Highlights
-
•
Hepatitis A vaccine is recommended for all people with HIV.
-
•
We studied hepatitis A immunization rates among people with HIV.
-
•
We observed low hepatitis A immunization rates in our study population.
-
•
CD4 count < 200 cells/µl and active substance use disorder were factors associated with longer time to vaccination.
-
•
Strategies are needed to improve hepatitis A immunization rates in our study population.
Keywords: hepatitis A, Human immunodeficiency virus, Vaccination, Immunization, Hepatitis
Abstract
Background
Studies have demonstrated low hepatitis A virus (HAV) vaccination rates among persons with HIV (PWH).
Methods
We conducted a retrospective study of persons entering HIV care at two clinics in Houston, Texas between 2010 and 2018. We defined those eligible for HAV vaccination as those who had no history of HAV vaccination and had a negative anti-HAV IgG at entry to care. Kaplan-Meier curves summarized time to receipt of HAV vaccines. The proportions of patients who received 1 and 2 HAV vaccines at 6, 12, and 24 months were estimated. Cox proportional hazards regression evaluated associations between patient characteristics and vaccination. Significant factors were included in a multivariable Cox proportional hazards model.
Results
Of 6,515 patients, 1372 were eligible for HAV vaccination. Of eligible patients, 29.2 % received 1 HAV vaccination at 6 months, 37.1 % at 12 months, and 47.8 % at 24 months. At 6 months, 10 % received 2 HAV vaccinations, 21.1 % at 12 months, and 33.4 % at 24 months. In multivariable analysis, men who have sex with men (adjusted HR 1.35, 95 % CI 1.06, 1.73) or those who had CD4 count ≥ 200 cells/µl (adjusted HR 2.52, 95 % CI 1.89, 3.37) had their second vaccination sooner than those who were not men who have sex with men or who had CD4 counts < 200 cells/µl, respectively. Patients > 50 years of age had their second vaccination sooner than those aged 30–50 years (adjusted HR 1.47, 95 % CI 1.08, 1.99). Those with active substance history had a longer time to second vaccination compared to those with no substance use history (adjusted HR 0.57, 95 % CI 0.40, 0.82).
Conclusions
HAV vaccination rates were low and highlight the need for effective solutions to address HAV immunization gaps in PWH, especially among young patients, those with active substance use disorders, and those with significant immunocompromise.
Introduction
Hepatitis A virus (HAV) vaccine is a highly effective tool to prevent hepatitis A infection, and it is recommended for all people with HIV (PWH) in the United States [1]. While HAV infection usually causes a self-limited illness and acute liver inflammation in the majority of persons, it may lead to more severe illness among PWH [2]. In addition to an extended time period of viremia and fecal excretion, PWH have an increased HAV load and the time to normalization of alanine aminotransferase levels may be prolonged [2]. Frequent co-infection with hepatitis B virus (HBV) and hepatitis C virus (HCV) in PWH further increases the risk of more severe illness due to HAV infection, including fulminant hepatitis [3]. Liver disease is among the leading non-AIDS causes of death in PWH [4], [5]. While HAV infection is most commonly acquired through ingestion of fecally contaminated food or water or through the fecal-oral route, infection may also occur through sexual transmission, particularly in men who have sex with men (MSM), and through injection drug use (IDU) [6], [7], [8], [9]. HAV outbreaks have increased worldwide over the past decade, notably among persons experiencing homelessness, those who inject drugs, MSM, and PWH [9], [10], [11], [12], [13], [14], [15], [16], [17], [18].
Previously, the US Centers for Disease Control and Prevention Advisory Committee on Immunization Practices (ACIP) recommended that PWH with other risk factors for becoming infected or developing severe HAV infection receive HAV vaccination: MSM, IDU, occupational risk, persons experiencing homelessness, chronic liver disease, and international travelers [19]. However, the ACIP updated the Recommended Adult Immunization Schedule in 2020 to include HIV as a risk factor for HAV infection and as an indication to receive the HAV vaccine [1]. Prior studies of HAV vaccination in PWH with additional risk factors for HAV infection have demonstrated that only 10–29 % of HAV susceptible patients received at least one dose of the HAV vaccine [20], [21], [22], with the exception of one study that showed HAV immunization rates over 60 % in an MSM population [23]. Prior to the addition of HIV as an indication for HAV vaccination by the ACIP in 2020, the 2009 update to the HIV Medicine Association (HIVMA) of the Infectious Disease Society of America primary care guidelines for the management of persons with HIV recommended consideration to giving HAV vaccine to all PWH [24].
