Abstract
Temporomandibular joint discs (TMJ discs) are unable to repair themselves in disease states, while induced stem cell differentiation is a common method to repair tissue defects. Nowadays, kinds of stem cells are attempted for tissue regeneration of TMJ disc, but these methods have several downsides, which limit their wide application. The proliferation and differentiation ability of human induced pluripotent stem cells (hiPSC) provides a new research direction for TMJ disc tissue regeneration. In this study, we investigated the feasibility of induced differentiation of hiPSC into TMJ disc cells in vitro and the differentiation efficiency of different methods to clarify the possibility and conditions of hiPSC application in TMJ disc tissue engineering. We collected sheep TMJ disc cells cultures for adding in hiPSC culture environment and treated hiPSC by both direct induction and Transwell co-culture for 7 days, 14 days and 21 days. The secretion of extracellular matrix in TMJ disc cells was detected by Sirius Red and Safranin O staining. Collagen Ⅰ and Collagen Ⅱ were qualitatively detected by immunohistochemical staining. The expression of extracellular matrix genes (type I collagen (COL1A1), type II collagen(COL2), glycosaminoglycan (GAG)), chondrogenic differentiation gene SOX9 and pluripotency gene OCT4 were detected by RT-qPCR. Our results showed that hiPSC had the ability to differentiate to TMJ disc cells by direct induction in TMJ disc cell culture medium and by Transwell co-culture method. The highest degree of differentiation was observed after 14 days of direct induction, while Transwell co-culture showed significant differentiation at different times and with different major directions. Meanwhile, Transwell co-culture not only differentiates hiPSC but also promotes the growth and proliferation of TMJ disc cells. Our study is valuable to investigate the possibility of differentiation of hiPSC toward TMJ disc cells and to determine the time of differentiation. It provides new ideas for the selection of seed cells for TMJ disc tissue engineering.
Keywords: Human induced pluripotent stem cells, Temporomandibular joint disc, Differentiation, In vitro
1. Introduction
Temporomandibular disorders (TMD) are the most common complex degenerative disorders of the maxillofacial region, the pathogenesis of which remains unknown [1]. Previous studies have shown that TMJ disc lesions are the first step in degenerative joint changes [2], with about 70 % of TMD patients presenting with abnormal disc position [3] and 5–15 % of patients with severe TMD presenting with disc perforation [4,5]. In contrast, the main component of TMJ disc is fibrocartilage [6], which cannot repair itself after injury due to the lack of nutritional support [7].
Tissue engineering provides a new approach to addressing the regeneration of TMJ discs [8,9]. The currently available TMJ disc tissue engineering seed cells are autologous chondrocytes, mesenchymal stem cells(MSCs), embryonic stem cells, and induced pluripotent stem cells(iPSCs). However, chondrocytes undergo dedifferentiation during in vitro passaging [10], and MSCs may have a progressive decrease in differentiation potential with age and alter cell phenotype during long-term passaging expansion [11]. The occurrence of embryonic stem cell teratomas and the immune reactions induced in living tissues have limited their application [12]. iPSCs have the advantages of vast source, strong differentiation ability, and individual specificity, and have shown promising applications in tissue engineering. For the application of iPSCs to TMJ disc tissue engineering is not clear, but it has been shown that synoviocytes and chondrocytes from TMJ osteoarthritis patients can be reprogrammed into iPSCs, which are then induced to differentiate into chondrocytes that deposit cartilage matrix [12]. It was shown that iPSCs could be successfully induced into chondrocyte-like cells after co-culturing iPSCs with primary articular chondrocytes under Transwell indirect co-culture conditions for 14 d. In addition, after injection of expanded and cultured iPSCs into the subcutaneous area of nude mice for 8–12 weeks, chondrocyte tissue formation could be observed under the microscope [13].
There is still a lack of effective clinical treatments for the repairing of tissue defects in TMJ disc lesions. TMJ discs are a tissue type that cannot repair itself in a damaged state. So far, the seed cells that have been found for tissue engineering are suboptimal. In that case, can hiPSC be applied to TMJ disc tissue engineering to achieve TMJ disc regeneration? This study was conducted to investigate the feasibility of differentiation of hiPSC toward TMJ disc cells and the efficiency of induced differentiation under different conditions, and to clarify the possibility and specific methods of hiPSC application in TMJ tissue engineering.
