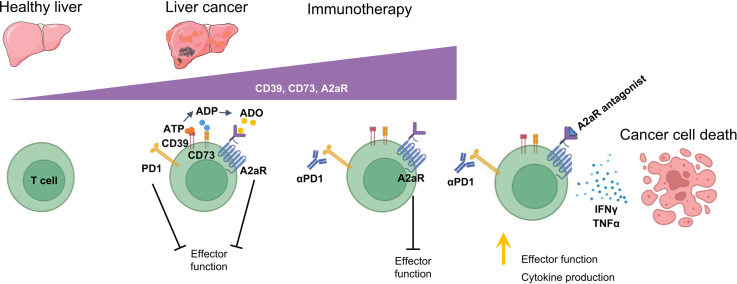
Abstract
Backgrounds & Aims
The efficacy of immune checkpoint inhibitor (ICI) therapy for liver cancer remains limited. As the hypoxic liver environment regulates adenosine signaling, we tested the efficacy of adenosine A2a receptor (A2aR) inhibition in combination with ICI treatment in murine models of liver cancer.
Methods
RNA expression related to the adenosine pathway was analyzed from public databases. Peripheral blood mononuclear cells of 13 patients with hepatocellular carcinoma (HCC) were examined by flow cytometry. The following murine cell lines were used: SB-1, RIL175, and Hep55.1c (liver cancer), CT26 (colon cancer), and B16–F10 (melanoma). C57BL/6 and BALB/c mice were used for orthotopic tumor models and were treated with SCH58261, an A2aR inhibitor, in combination with anti-PD1 therapy.
Results
RNA expression of ADORA2A in tumor tissues derived from patients with HCC was higher than in tissues from other cancer types. A2aR+ T cells in peripheral blood from patients with HCC were highly proliferative after immunotherapy. Likewise, in an orthotopic murine model, A2aR expression on T cells increased following anti-PD1 treatment, and the expression of A2aR on T cells increased more in tumor-bearing mice compared with tumor-free mice. The combination of SCH58261 and anti-PD1 led to activation of T cells and reductions in tumor size in orthotopic liver cancer models. In contrast, SCH58261 monotherapy was ineffective in orthotopic liver cancer models and the combination was ineffective in the subcutaneous tumor models tested. CD4+ T-cell depletion attenuated the efficacy of the combination therapy.
Conclusion
A2aR inhibition and anti-PD1 therapy had a synergistic anti-tumor effect in murine liver cancer models.
Impact and implications
Adenosine A2a receptor (A2aR)-expressing T cells in the liver increased in tumor-bearing mice and after anti-PD1 treatment. The combination of an A2aR inhibitor and anti-PD1 treatment had potent anti-tumor effects in two murine models of orthotopic liver cancer. Adenosine A2a receptor blockade promotes immunotherapy efficacy in murine models, highlighting putative clinical benefits for advanced stage liver cancer patients.
Keywords: Liver cancer, immunotherapy, adenosine receptor
Graphical abstract
Highlights
-
•
Adenosine-related genes are highly expressed in patients with liver cancer.
-
•
A2aR expression on T cells increases in tumor-bearing and ICI-treated mice.
-
•
A2aR blockade enhances the anti-tumor effect of ICIs.
Introduction
Liver cancer is the sixth most common cancer and the third leading cause of cancer-related death worldwide.1 There are two main types of primary liver cancer: cholangiocarcinoma and hepatocellular carcinoma (HCC). The most common type of liver tumor occurs due to metastasis from primary tumors outside the liver2 and these metastatic cancers often do not respond well to immune checkpoint inhibitor (ICI) therapy.3,4 While ICI therapy has become the standard of care for the treatment of primary liver cancer, the efficacy of mono-therapeutic agents remains limited.[5], [6], [7] Hypoxia, which is a well-known characteristic of liver cancer, likely contributes to poor ICI therapy outcomes.8 There are several factors contributing to hypoxia in liver tumors including: anatomy with the portal vein bringing 70% of the blood supply to the liver,9,10 fibrosis which impairs blood perfusion, and treatment-induced hypoxia with therapies such as trans-arterial chemoembolization (TACE).
A2a adenosine receptor (A2aR) antagonists represent an interesting tool to enhance anti-tumor immunity, especially in the context of ICI therapy and hypoxia.11 The A2aR has been shown to prevent excessive collateral tissue damage by overactive T cells and myeloid cells in non-infected but inflamed normal tissues of vital organs.12 In addition, A2aR antagonists have long been proposed as a therapeutic tool to unleash tumor-reactive T and natural killer (NK) cells.12,13 More recently Fong and colleagues described encouraging observations of tumor regression, disease control, and survival of patients with otherwise refractory renal cell cancer with progressive disease after treatment with an A2aR antagonist.14
Adenosine, the natural ligand for A2aR, is generated from extracellular ATP through the activity of CD39 and CD73.15 Modulating CD3916,17 and CD7318,19 have also been investigated to improve T cell function in cancer.20
Targeting the adenosine-A2aR-cAMP axis in combination with immunotherapy has been reported to enhance the anti-tumor effect in preclinical models. Indeed, the blockade of CD73 enhances the efficacy of anti-programmed death 1 (PD1) and anti-cytotoxic T-lymphocyte antigen 4 (CTLA4) treatment in subcutaneous tumor and chemically induced tumor models.21 The co-blockade of CD73 and A2aR inhibition has shown synergistic anti-tumor effects with A2aR considered a putative immunomodulatory molecule.22
In this study, we examined adenosine-related gene expression in liver cancer and investigated the efficacy of an A2aR inhibitor in preclinical murine liver cancer models.
