Highlights
-
•
Sonication increased antimicrobial activity of probiotic and yoghurt starter cultures.
-
•
Sonication improved survival of yoghurt cultures during cold storage.
-
•
Sonication increased peptide content, α-glucosidase and α-amylase inhibition of stirred yoghurt.
-
•
Sonication enhanced antioxidant and anticancer activities of stirred yoghurt.
Keywords: Stirred yoghurt, Starter cultures, Probiotic, Sonication, Functional and bioactive properties
Abstract
In this study, the effects of ultrasonicated Lactobacillus delbrueckii subsp. bulgaricus, Streptococcus thermophilus and Lactiplantibacillus plantarum AF1 (100 W, 30 kHz, 3 min) on the safety and bioactive properties of stirred yoghurt during storage (4 °C for 21 days) were investigated. The results showed that sonicated cultures were more effective in reducing pathogens than untreated ones. The highest antioxidant activity (DPPH and ABTS), α-glucosidase and α-amylase inhibition capacity were found in yoghurt containing sonicated probiotic + sonicated yoghurt starter cultures (P + Y + ). The highest amount of peptides (12.4 mg/g) was found in P + Y + yoghurts at the end of the storage time. There were not significant differences between the exopolysaccharide content of P + Y+ (17.30 mg/L) and P + Y- (17.20 mg/L) yoghurts, although it was significantly (P ≤ 0.05) higher than the other samples. The use of ultrasonicated cultures could enhance the safety of stirred yoghurt and improve its functional and bioactive properties.
1. Introduction
Due to their positive impact on health and nutritional value, there has been an increase in demand for fermented milk products [1].Yoghurt is the most widely consumed fermented dairy product worldwide due to its health benefits, high nutritional value and desirable sensory characteristics [2], [3].Commercial yoghurts are mainly divided into two groups based on texture structure: i) set-type and ii) stirred-type. The gel network of set yoghurt remains intact, while in stirred yoghurt, the acidified gel is broken during stirring process, obtaining a homogeneous and viscous product[4].
Yoghurt has been described as the most popular vehicle for delivering probiotic microorganisms, specially lactobacilli and bifidobacteria[5]. Lactic acid bacteria (LAB) are widely used as starter or adjunct cultures in the production of fermented dairy products and some of them show potential probiotic activity[6]. Among the different positive effects reported for lactic acid bacteria used for milk products fermentation,we cite pH reduction, increase of antioxidant activity, antimicrobial metabolites and exopolysaccharides production, flavor and aroma development, mycotoxins detoxification, development of vitamins and bioactive peptides, production of growth-promoting substances, α-amylase and α-glucosidase inhibition, enhancement of nutrients and bioactive compounds bioaccessibility as well as stimulation of the immune system [7], [8], [9], [10].
α-Amylase and α-glucosidase enzymes break down starch into glucose and lead to an increase in blood sugar levels. Therefore, inhibition of the activity of these two enzymes is considered an indirect indicator to evaluate the antidiabetic properties of fermented products. It has been reported that bioactive peptides produced by proteolytic systems of LAB strains are responsible for inhibiting α-amylase and α-glucosidase enzymes [11].
Lactiplantibacillus plantarum, as a heterofermentative LAB, is found in a range of fermented products including fermented fruit juice, meat, and dairy products. Some L. plantarum strains have been shown to possess probiotic properties [12]. Kurdish cheese, as one of the traditional Iranian cheeses, contains various native strains of LAB[13].
Ultrasound (US), as one of the novel green technologies, has been recently introduced to the food industry, and their usage is expanding[14]. Ultrasound is of great interest in research related to fermented dairy products, because this technique inhibits some undesirable microorganisms and inactivates some enzymes. Reduction of fermentation time, increase in cell growth and proliferation, acceleration of acidification and promotion of the activity of enzymes such as lactase and protease are some of the effects of ultrasounds in this area[15], [16], [17]. In connection with the use of ultrasound in yoghurt production, it has been reported that ultrasound promotes fat drop reduction and increases homogeneity, viscosity, whey coagulation, firmness, and water-holding capacity of yoghurt [18].Other uses of ultrasound are mentioned in the references [19].
L. plantarum AF1 is one of the native strains isolated from traditional Kurdish cheese that has been shown to have in vitro potential probiotic properties [13]. To date, no studies have been performed on the use of ultrasonicated L. plantarum AF1 in the production of stirred yoghurt. In the present work, the influence of ultrasound pre-treatment on microbiological and bioactive properties of probiotic stirred yoghurt was investigated following an experimental methodology. Several setups have been combined considering silent and sonicated conditions of starter cultures or/and probiotics. Based on the results, several mechanistic interpretations have been advanced according to the most plausible explanations found in literature.
2. Materials and methods
2.1. Microbial strains
Lactiplantibacillus plantarum AF1 was obtained from Department of Food Science and Technology, Fasa University (Fasa, Iran). The probiotic activity of this strain was confirmed previously by Hashemi, Shahidi, Mortazavi, Milani and Eshaghi [13]. The strain was reactivated in MRS broth (Oxoid, UK) at 37 °C for 24 h. Commercial lyophilized yoghurt starter culture, x-11, was purchased from Chr-Hansen (Horsholm, Denmark). The pathogenic bacteria including Escherichia coli PTCC1763, Staphylococcus aureus PTCC 1337, and Salmonella Typhi PTCC 1609 were purchased from the culture collection at Iranian Research Organization for Science and Technology (Tehran, Iran) and reactivated in nutrient broth (Oxoid) at 37 °C for 24 h. After reactivation, all strains were harvested by centrifugation at 5000 × g for 15 min at 4 °C and washed three times.
2.2. Yoghurt production
For yoghurt production, the x-11 starter (0.01 gr) was added to 500 mL sterilized skim milk according to the procedure provided by manufacturer. Then, 20 mL of the inoculated skim milk was added to 10000 mL of UHT milk (1.5 % fat; Pegah Company, Fars Province, Iran) and incubated at 40 °C for 8 h. Fermentation was stopped when the pH reached 4.6 by rapidly cooling the fermented milk in an ice bath to prevent excessive production of lactic acid. The yoghurt was then manually stirred using a stainless-steel bored disk with up-and-down movements for approximately one minute. After stirring, the sample was transferred to cups and stored at 4 °C. For probiotic samples, 7.5 log CFU/mL of L. plantarum AF1 were added to the UHT milk and co-cultured with yoghurt starter cultures. Milks inoculated with probiotic or yoghurt starters were subjected to an ultrasound (Hielscher; Germany; 100 W, 30 kHz) treatment for 3 min.Therefore, yoghurt samples including probiotic + yoghurt starter cultures (P-Y-), yoghurt starters (Y-), sonicated probiotic + yoghurt starter cultures (P + Y-), sonicated yoghurt starters (Y + ), probiotic + sonicated yoghurt starter cultures (P-Y + ) and sonicated probiotic + sonicated yoghurt starter cultures (P + Y + )were used. For each treatment, three replicates of the yoghurt production sequence were prepared. Finally, all yoghurt samples were stored at 4 °C for 21 days.
2.3. Enumeration of probiotic and yoghurt starter cells
Briefly, 5 g of each yoghurt sample were mixed with 45 mL of 0.1 % peptone water, followed by 10-fold dilutions. Then, 100 μL of the proper dilution were utilized to obtain the microbial counts. S. thermophilus was cultured aerobically in M17 agar (Oxoid) at 37 °C for 48 h, while L. delbrueckii subsp.bulgaricus was cultured anaerobically in MRS agar at 42 °C for 48 h. L. plantarum AF1was counted on MRS agar supplemented with 10 mg/mL vancomycin at 37 °C for 48 h.
2.4. Antimicrobial activity
In order to evaluate the evolution of the antimicrobial activity of yoghurt samples with the elapse of time, pathogenic bacteria were added to yoghurt samples separately (containing probiotic or not and sonicated or unsonicated samples) at 4.1 – 4.2 log CFU/mL before yoghurt fermentation. Enumeration of pathogenic bacteria was done at 0, 4 and 8 h after inoculation at 40 °C. Antimicrobial effect was also investigated during cold storage. The preparation of yoghurt samples for enumeration was carried out similarly to the procedure indicated in section 2.3. Baird Parker agar (Oxoid), Sorbitol MacConkey agar (Oxoid) and XLD medium (Oxoid) were used as appropriate mediums for S. aureus, E. coli and S. Typhi, respectively. Incubation for all pathogens was performed at 37 °C for 24 h.