We sought to understand the factors associated with HAV vaccination in Thomas Street Health Center (TSHC) and Harris Health Northwest HIV (NW HIV) Clinics in Houston, Texas and to determine the HAV vaccination rate of patients entering care in these clinics between 2010 and 2018. It was the practice in the two clinics in this study to administer HAV vaccine to all PWH during the study period based on the prior HIVMA recommendations. We hypothesized that those with additional risk factors for HAV acquisition or severe infection would be more likely to have received HAV vaccine than those without risk factors, as clinicians may have perceived them to be at higher risk for HAV infection or complications.
Methods
Study design and population
The primary objective of this study was to determine the proportions of PWH who received at least 1 and 2 HAV-containing vaccines (HAVRIX®; GlaxoSmithKline Biologicals, Rixensart, Belgium; VAQTA®, Merck & Co., Whitehouse Station, NJ; or TWINRIX®; GlaxoSmithKline Biologicals, Rixensart, Belgium) at 6, 12, and 24 months after entering care at the TSHC and NW HIV Clinics in Houston, TX. These clinics are part of the Harris County Health system and receive Ryan White funding. We also sought to evaluate patient demographic and clinical variables associated with HAV vaccine receipt.
The Baylor College of Medicine Institutional Review Board approved the study. We abstracted data by retrospective chart review from the electronic medical record (EMR). PWH ≥ 13 years of age who entered care as new patients with ≥ 1 clinic visits between January 1, 2010 and December 31, 2018 were included in the study. It is the standard practice in the clinics to check anti-HAV immunoglobulin G (IgG) on screening labs before the entry to care visit. Anti-HAV IgG screening rates prior to entry to care or within 90 days after entry to care and the number of patients eligible to receive a HAV-containing vaccine at entry to care were determined. We defined eligibility for HAV vaccine receipt to include all persons who were anti-HAV IgG negative and had not received prior HAV vaccination per medical record documentation. HAV and combination HAV-HBV combination vaccine administration dates were captured from the immunization tab in the EMR. During this study period, vaccinations were provided in the TSHC and NW HIV Clinics. Vaccinations were either administered during the routine clinic visits or at separate nurse visits for vaccination.
Substance use history, including IDU, and sexual history were abstracted from the EMR from a standardized questionnaire that is administered at patient entry to care. Risk factors for HAV infection in this study included history of chronic HBV and/or HCV infection, MSM, history of IDU, history of substance use, and history of hemophilia. We were unable to assess homelessness in this study due to limitations of data abstraction from the EMR. We identified chronic HBV infection, chronic HCV infection, and history of hemophilia by International Disease Classification (ICD)-9 and ICD-10 codes and by lab results for hepatitis B surface antigen and hepatitis C RNA.
Statistical analyses
We analyzed data using Stata 16 with the support of the Baylor College of Medicine Institute for Clinic and Translational Research (ICTR). Among patients eligible for HAV vaccine, Kaplan-Meier curves were used to summarize the time to receiving 1 HAV vaccine and time to receiving 2 HAV vaccines. The cumulative incidence (95 % CI) which is equivalent to the proportion of patients who received 1 and 2 HAV vaccines at 6, 12, and 24 months from entry to care was estimated. Patients were censored at either their last follow-up or at 24 months, whichever came first. Univariable Cox regression was used to test the associations between the time to receiving 1 or 2 HAV vaccinations and the following baseline characteristics: race/ethnicity, age category, sex at birth, being transgender, MSM, injection drug use, substance use disorder history, chronic hepatitis B, chronic hepatitis C, HIV viral load (VL) ≥ 200 copies/ml, CD4 ≥ 200 cells/µl, time period of entering care, and previous care elsewhere. Factors found significant at p < 0.05 were included in a multivariable Cox regression. To avoid collinearity, factors found to be correlated with each other (r ≥ |0.5|) were not included in the same model. The proportional hazards assumption was assessed by reviewing the scaled Shoenfeld residuals and log–log plots. Due to low frequencies, we combined Asian and other race/ethnicity categories in the survival analysis.
