed NIPS assay for autosomal and sex chromosome aneuploidy screening via low pass whole genome sequencing. Our primary outcome was to compare FF between those on anticoagulation (largely heparin-based products) compared to controls, and those on aspirin alone compared to controls. Secondary outcomes included differences in the rate of indeterminate results and total cfDNA concentration. Next, we conducted a nested case control study comparing those on anticoagulation to matched controls (1:2) to evaluate differences in cfDNA fragment size and GC content. Lastly, we compared chromosome level Z-scores (for chromosomes 13, 18, and 21) among those on anticoagulation with and without an indeterminate result to determine whether aneuploidy calling could have been affected by these parameters.
Study Population
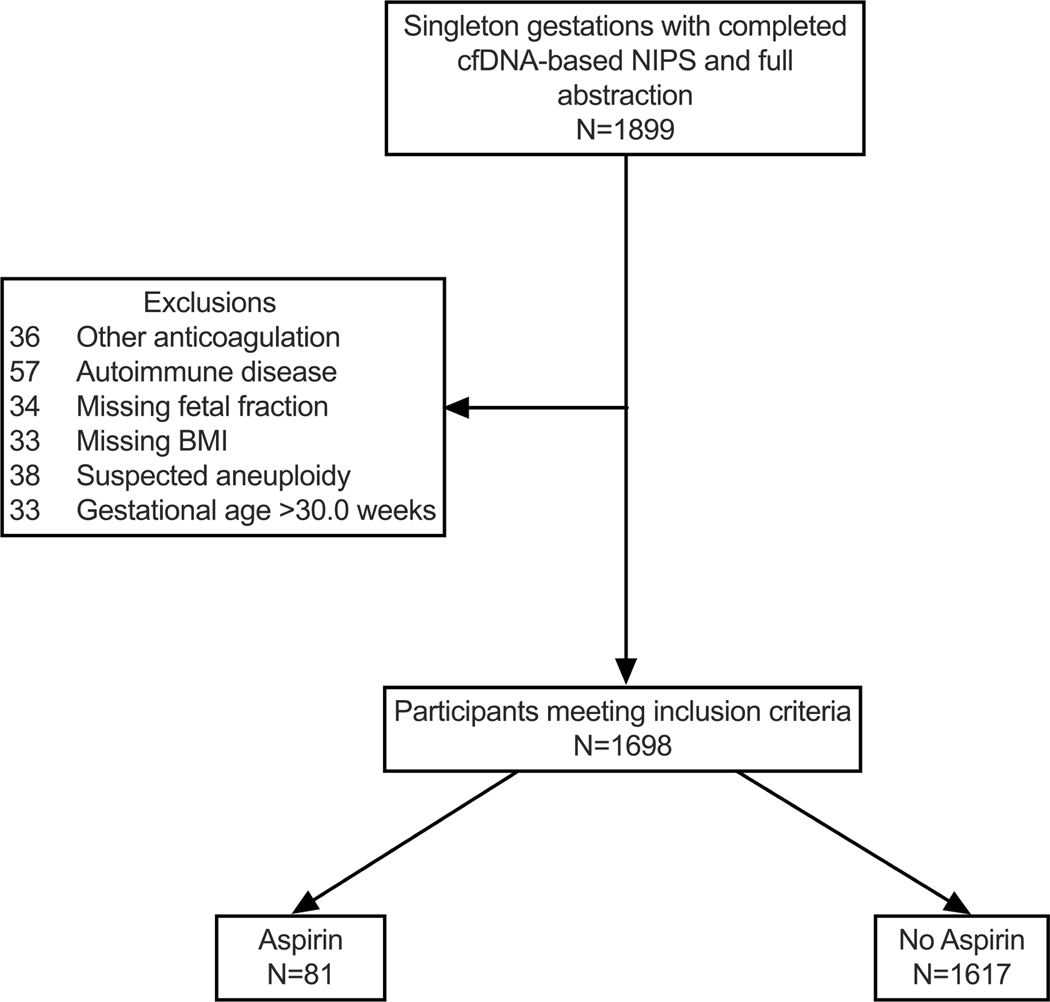
We included n=1899 patients with singleton pregnancies who completed NIPS (from May 2017 to 2021) for which medical record abstraction was complete. Demographic data were abstracted from the electronic health record. We excluded patients with autoimmune disease, suspected aneuploidy, missing body mass index (BMI), missing FF, or insufficient data to confirm or exclude anticoagulation use. Study groups included patients on anticoagulation (unfractionated heparin, LMWH, clopidogrel, fondaparinux) (n=29); patients on aspirin alone (without any other anticoagulant) (n=81); and controls (n=1600 in anticoagulant group and n=1617 in the aspirin group) [Figure 1a & 1b]. Although aspirin dosing was not confirmed for each case, virtually all patients meeting criteria for aspirin at our institution are prescribed 81mg daily. Because the gestational age at sample collection critically influences the FF, we restricted the control population to match the gestational age distribution in each group (10.0–29.2 weeks in the anticoagulation group and 10.0–33.0 weeks in the aspirin group), explaining the different size of the two control groups.