2. Materials and methods
2.1. Extraction and culture of sheep TMJ disc cells
With the approval of the Animal Ethics Committee of Lanzhou University (LZUKQ-2019-057), healthy fresh sheep heads aged 3–6 months were selected for this experiment. Sheep bilateral temporomandibular joint discs were isolated under aseptic conditions, clipped to 1 mm3 in 1 ml DMEM/F12 medium. The tissue blocks were digested with 7–8 ml type I collagenase in a constant temperature water bath shaker for 16 h (37 °C, 90 r/min), then DMEM/F12 culture solution was added to 10 ml and centrifuged at 1300 rpm for 13 min. After discarding the supernatant, add 10m1 DMEM/F12 medium and centrifuge at 1000 rpm for l0 minutes. Discard the supernatant, add 10 ml DMEM/FI2 medium, and centrifuge at 800 rpm for 8 min. Discard the supernatant, add complete culture medium (88 % DMEM/F12, 10 % FBS, 1 % Vitamin C, 1 % penicillin mixture), mix evenly, and inoculate cells to 25 cm2 sterile culture flasks in turn. The liquid volume of each flask was about 4–8 ml, and the first liquid changing occurred after 24 h. Change the medium liquid every other day until the primary cells grow to 80 % density of the field of view. 0.25 % Trypsin-EDTA was applied for digesting at 37 °C for 4 min, and the cells were passaged at a ratio of 1:4. The twice-generation sheep TMJ disc cells were used in this experiment.
2.2. Histological identification of sheep TMJ disc cells
After cell digestion and passaging, the cell suspension was gently added dropwise to a 20 × 20 mm sterile coverslip (about 1 × 104 cells) preplaced in a 6-well plate, and 1 ml of complete culture solution was gently added after 5h. When the cell density grew to 60 % of the field of view, the cells were rinsed 3 times with PBS buffer and fixed in paraformaldehyde at room temperature for 20 min. The cells were stained in the incubator for 40 min to detect the glycosaminoglycans with Safranin O staining. Meanwhile, the cells were stained in the incubator for an hour to detect the distribution of collagen fibers with Sirius Red staining. After staining, the PBS buffer was rinsed until the background was clean.
2.3. Culture of hiPSC and strategies of induced differentiation of hiPSC toward TMJ disc cells
The hiPSC lines (hNF-C1) were generated from human skin fibroblasts via Sendai virus, which was gifted by the Guangzhou Institutes of Biomedicine and Health (Guangzhou, China). hiPSC were cultured on Matrigel (DPBS buffer diluted at 1:80) coated 6-well plates, washed daily with DPBS buffer, and then fed by E8 medium. When the cells grew to 80 % in the field of view, they were digested with 0.5 mM EDTA for 3–4 min and then passaged at a ratio of 1:6.
Direct induced differentiation: sheep TMJ disc cell cultures were collected at the logarithmic growth phase and filtered through a 0.22 μm filter as induction conditioned medium for hiPSC differentiation. When hiPSC grew to 60%–70 % of the field of view in 6-well plates, they were washed twice with DPBS buffer, 2 ml of complete culture medium, and 1 ml of filtered conditioned medium were added to each well. FBS was added to bring the concentration to 15 %, and the medium was changed every other day and incubated for 7 days, 14 days, and 21 days respectively.
Transwell indirect co-culture induces hiPSC differentiation: TMJ disc cells were inoculated 24 h in advance at 1 × 104 per well in sterile 0.4 μm Transwell chambers. hiPSCs were cultured in E8 culture medium until the clonal mass grew to 60%–70 % of the field of view and co-cultured indirectly with TMJ disc cells.2 ml of complete culture solution was added to each well in the upper and lower chambers. FBS was added to the lower chamber to make the concentration 15 %, and the solution was changed every other day and cultured for 7 days, 14 days and 21 days respectively.
2.4. Histological staining (Sirius Red and Safranin O)
After 7 days, 14 days and 21 days of hiPSC culture respectively, 0.25 % trypsin-EDTA was used for digestion for 5–6 min. After full-blown and resuspension of cells, inoculate the cell suspension on sterile 20 × 20 mm coverslips (about 1 × 104 cells) preplaced in 6-well plates. When the cell density grew to 60 % of the field of view, the cells were rinsed 3 times with PBS buffer and fixed in paraformaldehyde at room temperature for 20 min. The cells were stained in the incubator for 40 min to detect the glycosaminoglycans with Safranin O staining. Meanwhile, the cells were stained in the incubator for an hour to detect the distribution of collagen fibers with Sirius Red staining. After staining, PBS buffer was used to rinse until the background was clean.