Results
Subject characteristics
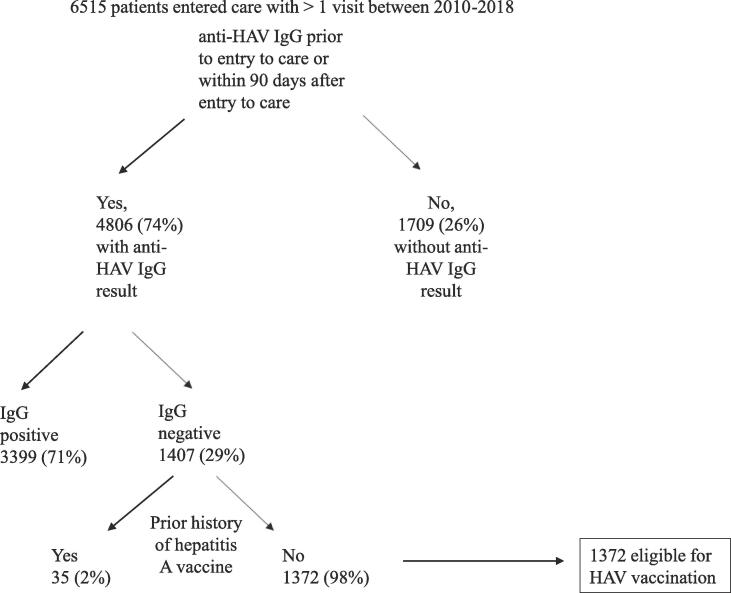
A total of 6,515 patients entered care and had at least 1 clinic visit between 2010 and 2018. Fig. 1 depicts the process by which 1372 patients were determined to be eligible for HAV vaccination. These 1372 patients had a negative anti-HAV IgG at entry to care in our clinics and had no history of HAV vaccination as documented in EMR. The average age at entry to care for the cohort eligible for HAV vaccination was 38.2 (SD 10.6) years (Table 1). We report combined race and ethnicity data, as this is the method by which the Ryan White HIV/AIDS Program collects and reports the data. The Black/African American population was the largest represented race/ethnicity in the cohort (70.5 %), followed by the Hispanic/Latino ethnicity (11.1 %), White (17.6 %), and Asian (0.7 %). The cohort was 67.4 % male at birth. Twelve (0.9 %) were transgender. At entry to care in our clinics, 65.5 % of patients had an absolute CD4 count ≥ 200 cells/µl; 79.7 % of patients had an HIV viral load (VL) ≥ 200 copies/mL and 37.7 % of patients reported HIV care elsewhere prior to entering care at our clinics.
Fig. 1.
Determination of cohort eligible for hepatitis A vaccination. HAV = hepatitis A virus, IgG = immunoglobulin G.
Table 1.
Characteristics of patients eligible for hepatitis A vaccination (n = 1372).
| n+ |
Percent (%) |
Mean (SD) |
|
|---|---|---|---|
| Age at Time of Entry to Care (years) | 38.2 (10.63) | ||
| Race/Ethnicity | |||
| White | 241 | 17.6 | |
| Black/African American | 967 | 70.5 | |
| Hispanic/Latinoa | 152 | 11.1 | |
| Asian | 10 | 0.7 | |
| Other | 2 | 0.1 | |
| Sex at Birth | |||
| Male | 925 | 67.4 | |
| Female | 447 | 32.6 | |
| Gender Identity | |||
| Male | 914 | 66.6 | |
| Female | 458 | 33.4 | |
| Transgender | |||
| No | 1360 | 99.1 | |
| Yes | 12 | 0.9 | |
| Year of Entry to Care | |||
| 2010–2014 | 899 | 65.5 | |
| 2015–2018 | 473 | 34.5 | |
|
CD4 count at Entry to Care N = 1353 |
|||
| < 200 | 467 | 34.5 | |
| ≥ 200 | 886 | 65.5 | |
|
HIV Viral Load at Entry to Care N = 1352 |
|||
| < 200 copies/mL | 274 | 20.3 | |
| ≥ 200 copies/mL | 1,078 | 79.7 |
N = Total number of patients in sample, n+ = frequency.
Hispanics/Latinos can be of any race.
Table 2 reports summary statistics of the cohort’s risk factors for HAV acquisition or severe infection: 6.2 % reported history of IDU; 4.7 % had history of chronic hepatitis B infection; 3.0 % had history of chronic hepatitis C infection; 37.7 % reported male-to-male sexual contact; and none had a history of hemophilia. Of the 1,214 patients for whom substance use history was available, 19.9 % had an active substance use disorder, and 18.6 % reported history of a substance use disorder. Among this cohort, 261 (19.0 %) had at least 2 of these risk factors for HAV acquisition or severe infection.