Figure(s) 1a&b: Flowchart of study population (a) anticoagulation group and (b) aspirin group.
Flow chart of the study populations.
cfDNA, cell-free DNA; NIPS, non-invasive prenatal screening
To evaluate differences in cfDNA fragment size and GC content, we performed a nested case-control study that included all cases of maternal anticoagulation use that were part of the primary analysis (n=29) with matched (1:2) controls (matched for gestational age at sample collection, maternal BMI, and fetal sex) (n=58). Among this nested case-control study, we also evaluate whether there were differences in chromosome level Z-scores for those on anticoagulation with an indeterminate result and matched cases on anticoagulation with a reportable result. Institutional Review Board approval was obtained (STUDY00005540) and data were collected and managed using Research Electronic Data Capture tools (Vanderbilt University, Nashville, TN).22,23
Sample Collection and Quantification of cfDNA
Samples were collected and processed as previously described.13,24,25 Briefly, plasma is isolated via centrifugation from whole blood collected in Streck (BCT1) tubes (Streck, La Vista, NE) and cfDNA is extracted using the QIAsymphony Circulating DNA Kit (Qiagen, Hilden, Germany). Total cfDNA concentration was measured by Qubit fluorometry (pg/μL). The Agilent TapeStation workflow, an automated electrophoresis system (Agilent, Santa Clara, CA) was used to assess the size and integrity of the DNA and functions as a critical quality control step throughout next-generation library preparation, hybridization capture, and sample pooling before sequencing.
Sequencing and Quantification of Fetal Fraction
Sequencing of cfDNA and FF determination were identical to as previously described.13,24,25 KAPA HyperPrep Kit (Roche Diagnostics, Indianapolis, IN) was used for adapter and index ligation for library preparation and amplification and purification was done by Agencourt AMPureXP (Brea, CA). Libraries were sequenced using an Illumina NextSeq 500 High Output 75 cycle kit (San Diego, CA) with a 37 bp paired-end read configuration. Samples were sequenced to an average depth of approximately 20 million paired-end reads, corresponding to an average genome depth of 0.5X. Reads were aligned to the human reference genome (hg19) with Bowtie (version 1.1.2). Run metrics were calculated with Picard (version 1.141). FF is calculated either by the percentage of reads that align to the Y chromosome or a custom bioinformatic algorithm based on the aggregate length distribution in sequencing reads for samples in which a Y chromosome was not present (i.e., female fetuses).26 An indeterminate result was defined as a FF of <4%. Failures owing to technical issues, including sample handling or sequencing failures, were excluded as these were related to collection or processing errors.
Statistical Analysis
Categorical variables were compared using chi-squared, Fisher exact, or Mann-Whitney tests. Continuous variables were tested for normality and by reviewing the quantile-quantile plot followed by comparisons using the t-test or Kruskal-Wallis tests as appropriate. Because total cfDNA concentration values were particularly skewed, this variable was log transformed, after which the distribution more closely approximated a normal distribution. For the log transformed total cfDNA concentration, geometric means with 95% confidence intervals (CIs) are reported. Multivariable logistic regression analysis was performed to determine the odds of an indeterminate result based on the presence or absence of anticoagulation use, adjusted for gestational age at sample collection, BMI at sample collection, and fetal sex.
For the nested case-control study, we compared demographic characteristics between groups as for the entire cohort. The cfDNA fragment size distribution and GC content between cases (anticoagulation use) and matched controls was plotted. Chromosome level absolute Z-scores between those on anticoagulation with an indeterminate result were compared to matched samples (for gestational age at draw and fetal sex) on anticoagulation with a reportable result using Wilcoxon rank-sum test. We chose to evaluate these three parameters (fragment size distribution, GC content, and Z-scores) as differences in these are proposed by others to influence aneuploidy calling among those on anticoagulation.9,17 Stata/SE (version 17.0; StataCorp, College Station, TX) was used for all statistical analyses.
RESULTS
Of 1629 NIPS results meeting inclusion criteria, 1.78% (n=29) were on anticoagulation with 76% (n=22) on prophylactic and 24% (n=7) on therapeutic anticoagulation. The groups were demographically similar (Table 1).