Materials and methods
Patients’ PBMCs and flow cytometry analysis
Peripheral blood mononuclear cells (PBMCs) from 13 patients were used for analyses. All patients were enrolled in a clinical trial at the NIH evaluating anti-CTLA4 therapy (tremelimumab) (NCT01853618). Clinical information is provided in Table 1. PBMCs were isolated from the blood as previously described.23 Briefly, blood was gradient-centrifuged using a lymphocyte separation medium (Ficoll Lonza), and the intermediate PBMC layer was frozen and stored in liquid nitrogen. All patients provided written informed consent, and the study design was consistent with the principles of the Helsinki Declaration. Multicolor flow cytometry was performed to study different immune cell subsets using the following antibodies (clone, manufacture): anti-CD19-BV421 (HIB19, BioLegend), anti-CD11c-BV510 (B-ly6, BD), anti-HLA-DR-BV785 (L243, BioLegend), anti-CD16-AF488 (3G8, BioLegend), anti-CD11b-PerCPCy5.5 (ICRF44, BioLegend), anti-CD3-AF700 (UCHT1, BD), anti-CD3-PECy7 (UCHT1, BD), anti-CD14-APC (M5E2, BD), anti-CD33-AF700 (WM53, BD), anti-CD56-APCFire750 (QA17A16, BioLegend), anti-FoxP3-PacificBlue (259D, BioLegend), anti-CD8-BV510 (SK1, BD), anti-CD45RA-BV650 (HI100, BioLegend), anti-CD4-BV785 (RPA- T4, BioLegend), anti-CCR7-FITC (150503, BD), anti-PD1-PE (EH12.1, BD), anti-CTLA4-PECy7 (L3D10, BioLegend), anti-Ki67-APC (Ki67, BioLegend),anti-CD39-BV605(A1, BioLegend), anti-CD73-PEDazzle594(AD2, BioLegend), and anti-A2A R-PE (7F6-G5-A2 Novus). Data for all samples were collected on a CytoFLEX LX flow cytometer (Beckman Coulter CytoFLEX Flow Cytometer) and analyzed using FlowJo software version 10.4.2 (BD Biosciences).
Table 1.
Patient characteristics (n = 13).
| Number | |
|---|---|
| Age, median (range) | 59 (42-76) |
| Sex | |
| Male | 11 |
| Female | 2 |
| Cirrhosis | |
| Yes | 10 |
| No | 3 |
| Etiology | |
| HBV | 4 |
| HCV | 5 |
| Non-B, non-C | 4 |
| Baseline Child-Pugh score | |
| 5 | 8 |
| 6 | 1 |
| 7 | 1 |
| BCLC stage | |
| B | 7 |
| C | 6 |
| Extrahepatic disease | |
| Yes | 3 |
| No | 10 |
| Prior sorafenib | |
| Yes | 6 |
| No | 7 |
Mice and cell lines
Six-to 10-week-old C57BL/6NCrl or BALB/C female mice were purchased from Charles River. The following murine tumor cell lines were used: Hep55.1c and RIL175 (HCC),24,25 SB1 (cholangiocarcinoma),26 CT26 (colon carcinoma)24 as well as B16–F10 (melanoma).25 Cells were cultured in RPMI 1640 GlutaMax with 10% fetal calf serum, ampicillin, and streptomycin. MTT assay (ab211091, Abcam) was performed according to the manufacturer’s protocol.
Mouse procedures and antibody treatment
Intrahepatic tumor injection was performed as described previously.27 In brief, 2.0x105 cells were resuspended in 20 ul of PBS mixed with Matrigel (Corning) and injected into the liver. A subcutaneous tumor was induced by injecting 1.0x106 cells into the left flank of each mouse as previously described.23 Treatment was initiated 5 days post tumor cell injection. The following antibodies were injected intraperitoneally into mice at a dose of 200 μg/mouse at the days indicated in the figures; anti-PD1 (clone 29F.1A12, BioXCell), anti-CD4 (clone GK1.5; BioXCell), and anti-CD8 (clone 2.43; BioXCell). The A2aR inhibitor (SCH58261, Sigma) was administered intraperitoneally at a concentration of 1 mg/kg body weight as indicated. All experiments were conducted according to local institutional guidelines and approved by the NIH Animal Care and Use Committee in Bethesda, MD.
Isolation of murine lymphocytes and flow cytometry
Lymphocytes from the liver and spleen of mice were isolated as previously described23 and analyzed by multicolor flow cytometry. Flow cytometry was performed on a CytoFLEX LX platform and results were analyzed using FlowJo software. The following antibodies were used in this study (clone, manufacture): anti-F4/80-Alexa Fluor 700 (BM8, BioLegend), anti-B220-Alexa Fluor 700 (RA3-6B2, BioLegend), anti-Cd11b-Alexa Fluor 700 (M1/70, BioLegend), anti-Cd3-Alexa Fluor 594 (17A2, BioLegend) anti-Cd4-BV605 (GK1.5, BioLegend), anti-Cd8-BV786 (53-6.7, BD), anti-Foxp3-BV421 (MF-14, BioLegend), anti-Cd62l-PerCP/Cy5.5 (MEL-14, BioLegend), anti-Cd44-BV510 (IM7, BioLegend), anti-NK1.1-BV510 (PK136, BioLegend), anti-A2Ar-FITC (7F6-G5-A2, NOVUS), anti-Cd73-APC (TY11.8, BioLegend), anti-Cd39-PECy7 (Duha59, BioLegend), anti-Cd19-PerCP Cy5.5(6D5, BioLegend), anti-PD1-APC/Cy7 (29F.1A12, BioLegend), anti-Cd107a- PE (1D4B, BD), anti-CD69-BV650 (H1.2F3, BioLegend), anti-Ifnγ-BV421 (XMG1.2, BioLegend), anti-Tnfα-PerCPCy5.5 (MP6-XT22, BioLegend), anti-GZMB-FITC (GB11, BioLegend), and anti-perforin-APC (S16009A, BioLegend).