2.5. Immunohistochemical staining of collagen Ⅰ and collagen Ⅱ
After 7 days, 14 days and 21 days of hiPSC culture respectively, 0.25 % trypsin-EDTA was used for digestion for 5–6 min. After full blown and resuspension of cells, inoculate the cell suspension on sterile 20 × 20 mm coverslips (about 1 × 104 cells) preplaced in 6-well plates. When the cell density grew to 60 % of the field of view, the cells were rinsed 3 times with PBS buffer and fixed in paraformaldehyde at room temperature for 20 min. The fixed cell crawls were washed 3 times with PBS buffer, treated with 0.2 % TritonX 100 to break the membrane, washed 3 times with PBS buffer, and closed with 5 % bovine serum albumin (BSA) for 20 min. After being washed 3 times with PBS buffer, they were incubated with anti-collagen I rabbit polyclonal antibody for 12 h at 40 °C, washed 3 times with PBS buffer and added with polymerized HRP-labeled anti-rabbit IgG. The condition of this process was incubation for 30 min at 37 °C. Samples were washed 3 times with PBS buffer and developed with DAB color developer for 5 min. Tap water was used to rinse thoroughly the cells, which were observed and photographed under an inverted microscope camera. The same staining method as the above step was performed with mouse anti-collagen II mouse monoclonal antibody.
2.6. Proliferation of sheep TMJ disc cells under indirect Transwell co-culture with hiPSC
Sheep TMJ disc cells were inoculated on 6-well plates at a density of 5 × 104. The supernatant was collected from the hiPSC culture fluid and added to the cultural environment of sheep TMJ disc cells. The amount of liquid was half of the complete medium. Another group of cells was added an equal amount of E8 medium. At the same time, sheep TMJ disc cells were Transwell co-cultured with hiPSC. The cell proliferation was measured after 24 h, 48 h, 72 h, 96 h and 120 h of culturing using the cell counting kit- 8 assay (CCK-8, Dojindo, Japan) according to the manufacturer's instructions. Six parallel wells were used for each group.
2.7. Detection of differentiated gene expression by RT-qPCR
The samples for RT-qPCR included cells obtained from direct induction and Transwell co-culture for 7 days, 14d days and 21 days. The mRNA was extracted and reverse transcribed to cDNA using the mRNA extraction kit and mRNA reverse transcription kit (Vazyme, America) instructions. Type I Collagen (COL1A1), type II Collagen (COL2), glycosaminoglycan (GAG), chondrogenic differentiation gene SOX9, and pluripotency gene OCT4 were detected by setting up the RT-qPCR reactions utilizing SYBR (Vazyme, America) in a quantitative RT-qPCR instrument (Rotor-Gene Q, Germany). In this experiment, we chose the following genes as indicators of the degree of cell differentiation: COL 1, COL2, GAG, SOX9, and OCT4. Among them, COL1, COL2, and GAG are the main components of the extracellular matrix of TMJ disc, which is divided into two directions of differentiation: fibroblasts and cartilage. When the expression of COL1 and GAG increased, we thought that IPSC differentiated towards fibroblasts. When the expression of COL2 is increased, we consider that IPSC differentiates towards chondrocytes. SOX9 is a key transcription factor in the process of chondrogenesis, which plays an important role in the proliferation and differentiation of chondrocytes by regulating the expression of a series of downstream factors in chondrogenic progenitor cells and articular chondrocytes in a stage-specific manner. OCT4 gene is considered to be a cellular pluripotency gene. Stem cells are pluripotent and therefore have a high expression of OCT4 gene, whereas after cell differentiation, cells lose their original ability to differentiate in multiple directions and the expression of OCT4 decreases. Therefore, after inducing IPSC differentiation we performed OCT4 expression assay to investigate whether IPSC had been differentiated. The primer sequences used in the experiment was listed below (Table 1).
Table 1.
Primers used in real-time RT-PCR.