Table 1:
Patient demographics for anticoagulation and aspirin groups with their respective control groups.
| Demographic Parameters | Anticoagulation (n=29) | No Anticoagulation (n=1600) | Aspirin (n=81) | No Aspirin (n=1617) |
|---|---|---|---|---|
| Age (years) | 36.5 ± 4.9 | 34.8 ± 4.9 | 36.5 ± 4.4 | 34.8 ± 5.0 |
| BMI at sample collection (kg/m2) | 27.4 ± 6.2 | 27.2 ± 6.5 | 30.8 ± 8.4 | 27.2 ± 6.5 |
| Gravidity | 3.2 ± 2.1 | 2.9 ± 1.9 | 3.3 ± 2.0 | 2.9 ± 1.9 |
| Gestational age at sample collection (weeks) | 14.0 ± 4.1 | 13.9 ± 3.9 | 14.5 ± 4.8 | 14.1 ± 4.3 |
| Female fetal sex | 11 (38.0) | 723 (46.0) | 37 (46.3) | 730 (45.1) |
| Indication for testing | ||||
| Advanced maternal age | 22 | 954 | 61 | 957 |
| First-line screen | 5 | 378 | 14 | 379 |
| Ultrasound abnormality | 1 | 159 | 3 | 171 |
| Abnormal serum screen | 1 | 82 | 3 | 83 |
| Other | 0 | 27 | 0 | 27 |
Data presented as mean ± standard deviation, N (%), or median (interquartile range)
BMI, body mass index
The mean FF for those on anticoagulation was significantly lower compared to controls (9.3% vs. 11.7%, p<0.01) (Table 2, Figure 2) and, correspondingly, the rate of an indeterminate result was significantly higher (17.2% vs. 2.7%, p<0.001) (Table 2). Additionally, the total cfDNA concentration was higher for those on anticoagulation compared to controls (218 pg/uL [95% CI 144.0–333.0] vs. 83.7 pg/uL [95% CI 80.9–86.6], p<0.001) (Table 2, Figure 3).
Table 2:
Cell-free DNA test characteristics by anticoagulation and aspirin status.
| Test Parameters | Anticoagulation (n=29) | No Anticoagulation (n=1600) | p-value | Aspirin (n=81) | No Aspirin (n=1617) | p-value |
|---|---|---|---|---|---|---|
| Fetal fraction (%) | 9.3 ± 5.2 | 11.7 ± 4.8 | <0.01 | 10.6 ± 4.8 | 11.8 ± 4.9 | 0.04 |
| Indeterminate result rate | 5 (17.2) | 43 (2.7) | <0.001 | 3 (3.7) | 44 (2.7) | 0.57 |
| Total cfDNA concentration (pg/uL) | 218 (144.0–330.0) | 83.7 (80.9–86.6) | <0.001 | 90.1 (80.1–101.4) | 83.8 (81.1–86.7) | 0.31 |
Data are mean ± standard deviation, N (%), or geometric median (95% CI)
cfDNA, cell-free DNA
Figure 2: Fetal fraction among those on anticoagulation, aspirin, and controls.
Comparisons of mean (± standard deviation) fetal fraction between those on anticoagulation and controls as well as those on aspirin alone and controls.
Figure 3: Total cfDNA concentration among those on anticoagulation, aspirin, and controls.
Comparisons of median (interquartile range) between those on anticoagulation and controls as well as those on aspirin alone and controls.
cfDNA, cell-free DNA
Logistic regression analysis demonstrated that for those on anticoagulation, the odds of an indeterminate result, after controlling for gestational age at sample collection, BMI at sample collection, and fetal sex, was more than 8-fold higher compared to controls (aOR 8.6, 95% CI 3.0–24.9, p<0.001).
For the aspirin group, n=81 met inclusion criteria with n=1617 controls. Notably, we excluded patients on other anticoagulants for this analysis. Demographics were similar across groups (Table 1). Although the mean FF was lower in those on aspirin (10.6% vs 11.8%, p=0.04) [Table 2, Figure 2], the indeterminate result rate and the total cfDNA concentration were not different (Table 2, Figure 3). Additionally, aspirin use was not associated with an increased odds (adjusted or unadjusted) of an indeterminate result (aOR 1.20, 95% CI 0.35–4.08, p=0.77).
For the nested case-control study comparing those on anticoagulation (n=29) to matched controls (n=58), demographic parameters were similar between groups (Table 3). No differences in the distribution of cfDNA fragment size (Figure 4) or GC content were observed (Figure 5). The median absolute Z-score was higher for chromosome 13 among those on anticoagulation with a reportable result compared to those on anticoagulation with an indeterminate result (p<0.01). No differences in absolute median Z-scores were noted for chromosomes 18 or 21 (both p>0.44) (Table 5).
Table 3:
Demographic parameters of the nested case control study
| Demographic Parameters | Anticoagulation (n=29) | Controls a (n=58) |
|---|---|---|
| Age (years) | 36.5 ± 4.9 | 34.7 ± 4.9 |
| BMI at sample collection (kg/m2) | 27.4 ± 6.2 | 26.5 ± 7.4 |
| Gestational age at sample collection (weeks) | 14.0 ± 4.1 | 14.0 ± 4.2 |
| Female fetal sex (%) | 11 (38%) | 22 (38%) |
| Mean fetal fraction | 9.3 ± 5.2 | 13.6 ± 4.8 |
Data are mean ± standard deviation, N (%)
matched for gestational age at sample collection, maternal body mass index at sample
collection, and fetal sex
Figure 4: Cell-free DNA fragment size distribution between those on anticoagulation and matched controls.