2.5. Determination of acidity and pH
The pH of yoghurt samples was measured by a digital pH-meter (Starter 2100F, OHAUS, USA). For the determination of the acidity, titration of yoghurt samples was carried out with 0.1 N NaOH, and data were expressed as % lactic acid (Eq. (1)[20].
| (1) |
2.6. Exopolysaccharide (EPS) content
Briefly, yoghurt samples were mixed with trichloroacetic acid (TCA) (20 % (w/v)) following centrifugation at 4000 × g for 20 min. NaOH was used for adjusting pH of supernatantat 6.8, and then boiling at 100 °C for 30 min was performed. Centrifugation at 4000 × g for 20 min was carried out and the obtained supernatant was mixed with 25 mL of cold absolute ethanol. After overnight incubation at 4 °C, centrifugation was carried out at 4000 × g for 20 min. Then, the pellet was thoroughly dissolved by Milli-Q water (10 mL) following sonication using the ultrasonic processor UP100H (Hielscher; Germany; 100 W, 30 kHz) at ambient temperature for 30 min. Next, the solution was dialyzed using a membrane with a molecular mass cut–off value of 13,000 Da against tap water. The quantification of EPS content was determined by the phenol–sulphuric technique and was expressed as glucose equivalent[21].
2.7. Measurement of peptide content
Yoghurt samples were centrifuged at 4000 × g for 15 min and the obtained supernatants were treated with HCl (1 mol/L) or NaOH (1 mol/L) to adjust pH at 4.6. Centrifugation was performed again and 2.5 mL of the obtained clear supernatant were added to 2.5 mL of TCA (10 %). The solution was centrifuged at 4000 × g for 15 min. After that, the obtained supernatant was added to 5 mL of biuret reactive.After 20 min, the peptide content was calculated using spectrophotometric measurement at 540 nm (UV/visible, Philips, Cambridge, UK). Casein was utilized as a standard solution [22], [23].
2.8. α-Amylase inhibition assay
After centrifugation of samples at 4000 × g for 15 min, the supernatants were supplemented with HCl (1 M) or NaOH (1 M) to adjust pH at 4.6. After centrifugation, the clear supernatant (100 µL) was mixed with 100 µL of α-amylase and incubation was performed at 37 °C for 5 min.The initiation of the reaction was carried out using 250 µL of 1 % starch at 37 °C for 5 min and termination was done by the addition of 200 μL 1 % 3,5-dinitrosalicylic acid and 12 % sodium potassium tartrate in 0.4 M NaOH. The absorbance was read at 540 nm for measurement of α-amylase activity and the solution without substrate was considered as blank[24]. The inhibition percentage was calculated as follows (Eq. (2):
| (2) |
2.9. α-Glucosidase inhibition assay
To measure α-glucosidase inhibition, the clear supernatant was obtained similar to α-amylase inhibition assay. Then, 50 µL of the clear supernatant was mixed with α-glucosidase and incubation was done at 37 °C for 10 min. The initiation of the reaction was performed by the addition of 5 mM p-nitrophenyl⍺-D-glucopyranoside (50 µL) at 30 °C for 30 min and termination was performed using 1 mL of 0.1 M Na2CO3. ⍺-Glucosidase activity was calculated at 400 nm and the solution without substrate was considered as blank [24]. The inhibition percentage was calculated as follows (Eq. (3):
| (3) |
2.10. Antioxidant assays
For determining the DPPH radical scavenging activity, samples were supplemented with methanol following centrifugation at 3500 × g for 20 min. The obtained supernatant was mixed with DPPH (1 mL) and methanol (1.5 mL). After that, the solution was kept at room temperature for 30 min and the absorbance was read at 517 nm[25].
On the other hand, to evaluate ABTS radical scavenging activity, briefly, 10 µL of diluted sample in water was supplemented with 1 mL of diluted ABTS radical solution in 5 mmol/ L of phosphate-buffered saline following shaking for 15 s at ambient temperature. The absorbance was read at 734 nm and Trolox was utilized as a standard [26].
2.11. Cytotoxicity assay
Caco-2 cells were cultured in a 96-well plate at 1 × 103 cells following incubation for 24 h. Yoghurt samples were centrifuged at 3500 × g for 15 min and 25 μL of the filtered supernatant (0.22 μm) was added to each well following incubation at 37 °C for 72 h. Afterward, Abnova Cell Cytotoxicity Assay Kit (KA4151, Jhongli, Taiwan) was utilized, and incubation was carried out at 37 °C for 6 h following determination of the absorbance at 570 nm and 605 nm [27].
2.12. Statistical analysis
Statistical analyses (One-way ANOVA, Duncan, Repeated Measures and Paired Samples T Test) were carried out by the SPSS package program (v. 20.0 for Windows, SPSS Inc., Chicago, IL, USA).
3. Results and discussion
3.1. The viability of L. bulgaricus, s. Thermophilus and L. Plantarum in yoghurt during cold storage
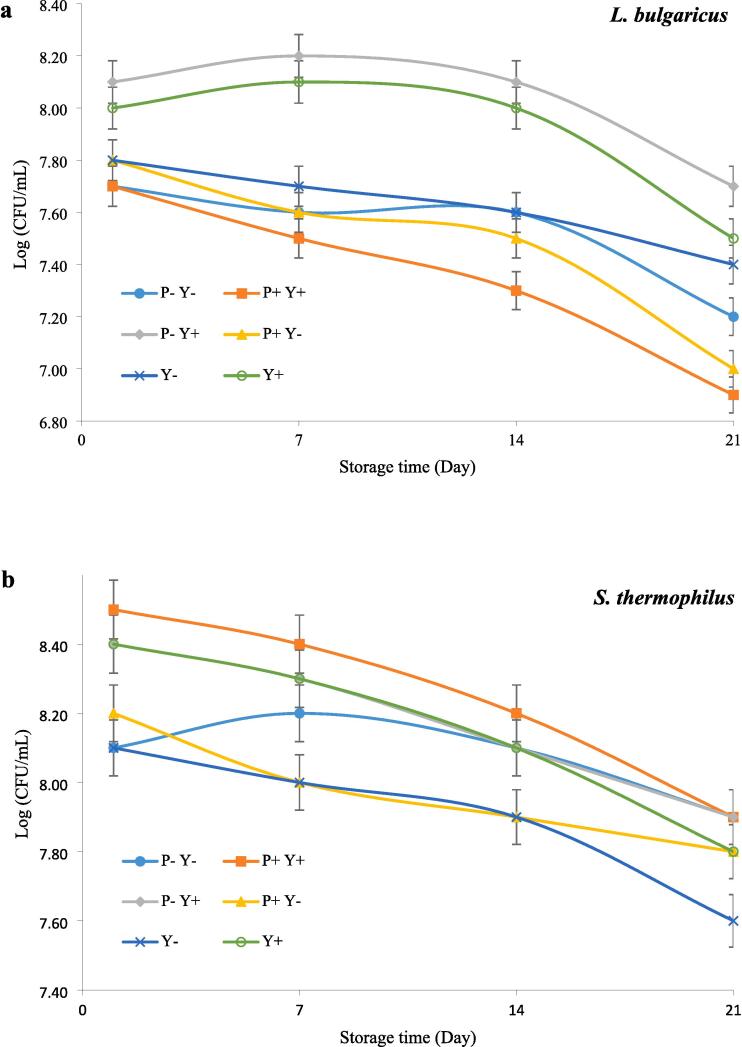
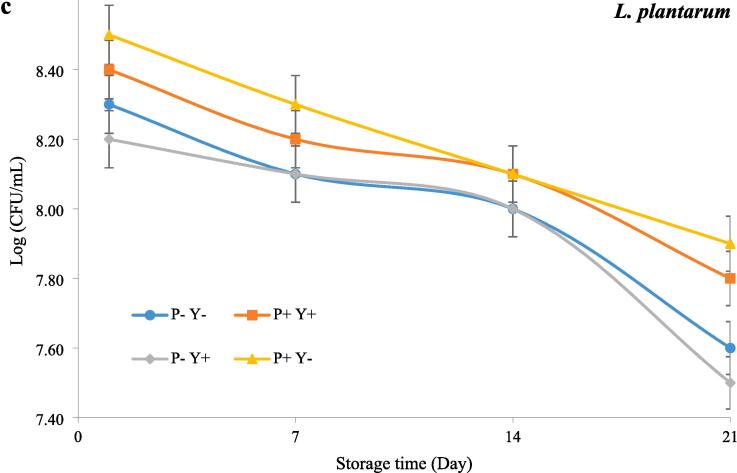
Fig. 1 shows the effect of ultrasound treatment on the viability of traditional yoghurt cultures and L. plantarum during cold storage. The number of LAB in all samples appeared to decrease with the elapse of storage time. This decrease was significant (P ≤ 0.05) at the end of the storage period, compared to the starting point.The number of L. bulgaricus in P-Y + sample was significantly (P ≤ 0.05) higher than the other samples except for Y + sample (Fig. 1a). The P + Y + sample showed the highest S. thermophilus countand the lowest number of L. bulgaricus while the lowest survival rate of S. thermophilus was also observed in Y- sample during storage (Fig. 1b). Moreover, it was also found that P + Y- and P-Y + yoghurts contained the highest and lowest counts of L. plantarum, respectively (Fig. 1c).