RNA sequencing analysis using a public database
ADORA1, ADORA2A, ADORA2B, ADORA3, ENTPD1, and NT5E expression were analyzed from different tumor tissues using TCGA (The Cancer Genome Atlas) datasets.[28], [29], [30]
Statistical analysis
For statistical analyses, PRISM (GraphPad, Ver8.4.3.) software was used. For the comparison of two groups, a Student’s t-test (for the parametric test) and the Mann-Whitney U test (for the non-parametric test) were used. For multiple comparisons, one-way ANOVA and Tukey’s post hoc test (for the parametric test) and Kruskal-Wallis and Dunn’s post hoc test (for the non-parametric test) were utilized. For multiple comparisons in two categorical variables, two-way ANOVA with a post hoc Sidak test was used. All statistical information is described in the Fig. legends.
Results
A2aR is highly expressed in tumor tissue from patients with HCC and on T cells following ICI treatment
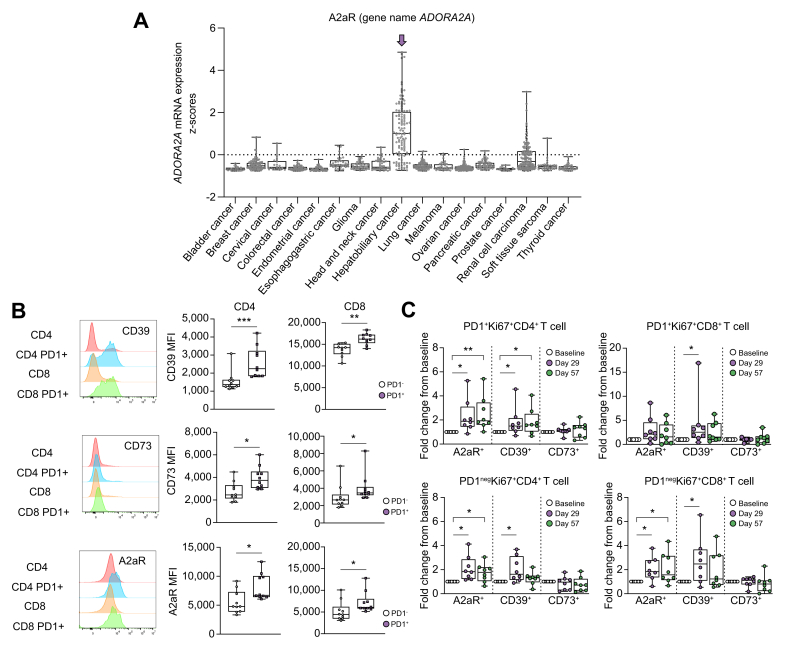
To examine the adenosine pathway in liver cancer tissue, we analyzed the mRNA expression of adenosine receptors and enzymes that metabolize and bind adenosine.15 CD39 and CD73 are enzymes that degrade ATP to ADP, and ADP to adenosine, respectively.16,18 The expression of ADORA1 (encoding the adenosine A1 receptor), ADORA2A (encoding the adenosine A2a receptor), ADORA2B (encoding the adenosine A2b receptor), ADORA3 (encoding the adenosine A3 receptor), ENTPD1 (encoding CD39), and NT5e (encoding CD73) in different types of cancers were analyzed using TCGA datasets.29 ADORA2A expression was highest in hepatobiliary cancers (Fig. 1A), while expression levels of ENTPD1 and NT5e were similar to those in other cancers (Fig. S1A). The expression levels of other adenosine receptors such as ADORA1, ADORA2B, and ADORA3 in hepatobiliary tumor tissue were also similar to those in other types of cancer (Fig. S1A).
Fig. 1.
A2aR expression is increased in hepatobiliary cancer and ICI therapy increases adenosine-related protein expression in T cells.
(A) The expression level of ADORA2A in tumor tissue of each cancer type was analyzed using TCGA datasets. (B–C) PBMCs of 13 patients with HCC who underwent immunotherapy were analyzed with flow cytometry at baseline, the first day of the second course (C2D1 day 29), and the first day of the third course (C3D1 day 57). The MFI of CD39, CD73, and A2aR of CD4 and CD8 cells with or without PD1 expression at baseline (B). Fold changes in the frequency of each T-cell subset from baseline to day 29 and day 57 were calculated for eight patients (C). ∗p <0.05, ∗∗p <0.01, ∗∗∗p <0.001 (B) Mann-Whitney U test (C) Kruskal-Wallis and Dunn’s post hoc test. HCC, hepatocellular carcinoma; ICI, immune checkpoint inhibitor; MFI, mean fluorescence intensity; PBMCs, peripheral blood mononuclear cells; TCGA, The Cancer Genome Atlas.
Next, we analyzed A2aR, CD39, and CD73 expression on different immune cell subsets in PBMCs from 13 patients with HCC (Table 1) who were enrolled in a clinical trial testing the combination of anti-CTLA4 plus locoregional therapies (NCT01853618, Fig. S1B).23 CD39 expression was highest on CD8+ T cells. CD73 expression was highest on B cells and A2aR expression were similar on all immune cells analyzed with the highest expression on regulatory T cells (Tregs) (Fig. S1C). CD39, CD73, and A2aR expression were higher on PD1+CD4+T cells and PD1+CD8+T cells than on PD-1negCD4+T cells and PD1negCD8+T cells at baseline (Fig. 1B). Next, peripheral T cells from eight paired samples were examined before treatment, after one dose of anti-CTLA4 (C2D1 d29), and after two doses of anti-CTLA4 and TACE (C3D1 d57). T-cell proliferation was examined by Ki67 staining of T cells, and PD1+ and PD1neg cells were analyzed separately. As shown in Fig. 1C, the largest increase in proliferating cells was seen in A2aR+ T cells (both PD1+ and PD1neg CD4+ and CD8+ T cells). Proliferating A2aR+ PD1+CD4+ T cells remained elevated after two doses of anti-CTLA treatment and TACE. The proliferation of both CD39+ T cells also increased upon anti-CTLA4 treatment (Fig. 1C). In contrast the frequency of Ki67+A2aRneg T cells did not change upon treatment (Fig. S1D). Collectively, these results suggest that ICI therapy induces the proliferation of peripheral T cells expressing adenosine-related proteins in HCC.