Distribution of cfDNA fragment size in base pairs among those on anticoagulation (n=29, blue line) and a representative control group (n=58, red line) matched for gestational age at draw, body mass index at draw, and fetal sex.
bp, base pairs
Figure 5: GC content between those on anticoagulation and matched controls.
GC content by fraction of reads among those on anticoagulation (n=29, blue line) and a representative control group (n=58, red line) matched for gestational age at draw, body mass index at draw, and fetal sex.
Table 5:
Absolute chromosome level Z-scores for those with an indeterminate and a reportable result on anticoagulation.
| Parameters | Result Status | ||
|---|---|---|---|
| Reportablea (n=5) | Indeterminate (1st Draw) (n=5) | p-value | |
| Gestational age at NIPT (weeks) | 12.2 ± 0.7 | 11.9 ± 0.2 | 0.45 |
| Chromosome 13 Z-score | 0.18 ± 0.04 | 0.07 ± 0.04 | <0.01 |
| Chromosome 18 Z-score | 0.12 ± 0.06 | 0.11 ± 0.07 | 0.84 |
| Chromosome 21 Z-score | 0.10 ± 0.10 | 0.20 ± 0.08 | 0.14 |
Matched for gestational age at draw and fetal sex
COMMENT
Principal Findings
Maternal anticoagulation use, independent of autoimmune disease, is associated with lower FF, increased total cfDNA concentration, and an increased likelihood of indeterminate results. No substantial differences were noted in cfDNA fragment size or GC content based on anticoagulation use. Although median Z-scores for chromosome 13 were slightly higher for those on anticoagulation with sufficient FF, this difference did not influence designation of a result as reportable, and no differences were noted for chromosomes 18 or 21.
Results in the Context of What is Known
Maternal factors such as obesity24,27,28 and autoimmune disease13 are known to influence NIPS test characteristics. Although previous reports suggest that maternal anticoagulation use influences NIPS assays (trend toward low FF and higher rates of indeterminate results), these studies were limited by inclusion of coexisting maternal autoimmune disease, other confounders, small sample sizes, and for some, an in vitro design.9,10,14,16–18,29 Our study demonstrates that maternal anticoagulation use alone can influence NIPS, and clarifies that lower FF, likely due to dilution from maternal sources of cfDNA based on increased cfDNA quantities, is a contributor. Although the lower FF is an important observation, the significant difference in the rate of an indeterminate result is clinically relevant as it indicates that results are more frequently not able to be generated for this population.
Mechanisms contributing to this effect are not yet known. Most studies that report higher indeterminate result rates with anticoagulation relate this to lower FF;10,14–16 however, two notable studies suggest differing etiologies.9,17 In one small study, it was hypothesized that anticoagulation (particularly LWMH) resulted in an increase in shorter cfDNA fragments with higher GC content, driven by increased apoptosis, and that this contributed to aberrant Z-scores that could lead to false positive aneuploidy calling.9 The reported FF in each of these five cases was over 4% and although it’s unclear what cutoff is used by that specific lab, in most cases, these would not have been called as indeterminate due to low FF. Studies demonstrating the apoptotic potential of heparin-derived products are largely in vitro with varied effects based on cell type.30–32 Interestingly, other studies (animal and cell culture) suggest that heparin-derived products inhibit apoptosis.33–36 Nakamura et al also investigated NIPS results of those on anticoagulation (n=23, many with concomitant autoimmune disease) compared to controls.17 Although those on anticoagulation had a higher nonreportable result rate (8.7% [n=2/23] vs. 0.15% [n=4/2628]), this was due to “borderline Z-scores” and not low FF. For their assay, they also designate a FF of <4% as indeterminate. These investigators also reported a shorter peak fragment size (only 1bp difference), increase in GC content, and a difference in mean Z-scores for chromosome 13, 18, and 21, this latter finding being the reason for the nonreportable result in their 2 cases. An explanation for these findings, particularly Z-score differences, however, remains unclear.
We also investigated cfDNA fragment size distribution and GC content and noted no difference between those on anticoagulation and matched controls, including no appreciable differences in the proportion of fragment sizes (Figure 4) and overlapping curves of GC content distribution (Figure 5). This contrasts with the Grömminger et al report and the study by Nakamura et al. It is not immediately clear what mechanisms affecting the GC content of cfDNA, a feature intrinsic to the DNA sequence, would be related to maternal anticoagulation use. Additionally, GC correction is standard practice for next generation sequencing pipelines,37 including ours; thus, the stark differences observed in other reports are intriguing. The details of the methodologies utilized by these other assays, accessible through referenced papers, indicates that they are largely consistent with our laboratory procedures, however, some differences in library preparation techniques and bioinformatic analyses may partially explain some of the observed differences. Conversely, our data demonstrates that anticoagulation use does not influence our cfDNA extraction, library preparation, or sequencing, in that our methodology is not preferentially selecting GC rich fragments, and subsequently influencing test results. Ultimately, all our cases of indeterminate results on anticoagulation were due to FF <4%, which can be explained by our finding of an increase in the total amount of cfDNA in the samples (possible dilution from maternal sources). Although we specifically either excluded or controlled for factors known to influence the FF, it’s possible that other mechanism, aside from increased total cfDNA, are at play.