Fig. 1.
The effect of ultrasound treatment on the viability of L. bulgaricus(a), S. thermophilus (b) and L. plantarum (c) in stirred yoghurt during cold storage. (P-Y-: probiotic + yoghurt starter cultures, Y-: yoghurt starters, P + Y-: sonicated probiotic + yoghurt starter cultures, Y+: sonicated yoghurt starters, P-Y+: probiotic + sonicated yoghurt starter cultures, P + Y+: sonicated probiotic + sonicated yoghurt starter cultures).
The decreased LAB growth during the cold storage period can be due to the production and accumulation of lactic acid. Moreover, the different viability of various Lactiplantibacillus strains during cold storage of yoghurt may be due to differences in their growth rate and acidification capacity[28]. Moreover, lactic acid bacteria generate diverse antimicrobial peptides and proteins (bacteriocins) to boost their competitive edge against closely related species.For example, according to Settachaimongkon, van Valenberg, Gazi, Nout, van Hooijdonk, Zwietering and Smid [29], co-culture of sublethally precultured L. plantarum WCFS1 with yoghurt starter cultures significantly impaired the survival of L. delbrueckii subsp. bulgaricus, while no adverse effect on the survival of S. thermophilus has been recorded.They possibly attributed the adverse effect of stress-adapted L. plantarum WCFS1 on the survival of L.delbrueckii subsp.bulgaricus to the induction of plantaricins production.
Based on previous studies, mixing S. thermophilus with cultures such as L. bulgaricus, L. rhamnosus, L. acidophilus and L. casei causes better growth and higher survival of this bacterium, which is attributed to the higher proteolytic activity and lactose consumption of this strain compared to some strains[30].The elimination of cell bunches in microbial cultures, leading to increased nutrient utilization; the elevation of cell membrane permeability, resulting in enhanced membrane absorption of nutrients and, consequently, increased cellular growth and proliferation; and the improvement of culture medium conditions, transforming it into an optimal environment for the growth and multiplication of cells, are cited as factors that accentuate the positive effects of ultrasound on microbial metabolism[31].In general, the positive effect of ultrasound on LAB growth can be due to the release of some growth-promoting components, as well as the slight rise in the local temperature within the sample inducing LAB activation and the creation of anaerobic conditions due to ultrasonic degassing[16], [32]. Shokri, Terefe, Shekarforoush and Hosseinzadeh [33]demonstrated that the growth rate of Lactobacillus brevis increases when subjected to ultrasonication compared to non-ultrasonicated samples. Similarly, Dahroud, Mokarram, Khiabani, Hamishehkar, Bialvaei, Yousefi and Kafil [34] reported an enhanced growth rate of Lactobacillus casei subsp. casei when exposed to ultrasound compared to untreated samples. It has been suggested that this increase in growth rate may be attributed to the impact of ultrasound on the medium and microorganisms, such as enhanced mass transfer through micro-mixing and improved transport of nutrients from the outside to the inside of the cell[35]. It has been shown that the response of microorganisms to ultrasound is strain-dependent and therefore it can be expected that ultrasound affects the survival of different LAB to different extents[36]. Moreover, the influence of ultrasound treatment on the growth and viability of microorganisms is affected by their physical and biological properties such as size, growth phase, capsule thickness, etc.[37].
3.2. The inhibition of pathogenic bacteria in yoghurt during incubation
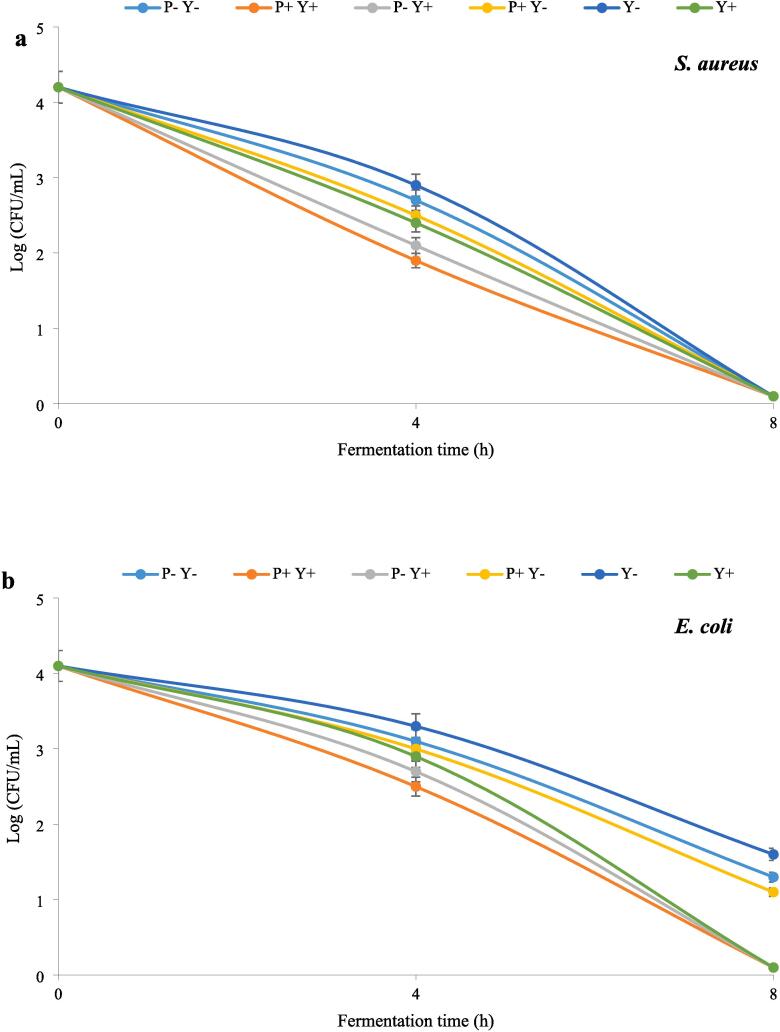
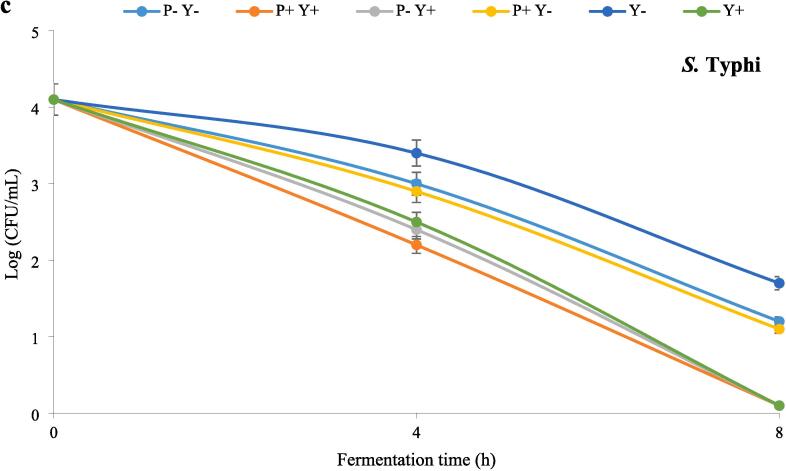
The effects of ultrasound treatments on S. aureus, E. coli and S. Typhi in stirred yoghurt during 8 h incubation period at 40 °C are shown in Fig. 2a-c, respectively. According to the figures, the number of pathogens decreased significantly (P ≤ 0.05) with the elapse of fermentation time. The highest and lowest number of pathogens were found in Y- and Y + P + samples, respectively. In general, samples containing ultrasonicated yoghurt cultures (L. bulgaricus, S. thermophilus) were more effective in inhibiting pathogens, which is attributed to the shear forces created by the acoustic cavitation bubbles[38]. No significant differences were observed in the number of S. aureus among different samples at the end of the fermentation period (8 h).
Fig. 2.
The effect of ultrasound treatment on pathogenic bacteria in stirred yoghurt during incubation, S. aureus (a), E. coli (b) and S.Typhi (c). (P-Y-: probiotic + yoghurt starter cultures, Y-: yoghurt starters, P + Y-: sonicated probiotic + yoghurt starter cultures, Y+: sonicated yoghurt starters, P-Y+: probiotic + sonicated yoghurt starter cultures, P + Y+: sonicated probiotic + sonicated yoghurt starter cultures).