A2aR expression on hepatic T cells increases in mice with orthotopic tumors
To experimentally investigate the adenosine pathway in murine liver cancer models, we first profiled A2aR, CD39, and CD73 expressions on T cells from the liver, spleen, and blood of naïve, tumor-free mice. Expression of A2aR, CD39, and CD73 on CD4+ T cells was higher in liver-derived T cells compared to blood-derived and splenic T cells (Fig. S2A). Similarly, the number of CD39+CD8+ and A2aR+CD8+ T cells was higher in the liver than in the spleen, whereas most of the CD8+ T cells from the liver, spleen, and blood expressed CD73 (Fig. S2A). In liver tissue, A2aR expression on T cells was higher in effector CD44+ CD62Lneg CD4+ and CD8+ T cells compared to their naïve CD44neg CD62L+ T cell counterparts (Fig. S2B). Next, we investigated adenosine-related genes in primary cancer cell lines. The expression of A2aR, CD39, and CD73 was examined in three different primary murine liver tumor cell lines (SB-1, RIL 175, and Hep55.1c cells), and murine cell lines derived from tumors, which commonly metastasize to the liver: melanoma (B16–F10) and colorectal cancer (CT26). The highest CD73 expression was found on SB-1 cells (Fig. S2C). There were no differences in A2aR and CD39 expression on the cancer cell lines tested.
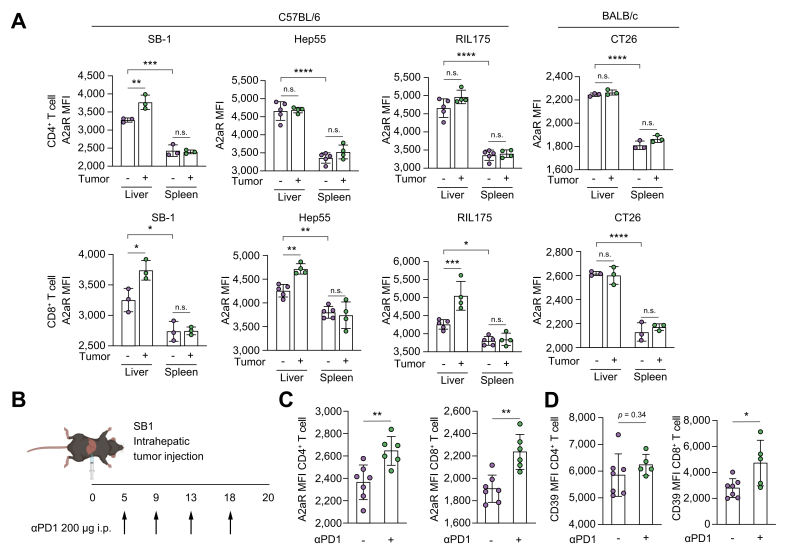
To investigate the change of A2aR expression on T cells from tumor-bearing mice, we analyzed A2aR expression on CD4+ and CD8+ T cells from C57BL/6 mice and BALB/c mice after orthotopic injection of three different liver tumor cell lines. A2aR expression was higher on hepatic CD8+ T cells derived from liver tumor-bearing mice and on hepatic CD4+ T cells from SB1 tumor-bearing mice. However, A2aR expression did not change on CD4+ and CD8+ T cells from BALB/c mice after injection with CT26 colon cancer cells (Fig. 2A). These results suggest that A2aR expression on T cells increases in the liver of tumor-bearing mice.
Fig. 2.
Orthotopic tumors and anti-PD1 treatment increase the expression of adenosine-related proteins in hepatic T cells in murine models.
(A) SB-1 cell, Hep55.1c cell, and RIL175 cells were injected into the livers of C57BL/6 female mice. CT26 cells were injected into the livers of BALB/C female mice. The lymphocytes of the liver and spleen were isolated and analyzed with multicolor flow cytometry. The MFI of A2aR in CD4 and CD8 cells was analyzed; n = 3-5/group. (B-D) SB-1 cells were injected into the liver of C57BL/6 female mice and treated with anti-PD1. (B) The experimental settings. (C-D) The lymphocytes of the liver and spleen were isolated and analyzed with multicolor flow cytometry. The MFI of A2aR and CD39 in CD4 and CD8 cells was analyzed. n = 6-7/group; n.s., p >0.05; ∗p <0.05; ∗∗p <0.01; ∗∗∗p <0.005; ∗∗∗p <0.001. (A) Two-way ANOVA with post hoc Sidak’s test. (C-D) Mann-Whitney U test. MFI, mean fluorescence intensity.
The A2aR expression increases upon treatment with ICI therapy
Next, we investigated A2aR expression on T cells in mice treated with ICI. SB1 cells were injected into the liver of mice and treated with anti-PD-1 therapy (Fig. 2B). A2aR expression on hepatic CD4+ and CD8+ T cells increased in tumor-bearing mice after anti-PD1 therapy (Fig. 2C). Similarly, the expression of CD39 on CD8+ T cells increased in liver tumor-bearing mice after treatment (Fig. 2D). In contrast, A2aR expression on CD4+ and CD8+ T cells did not change when C57BL/6 naïve, tumor-free mice were treated with anti-PD-1 (Fig. S3).