As low FF may itself mask whether anticoagulation leads to falsely elevated Z-scores, we evaluated Z-scores for those on anticoagulation with reportable results (FF ≥4%) and those with indeterminate results (FF <4%) (Table 4) and found no aberrations in Z-score profiles for chromosomes 18 or 21 (Table 5) with subsequently no concern for false positive aneuploidy calling. Although the absolute Z-score for chromosome 13 was slightly higher in those on anticoagulation with a reportable result, this difference did not influence our ability to adjudicate risk of aneuploidy and generation of a reportable result, and notably did not contribute to any false positive results.
Table 4:
Clinical details of those on anticoagulation with and without an indeterminate result.
| Patient | Anticoagulant | Gestational age at draw (weeks) | FF | cfDNA concentration (pg/uL) | Fetal sex | Details of repeat testing |
|---|---|---|---|---|---|---|
| 1 | Therapeutic LMWH | 11.8 | 3.8% | 609 | M | 3 weeks later; FF 6%, screen negative |
| 2 | Therapeutic LMWH | 11.7 | 3.4% | 1070 | M | 1 week later, FF 2.1%, indeterminate |
| 3 | Clopidogrel + ASA | 12.1 | 2.4% | 106 | M | 1 week later, FF 3.3%, indeterminate |
| 4 | Prophylactic LMWH + ASA | 12.1 | 2.8% | 445 | M | Not done |
| 5 | Prophylactic LMWH | 11.7 | 3.8% | 101 | M | 3 weeks later, FF 5%, screen negative |
| 6 | Prophylactic LMWH + ASA | 11.6 | 13% | 109 | M | N/A |
| 7 | Prophylactic LMWH + ASA | 12.9 | 7% | 236 | M | N/A |
| 8 | Prophylactic LMWH + ASA | 11.3 | 11% | 41.5 | M | N/A |
| 9 | Prophylactic LMWH + ASA | 12.7 | 5% | 208 | M | N/A |
| 10 | Prophylactic LMWH | 12.6 | 12% | 327 | M | N/A |
Patients 1–5: initially had an indeterminate result, Patients 6–10 had a reportable result FF, fetal fraction; cfDNA, cell-free DNA; LMWH, low molecular weight heparin; ASA, aspirin; N/A, not applicable
Our study excluded cases with suspected aneuploidy as aneuploidy itself can influence the FF, and differences in FF was our primary outcome. On review of these exclusions, there were 2 cases in the anticoagulation group excluded for suspected aneuploidy: one with a suspected sex chromosome aneuploidy (XXX) that had cord blood karyotype (results not accessible as this occurred at an outside institution); the second with suspected Trisomy 21 that was confirmed on products of conception following dilation and evacuation. There were no screen positive cases of Trisomy 13 or 18 among those on anticoagulation.
Clinical Implications
CfDNA-based NIPS is widely used and our understanding of biological factors influencing test performance and results continues to evolve as does our counseling and management following possible test failure. As an indeterminate result can raise concern for aneuploidy, assessment of maternal factors that may skew test results when considering management steps is important. Given that heparin products are not uncommonly used in pregnancy, understanding that this medication can influence FF is relevant information for the clinical provider and patient.
Aspirin is also increasingly prescribed in pregnancy,20 including in the setting of advanced maternal age, itself a common indication for NIPS. As aspirin influences coagulation parameters to a degree, understanding that at least at low doses (81 mg daily) it does not affect NIPS test characteristics or increase the likelihood of an indeterminate result is relevant to clinical providers and for future studies evaluating heparin-products since aspirin is commonly co-prescribed.
Research Implications
Our findings build on the available literature which implicates that anticoagulation influences NIPS test characteristics, here clarifying that anticoagulants contribute to low FF, independent of maternal autoimmune disease. Based on others’ reports, we also evaluated discrete test characteristics proposed to be influenced by anticoagulation use and noted that changes in these parameters were not evident, or, if present, were subtle, such that clinical aneuploidy calling was not affected. As such, future research should focus on mechanisms explaining lower FF when anticoagulation is present and ultimately developing laboratory level modifications that could minimize these effects to optimize the likelihood of a reportable result. Whether deferring an anticoagulation dose or scheduling a blood draw prior to administration of a dose can be safely considered, specifically considering potentially increased maternal risks, deserves further investigation.