P + Y + yoghurt contained the lowest number of S. aureus in the fourth hour of fermentation, while the highest number of S. aureus was observed in Y- sample. The highest and lowest number of E. coli were also detected in Y- and P + Y + yoghurts, respectively. At the end of fermentation, the number of E. coli in P + Y+, P-Y + and Y + samples was significantly (P ≤ 0.05) lower than the other three samples. After the start of fermentation, the number of S. Typhi in Y-yoghurt was significantly higher than others. At mid-fermentation time (4 h), the number of S. Typhi in P + Y + sample was significantly (P ≤ 0.05) lower than the other yoghurts (except P-Y + yoghurt).At the end of fermentation, the number of S. Typhi in P + Y+, P-Y + and Y + samples was significantly (P ≤ 0.05) lower than the other samples.
Microbial tests did not detect any pathogens during cold storage, which could be due to post-acidification and increased lactic acid concentration. The main reason for the inhibitory effects of lactic acid bacteria against pathogens is the production of lactic acid. Additionally, the production of other metabolites such as bacteriocins, hydrogen peroxide, diacetyl and short-chain fatty acids are also involved in the antimicrobial activity of these bacteria [39].
The ability of the sonicated L. bulgaricus and S. thermophilus to produce higher amounts of lactic acid could be the reason for further reduction of pathogens in samples containing these LAB. Ultrasound can stimulate the growth of lactic acid bacteria and reduce the pH of the environment by accelerating the hydrolysis of lactose, which makes the environment unsuitable for the growth of pathogens [40].Ultrasound can lead to the release of bioactive peptides. The antimicrobial peptides released by LAB probiotics kill the pathogenic organisms [41].
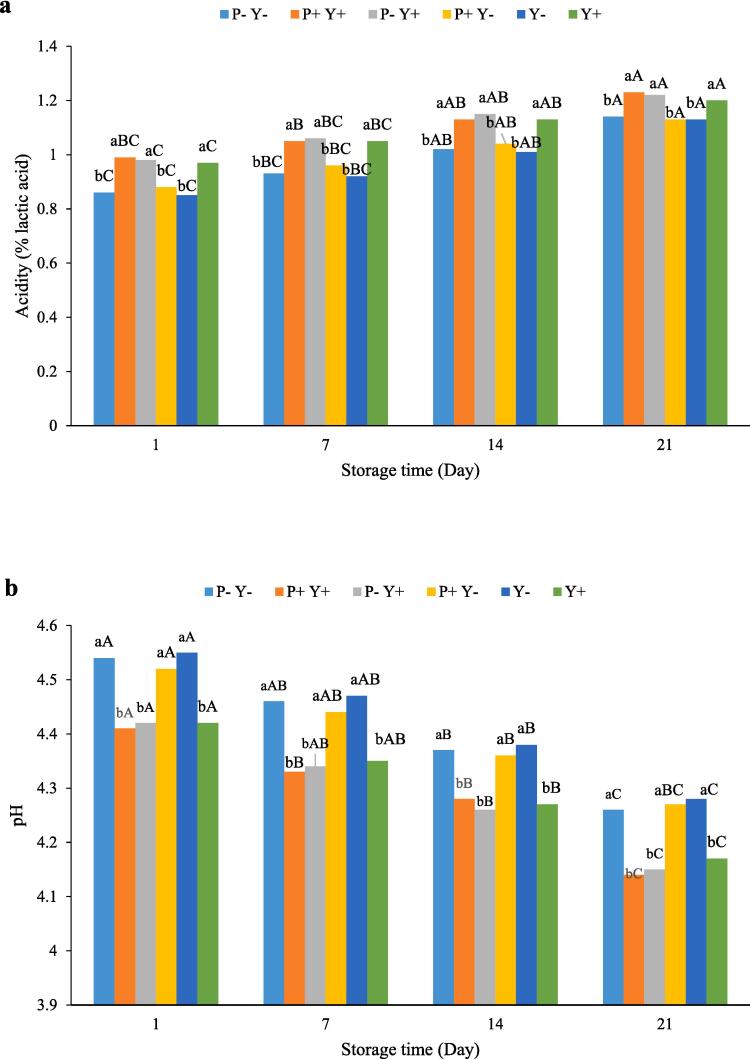
3.3. Changes in acidity and pH of yoghurt during cold storage
Quantitative changes in titratable acidity and pH of yoghurts during cold storage are shown in Fig. 3a and 3b, respectively.Accordingly, the acidity of the samples increased with the elapse of storage time, so that yoghurts had significantly (P ≤ 0.05) higher acidity at the end of cold storage than at the beginning of it. Sonication of yoghurt cultures and L. plantarum increased the acid production in the samples containing them. Yoghurts containing Y + starters had the highest amount of acidity, while the lowest acidity was observed in Y- sample.
Fig. 3.
The effect of ultrasound treatment on the acidity (a) and pH (b) of stirred yoghurt during cold storage.(P-Y-: probiotic + yoghurt starter cultures, Y-: yoghurt starters, P + Y-: sonicated probiotic + yoghurt starter cultures, Y+: sonicated yoghurt starters, P-Y+: probiotic + sonicated yoghurt starter cultures, P + Y+: sonicated probiotic + sonicated yoghurt starter cultures).
The pH values of the yoghurts varied between 4.14 (P + Y + sample on day 21) to 4.55 (Y- sample on day 1). Increasing the storage time led to a significant (P ≤ 0.05) decrease in the pH values of the yoghurts. Although the difference between the pH of P + Y+, P-Y + and Y + yoghurts was not significant, their pH was significantly (P ≤ 0.05) lower than that of other samples. In general, the ultrasonicated cultures further reduced the pH of the yoghurts, and among them, ultrasonicated L. bulgaricus and S. thermophilus were more effective in reducing pH of samples than L. plantarum.
During lactic fermentation, lactose is hydrolyzed intracellularly to glucose and galactose by β-galactosidase. These monosaccharides are subsequently converted into lactic acid.Ultrasonication of bacterial cells can lead to the release of β-galactosidase[42], through a process known as the ultrasound switching effect [43], [44]. β-galactosidase enzymes released from ultrasonicated bacterial cells provide more glucose and galactose to LAB, leading to more lactic acid production[32]. It has been shown that the increase in lactose hydrolysis in sonicated fermentation depends on the bacterial strains used.Various survival rates of LAB strains cause them to show different inherent abilities to hydrolyse lactose[36]. Moreover, the increase in acidity can be affected by different ultrasound parameters.
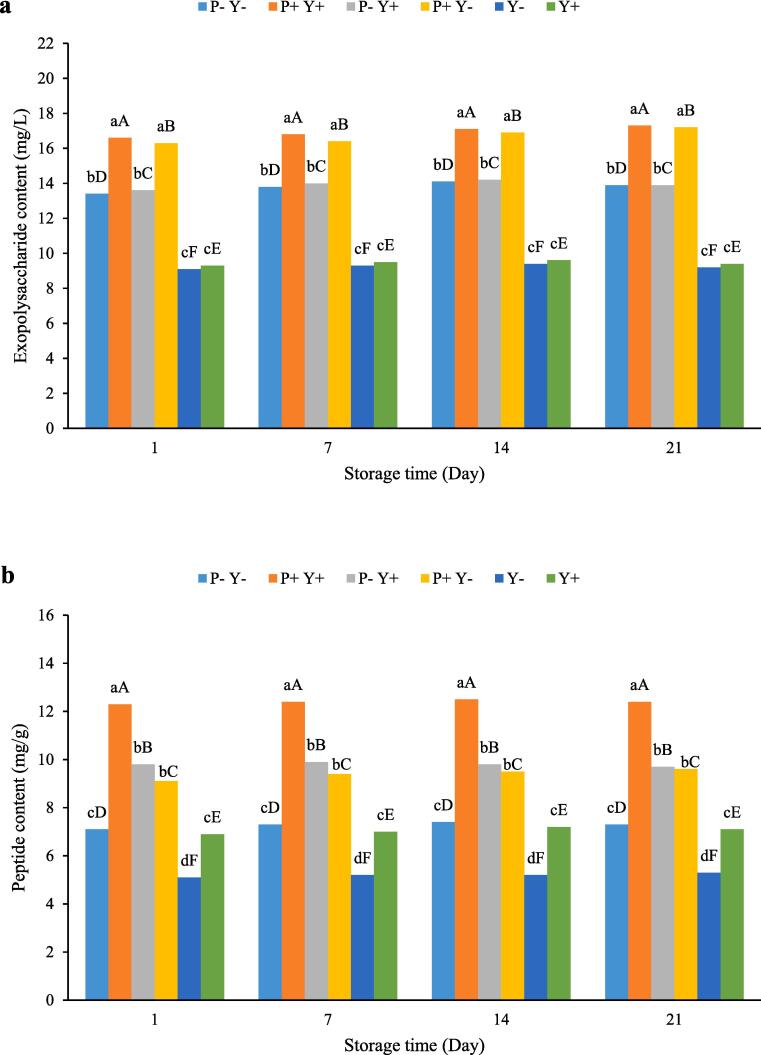
3.4. EPS and peptide contents of yoghurt during cold storage
The EPS changes in various samples as a function of storage time is shown in Fig. 4a. As can be seen, the EPS concentration did not change significantly in any of the yoghurt samples during the cold storage period. At all storage times, the highest amount of EPS was found in P + Y + and P + Y-, followed by P-Y + and P-Y- yoghurts, whereas the lowest amount was measured in Y + and Y- samples.