Table 2.
Patient risk factors for hepatitis A acquisition and severe infection*.
| n+ |
Percent (%) |
|
|---|---|---|
|
History of Injection Drug Use N = 1372 |
||
| No | 1287 | 93.8 |
| Yes | 85 | 6.2 |
|
History of Substance Abuse N = 1214 |
||
| None | 747 | 61.5 |
| Yes, active | 241 | 19.9 |
| Yes, not active | 226 | 18.6 |
|
Male-to-male sexual contact N = 1372 |
||
| No | 855 | 62.3 |
| Yes | 517 | 37.7 |
|
Chronic HBV Infection1 N = 1372 |
||
| No | 1307 | 95.3 |
| Yes | 65 | 4.7 |
|
Chronic HCV Infection1 N = 1372 |
||
| No | 1331 | 97.0 |
| Yes | 41 | 3.0 |
|
History of Hemophilia1 N = 1372 |
||
| No |
1372 | 100 |
N = Total number of patients in sample, n+ = frequency.
HAV = hepatitis A virus.
by International Disease Classification (ICD-9) and ICD-10 codes, hepatitis B surface antigen and HCV RNA.
Excluding HIV, which was later added as a risk factor for HAV and indication for HAV vaccination[1].
HAV vaccination rates
At six months from entry to care, 29.2 % (95 % CI 26.7, 31.9 %) of patients had received 1 dose of HAV vaccination, 37.1 % of patients (95 % CI 34.4, 40.0 %) at 12 months, and 47.8 % of patients (95 % CI 44.8, 50.8) at 24 months (Table 3). At six months from entry to care, 10.0 % (95 % CI 8.4, 11.9 %) of patients had received at least two doses of HAV vaccines, 21.2 % (95 % CI 18.9, 23.7 %) of patients at 12 months, and 33.4 % (95 % CI 30.5, 36.4 %) of patients at 24 months (Table 4). Of the 538 patients who received at least one HAV vaccine in this study, 283 (52.6 %) received combined HAV-HBV vaccine.
Table 3.
Proportion of patients who received 1 hepatitis A vaccination at specified time.
| Months from entry to care | At risk | N received vaccine | Cumulative incidence1 | 95 % C.I. |
|---|---|---|---|---|
| 0 | 1372 | |||
| 6 | 763 | 361 | 0.292 | 0.267–––0.319 |
| 12 | 616 | 81 | 0.371 | 0.344–––0.400 |
| 24 | 426 | 96 | 0.478 | 0.448–––0.508 |
equivalent to proportion of patients who received 1 HAV vaccination at specified time.
Table 4.
Proportion of patients who received 2 hepatitis A vaccinations at specified time.
| Months from entry to care | At risk | N received vaccine | Cumulative incidence1 | 95 % C.I. |
|---|---|---|---|---|
| 0 | 1372 | |||
| 6 | 963 | 115 | 0.100 | 0.084––0.119 |
| 12 | 764 | 113 | 0.212 | 0.188–––0.237 |
| 24 | 532 | 109 | 0.334 | 0.305–––0.364 |
equivalent to proportion of patients who received 2 HAV vaccinations at specified time.
Subject characteristics and HAV vaccination rates
In univariable analysis using Cox regression, race/ethnicity, gender, injection drug use, chronic hepatitis B infection, and chronic hepatitis C infection, receiving prior care elsewhere, and having more than one risk factor for severe hepatitis A infection (risks outlined in Table 2) were not associated with time to receiving 1 HAV vaccine. Age > 50 years compared to 30–50 (p = 0.009), being MSM (p = 0.001), and having CD4 ≥ 200 copies/µl (p < 0.001) were associated with shorter time to first HAV vaccine dose (Table 5). Having an active substance use disorder (p = 0.016), entering care between 2015 and 2018 (p = 0.037), and HIV VL ≥ 200 copies/ml (p = 0.010) were associated with longer time to vaccination (Table 5). In multivariable analysis, MSM (adjusted HR 1.39, 95 % CI: 1.15, 1.68) or those who had a CD4 count ≥ 200 cells/uL (adjusted HR 2.40, 95 % CI: 1.92, 2.99) had their first vaccination sooner than those who were not MSM or had a CD4 count < 200 cells/uL, respectively (Table 6). Age (p = 0.058), time period of entry to care (p = 0.061), substance use history (p = 0.110) and viral load (p = 0.672) were not associated with time to first vaccination in the multivariable model (Table 6).