| gene | primer sequences 5′-3′ | primer sequences 3′-5′ |
|---|---|---|
| COL1A1 | TGGCAAAGATGGACTCAACG | TCACGGTCACGAACCACATT |
| COL2 | CAGATGACCTTCCTACGCCT | TCTTCTGTGACCGGTACTCG |
| GAG | TCCGCTGGTCTGATGGACAC | CCAGATCATCACTACGCAGTC CTC |
| SOX9 | CCCCAACAGATCGCCTACAG | GAGTTCTGGTCGGTGTAGTC |
| OCT4 | CCTCACTTCACTGCACTGTA | CAGGTTTTCTTTCCCTAGCT |
| ACTB | CCCAGAGCAAGAGAGG | GTCCAGACGCAGGATG |
2.8. Statistical analysis
Statistical analyses were conducted using SPSS 20.0 (IBM) software. All the data were represented as mean ± standard deviation (SD). Normality test and homogeneity test of variance were first conducted. If the data conformed to normal distribution and did not conform to homogeneity of variance, which test was used for population mean comparison and Dunnett T3 test was used for pair-to-pair comparison between groups; If the data does not conform to normal distribution and homogeneity of variance, the Mann-Whitney U test was used for pair-to-pair comparison between groups. A p-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Cell morphology and histological staining of sheep TMJ disc cells
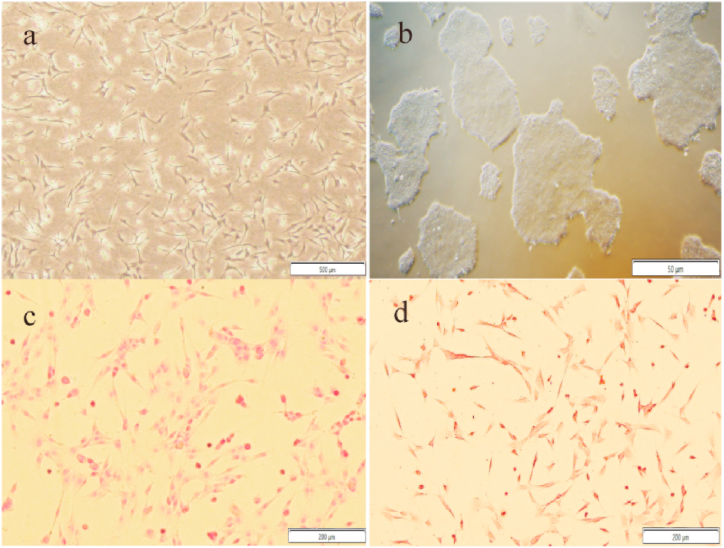
Sheep TMJ disc cells are mixed cells, mainly consisting of long spindle-shaped fibroblasts and polygonal or round chondrocytes, with single-cell growth features and a star network distribution (Fig. 1a). hiPSCs are more tightly connected and have a nest-like shape (Fig. 1b). The results of Sirius Red and Safranin O staining of sheep TMJ disc cells showed that the cells were stained pink and the cell nucleus was darker than the cytoplasmic staining. This histological staining indicated that the extracellular matrix(ECM) of sheep TMJ disc cells was rich in collagen fibers and glycosaminoglycans (Fig. 1c and d).
Fig. 1.
Cell morphology of sheep TMJ disc cells(a) and hiPSC(b). Cell samples were stained with Sirius Red (c, d). Scale bars, 500 μm(a), 50 μm(b), and 200 μm (c, d). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.2. Morphological observation and staining results of hiPSC after induction of differentiation
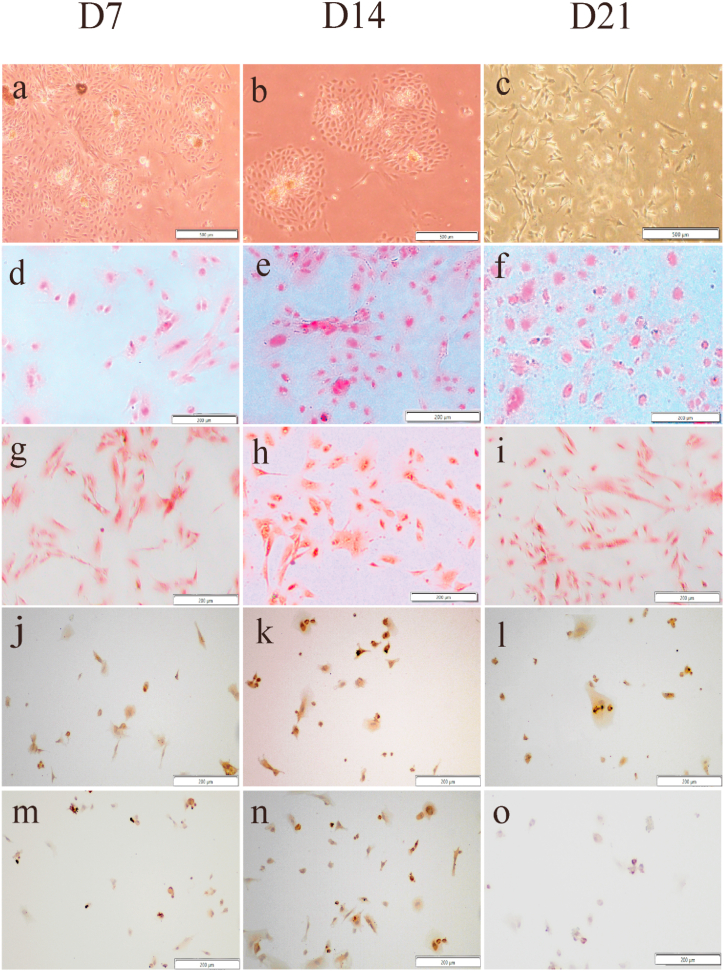
After direct induction and Tranwell co-culture for 7 days, 14 days and 21 days, hiPSC changed from aggregated growth to single-cell growth, and a stellate reticular field similar to that of sheep TMJ disc cells with long spindle and polygonal cell morphology could be seen (Fig. 2 a-c and Fig. 3 a-c). Sirius Red and Safranin O staining showed that these cells could be stained pink after induced differentiation, and the cell nucleus was stained darker than the cytoplasm. Collagen fibers and glycosaminoglycans appeared in the ECM of induced cells, and the staining results indicated that hiPSC had differentiated toward TMJ disc cells (Fig. 2 d-i and Fig. 3 d-i). In the immunohistochemical staining results, Collagen Ⅰ was positive 7 days, 14 days and 21 days after direct induction. In the Transwell co-culture condition, positive Collagen Ⅰ was observed at the time of 14 days and 21 days (Fig. 2 j-l, Fig. 3 k-l). However, Collagen II was positive at 14 days after direct induction (Fig.s. 2n), 7 and 14 days after indirect co-culture (Fig. 3m and n), compared than that of 7 days and 21 days after in direct induction (Fig. 2m and o) and 21 days in indirect co-culture (Fig. 3o).