Fig. 4.
The effect of ultrasound treatment on exopolysaccharide (a) and peptide (b) contents of stirred yoghurt during cold storage. (P-Y-: probiotic + yoghurt starter cultures, Y-: yoghurt starters, P + Y-: sonicated probiotic + yoghurt starter cultures, Y+: sonicated yoghurt starters, P-Y+: probiotic + sonicated yoghurt starter cultures, P + Y+: sonicated probiotic + sonicated yoghurt starter cultures). Different letters indicate significant differences (P ≤ 0.05). Lowercase letters: differences between different samples in the same time conditions. Uppercase letters: differences between the same samples at different storage times.
Fermentation conditions, culture medium composition, microbial growth phase and strain type affect the production and the physicochemical composition of EPS [45]. The production of EPS by L. bulgaricus strains has been reported previously[46]. For instance, Khadem, Tirtouil, Drabo and Boubakeur [47] also showed that S. thermophilus strain had important potential to produce EPS. They reported that using ultrasound for a certain period of time could increase the production of EPS by this strain. On the other hand, Hashemi and Gholamhosseinpour [48] reported that ultrasonication had a growth stimulating effect on L. plantarum LP3 and LU5 strains and increased the amount of EPS produced by facilitating their growth.
Fig. 4b illustrates the peptide content of yoghurts over 21 d storage period. Based on the figure, storage time had no significant effect on the peptide concentration of samples.The peptide content of the samples varied between 5.1 (mg/g) on the first day for Y- yoghurt to 12.5 (mg/g) on day 14 for P + Y + yoghurt. The peptide content of P + Y + yoghurt was significantly (P ≤ 0.05) higher than that of other samples, while the lowest peptide content was found in sample Y-.
Increased levels of bioactive peptides under the influence of ultrasound treatment have been reported in various studies[49], [50]. It has been reported that the formation, expansion and collapse of cavitation bubble, microstreaming, sonochemical reactions and particularly water sonolysis during the use of ultrasound treatment can lead to physicochemical changes in proteins and peptides [51].Ozuna, Paniagua-Martínez, Castaño-Tostado, Ozimek and Amaya-Llano [52] reported that ultrasound-induced cavitation promotes a greater accessibility to proteolytic enzymes by degrading the tertiary and quaternary structures of proteins, leading to the release of bioactive peptides.Wali, Ma, Shahnawaz, Hayat, Xiaong and Jing [49] stated that ultrasound of protein before enzymatic hydrolysis improved the release of bioactive peptides due to the protein unfolding and enhanced accessibility of enzymes, although the purity and structure of the protein should also be considered.
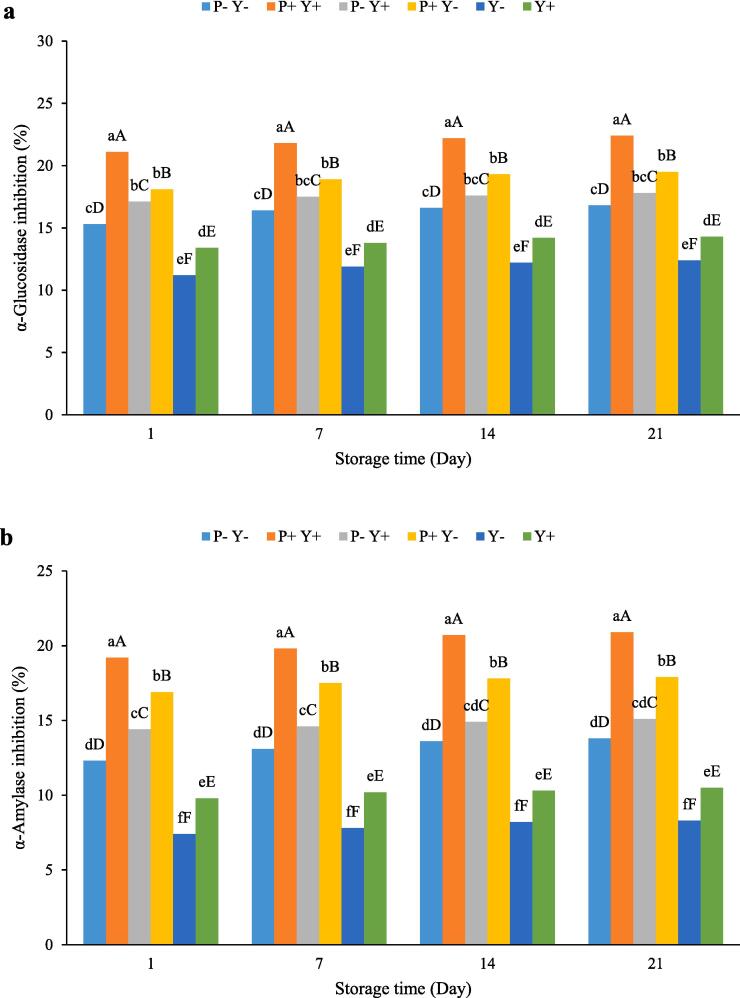
3.5. α-Glucosidase and α-amylase inhibitory activities of yoghurt during cold storage
Results of α-glucosidase and α-amylase inhibitory activities are shown in Fig. 5a-b, respectively. As can be observed, the changes in α-glucosidase and α-amylase inhibition activities of yoghurts during the cold storage period were not significant. The highest α-glucosidase and α-amylase inhibition capacity was related to P + Y + sample, followed by P + Y-, P-Y+, P-Y-, Y + and Y- yoghurts. Also, the α-amylase inhibition activities of all yoghurts (except P-Y + and P-Y- samples on the 14 and 21 days of cold storage) were significantly (P ≤ 0.05) different from each other (Fig. 5b).
Fig. 5.
The effect of ultrasound treatment on α-glucosidase (a) and α-amylase (b) inhibition of stirred yoghurt during cold storage. (P-Y-: probiotic + yoghurt starter cultures, Y-: yoghurt starters, P + Y-: sonicated probiotic + yoghurt starter cultures, Y+: sonicated yoghurt starters, P-Y+: probiotic + sonicated yoghurt starter cultures, P + Y+: sonicated probiotic + sonicated yoghurt starter cultures). Different letters indicate significant differences (P ≤ 0.05). Lowercase letters: differences between different samples in the same time conditions. Uppercase letters: differences between the same samples at different storage times.
Bioactive peptides produced by proteolytic enzymes of LAB strains inhibit the activities of α-glucosidase and α-amylase enzymes[11]. In addition, the bioactivity of EPS produced by LAB has been shown to help inhibiting these enzymes [53].
For instance, various previous studies have reported the effect of ultrasound on increasing the inhibition of α-glucosidase and α-amylase enzymes[48], [54]. Ultrasound destroys the LAB cell wall, releasing intracellular proteases out of the cell through the microjetting and shockwaves processes accompanying the collapse of acoustic cavitation bubbles. Bioactive peptides resulting from the proteolytic activity of these enzymes are able to inhibit the activity of α-glucosidase and α-amylase. Ultrasonication can further enhance the subsequent activity of the released intracellular enzymes[54].