Table 5.
Univariable Cox proportional hazards regression for time to completing 1 hepatitis A vaccination.
| Hazards ratio | 95 % C.I. | p value | |
|---|---|---|---|
| Race/Ethnicity | 0.096 | ||
| White | Reference | ||
| Black | 0.81 | 0.65––1.02 | 0.073 |
| Hispanic | 1.03 | 0.75 – 1.40 | 0.856 |
| Other | 0.50 | 0.16 – 1.57 | 0.233 |
| Age category | 0.011 | ||
| 30–50 years | Reference | ||
| < 30 years | 1.24 | 1.02–1.51 | 0.032 |
| >50 years | 1.37 | 1.08–1.73 | 0.009 |
| Female at birth | 0.96 | 0.80–1.15 | 0.653 |
| Transgender | 2.24 | 1.00–5.02 | 0.050 |
| MSM | 1.33 | 1.12–1.57 | 0.001 |
| Injection drug use | 1.10 | 0.78–1.56 | 0.580 |
| Substance use | 0.043 | ||
| None | Reference | ||
| Yes, active | 0.73 | 0.57–0.94 | 0.016 |
| Yes, not active | 1.02 | 0.81–1.28 | 0.880 |
| Chronic hepatitis B | 1.02 | 0.69–1.50 | 0.934 |
| Chronic hepatitis C | 1.43 | 0.92–2.21 | 0.108 |
| HIV VL ≥ 200 copies/ml | 0.76 | 0.62–0.94 | 0.010 |
| CD4 ≥ 200 cells/µl | 2.38 | 1.95–2.91 | <0.001 |
| Entered care 2015–2018 | 0.82 | 0.68–0.99 | 0.037 |
| Previous care elsewhere | 1.05 | 0.88–1.26 | 0.572 |
| More than 1 risk factor* | 1.18 | 0.96–1.46 | 0.116 |
MSM = men who have sex with men.
VL = viral load.
risk factors considered here are those listed in Table 2.
Table 6.
Multivariable Cox proportional hazards regression for time to completing 1 hepatitis A vaccination.
| Hazards ratio | 95 % C.I. | p value | |
|---|---|---|---|
| Age category | 0.058 | ||
| 30–50 years | Reference | ||
| <30 years | 0.97 | 0.78–1.21 | 0.781 |
| >50 years | 1.33 | 1.03–1.70 | 0.026 |
| MSM | 1.39 | 1.15–1.68 | 0.001 |
| Substance use | 0.110 | ||
| None | Reference | ||
| Yes, active | 0.76 | 0.59–0.99 | 0.040 |
| Yes, not active | 1.00 | 0.79–1.27 | >0.999 |
| HIV VL > 200 copies/ml | 0.95 | 0.75–1.20 | 0.672 |
| CD4 ≥ 200 cells/µl | 2.40 | 1.92–2.99 | <0.001 |
| Entered care 2015–2018 | 0.82 | 0.67–1.01 | 0.061 |
MSM = men who have sex with men.
VL = viral load.
In univariable using Cox regression, age > 50 years compared to 30–50 (p = 0.001), being transgender (p = 0.024), MSM (p = 0.014), and having CD4 ≥ 200 copies/µl (p < 0.001) were associated with shorter time to completion of 2 HAV vaccines (Table 7). In multivariable analysis, MSM (adjusted HR 1.35, 95 % CI: 1.06, 1.73) or those who had a CD4 count ≥ 200 cells/uL (adjusted HR 2.52, 95 % CI: 1.89, 3.37) had their second vaccination sooner than those who were not MSM or had a CD4 count less than 200 cells/uL, respectively (Table 8). Patients older than 50 years had their second vaccination sooner than those aged 30–50 (adjusted HR 1.47, 95 % CI: 1.08, 1.99). Those with an active substance use history had a longer time until their second vaccination compared to those with no substance use history (adjusted HR 0.57, 95 % CI: 0.40, 0.82). The time period of entry to care (p = 0.125), viral load (p = 0.403), and being transgender (p = 0.115) were not associated with the time to second vaccination in the multivariable model.