Fig. 2.
Cell morphology and histological staining results of direct induction. The hiPSCs were induced in conditioning medium for 7 days (Fig.s. 2a), 14 days (Fig. 2b) and 1days (Fig. 2c). Cell samples were stained with Sirius Red (Fig. 2 d-f) and Safranin O (Fig. 2 g-i). Collagen Ⅰ (Fig. 2 j-l) and collagen Ⅱ (Fig. 2 m-o) were detected by immunohistochemical staining. Scale bars, 500 μm(a-c) and 200 μm(d-o). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
Cell morphology and histological staining results of Transwell co-culture induction. The hiPSCs were co-cultured with sheep TMJ disc cells conditioning medium for 7 days (Fig.s. 3a),14 days (Fig. 3b) and 21days (Fig. 3c). Cell samples were stained with Sirius Red (Fig. 3 d-f) and Safranin O (Fig. 3 g-i). Collagen Ⅰ (Fig. 3 j-l) and collagen Ⅱ (Fig. 3 m-o) were detected by immunohistochemical staining. Scale bars, 500 μm(a-c) and 200 μm(d-o). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.3. Proliferation of sheep TMJ disc cells under indirect Transwell co-culture with hiPSC
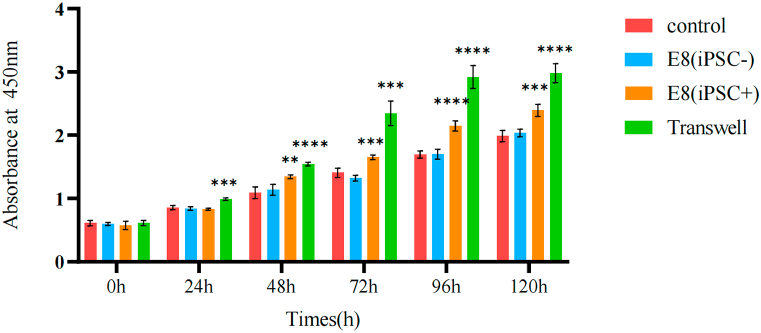
The results (Fig. 4) showed that the addition of E8 medium merely had essentially no effect on the proliferation of sheep TMJ disc cells. After the addition of supernatant from the culture medium of hiPSC, the proliferation status of sheep TMJ disc cells increased after 48 h compared with the regular culture group with increasing culture time. In contrast, the proliferation status of sheep TMJ disc cells increased after 24 h compared with the regular culture group under indirect Transwell co-culture conditions with hiPSC.
Fig. 4.
Cell proliferation capacity of sheep TMJ disc cells in culture mediums. The viability of sheep TMJ disc cells at 24-h intervals over a five-day culture period using a CCK8 regent at a 450 nm wave length. ** indicates p<0.01,*** indicates p<0.001,**** indicates p<0.0001 vs control group.