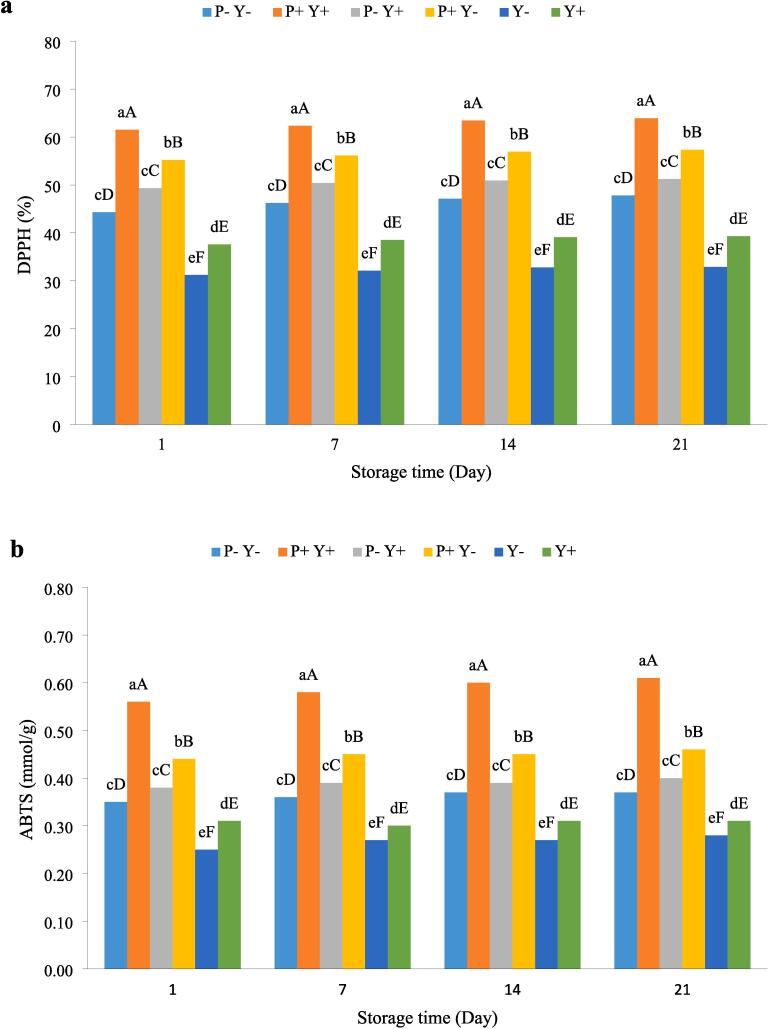
3.6. The antioxidant and cytotoxicity activities of yoghurt during cold storage
The radical scavenging activity of yoghurts during 3 weeks of storage, measured by the DPPH method, is shown in Fig. 6a. The results showed a non-significant increase in the antioxidant activity of yoghurts as the storage time progressed. At all storage times, P + Y + yoghurt showed the highest antioxidant activity compared with that of the others, followed by P + Y-, P-Y+, P-Y-, Y + and Y- samples. The antioxidant activities of P + Y+, P + Y-, P-Y+, Y + and Y- yoghurts were significantly (P ≤ 0.05) different from each other.
Fig. 6.
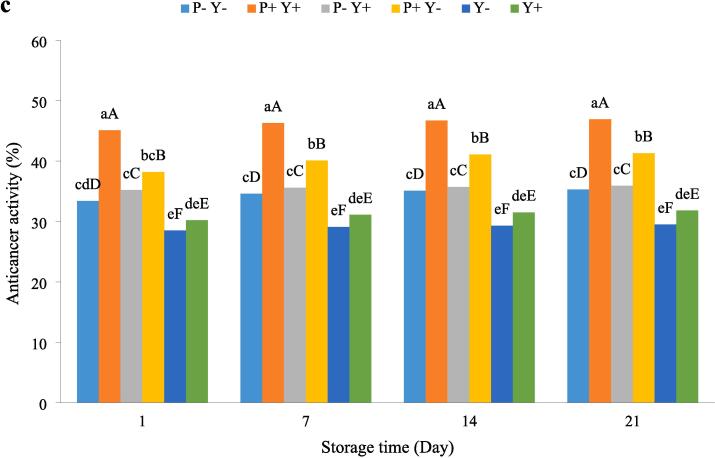
The effect of ultrasound treatment on antioxidant (DPPH (a) and ABTS (b)) and cytotoxicity against cancer cells (c) activities of stirred yoghurt during cold storage. (P-Y-: probiotic + yoghurt starter cultures, Y-: yoghurt starters, P + Y-: sonicated probiotic + yoghurt starter cultures, Y+: sonicated yoghurt starters, P-Y+: probiotic + sonicated yoghurt starter cultures, P + Y+: sonicated probiotic + sonicated yoghurt starter cultures). Different letters indicate significant differences (P ≤ 0.05). Lowercase letters: differences between different samples in the same time conditions. Uppercase letters: differences between the same samples at different storage times.
The measured antioxidant activity of yoghurts by the ABTS method is presented in Fig. 6b. The antioxidant activity measured by ABTS method showed a similar trend to that of DPPH method. The antioxidant activities of P-Y + and P-Y-yoghurts had significant differences (P ≤ 0.05) compared to those of P + Y+, P-Y-, Y- and Y + samples.
An increased antioxidant activity during milk fermentation has been reported previously [55], being cell lysis products, extracellular metabolites, protein peptides and hydrolysed milk constituents proposed as the main causes of this increase. The proteolytic activity of lactic acid bacteria increases the levels of antioxidant peptides and free amino acids and thus improves the overall antioxidant properties of the product[12]. Balakrishnan and Agrawal [55] stated that the reason for the significant increase in antioxidant activity of fermented milk compared to non-fermented milk is the existence of peptides from protein hydrolysis in the former.
Various studies have shown that ultrasonication increases the antioxidant capacity of products. In their research, Uluko, Zhang, Liu, Tsakama, Lu and Lv [50]pointed out the ability of ultrasonic treatment to produce peptides with antioxidant properties and stated that ultrasonicated samples had higher antioxidant capacities than untreated samples. In addition, the antioxidant activity of β-lactoglobulin has been attributed to the existence of surface sulfhydryl groups in this protein[56]. Increasing the ultrasound amplitude increases the antioxidant activity by partially degrading inter- and intra-molecular hydrogen bonds of the β-lactoglobulin molecule[57].
The changes in the cytotoxicity against cancer cells of yoghurt samples during cold storage period are given in Fig. 6c. In general, the increase of cytotoxicity against cancer cells of the samples during storage was not significant. During the storage period, the highest and lowest cytotoxicity against cancer cellswere obtained for P + Y+ (45.10–46.90 %) and Y- (28.5–29.5 %) samples, respectively. The antioxidant activities of P + Y + and P + Y- yoghurts were significantly (P ≤ 0.05) higher than those of other samples. The proteolytic and acidification activities of lactic acid bacteria as well as fermentation conditions are among the main factors affecting the anticancer (cytotoxicity against cancer cells) properties of fermented dairy products[57]. It is thought that most LAB strains exhibit their anti-proliferative effects by inducing apoptosis and necrosis, although the exact mechanism of the anticancer effects of the fractions produced by these bacteria has not yet been fully elucidated[58].
Ultrasound treatment enhances the anticancer capacity of products due to its effect on increasing the amount of peptides[16], [51].Sah, Vasiljevic, McKechnie and Donkor [59] have stated that the anticancer activities of natural and synthetic anticancer peptides are influenced by important factors such as net charge, hydrophobicity,amphipathicity, oligomerization ability, and secondary structure in the membrane.
4. Conclusions
In this investigation, the impacts of ultrasound treatment and storage time on LAB viability, safety and bioactive attributes of stirred yoghurt were explored. The findings revealed that ultrasonication of the inoculated milk fostered the proliferation of LAB, leading to a reduction in the population of pathogenic microorganisms during the fermentation by 60 % to 97 %, depending on the type of pathogen. Although the inactivation was not complete, it significantly enhanced the initial safety of the product. Throughout storage, the LAB counts and pH of all samples exhibited a significant decrease (P ≤ 0.05), while the bioactive properties of yoghurts were insignificantly affected by the storage period. The P + Y + yoghurt showed significantly higher levels (P ≤ 0.05) of peptides, antioxidant activity, α-glucosidase and α-amylase inhibition, EPS content, and cytotoxicity against cancer cells compared to other samples. Ultrasound, by stimulating the growth of LAB, heightened their activity during cold storage. The accumulation of lactic acid produced by these bacteria during cold storage resulted in the complete inhibition of pathogens, ensuring the safety of the final product. In conclusion, ultrasound treatment can be considered a valuable technique for enhancing safety and improving the bioactive properties of stirred yoghurt. As a future approach, it is recommended to investigate the effect of pulsed ultrasound on the activity of LAB and the bioactive properties of fermented foods.