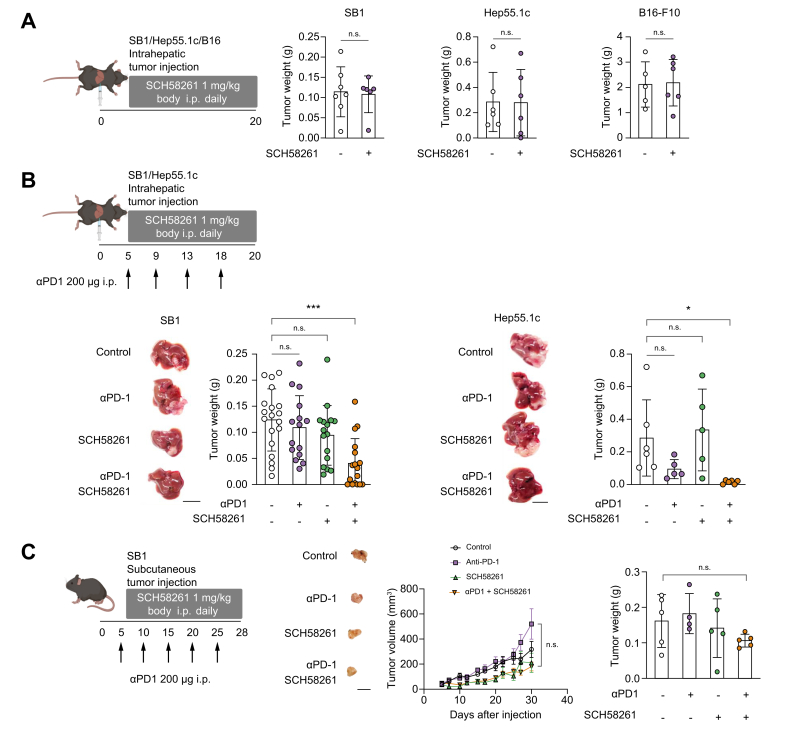
The combination of SCH58261 and anti-PD1 therapy is effective in mice with orthotopic liver tumors
Based on the observation that A2aR expression increased in T cells in tumor-bearing mice, we investigated the effect of a potent and selective A2aR antagonist, SCH58261,[31], [32], [33] in tumor bearing mice. First, we excluded a direct effect of SCH58261 on tumor cells in vitro. Incubation of different tumor cells with the A2aR inhibitor affected cell viability only at micromolar concentrations of SCH58261, indicating a lack of a direct effect on tumor cell proliferation (Fig. S4). Next, we tested SCH58261 treatment in mice with intrahepatic SB1, Hep55.1c, or B16–F10 tumors. Mice were treated 5 days after cell line injection for a total of 15 days. Similar to our in vitro studies, SCH58261 monotherapy had no effect on tumor growth (Fig. 3A). Based on the observation that A2aR expression on T cells changed upon anti-PD1 treatment in mice with orthotopically growing tumors (Fig. 2C), we tested the combination of SCH58261 plus anti-PD1 treatment. Combined SCH58261 plus anti-PD1 had a stronger effect on growth of intrahepatic tumors than individual treatment alone in both tumor models (Fig. 3B). Next, we asked if this effect was also found in mice with subcutaneous tumors. SB1 cells were injected subcutaneously into C57BL/6 mice and treated with SCH58261 plus an anti-PD1 antibody (Fig. 3C). Neither single agent treatments nor the combination of SCH58261 plus an anti-PD1 antibody significantly affected growth of subcutaneous tumors (Fig. 3C). Collectively, these results suggest that A2aR blockade can have synergistic effects with anti-PD1 therapy in murine models of orthotopic liver cancer.
Fig. 3.
Blocking A2aR activity promotes anti-PD1 therapy against liver tumors.
(A) SB1, Hep55.1c, or B16–F10 cells were injected into the livers of C57BL/6 mice and treated with daily intraperitoneal injections of SCH58261 from 5 days after injection. Mice were analyzed 20 days after tumor injection; n = 5-7/group. (B) SB1 cells or Hep55.1c cells were injected into the liver of C57BL/6 mice and treated with SCH58261, anti-PD1, or combination therapy from 5 days after injection. The representative macro tumor image is shown. The weight of each group is shown in the right panel. For the SB-1 tumor model, n = 5-7/group/experiment and the experiment was repeated three times. For the Hep55.1c model, n = 5-6. (C) SB1 cells were injected subcutaneously into C57BL/6 mice and treated with SCH58261, anti-PD1, or combination therapy from 5 days after injection. The representative macro tumor image is shown in the middle. The tumor volume and weight of each group are shown in the right panel; n = 5/group and the experiment was repeated twice. Scale bar: 1 cm. n.s., p >0.05; ∗p <0.05; ∗∗p <0.01; ∗∗∗p <0.001. (A) Mann-Whitney U test (B) (C) Kruskal-Wallis and Dunn’s post hoc test.