3.4. Detection of mRNA expression levels of differentiated genes under different conditions and induction times using RT-qPCR
To evaluate the effect of different induction conditions and time on hiPSC differentiation, the relative expression levels of COL1A1, COL2, GAG, SOX9 and OCT4 were examined using RT-qPCR. Compared with hiPSC, COL1A1 mRNA expression levels were significantly increased at 7 days, 14 days and 21 days with direct induction and 14 days and 21 days of co-culture, with the highest levels at 14 days with both direct induction and co-culture (Fig. 5a). COL2 mRNA expression levels were significantly increased at 14 days with direct induction and 7 days and 14 days with co-culture, while the highest level appearing at 14 days with co-culture (Fig. 5b). GAG mRNA expression levels were significantly increased at 7 days, 14 days and 21 days with direct induction and 14 days and 21 days with co-culture, with the highest levels at 14 days of both direct induction and co-culture (Fig. 5c). SOX9 mRNA expression levels were significantly increased at 7 days, 14 days of direct induction and 7 days, 14 days and 21 days of co-culture. The highest level showed up at 14 days with both of direct induction and co-culture (Fig. 5c). The expression level of OCT4 mRNA significantly decreased after direct induction and co-culture at 7 days, 14 days, and 21 days (Fig. 5e). The above results showed that hiPSC pluripotency was significantly reduced under different induction conditions and time. Significant differentiation was observed at each detected time, but co-culture was more efficient than direct induction differentiation at the same differentiation time. The highest differentiation efficiency was observed at 14 days during direct induction, with significant direction toward both fibroblasts and chondrocytes. The chondrocyte direction was predominant at 7 days under co-culture conditions. The highest differentiation efficiency was observed at 14 days under co-culture condition, with significant differentiation in both directions.
Fig. 5.
The relative expression levels of COL1A1(a), COL2(b), GAG(c), SOX9(d) and OCT4(e)conditioning medium was detected using RT-qPCR. The expression of these genes in hiPSCs before differentiation was standardized to 1. (# indicates p<0.05, ## indicates p<0.01, ### indicates p<0.001, #### indicates p<0.0001, ns indicates no statistical difference vs. hiPSC; * indicates p<0.05, ** indicates p<0.01, *** indicates p<0.001, **** indicates p<0.0001).
4. Discussion
TMJ disc, the joint structure between the mandible and the temporal bone, is associated with 70 % of TMD development. Because of the lack of self-nutritional support, the TMJ disc cannot repair itself after suffering from injury. In previous tissue engineering studies for TMJ disc regeneration, there are still some obstacles regarding cell sources, scaffold materials and growth factor modulation. Many problems with the TMJ disc tissue engineering seed cells have been identified so far. In recent years, research on MSCs has expanded to include bone marrow [14], adipose [15], and synovial fluid [16], and studies on the application of various MSCs to repair TMJ disc defects are continuously increasing. However, some unresolved limitations remain, such as the low amount of healthy tissue for bone marrow and synovial synovial MSCs and the high requirements for cytokine during the chondrogenic differentiation process of adipose MSCs [17]. iPSCs have vital accessibility and the ability to proliferate and differentiate, which can be induced to differentiate into components of TMJ disc cells. Therefore, the application of iPSCs in TMJ disc tissue engineering was taken as a highlight in this experiment to explore the specific conditions and methods. Sheep are common animal model species for TMJ tissue engineering, with reasonable cost and similar shape, size and function to human TMJ. Therefore, this experiment used young sheep TMJ disc cells and hiPSC as co-culture subjects.
Under the conditions of the cell co-culture method, different types of cells are grown in the same cultural environment, and cell-to-cell interactions are achieved through direct and indirect contact. Previous studies have found that cells in Transwell co-culture condition can provide differentiation regulatory signals to another type of cells through the production of growth factors [18]. In this study, we collected sheep TMJ disc cell cultures as the conditioned medium for hiPSC differentiation. Direct induction and Transwell co-culture methods were used to induce hiPSC differentiation by cytokines secreted by TMJ disc cells. The differentiation ability and efficiency of hiPSC under different conditions were investigated. The results showed that the cell morphology and growth characteristics of hiPSC were very similar to TMJ disc cells after direct induction and co-culture by TMJ disc cell culture medium. In the meantime, hiPSC expressed TMJ disc cell-specific ECM (collagen fibrils and glycosaminoglycans) after induction. RT-qPCR results showed that COL1A1, COL2, GAG, and SOX9 gene expression was highest at 14 days of direct induction, and the overall differentiation efficiency was best at the fourteenth day. Transwell co-culture was dominated by chondrogenic direction differentiation at 7 days and both directions at 14 days. The overall differentiation in co-culture condition was higher than the direct induction group. This is in agreement with the results of immunohistochemical staining. Expression of SOX9 improved with increasing induction time. However, since the relative expression of COL2 mRNA was not significantly found at 21 days of co-culture, it cannot be assumed that hiPSC differentiated in the chondrocyte direction under this induction condition. Because hiPSC may have differentiated in other directions, such as osteogenesis and lipogenesis at that time, further investigation is needed. The proliferative capacity of sheep TMJ disc cells were elevated compared to conventional culture during the co-culture time of 24–120 h with hiPSC. Similarly, the addition of supernatant from hiPSC culture fluid also promoted the growth of sheep TMJ disc cells. In contrast, the proliferation capacity of sheep TMJ disc cells was not significantly altered by the addition of regular hiPSC medium to the sheep TMJ disc cell culture medium. This suggests that the process of hiPSC growth metabolism may produce some growth factors that can promote the proliferation of sheep TMJ disc cells. In other words, our indirect co-culture model of sheep TMJ disc cells with hiPSC established by Transwell is reasonable.