CRediT authorship contribution statement
Aliakbar Gholamhosseinpour: Conceptualization, Data curation, Investigation, Methodology, Writing – review & editing. Seyed Mohammad Bagher Hashemi: . Fatemeh Safari: Data curation, Formal analysis, Investigation, Methodology, Software. Kaouther Kerboua: Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Shiby V., Mishra H. Fermented milks and milk products as functional foods—A review. Crit. Rev. Food Sci. Nutr. 2013;53:482–496. doi: 10.1080/10408398.2010.547398. [DOI] [PubMed] [Google Scholar]
- 2.N.H. El-Abbadi, M.C. Dao, S.N. Meydani, Yogurt: role in healthy and active aging, The American journal of clinical nutrition, 99 (2014) 1263S-1270S. [DOI] [PMC free article] [PubMed]
- 3.Savaiano D.A., Hutkins R.W. Yogurt, cultured fermented milk, and health: A systematic review. Nutr. Rev. 2021;79:599–614. doi: 10.1093/nutrit/nuaa013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li S., Ye A., Singh H. Effects of seasonal variations on the quality of set yogurt, stirred yogurt, and Greek-style yogurt. J. Dairy Sci. 2021;104:1424–1432. doi: 10.3168/jds.2020-19071. [DOI] [PubMed] [Google Scholar]
- 5.Nyanzi R., Jooste P.J., Buys E.M. Invited review: Probiotic yogurt quality criteria, regulatory framework, clinical evidence, and analytical aspects. J. Dairy Sci. 2021;104:1–19. doi: 10.3168/jds.2020-19116. [DOI] [PubMed] [Google Scholar]
- 6.Bintsis T. Lactic acid bacteria as starter cultures: An update in their metabolism and genetics. AIMS Microbiology. 2018;4:665. doi: 10.3934/microbiol.2018.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ağagündüz D., Şahin T.Ö., Ayten Ş., Yılmaz B., Güneşliol B.E., Russo P., Spano G., Özogul F. Lactic acid bacteria as pro-technological, bioprotective and health-promoting cultures in the dairy food industry. Food Biosci. 2022 [Google Scholar]
- 8.Feng T., Wang J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: A systematic review. Gut Microbes. 2020;12:1801944. doi: 10.1080/19490976.2020.1801944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elsanhoty R.M., Salam S.A., Ramadan M.F., Badr F.H. Detoxification of aflatoxin M1 in yoghurt using probiotics and lactic acid bacteria. Food Control. 2014;43:129–134. [Google Scholar]
- 10.Kumar N., Puri N., Marotta F., Dhewa T., Calabrò S., Puniya M., Carter J. Diabesity: an epidemic with its causes, prevention and control with special focus on dietary regime. Functional Foods in Health and Disease. 2017;7:1–16. [Google Scholar]
- 11.Ayyash M., Al-Nuaimi A.K., Al-Mahadin S., Liu S.-Q. In vitro investigation of anticancer and ACE-inhibiting activity, α-amylase and α-glucosidase inhibition, and antioxidant activity of camel milk fermented with camel milk probiotic: A comparative study with fermented bovine milk. Food Chem. 2018;239:588–597. doi: 10.1016/j.foodchem.2017.06.149. [DOI] [PubMed] [Google Scholar]
- 12.Gholamhosseinpour A., Hashemi S.M.B. Ultrasound pretreatment of fermented milk containing probiotic Lactobacillus plantarum AF1: Carbohydrate metabolism and antioxidant activity. J. Food Process Eng. 2019;42:e12930. [Google Scholar]
- 13.Hashemi S.M.B., Shahidi F., Mortazavi S.A., Milani E., Eshaghi Z. Potentially probiotic Lactobacillus strains from traditional Kurdish cheese. Probiotics Antimicrob. Proteins. 2014;6:22–31. doi: 10.1007/s12602-014-9155-5. [DOI] [PubMed] [Google Scholar]
- 14.Kiani H., Karimi F., Labbafi M., Fathi M. A novel inverse numerical modeling method for the estimation of water and salt mass transfer coefficients during ultrasonic assisted-osmotic dehydration of cucumber cubes. Ultrason. Sonochem. 2018;44:171–176. doi: 10.1016/j.ultsonch.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Guimarães J.T., Balthazar C.F., Scudino H., Pimentel T.C., Esmerino E.A., Ashokkumar M., Freitas M.Q., Cruz A.G. High-intensity ultrasound: A novel technology for the development of probiotic and prebiotic dairy products. Ultrason. Sonochem. 2019;57:12–21. doi: 10.1016/j.ultsonch.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Gholamhosseinpour A., Hashemi S.M.B., Raoufi Jahromi L., Sourki A.H. Conventional heating, ultrasound and microwave treatments of milk: Fermentation efficiency and biological activities. Int. Dairy J. 2020;110 [Google Scholar]
- 17.Mota M.J., Lopes R.P., Koubaa M., Roohinejad S., Barba F.J., Delgadillo I., Saraiva J.A. Fermentation at non-conventional conditions in food-and bio-sciences by the application of advanced processing technologies. Crit. Rev. Biotechnol. 2018;38:122–140. doi: 10.1080/07388551.2017.1312272. [DOI] [PubMed] [Google Scholar]
- 18.Carrillo-Lopez L.M., Garcia-Galicia I.A., Tirado-Gallegos J.M., Sanchez-Vega R., Huerta-Jimenez M., Ashokkumar M., Alarcon-Rojo A.D. Recent advances in the application of ultrasound in dairy products: Effect on functional, physical, chemical, microbiological and sensory properties. Ultrason. Sonochem. 2021;73 doi: 10.1016/j.ultsonch.2021.105467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farahmandfar R., Safari R., Ahmadi vavsari F., Bakhshandeh T. The effect of a jwain (Trachyspermum ammi) extracted by ultrasound-assisted solvent on quality properties of silver carp (Hypophthalmichthys molitrix) surimi stored at 4C. J. Food Process. Preserv. 2016;40:291–297. [Google Scholar]
- 20.Jayarathna S., Priyashantha H., Johansson M., Vidanarachchi J.K., Jayawardana B.C., Liyanage R. Probiotic enriched fermented soy-gel as a vegan substitute for dairy yoghurt. J. Food Process. Preserv. 2021;45:e15092. [Google Scholar]
- 21.Amatayakul T., Halmos A., Sherkat F., Shah N. Physical characteristics of yoghurts made using exopolysaccharide-producing starter cultures and varying casein to whey protein ratios. Int. Dairy J. 2006;16:40–51. [Google Scholar]
- 22.Lu W., Ren G.-P., Song J.-M. Determination of Content of Peptides in Protein Hydrolysates [J] Food Sci. 2005;7:039. [Google Scholar]
- 23.Huang G., Chen S., Tang Y., Dai C., Sun L., Ma H., He R. Stimulation of low intensity ultrasound on fermentation of skim milk medium for yield of yoghurt peptides by Lactobacillus paracasei. Ultrason. Sonochem. 2019;51:315–324. doi: 10.1016/j.ultsonch.2018.09.033. [DOI] [PubMed] [Google Scholar]
- 24.Kim Y.-M., Wang M.-H., Rhee H.-I. A novel α-glucosidase inhibitor from pine bark. Carbohydr. Res. 2004;339:715–717. doi: 10.1016/j.carres.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Y.H. Pyo, T.C. Lee, Y.C. Lee, Effect of lactic acid fermentation on enrichment of antioxidant properties and bioactive isoflavones in soybean, Journal of food science, 70 (2005) S215-S220.
