Abstract
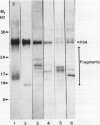
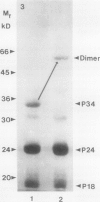
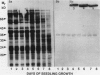
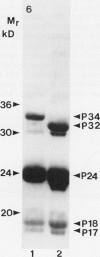
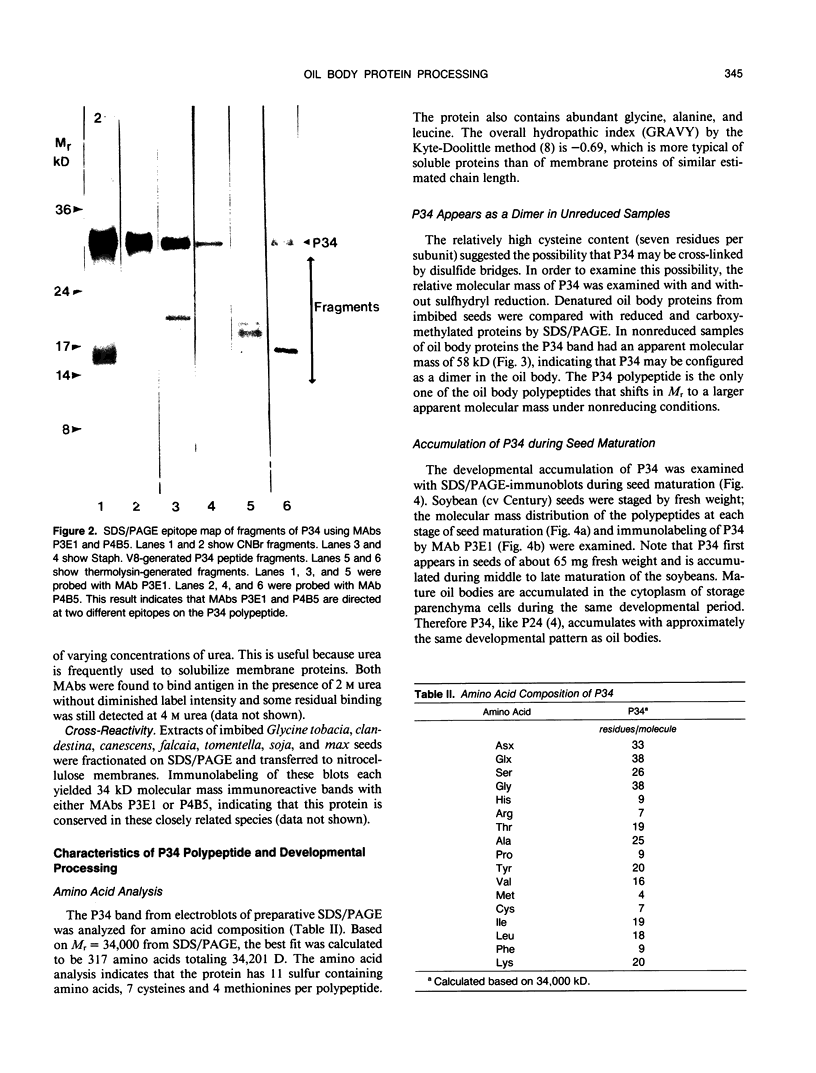
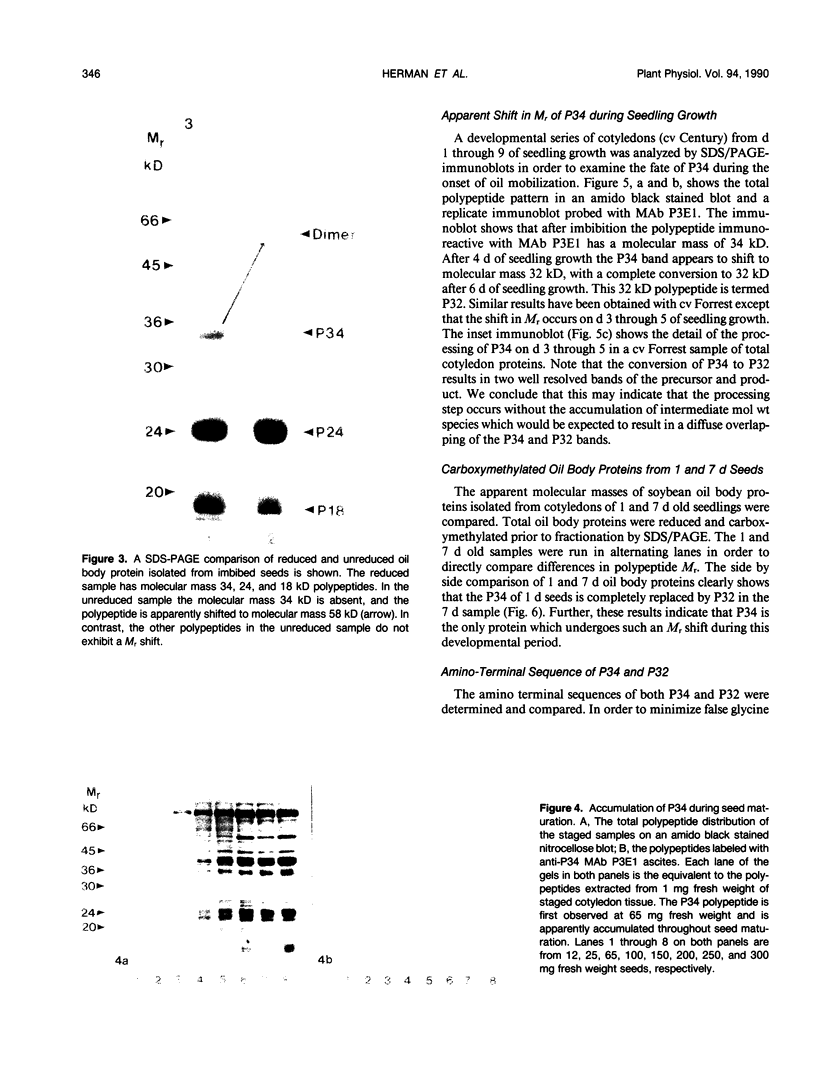
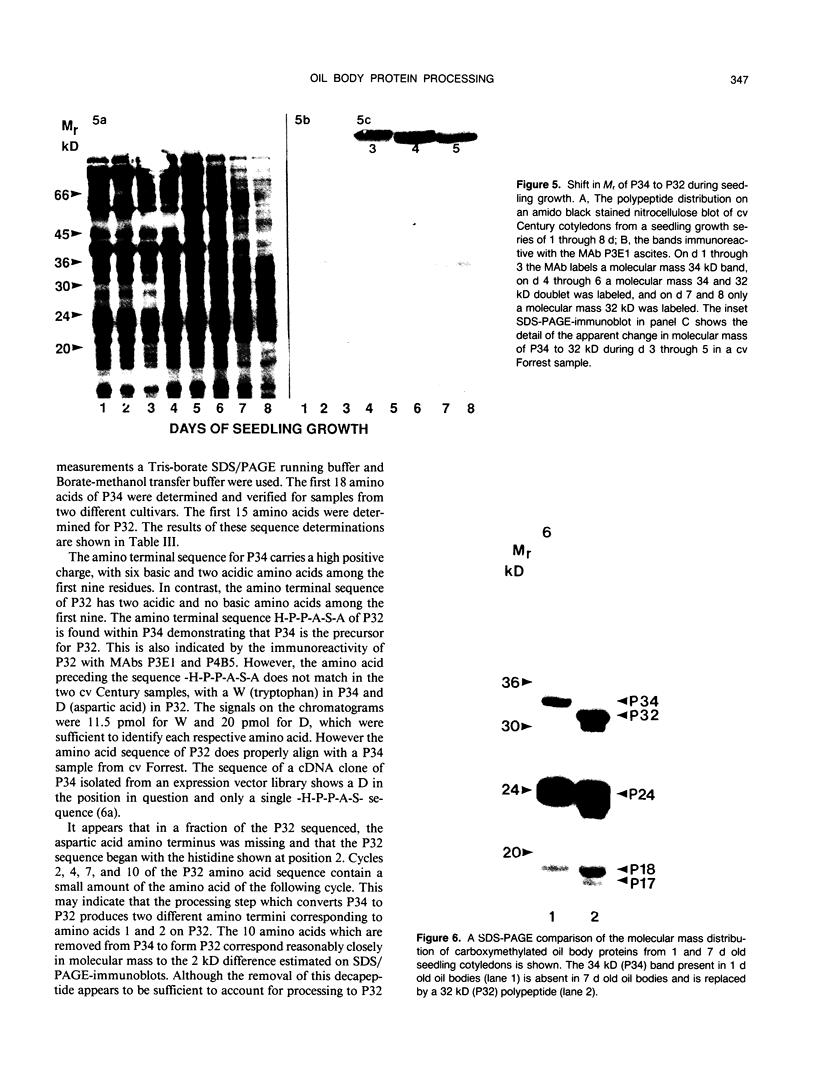
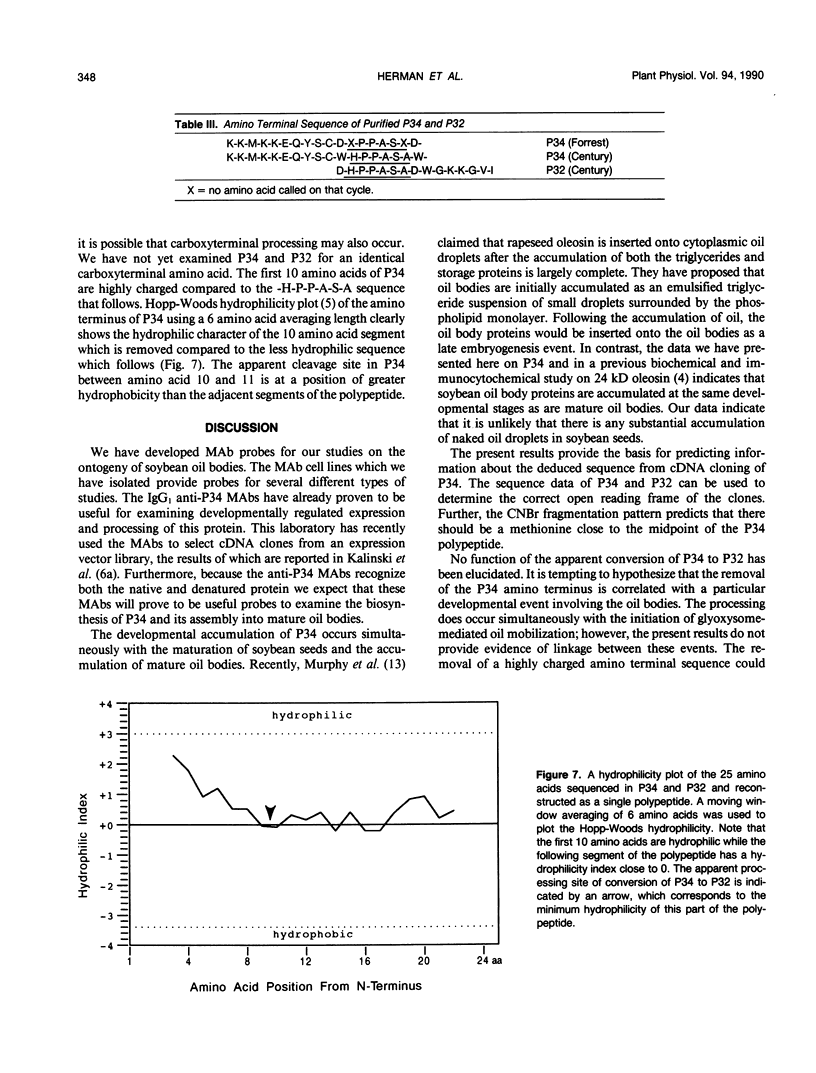
The membrane surrounding the oil body contains several different specific polypeptides. To study the biosynthesis and posttranslational modification of these polypeptides we have prepared monoclonal antibodies against purified oil bodies of soybean (Glycine max). Three of the five monoclonals selected recognize a molecular mass 34 kilodalton protein (P34). Epitope mapping of CNBr and proteolytic fragments of P34 indicates that two of the anti-P34 monoclonal antibodies are directed at different epitopes. P34 is accumulated during seed maturation at the same time as the reserve proteins and oil. SDS/PAGE-immunoblots of germinating soybean seed cotyledons indicate that the protein is initially present as a molecular mass 34 kilodalton polypeptide and is processed to molecular