Strengths and Limitations
Strengths of this study include a relatively large sample size of anticoagulation use without concomitant autoimmune disease; the use of a single platform with uniform collection, processing, sequencing, and bioinformatic procedures; access to detailed medical and obstetric clinical data; and access to laboratory level data. The relationship with our laboratory provides us with access to specific assay parameters (cfDNA concentration, cfDNA fragment size, GC content, chromosome level Z-scores, etc.), not otherwise accessible to ordering providers, which we have found crucial to best understand implications of various maternal or fetal factors on NIPS test characteristics. This allowed for verification that low FF accounted for each indeterminate result among those on anticoagulation and not any other pre-analytic or analytic parameter, such as aberrant or unusual Z-scores. Lastly, we excluded parameters known to influence the FF (aneuploidy, autoimmune disease) and controlled for relevant confounders (gestational age at draw, BMI, and fetal sex). Fetal sex is not only important to align with current research guidelines,38,39 but also because the FF is most precise in pregnancies with male fetuses with our analytic approach.
Study limitations include the retrospective design and the inability to examine the effects of anticoagulants by type or dosing. As we derived samples and data from a clinical screening program, we were not able to measure the effect of timing of anticoagulation administration.
Conclusions
Maternal anticoagulation use, and not aspirin, is associated with higher likelihood of an indeterminate result on NIPS due to low FF. This is likely a result of correspondingly higher amounts of total cfDNA in the sample. In our assay that employs massively parallel sequencing for generation of results, anticoagulation did not substantially influence sequencing-related parameters, such as cfDNA fragment size, GC content, or chromosome level Z-scores, and did not result in any false positive results.
Supplementary Material
HIGHLIGHTS.
Maternal anticoagulation contributes to lower fetal fraction and higher rates of indeterminate results on noninvasive prenatal screening.
Aspirin does not affect cfDNA based aneuploidy screening results.
Low FF, and not differences in cfDNA fragment size, GC content, or chromosome level Z-scores, was the underlying reason for indeterminate results.
AJOG AT A GLANCE.
A: Why was this study conducted?
Cell-free DNA-based noninvasive prenatal screening results can be affected by biological factors.
The effect of anticoagulation and aspirin use is poorly understood.
B: What are the key findings?
Among those on anticoagulation (independent of autoimmune disease), fetal fraction was lower, with higher total cfDNA concentration, and indeterminate result rates.
Sequencing-level factors such as fragment size, GC content, and Z-scores were not different and did not explain the results.
Aspirin use alone was not associated with an increased rate of indeterminate results.
C: What does this study add to what is already known?
Anticoagulation, independent of autoimmune disease, increases the likelihood of indeterminate results.
Indeterminate results were due to low fetal fraction and not other sequencing-related differences.
Aspirin alone does not affect the indeterminate result rate.
FUNDING:
UL1 TR002319 (National Center for Advancing Translational Sciences), K08HL150169 NIH/NHLBI (Shree)
None of the above listed funders were involved in conduct of the research, preparation of this article, study design, collection, analysis, or interpretation of results. Additionally, they were not involved in the writing of the report or in the decision to submit the article for publication.
Footnotes
CONDENSATION PAGE
TWEETABLE STATEMENT
Maternal anticoagulation leads to more indeterminate results due to low fetal fraction
DISCLOSURE STATEMENT: The authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Yaron Y. The implications of non-invasive prenatal testing failures: a review of an under-discussed phenomenon. Prenat Diagn. May 2016;36(5):391–6. doi: 10.1002/pd.4804 [DOI] [PubMed] [Google Scholar]