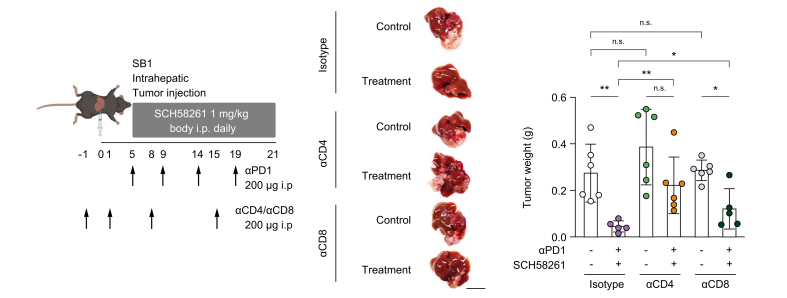
SCH58261 has a synergistic effect with immune checkpoint therapy modulated by CD4+ T cells
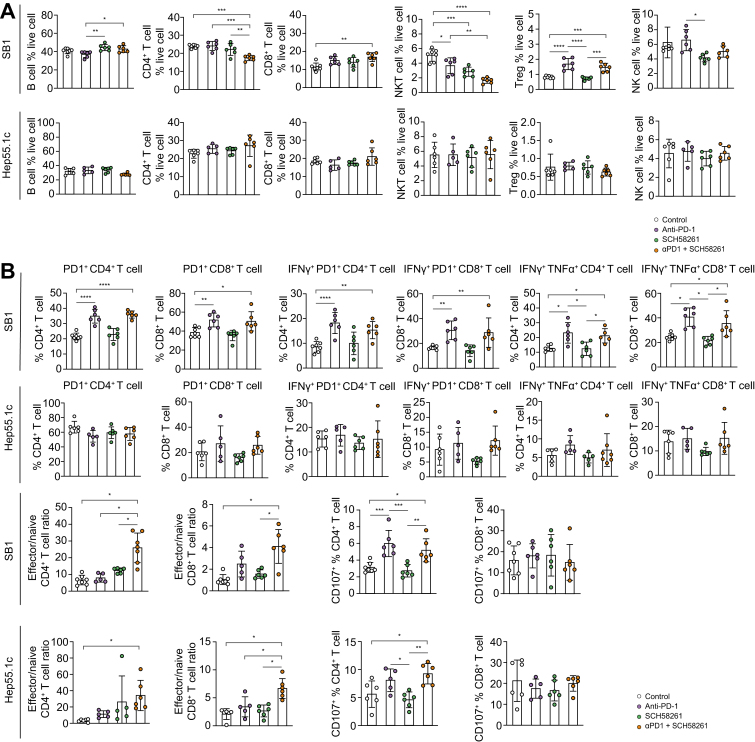
To better understand the mechanism of SCH58261 and anti-PD1 treatment’s efficacy, we studied immune cells isolated from tumor-bearing mice after treatment with the combination of SCH58261 and an anti-PD1 antibody. Both SB1 and Hep55.1c intrahepatic tumors were reduced in size after treatment with SCH58261 plus anti-PD1 (Fig. 3B), however, there were no common quantitative differences in B cells, CD4+ and CD8+ T cells, NKT cells, regulatory T cells and NK cells found in RIL175 and Hep55.1c tumor models (Fig. 4A). Next, we studied CD107 expression and cytokine release by T cell to assess T-cell function. CD107+ CD4+ T cells in the liver increased upon ICI treatment in both tumor models, but SCH58261 treatment had no additional effect. Similar results were observed when we looked at IFNγ+TNFα+ CD4+ and CD8+ T cells (Fig. 4B). Finally, we analyzed the ratio of effector:naïve T cells. The ratio of CD44+CD62Lneg effector T cells and CD44negCD62L+ naïve T cells were calculated both for hepatic CD4+ and CD8+ T cells. The effector CD4+ T cell and CD8+ T cell population significantly increased in the combination therapy group in the liver of both orthotopic models but did not increase in mice which had received anti-PD1 or SCH58261 monotherapy (Fig. 4B). Interestingly, this increase in the ratio of effector:naïve T cells after anti-PD1 plus SCH58621 treatment was not observed in tumor-infiltrating lymphocytes from subcutaneous tumors (Fig. S5). Finally, we performed CD4+ T cell and CD8+ T-cell depletion to verify which cell type was needed to exert anti-tumor immunity in mice treated with the combination. While both depletion of CD8+ T cells and CD4+ T cells ameliorated the effect of combination therapy, depletion of CD4+ T cells had a stronger effect on tumor growth (Fig. 5). Based on these results, we suggest that SCH58261 increases effector T cells and activates T cells synergistically with anti-PD1 in a CD4+ T cell-dependent manner.
Fig. 4.
A2aR blockade + ICI therapy against liver cancer increase effector T cell frequency in the liver.
(A,B) SB1 or Hep55.1c cells were injected into the livers of C57BL/6 mice and treated with SCH58261, anti-PD1, or combination therapy from 5 days after injection. (A) The pan-immune cell population was analyzed with flow cytometry. The cytokine production and subsets of T cells were analyzed with flow cytometry. Manual gating for each cell subset is shown in (B); n = 6-7/group with experiments repeated twice. n.s., p >0.05; ∗p <0.05; ∗∗p <0.01; ∗∗∗p <0.001. (A-B) Kruskal-Wallis and Dunn’s post hoc test. NK, natural killer; Treg, regulatory T cell.
Fig. 5.
CD4+T cells are required for effective A2aR blockade + ICI therapy against liver cancer.
SB1 cells were injected into the livers of C57BL/6 mice and treated with combination therapy with or without anti-CD4 antibody or anti-CD8 antibody. The representative liver image and tumor weight are shown. Scale bar:1 cm; n = 5-6/group. n.s., p >0.05; ∗p <0.05; ∗∗p <0.01; ∗∗∗p <0.001. Kruskal-Wallis and Dunn’s post hoc test. ICI, immune checkpoint inhibitor.
Discussion
This study demonstrates that the adenosine synthesis proteins, CD39 and CD73, and receptor (A2aR) are overexpressed in T cells from human PBMCs after immunotherapy. Hepatic A2aR-expressing T cells increased in tumor-bearing mice and after anti-PD1 treatment. A2aR inhibitor and anti-PD1 treatment revealed potent anti-tumor effects together in two murine liver cancer models.