Table 7.
Univariable Cox proportional hazards regression for time to completing 2 hepatitis A vaccinations.
| Hazards ratio | 95 % C.I. | p value | |
|---|---|---|---|
| Race/Ethnicity | 0.164 | ||
| White | Reference | ||
| Black | 0.74 | 0.56–0.97 | 0.032 |
| Hispanic | 0.88 | 0.60–1.31 | 0.538 |
| Other | 0.79 | 0.25–2.51 | 0.686 |
| Age category | 0.005 | ||
| 30–50 years | Reference | ||
| <30 years | 1.20 | 0.93–1.54 | 0.165 |
| >50 years | 1.59 | 1.20–2.12 | 0.001 |
| Female at birth | 1.06 | 0.85–1.33 | 0.618 |
| Transgender | 2.77 | 1.14–6.69 | 0.024 |
| MSM | 1.31 | 1.06–1.63 | 0.014 |
| Injection drug use | 1.14 | 0.74–1.76 | 0.543 |
| Substance use | 0.004 | ||
| None | Reference | ||
| Yes, active | 0.56 | 0.39–0.80 | 0.001 |
| Yes, not active | 1.05 | 0.79–1.40 | 0.728 |
| Chronic hepatitis B | 0.75 | 0.44–1.28 | 0.292 |
| Chronic hepatitis C | 1.42 | 0.83–2.42 | 0.203 |
| HIV VL ≥ 200 copies/ml | 0.74 | 0.57–0.97 | 0.027 |
| CD4 ≥ 200 cells/µl | 2.55 | 1.96–3.32 | <0.001 |
| Entered care 2015–2018 | 0.74 | 0.58–0.95 | 0.016 |
| Previous care elsewhere | 0.88 | 0.70–1.11 | 0.271 |
| More than 1 risk factor* | 1.09 | 0.83–1.42 | 0.550 |
MSM = men who have sex with men.
VL = viral load.
risk factors considered here are those listed in Table 2.
Table 8.
Multivariable Cox proportional hazards regression for time to completing 2 hepatitis A vaccinations.
| Hazards ratio | 95 % C.I. | p value | |
|---|---|---|---|
| Age category | 0.037 | ||
| 30–50 years | Reference | ||
| <30 years | 0.99 | 0.74–1.31 | 0.922 |
| >50 years | 1.47 | 1.08–1.99 | 0.014 |
| MSM | 1.35 | 1.06–1.73 | 0.015 |
| Transgender | 2.24 | 0.82–6.12 | 0.115 |
| Substance use | 0.009 | ||
| None | Reference | ||
| Yes, active | 0.57 | 0.40–0.82 | 0.003 |
| Yes, not active | 1.01 | 0.76–1.35 | 0.934 |
| HIV VL ≥ 200 copies/ml | 0.89 | 0.67–1.18 | 0.403 |
| CD4 ≥ 200 cells/µl | 2.52 | 1.89–3.37 | <0.001 |
| Entered care 2015–2018 | 0.81 | 0.62–1.06 | 0.125 |
MSM = men who have sex with men.
VL = viral load.
Discussion
Our study demonstrated low HAV vaccination rates among PWH over 24 months after entry to HIV care at two clinics in Houston, Texas, despite high prevalence of additional factors for HAV infection. PWH are at risk for severe hepatitis A infection [2]. In addition, many PWH have underlying liver disease, which puts them at greater risk for severe hepatitis A infection and resultant disease [3], [4], [5]. Our findings of low HAV vaccination rates in PWH align with earlier studies and show that no improvements in vaccination occurred, at least within 2 years of entry to HIV care [20], [21], [22]. A retrospective, cross-sectional analysis by Tedaldi et al. of 9 clinic sites, spanning seven US cities, participating in the HIV Outpatient Study examined HAV vaccination rates in 2002. Of the 716 patients eligible for HAV vaccination, only 23.3 % received ≥ 1 dose of HAV vaccine, and, of those who received ≥ 1 dose of HAV vaccine, only 53.9 % received ≥ 2 doses (12.6 % of the overall population received two doses) [20]. Of note, the study by Tedaldi et al. evaluated HAV vaccination rates in PWH who had other risk factors for HAV infection that included whether they were HCV co-infected, were hepatitis B surface antigen positive, or had IDU or male-to-male sexual contact as a risk factor for HIV [12].