- 2.Tjoa ML, Cindrova-Davies T, Spasic-Boskovic O, Bianchi DW, Burton GJ. Trophoblastic oxidative stress and the release of cell-free feto-placental DNA. Am J Pathol. Aug 2006;169(2):400–4. doi: 10.2353/ajpath.2006.060161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lo YM, Tein MS, Lau TK, et al. Quantitative analysis of fetal DNA in maternal plasma and serum: implications for noninvasive prenatal diagnosis. Am J Hum Genet. Apr 1998;62(4):768–75. doi: 10.1086/301800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canick JA, Palomaki GE, Kloza EM, Lambert-Messerlian GM, Haddow JE. The impact of maternal plasma DNA fetal fraction on next generation sequencing tests for common fetal aneuploidies. Prenat Diagn. Jul 2013;33(7):667–74. doi: 10.1002/pd.4126 [DOI] [PubMed] [Google Scholar]
- 5.Ashoor G, Syngelaki A, Poon LC, Rezende JC, Nicolaides KH. Fetal fraction in maternal plasma cell-free DNA at 11–13 weeks’ gestation: relation to maternal and fetal characteristics. Ultrasound Obstet Gynecol. Jan 2013;41(1):26–32. doi: 10.1002/uog.12331 [DOI] [PubMed] [Google Scholar]
- 6.Nygren AO, Dean J, Jensen TJ, et al. Quantification of fetal DNA by use of methylation-based DNA discrimination. Clin Chem. Oct 2010;56(10):1627–35. doi: 10.1373/clinchem.2010.146290 [DOI] [PubMed] [Google Scholar]
- 7.Deng C, Liu S. Factors Affecting the Fetal Fraction in Noninvasive Prenatal Screening: A Review. Front Pediatr. 2022;10:812781. doi: 10.3389/fped.2022.812781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bianchi DW, Chudova D, Sehnert AJ, et al. Noninvasive Prenatal Testing and Incidental Detection of Occult Maternal Malignancies. JAMA. Jul 14 2015;314(2):162–9. doi: 10.1001/jama.2015.7120 [DOI] [PubMed] [Google Scholar]
- 9.Grömminger S, Erkan S, Schöck U, et al. The influence of low molecular weight heparin medication on plasma DNA in pregnant women. Prenat Diagn. Nov 2015;35(11):1155–7. doi: 10.1002/pd.4668 [DOI] [PubMed] [Google Scholar]
- 10.Burns W, Koelper N, Barberio A, et al. The association between anticoagulation therapy, maternal characteristics, and a failed cfDNA test due to a low fetal fraction. Prenat Diagn. Nov 2017;37(11):1125–1129. doi: 10.1002/pd.5152 [DOI] [PubMed] [Google Scholar]
- 11.Revello R, Sarno L, Ispas A, Akolekar R, Nicolaides KH. Screening for trisomies by cell-free DNA testing of maternal blood: consequences of a failed result. Ultrasound Obstet Gynecol. Jun 2016;47(6):698–704. doi: 10.1002/uog.15851 [DOI] [PubMed] [Google Scholar]
- 12.Palomaki GE, Kloza EM. Prenatal cell-free DNA screening test failures: a systematic review of failure rates, risks of Down syndrome, and impact of repeat testing. Genet Med. Nov 2018;20(11):1312–1323. doi: 10.1038/gim.2018.22 [DOI] [PubMed] [Google Scholar]
- 13.MacKinnon HJ, Kolarova TR, Katz R, et al. The impact of maternal autoimmune disease on cell-free DNA test characteristics. Am J Obstet Gynecol MFM. Nov 2021;3(6):100466. doi: 10.1016/j.ajogmf.2021.100466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma GC, Wu WJ, Lee MH, Lin YS, Chen M. Low-molecular-weight heparin associated with reduced fetal fraction and subsequent false-negative cell-free DNA test result for trisomy 21. Ultrasound Obstet Gynecol. Feb 2018;51(2):276–277. doi: 10.1002/uog.17473 [DOI] [PubMed] [Google Scholar]
- 15.Suzumori N, Sekizawa A, Takeda E, et al. Classification of factors involved in nonreportable results of noninvasive prenatal testing (NIPT) and prediction of success rate of second NIPT. Prenat Diagn. Jan 2019;39(2):100–106. doi: 10.1002/pd.5408 [DOI] [PubMed] [Google Scholar]
- 16.Dabi Y, Guterman S, Jani JC, et al. Autoimmune disorders but not heparin are associated with cell-free fetal DNA test failure. J Transl Med. Dec 3 2018;16(1):335. doi: 10.1186/s12967-018-1705-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura N, Sasaki A, Mikami M, et al. Nonreportable rates and cell-free DNA profiles in noninvasive prenatal testing among women with heparin treatment. Prenat Diagn. Jun 2020;40(7):838–845. doi: 10.1002/pd.5695 [DOI] [PubMed] [Google Scholar]
- 18.Kuhlmann-Capek M, Chiossi G, Singh P, et al. Effects of medication intake in early pregnancy on the fetal fraction of cell-free DNA testing. Prenat Diagn. Apr 2019;39(5):361–368. doi: 10.1002/pd.5436 [DOI] [PubMed] [Google Scholar]
- 19.Awtry EH, Loscalzo J. Aspirin. Circulation. Mar 14 2000;101(10):1206–18. doi: 10.1161/01.cir.101.10.1206 [DOI] [PubMed] [Google Scholar]
- 20.Davidson KW, Barry MJ, Mangione CM, et al. Aspirin Use to Prevent Preeclampsia and Related Morbidity and Mortality: US Preventive Services Task Force Recommendation Statement. Jama. Sep 28 2021;326(12):1186–1191. doi: 10.1001/jama.2021.14781 [DOI] [PubMed] [Google Scholar]