- 26.Virtanen T., Pihlanto A., Akkanen S., Korhonen H. Development of antioxidant activity in milk whey during fermentation with lactic acid bacteria. J. Appl. Microbiol. 2007;102:106–115. doi: 10.1111/j.1365-2672.2006.03072.x. [DOI] [PubMed] [Google Scholar]
- 27.Elfahri K., Vasiljevic T., Yeager T., Donkor O. Anti-colon cancer and antioxidant activities of bovine skim milk fermented by selected Lactobacillus helveticus strains. J. Dairy Sci. 2016;99:31–40. doi: 10.3168/jds.2015-10160. [DOI] [PubMed] [Google Scholar]
- 28.Hashemi S.M.B., Gholamhosseinpour A., Abedi E. Biopreservative potential of Lactobacillus strains in yoghurt dessert. J. Food Meas. Charact. 2021;15:1634–1643. [Google Scholar]
- 29.Settachaimongkon S., van Valenberg H.J., Gazi I., Nout M.R., van Hooijdonk T.C., Zwietering M.H., Smid E.J. Influence of Lactobacillus plantarum WCFS1 on post-acidification, metabolite formation and survival of starter bacteria in set-yoghurt. Food Microbiol. 2016;59:14–22. doi: 10.1016/j.fm.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen H.T.H., Ong L., Lefèvre C., Kentish S.E., Gras S.L. The microstructure and physicochemical properties of probiotic buffalo yoghurt during fermentation and storage: a comparison with bovine yoghurt. Food Bioproc. Tech. 2014;7:937–953. [Google Scholar]
- 31.Behzadnia A., Moosavi-Nasab M., Tiwari B.K., Setoodeh P. Lactobacillus plantarum-derived biosurfactant: Ultrasound-induced production and characterization. Ultrason. Sonochem. 2020;65 doi: 10.1016/j.ultsonch.2020.105037. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen T.M.P., Lee Y.K., Zhou W. Stimulating fermentative activities of bifidobacteria in milk by highintensity ultrasound. Int. Dairy J. 2009;19:410–416. [Google Scholar]
- 33.Shokri S., Terefe N.S., Shekarforoush S.S., Hosseinzadeh S. Ultrasound-assisted fermentation for enhancing metabolic and probiotic activities of LactoBacillus brevis. Chemical Engineering and Processing-Process Intensification. 2021;166 [Google Scholar]
- 34.Dahroud B.D., Mokarram R.R., Khiabani M.S., Hamishehkar H., Bialvaei A.Z., Yousefi M., Kafil H.S. Low intensity ultrasound increases the fermentation efficiency of Lactobacillus casei subsp. casei ATTC 39392. Int. J. Biol. Macromol. 2016;86:462–467. doi: 10.1016/j.ijbiomac.2016.01.103. [DOI] [PubMed] [Google Scholar]
- 35.Bolívar-Jacobo N.A., Reyes-Villagrana R.A., Espino-Solís G.P., Rentería-Monterrubio A.L., Arévalos-Sánchez M.M., Sánchez-Vega R., Santellano-Estrada E., Chávez-Flores D., Chávez-Martínez A. The Effects of a High-Intensity Ultrasound on the Fermentative Activity and Kinetic Growth of Lactobacillus Acidophilus and Lactobacillus Helveticus. Fermentation. 2023;9:356. [Google Scholar]
- 36.Abesinghe A., Islam N., Vidanarachchi J., Prakash S., Silva K., Karim M. Effects of ultrasound on the fermentation profile of fermented milk products incorporated with lactic acid bacteria. Int. Dairy J. 2019;90:1–14. [Google Scholar]
- 37.Gao S., Lewis G.D., Ashokkumar M., Hemar Y. Inactivation of microorganisms by low-frequency high-power ultrasound: 1. Effect of Growth Phase and Capsule Properties of the Bacteria. Ultrasonics Sonochemistry. 2014;21:446–453. doi: 10.1016/j.ultsonch.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Dai J., Bai M., Li C., Cui H., Lin L. Advances in the mechanism of different antibacterial strategies based on ultrasound technique for controlling bacterial contamination in food industry. Trends Food Sci. Technol. 2020;105:211–222. [Google Scholar]
- 39.Ewe J.-A., Wan-Abdullah W.-N., Alias A.K., Liong M.-T. Effects of ultrasound on growth, bioconversion of isoflavones and probiotic properties of parent and subsequent passages of Lactobacillus fermentum BT 8633 in biotin-supplemented soymilk. Ultrason. Sonochem. 2012;19:890–900. doi: 10.1016/j.ultsonch.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 40.Ojha K.S., Mason T.J., O’Donnell C.P., Kerry J.P., Tiwari B.K. Ultrasound technology for food fermentation applications. Ultrason. Sonochem. 2017;34:410–417. doi: 10.1016/j.ultsonch.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 41.Srinivash M., Krishnamoorthi R., Mahalingam P.U., Malaikozhundan B., Keerthivasan M. Probiotic potential of exopolysaccharide producing lactic acid bacteria isolated from homemade fermented food products. Journal of Agriculture and Food Research. 2023;11 [Google Scholar]
- 42.Nguyen T.M.P., Lee Y.K., Zhou W. Effect of high intensity ultrasound on carbohydrate metabolism of bifidobacteria in milk fermentation. Food Chem. 2012;130:866–874. [Google Scholar]
- 43.Sangwan V., Tomar S.K., Ali B., Singh R.R., Singh A.K. Production of β-galactosidase from streptococcus thermophilus for galactooligosaccharides synthesis. J. Food Sci. Technol. 2015;52:4206–4215. doi: 10.1007/s13197-014-1486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.H. Nomura, S. Koda, What Is Sonochemistry?, in: Sonochemistry and the acoustic bubble, Elsevier, 2015, pp. 1-9.
- 45.Yilmaz M., Dertli E., Toker O., Tatlisu N., Sagdic O., Arici M. Effect of in situ exopolysaccharide production on physicochemical, rheological, sensory, and microstructural properties of the yogurt drink ayran: an optimization study based on fermentation kinetics. J. Dairy Sci. 2015;98:1604–1624. doi: 10.3168/jds.2014-8936. [DOI] [PubMed] [Google Scholar]
- 46.Bancalari E., Gatti M., Bottari B., Mora D., Arioli S. Disclosing Lactobacillus delbrueckii subsp. bulgaricus intraspecific diversity in exopolysaccharides production. Food Microbiol. 2021 doi: 10.1016/j.fm.2021.103924. [DOI] [PubMed] [Google Scholar]
- 47.Khadem H., Tirtouil A.M., Drabo M.S., Boubakeur B. Ultrasound conditioning of Streptococcus thermophilus CNRZ 447: growth, biofilm formation, exopolysaccharide production, and cell membrane permeability. Biotechnologia. 2020;101:159–165. [Google Scholar]
- 48.Hashemi S.M.B., Gholamhosseinpour A. Effect of ultrasonication treatment and fermentation by probiotic Lactobacillus plantarum strains on goat milk bioactivities. Int. J. Food Sci. Technol. 2020;55:2642–2649. [Google Scholar]
- 49.Wali A., Ma H., Shahnawaz M., Hayat K., Xiaong J., Jing L. Impact of power ultrasound on antihypertensive activity, functional properties, and thermal stability of rapeseed protein hydrolysates. J. Chem. 2017;2017 [Google Scholar]
- 50.Uluko H., Zhang S., Liu L., Tsakama M., Lu J., Lv J. Effects of thermal, microwave, and ultrasound pretreatments on antioxidative capacity of enzymatic milk protein concentrate hydrolysates. J. Funct. Foods. 2015;18:1138–1146. [Google Scholar]
- 51.Kadam S.U., Tiwari B.K., Álvarez C., O'Donnell C.P. Ultrasound applications for the extraction, identification and delivery of food proteins and bioactive peptides. Trends Food Sci. Technol. 2015;46:60–67. [Google Scholar]
- 52.Ozuna C., Paniagua-Martínez I., Castaño-Tostado E., Ozimek L., Amaya-Llano S.L. Innovative applications of high-intensity ultrasound in the development of functional food ingredients: Production of protein hydrolysates and bioactive peptides. Food Res. Int. 2015;77:685–696. [Google Scholar]
- 53.Al-Dhaheri A.S., Al-Hemeiri R., Kizhakkayil J., Al-Nabulsi A., Abushelaibi A., Shah N.P., Ayyash M. Health-promoting benefits of low-fat akawi cheese made by exopolysaccharide-producing probiotic Lactobacillus plantarum isolated from camel milk. J. Dairy Sci. 2017;100:7771–7779. doi: 10.3168/jds.2017-12761. [DOI] [PubMed] [Google Scholar]
- 54.Kwiatkowska B., Bennett J., Akunna J., Walker G.M., Bremner D.H. Stimulation of bioprocesses by ultrasound. Biotechnol. Adv. 2011;29:768–780. doi: 10.1016/j.biotechadv.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 55.Balakrishnan G., Agrawal R. Antioxidant activity and fatty acid profile of fermented milk prepared by Pediococcus pentosaceus. J. Food Sci. Technol. 2014;51:4138–4142. doi: 10.1007/s13197-012-0891-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li M., Ma Y., Ngadi M.O. Binding of curcumin to β-lactoglobulin and its effect on antioxidant characteristics of curcumin. Food Chem. 2013;141:1504–1511. doi: 10.1016/j.foodchem.2013.02.099. [DOI] [PubMed] [Google Scholar]
- 57.Ma'mon M.H., Nuirat A., Zihlif M.A., Taha M.O. Exploring the influence of culture conditions on kefir's anticancer properties. J. Dairy Sci. 2018;101:3771–3777. doi: 10.3168/jds.2017-13539. [DOI] [PubMed] [Google Scholar]
- 58.Liu C.-F., Pan T.-M. In vitro effects of lactic acid bacteria on cancer cell viability and antioxidant activity. J. Food Drug Anal. 2010;18 [Google Scholar]
- 59.Sah B.N.P., Vasiljevic T., McKechnie S., Donkor O. Identification of anticancer peptides from bovine milk proteins and their potential roles in management of cancer: a critical review. Compr. Rev. Food Sci. Food Saf. 2015;14:123–138. doi: 10.1111/1541-4337.12126. [DOI] [PubMed] [Google Scholar]