One interesting result from our study was the observation that A2aR expression on T cells following anti-PD1 antibody therapy only increased in tumor-bearing mice. Furthermore, A2aR antagonist monotherapy did not show anti-tumor effects, in line with a study by Willingham and colleagues, which reported that single-agent A2aR inhibition lacked an anti-tumor effect without ICI treatment.34 Ohta and colleagues also showed that single-agent A2aR inhibition did not have an anti-tumor effect in the absence of tumor-specific T-cell transfer,13 while a recent clinical trial showed that an A2aR antagonist lacked anti-tumor effects without tumor-specific T-cell infiltration or enhancement of adenosine signaling.14 Based on these results, we propose that expansion of A2aR-expressing effector T cells by ICI treatment might be necessary for A2aR inhibition to exhibit anti-tumor effects.
Overall, the expression of genes involved in adenosine signaling was rather low in HCC. This may be because we used bulk RNA sequencing datasets from tumor tissue, which may have contained only a limited number of immune cells, which is a potential limitation of this study.
The efficacy of A2aR antagonists for murine cancer therapy has been described in renal cancer by Fong et al., wherein they describe CD8+ T-cell recruitment following A2aR inhibitor treatment. They also conducted a clinical trial showing that treatment efficacy was related to the adenosine-related gene expression profile before treatment.14 The combination of an A2aR antagonist and immunotherapy has been shown to have a synergistic effect on a subcutaneous colon cancer model by activating CD8 cells and blocking Treg activity in the tumor.35 Furthermore, the inhibition of CD73 and A2aR reduced tumor initiation, growth, and metastasis in a melanoma model by recruiting effector lymphocytes and increasing IFNγ expression.22
Herein, we describe the A2aR expression on hepatic T cells when an intrahepatic tumor is present and following anti-PD1 treatment. The combination effect with anti-PD1 therapy was only seen in the liver orthotopic model while CD4+ T-cell depletion attenuated this effect. Overall, this data suggests that in liver tumors where anti-PD1 therapy or other ICIs are ineffective, the addition of A2aR blockade may disinhibit adenosine-mediated T-cell immunosuppression. On the other hand, A2aR agonists have been reported to increase Treg proliferation and immunosuppressive function,36 though an A2aR inhibitor did not affect the number of Tregs in the liver of the orthotopic liver tumor model (Fig. 4A). This suggests that A2aR inhibition directly disinhibits and expands effector T cells in murine liver tumors by attenuating the adenosine-A2AR-cAMP axis.
Currently, there are limited treatment options for hepatobiliary cancers and many cancers are not responsive to immunotherapy. We show that targeting the adenosine pathway is mechanistically promising for clinical application. Intriguingly, an A2aR antagonist is already used for the treatment of Parkinson’s disease, indicating a promising safety profile for early clinical trials.37
In conclusion, A2aR is highly expressed in human liver cancer and immune therapy increases the A2aR expression of T cells. We demonstrate that an A2aR antagonist enhanced the anti-tumor effect of anti-PD1 therapy in tumor-bearing mice, highlighting a putative therapeutic target for clinical application in hepatobiliary cancers and potentially cancers that have metastasized to the liver.
Financial support
BR was supported by the International Liver Cancer Association (ILCA) Fellowship Award 2021. S.W. was funded by the Deutsche Forschungsgemeinschaft (WA-4610/1-1). K. B. received support through an iCURE-NCI fellowship. T.F.G. was supported by the Intramural Research Program of the NIH, NCI (ZIA BC 011345).
Authors’ contributions
Conceptualization: YM, JDM, CM, TFG. Methodology: YM, JDM, CM, TFG, BR, KCB, SW, JCM, CF, XC. Investigation: YM, JDM. Visualization: YM, JDM, TFG. Funding acquisition: TFG, MC. Project administration: YM, JDM, CM, TFG. Supervision: TFG, MC. Writing – original draft: YM, TFG. Writing – review & editing: YM, JDM, CM, KCB, BR, RB, BLG, SW, JCM, CF, XC, TFG.
Data availability statement
Data analyzed in the study are available from the corresponding author by request.
Conflict of interest
The authors declare no competing interests.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100959.
Supplementary data
The following are the supplementary data to this article:
:
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Budczies J., von Winterfeld M., Klauschen F., et al. The landscape of metastatic progression patterns across major human cancers. Oncotarget. 2015;6:570–583. doi: 10.18632/oncotarget.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu J., Green M.D., Li S., et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med. 2021;27:152–164. doi: 10.1038/s41591-020-1131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tumeh P.C., Hellmann M.D., Hamid O., et al. Liver metastasis and treatment outcome with anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res. 2017;5:417–424. doi: 10.1158/2326-6066.CIR-16-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finn R.S., Qin S., Ikeda M., et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 6.Llovet J.M., Castet F., Heikenwalder M., et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022;19:151–172. doi: 10.1038/s41571-021-00573-2. [DOI] [PubMed] [Google Scholar]
- 7.Abou-Alfa G.K., Lau G., Kudo M., et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 2022;1 doi: 10.1056/EVIDoa2100070. [DOI] [PubMed] [Google Scholar]
- 8.Mo Z., Liu D., Rong D., et al. Hypoxic characteristic in the immunosuppressive microenvironment of hepatocellular carcinoma. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.611058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morse M.A., Sun W., Kim R., et al. The role of angiogenesis in hepatocellular carcinoma. Clin Cancer Res. 2019;25:912–920. doi: 10.1158/1078-0432.CCR-18-1254. [DOI] [PubMed] [Google Scholar]