- 21.Practice Bulletin No. 132: Antiphospholipid syndrome. Obstet Gynecol. Dec 2012;120(6):1514–21. doi: 10.1097/01.AOG.0000423816.39542.0f [DOI] [PubMed] [Google Scholar]
- 22.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. Apr 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. Jul 2019;95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shree R, Kolarova TR, MacKinnon HJ, et al. Low fetal fraction in obese women at first trimester cell-free DNA based prenatal screening is not accompanied by differences in total cell-free DNA. Prenat Diagn. Sep 2021;41(10):1277–1286. doi: 10.1002/pd.6023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shree R, Kolarova TR, MacKinnon HJ, Lockwood CM, Chandrasekaran S. Association of fetal fraction with hypertensive disorders of pregnancy incidence and disease severity. Am J Obstet Gynecol MFM. Sep 2022;4(5):100671. doi: 10.1016/j.ajogmf.2022.100671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu SC, Chan KC, Zheng YW, et al. Size-based molecular diagnostics using plasma DNA for noninvasive prenatal testing. Proc Natl Acad Sci U S A. Jun 10 2014;111(23):8583–8. doi: 10.1073/pnas.1406103111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livergood MC, LeChien KA, Trudell AS. Obesity and cell-free DNA “no calls”: is there an optimal gestational age at time of sampling? Am J Obstet Gynecol. Apr 2017;216(4):413.e1–413.e9. doi: 10.1016/j.ajog.2017.01.011 [DOI] [PubMed] [Google Scholar]
- 28.Rolnik DL, Yong Y, Lee TJ, Tse C, McLennan AC, da Silva Costa F. Influence of Body Mass Index on Fetal Fraction Increase With Gestation and Cell-Free DNA Test Failure. Obstet Gynecol. Aug 2018;132(2):436–443. doi: 10.1097/aog.0000000000002752 [DOI] [PubMed] [Google Scholar]
- 29.Hui L, Bethune M, Weeks A, Kelley J, Hayes L. Repeated failed non-invasive prenatal testing owing to low cell-free fetal DNA fraction and increased variance in a woman with severe autoimmune disease. Ultrasound Obstet Gynecol. Aug 2014;44(2):242–3. doi: 10.1002/uog.13418 [DOI] [PubMed] [Google Scholar]
- 30.Erduran E, Zaman T, Deger O, Tekelioglu Y, Bahadir A. In vitro determination of apoptotic effect of heparin on lymphoblasts by using flow cytometric DNA analysis and measurements of caspase-9 activation and cytochrome C level. J Pediatr Hematol Oncol. Jan 2012;34(1):e26–9. doi: 10.1097/MPH.0b013e318228177f [DOI] [PubMed] [Google Scholar]
- 31.Erduran E, Tekelioğlu Y, Gedik Y, Yildiran A. Apoptotic effects of heparin on lymphoblasts, neutrophils, and mononuclear cells: results of a preliminary in vitro study. Am J Hematol. Jun 1999;61(2):90–3. doi: [DOI] [PubMed] [Google Scholar]
- 32.Manaster J, Chezar J, Shurtz-Swirski R, et al. Heparin induces apoptosis in human peripheral blood neutrophils. Br J Haematol. Jul 1996;94(1):48–52. doi: 10.1046/j.1365-2141.1996.6202063.x [DOI] [PubMed] [Google Scholar]
- 33.Bose P, Black S, Kadyrov M, et al. Heparin and aspirin attenuate placental apoptosis in vitro: implications for early pregnancy failure. Am J Obstet Gynecol. Jan 2005;192(1):23–30. doi: 10.1016/j.ajog.2004.09.029 [DOI] [PubMed] [Google Scholar]
- 34.Tamaru S, Kajihara T, Mizuno Y, et al. Heparin prevents oxidative stress-induced apoptosis in human decidualized endometrial stromal cells. Med Mol Morphol. Dec 2019;52(4):209–216. doi: 10.1007/s00795-019-00220-x [DOI] [PubMed] [Google Scholar]
- 35.Quan D, Li L, Zuo M. Efficacy of Low Molecular Heparin on Preeclampsia by Inhibiting Apoptosis of Trophoblasts via the p38MAPK Signaling Pathway. Comput Math Methods Med. 2021;2021:3337514. doi: 10.1155/2021/3337514 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.de Jesus-Silva SG, de Moraes Silva MA, Carbonel AAF, et al. Heparin Attenuates Visceral Apoptosis in a Swine Model of Hemorrhagic Shock and Reperfusion Injury. Ann Vasc Surg. Aug 2020;67:449–460. doi: 10.1016/j.avsg.2020.01.106 [DOI] [PubMed] [Google Scholar]
- 37.Benjamini Y, Speed TP. Summarizing and correcting the GC content bias in high-throughput sequencing. Nucleic Acids Res. May 2012;40(10):e72. doi: 10.1093/nar/gks001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller LR, Marks C, Becker JB, et al. Considering sex as a biological variable in preclinical research. Faseb j. Jan 2017;31(1):29–34. doi: 10.1096/fj.201600781R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arnegard ME, Whitten LA, Hunter C, Clayton JA. Sex as a Biological Variable: A 5-Year Progress Report and Call to Action. J Womens Health (Larchmt). Jun 2020;29(6):858–864. doi: 10.1089/jwh.2019.8247 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.