The present study has some limitations since the changes in ECM protein levels under various induction conditions were not explored. In addition, previous studies have confirmed that TMJ disc can regulate endogenous cell differentiation through growth factors such as BMP, TGF-β, FGF, and IGF [[19], [20]], so we should further explore the regulatory mechanism of iPSCs differentiation toward TMJ disc cells.
5. Conclusion
In summary, the possibility of differentiation of hiPSC to TMJ disc cells was clarified by both direct induction of culture medium collected from TMJ disc cells and co-culture by Transwell. By histological staining and RT-qPCR differentiation gene expression assay after 7 days, 14 days and 21 days of culture, 14 days of co-culture was taken as the optimal induction time. This is because there is a clear differentiation of hiPSC toward both fibroblasts and chondrocytes at this time. Furthermore, hiPSC differentiated significantly toward chondrocytes at 7 days of Transwell co-culture, so this condition can be used as the induction condition for chondrogenic differentiation. Our results are an important guideline for the induction of hiPSC differentiation toward TMJ disc cells under in vitro conditions and provide a new approach for repairing tissue defects in TMJ disc. Surprisingly, Transwell co-culture not only differentiates hiPSC but also promotes the growth and proliferation of TMJ disc cells. In the future, we will add protein level assay and change the induction conditions to explore further and provide new strategies for the applying of iPSCs in TMJ disc tissue engineering. However, this study is still in the exploratory stage of the application of hiPSC to TMJ disc tissue engineering. In future studies, we can add the protein level assays of collagen and glycosaminoglycan in the extracellular matrix of TMJ disc as well as the cell proliferation ability assay, combined with the gene expression to comprehensively evaluate the differentiation efficiency and direction of hiPSC under different induction conditions.
6. Data availability statements
The data generated in the present study are included in the figures and tables of this article.
7. Ethics approval and consent to participate
Not applicable.
CRediT authorship contribution statement
Yiqing Zhao: Writing – original draft, Data curation, Conceptualization. Ce Li: Writing – review & editing, Formal analysis, Data curation. Siyang Hu: Writing – review & editing, Formal analysis, Data curation. Chunya Wang: Methodology, Investigation. Xueru Bian: Methodology, Investigation. Hong Kang: Writing – review & editing, Project administration, Funding acquisition, Conceptualization. Ping Zhou: Visualization, Software, Resources. Guangjie Bao: Writing – review & editing, Resources, Funding acquisition, Formal analysis.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Hong Kang reports article publishing charges and equipment, drugs, or supplies were provided by Lanzhou University. Guangjie Bao reports equipment, drugs, or supplies was provided by Northwest Minzu University. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Natural Science Foundation of Gansu Province (21JR7RA161) and the Science and Technology Program of Gansu Province (22YF7GA005).
Contributor Information
Hong Kang, Email: kanghong@lzu.edu.cn.
Ping Zhou, Email: zhoup@lzu.edu.cn.
Guangjie Bao, Email: yxbgj@xbmu.edu.cn.