- 10.Cramer T., Vaupel P. Severe hypoxia is a typical characteristic of human hepatocellular carcinoma: scientific fact or fallacy? J Hepatol. 2022;76:975–980. doi: 10.1016/j.jhep.2021.12.028. [DOI] [PubMed] [Google Scholar]
- 11.Sitkovsky M.V. Lessons from the A2A adenosine receptor antagonist-enabled tumor regression and survival in patients with treatment-refractory renal cell cancer. Cancer Discov. 2020;10:16–19. doi: 10.1158/2159-8290.CD-19-1280. [DOI] [PubMed] [Google Scholar]
- 12.Ohta A., Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 13.Ohta A., Gorelik E., Prasad S.J., et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci. 2006;103:13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fong L., Hotson A., Powderly J.D., et al. Adenosine 2A receptor blockade as an immunotherapy for treatment-refractory renal cell cancer. Cancer Discov. 2020;10:40–53. doi: 10.1158/2159-8290.CD-19-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatfield S.M., Sitkovsky M.V. Antihypoxic oxygenation agents with respiratory hyperoxia to improve cancer immunotherapy. J Clin Invest. 2020;130:5629–5637. doi: 10.1172/JCI137554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deaglio S., Dwyer K.M., Gao W., et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun X., Wu Y., Gao W., et al. CD39/ENTPD1 expression by CD4+Foxp3+ regulatory T cells promotes hepatic metastatic tumor growth in mice. Gastroenterology. 2010;139:1030–1040. doi: 10.1053/j.gastro.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin D., Fan J., Wang L., et al. CD73 on tumor cells impairs antitumor T-cell responses: a novel mechanism of tumor-induced immune suppression. Cancer Res. 2010;70:2245–2255. doi: 10.1158/0008-5472.CAN-09-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L., Fan J., Thompson L.F., et al. CD73 has distinct roles in nonhematopoietic and hematopoietic cells to promote tumor growth in mice. J Clin Invest. 2011;121:2371–2382. doi: 10.1172/JCI45559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia C., Yin S., To K.K.W., et al. CD39/CD73/A2AR pathway and cancer immunotherapy. Mol Cancer. 2023;22:44. doi: 10.1186/s12943-023-01733-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allard B., Pommey S., Smyth M.J., et al. Targeting CD73 enhances the antitumor activity of anti-PD-1 and anti-CTLA-4 mAbs. Clin Cancer Res. 2013;19:5626–5635. doi: 10.1158/1078-0432.CCR-13-0545. [DOI] [PubMed] [Google Scholar]
- 22.Young A., Ngiow S.F., Barkauskas D.S., et al. Co-Inhibition of CD73 and A2AR adenosine signaling improves anti-tumor immune responses. Cancer Cell. 2016;30:391–403. doi: 10.1016/j.ccell.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 23.Duffy A.G., Ulahannan S.V., Makorova-Rusher O., et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545–551. doi: 10.1016/j.jhep.2016.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wabitsch S., McCallen J.D., Kamenyeva O., et al. Metformin treatment rescues CD8(+) T-cell response to immune checkpoint inhibitor therapy in mice with NAFLD. J Hepatol. 2022;77:748–760. doi: 10.1016/j.jhep.2022.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma C., Fu Q., Diggs L.P., et al. Platelets control liver tumor growth through P2Y12-dependent CD40L release in NAFLD. Cancer Cell. 2022;40:986–998.e5. doi: 10.1016/j.ccell.2022.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diggs L.P., Ruf B., Ma C., et al. CD40-mediated immune cell activation enhances response to anti-PD-1 in murine intrahepatic cholangiocarcinoma. J Hepatol. 2021;74:1145–1154. doi: 10.1016/j.jhep.2020.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown Z.J., Heinrich B., Greten T.F. Mouse models of hepatocellular carcinoma: an overview and highlights for immunotherapy research. Nat Rev Gastroenterol Hepatol. 2018;15:536–554. doi: 10.1038/s41575-018-0033-6. [DOI] [PubMed] [Google Scholar]
- 28.Cerami E., Gao J., Dogrusoz U., et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan-cancer analysis of whole genomes. Nature. 2020;578:82–93. doi: 10.1038/s41586-020-1969-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao J., Aksoy B.A., Dogrusoz U., et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ongini E., Fredholm B.B. Pharmacology of adenosine A2A receptors. Trends Pharmacol Sci. 1996;17:364–372. [PubMed] [Google Scholar]
- 32.Yang M., Soohoo D., Soelaiman S., et al. Characterization of the potency, selectivity, and pharmacokinetic profile for six adenosine A2A receptor antagonists. Naunyn Schmiedebergs Arch Pharmacol. 2007;375:133–144. doi: 10.1007/s00210-007-0135-0. [DOI] [PubMed] [Google Scholar]
- 33.Beavis P.A., Divisekera U., Paget C., et al. Blockade of A2A receptors potently suppresses the metastasis of CD73+ tumors. Proc Natl Acad Sci U S A. 2013;110:14711–14716. doi: 10.1073/pnas.1308209110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willingham S.B., Ho P.Y., Hotson A., et al. A2AR antagonism with CPI-444 induces antitumor responses and augments efficacy to anti-PD-(L)1 and anti-CTLA-4 in preclinical models. Cancer Immunol Res. 2018;6:1136–1149. doi: 10.1158/2326-6066.CIR-18-0056. [DOI] [PubMed] [Google Scholar]
- 35.Leone R.D., Sun I.M., Oh M.H., et al. Inhibition of the adenosine A2a receptor modulates expression of T cell coinhibitory receptors and improves effector function for enhanced checkpoint blockade and ACT in murine cancer models. Cancer Immunol Immunother. 2018;67:1271–1284. doi: 10.1007/s00262-018-2186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohta A., Kini R., Ohta A., et al. The development and immunosuppressive functions of CD4(+) CD25(+) FoxP3(+) regulatory T cells are under influence of the adenosine-A2A adenosine receptor pathway. Front Immunol. 2012;3:190. doi: 10.3389/fimmu.2012.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng J., Zhang X., Zhen X. Development of adenosine A(2A) receptor antagonists for the treatment of Parkinson's disease: a recent update and challenge. ACS Chem Neurosci. 2019;10:783–791. doi: 10.1021/acschemneuro.8b00313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
:
Data Availability Statement
Data analyzed in the study are available from the corresponding author by request.