mass 32 kilodalton on the fourth through sixth days of seedling growth simultaneously with the onset of oil mobilization. A comparison of reduced and carboxymethylated oil body proteins with nonreduced proteins by SDS/PAGE indicates that P34 exists in vivo as a dimer of molecular mass 58 kilodalton. Comparing the amino terminal sequences of P34 and P32 indicates that their difference is at least in part due to the removal of the amino terminus of P34. The amino terminal sequences of P34 and P32 were aligned to show that the transition of P34 to P32 was accompanied by the removal of a hydrophilic decapeptide (KKMKKEQYSC) at the amino terminus of P34. Hopp-Woods hydrophilicity analysis of the deleted amino terminus of P34 shows that it is more hydrophilic and charged than the sequence of the protein which immediately follows.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Civin C. I., Banquerigo M. L. Rapid, efficient cloning of murine hybridoma cells in low gelation temperature agarose. J Immunol Methods. 1983 Jun 24;61(1):1–8. doi: 10.1016/0022-1759(83)90002-9. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinski A., Weisemann J. M., Matthews B. F., Herman E. M. Molecular cloning of a protein associated with soybean seed oil bodies that is similar to thiol proteases of the papain family. J Biol Chem. 1990 Aug 15;265(23):13843–13848. [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LeGendre N., Matsudaira P. Direct protein microsequencing from Immobilon-P Transfer Membrane. Biotechniques. 1988 Feb;6(2):154–159. [PubMed] [Google Scholar]
- Moreau R. A., Liu K. D., Huang A. H. Spherosomes of Castor Bean Endosperm: MEMBRANE COMPONENTS, FORMATION, AND DEGRADATION. Plant Physiol. 1980 Jun;65(6):1176–1180. doi: 10.1104/pp.65.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. J., Cummins I., Kang A. S. Synthesis of the major oil-body membrane protein in developing rapeseed (Brassica napus) embryos. Integration with storage-lipid and storage-protein synthesis and implications for the mechanism of oil-body formation. Biochem J. 1989 Feb 15;258(1):285–293. doi: 10.1042/bj2580285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu R., Wang S. M., Lin Y. H., Vance V. B., Huang A. H. Characteristics and biosynthesis of membrane proteins of lipid bodies in the scutella of maize (Zea mays L.). Biochem J. 1986 Apr 1;235(1):57–65. doi: 10.1042/bj2350057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick D., Sartoris D. J. Diagnosing internal derangement of the knee with magnetic resonance imaging. West J Med. 1989 Jun;150(6):682–683. [PMC free article] [PubMed] [Google Scholar]
- Slack C. R., Bertaud W. S., Shaw B. D., Holland R., Browse J., Wright H. Some studies on the composition and surface properties of oil bodies from the seed cotyledons of safflower (Carthamus tinctorius) and linseed (Linum ustatissimum). Biochem J. 1980 Sep 15;190(3):551–561. doi: 10.1042/bj1900551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance V. B., Huang A. H. The major protein from lipid bodies of maize. Characterization and structure based on cDNA cloning. J Biol Chem. 1987 Aug 15;262(23):11275–11279. [PubMed] [Google Scholar]
- Yatsu L. Y., Jacks T. J. Spherosome membranes: half unit-membranes. Plant Physiol. 1972 Jun;49(6):937–943. doi: 10.1104/pp.49.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]