References
- 1.Shu W., Liu L., Bao G., Kang H. Tissue engineering of the temporomandibular joint disc: current status and future trends. Int. J. Artif. Organs. 2015;38(2):55–68. doi: 10.5301/ijao.5000393. [DOI] [PubMed] [Google Scholar]
- 2.Matuska A.M., Muller S., Dolwick M.F., McFetridge P.S. Biomechanical and biochemical outcomes of porcine temporomandibular joint disc deformation. Arch. Oral Biol. 2016;64:72–79. doi: 10.1016/j.archoralbio.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solberg W.K., Woo M.W., Houston J.B. Prevalence of mandibular dysfunction in young adults. J. Am. Dent. Assoc. 1979;98(1):25–34. doi: 10.14219/jada.archive.1979.0008. [DOI] [PubMed] [Google Scholar]
- 4.Muñoz-Guerra M.F., Rodríguez-Campo F.J., Escorial Hernández V., Sánchez-Acedo C., Gil-Díez Usandizaga J.L. Temporomandibular joint disc perforation: long-term results after operative arthroscopy. J. Oral Maxillofac. Surg. : Official Journal of the American Association of Oral and Maxillofacial Surgeons. 2013;71(4):667–676. doi: 10.1016/j.joms.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Kuribayashi A., Okochi K., Kobayashi K., Kurabayashi T. MRI findings of temporomandibular joints with disk perforation. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2008;106(3):419–425. doi: 10.1016/j.tripleo.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 6.Aryaei A., Vapniarsky N., Hu J.C., Athanasiou K.A. Recent tissue engineering advances for the treatment of temporomandibular joint disorders. Curr. Osteoporos. Rep. 2016;14(6):269–279. doi: 10.1007/s11914-016-0327-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Undt G., Jahl M., Pohl S., Marlovits S., Moser D., Yoon H.-H., Frank J., Lang S., Czerny C., Klima G., Gentleman E., Ewers R. Matrix-associated chondrocyte transplantation for reconstruction of articulating surfaces in the temporomandibular joint: a pilot study covering medium- and long-term outcomes of 6 patients. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology. 2018;126(2):117–128. doi: 10.1016/j.oooo.2018.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francis S.L., di Bella C., Wallace G.G., Choong P.F.M. Cartilage tissue engineering using stem cells and bioprinting technology-barriers to clinical translation. Frontiers in Surgery. 2018;5:70. doi: 10.3389/fsurg.2018.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandes H.R., Gaddam A., Rebelo A., Brazete D., Stan G.E., Ferreira J.M.F. Bioactive glasses and glass-ceramics for healthcare applications in bone regeneration and tissue engineering. Materials. 2018;11(12) doi: 10.3390/ma11122530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benya P.D., Padilla S.R., Nimni M.E. Independent regulation of collagen types by chondrocytes during the loss of differentiated function in culture. Cell. 1978;15(4):1313–1321. doi: 10.1016/0092-8674(78)90056-9. [DOI] [PubMed] [Google Scholar]
- 11.Payne K.A., Didiano D.M., Chu C.R. Donor sex and age influence the chondrogenic potential of human femoral bone marrow stem cells. Osteoarthritis Cartilage. 2010;18(5):705–713. doi: 10.1016/j.joca.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi Kazutoshi, Tanabe Koji, Ohnuki Mari, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi Kazutoshi, Yamanaka Shinya. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 14.Tetè G., D'Orto B., Nagni M., Agostinacchio M., Polizzi E., Agliardi E. Role of induced pluripotent stem cells (IPSCS) in bone tissue regeneration in dentistry: a narrative review. J. Biol. Regul. Homeost. Agents. 2020;34(6 Suppl. 3):1–10. [PubMed] [Google Scholar]
- 15.Chen K., Man C., Zhang B., Hu J., Zhu S.S. Effect of in vitro chondrogenic differentiation of autologous mesenchymal stem cells on cartilage and subchondral cancellous bone repair in osteoarthritis of temporomandibular joint. Int. J. Oral Maxillofac. Surg. 2013;42(2):240–248. doi: 10.1016/j.ijom.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 16.Wu L., Cai X., Zhang S., Karperien M., Lin Y. Regeneration of articular cartilage by adipose tissue derived mesenchymal stem cells: perspectives from stem cell biology and molecular medicine. J. Cell. Physiol. 2013;228(5):938–944. doi: 10.1002/jcp.24255. [DOI] [PubMed] [Google Scholar]
- 17.Huang Y.-Z., Xie H.-Q., Silini A., Parolini O., Zhang Y., Deng L., Huang Y.-C. Mesenchymal stem/progenitor cells derived from articular cartilage, synovial membrane and synovial fluid for cartilage regeneration: current status and future perspectives. Stem Cell Reviews and Reports. 2017;13(5):575–586. doi: 10.1007/s12015-017-9753-1. [DOI] [PubMed] [Google Scholar]
- 18.Hennig T., Lorenz H., Thiel A., Goetzke K., Dickhut A., Geiger F., Richter W. Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGFβ receptor and BMP profile and is overcome by BMP-6. J. Cell. Physiol. 2007;211(3):682–691. doi: 10.1002/jcp.20977. [DOI] [PubMed] [Google Scholar]
- 19.Goers L., Freemont P., Polizzi K.M. Co-culture systems and technologies: taking synthetic biology to the next level. J. R. Soc., Interface. 2014;11(96) doi: 10.1098/rsif.2014.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Acri T.M., Shin K., Seol D., Laird N.Z., Song I., Geary S.M., Chakka J.L., Martin J.A., Salem A.K. Tissue engineering for the temporomandibular joint. Adv. Healthcare Mater. 2019;8(2) doi: 10.1002/adhm.201801236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated in the present study are included in the figures and tables of this article.