An evaluation of hepatitis prevention services for MSM with HIV infection conducted from 2004 to 2007 in 8 HIV clinics in 6 US cities among 1329 patients reported that 47 % percent of MSM with HIV infection were screened for anti-HAV IgG. Among those eligible for HAV vaccination, 29 % received at least one HAV vaccine dose [21]. Another study examining HAV vaccination rates among PWH with other risk factors for HAV infection (MSM and IDU) utilized the Medical Monitoring Project, a national surveillance database of persons receiving HIV medical care in the US, from 2009 to 2013. Of their study population who were eligible for HAV vaccination, only 10 % received vaccination within 12 months of observation [22]. A more recent study that evaluated HAV vaccination rates among MSM with HIV infection at the University of Nebraska Medical Center HIV clinic reported over 60 % completion of HAV vaccine series [23].
Although it had generally been the practice at our clinics to screen for anti-HAV IgG and to vaccinate all PWH susceptible for HAV, we observed that of the 6515 patients who entered care during our study period, 1709 (26 %) did not have anti-HAV IgG checked prior to care or within 90 days of entry to care. Of those who did have anti-HAV IgG checked, 71 % had a positive test result. We analyzed whether those who were “high risk” for HAV infection (MSM, injection drug use, chronic hepatitis B or C infection, and hemophilia) in the population of 6515 patients entering care during the study period were more likely to have had anti-HAV IgG checked than those who did not have one of these risk factors. However, we did not find an association between checking anti-HAV IgG and being high risk for HAV acquisition or more severe disease. Thus, it does not appear that providers were only checking anti-HAV IgG on those who had risk factors for HAV vaccination. Some of the 1709 patients who entered care during 2010–2018 and did not have an anti-HAV IgG test may have been candidates for HAV vaccination. However, as 71 % of the population tested for anti-HAV IgG were seropositive, we noted that including those who never had a anti-HAV IgG test performed might mean including many people who were not eligible for vaccination. We did perform univariable and multivariable analyses evaluating for factors associated with time to vaccination among the cohort of those who did not have anti-HAV IgG and those who were anti-HAV-IgG negative and had never received HAV vaccine and found that the anti-HAV IgG not being performed (compared to being performed and with a negative result) was associated with longer time to completing both 1 (HR 0.35 95 % CI 0.30, 0.40, p < 0.001) and 2 HAV vaccinations (HR 0.40 95 % CI 0.33, 0.48, p < 0.001). In multivariable analysis when controlling for other significant variables for time to completing 1 and 2 HAV vaccinations, having anti-HAV not performed versus a negative test result was also associated with longer time to vaccination.
While we hypothesized that we would see associations between time to hepatitis A vaccination and risk factors for HAV acquisition or severe disease (i.e. hepatitis B or C infections, injection drug use, or male-to-male sexual contact), we only observed an association with faster time to HAV vaccination among MSM patients. Receipt of at least 1 dose of HAV vaccine over a 24-month period was more likely to occur in PWH who had a CD4 ≥ 200 cells/µl and MSM. The 2020 CDC ACIP guidelines recommend HAV vaccination for all PWH, a recommendation that may streamline the approach of vaccinating PWH against HAV. Future studies would be needed to assess the changes in HAV vaccination rates in PWH as a result of these recommendations.
The finding of CD4 count ≥ 200 cells/µl being associated with faster time to vaccine receipt may reflect a provider’s decision to delay immunization until immune reconstitution has occurred among those with CD4 < 200 cells/µl during this study period. We also noted that CD4 count ≥ 200 cells/µl was associated with faster time to hepatitis B immunization in a study conducted in our clinics [25]. A study of immunization rates among PWH at the University of Nebraska Medical Center HIV clinic had similar findings. The authors found that CD4 ≤ 200 cell/ µl (adjusted OR 9.44, 95 % CI 2.14–41.57) was associated with not being up to date with all vaccinations in a multivariable analysis [23]. Given the preponderance of additional HAV risk factors in our population, the fact that HIV has been identified as a risk factor for HAV infection, and that even 2 years after care entry the majority of these patients remain unvaccinated, we propose that vaccinating upon entry to care, just like ART initiation, should not be delayed. This recommendation is in accordance with the ACIP recommendations that state HAV vaccination should not be delayed until the CD4 count reaches a particular threshold because of the sustained risk for HAV exposure created by missed vaccination opportunities [1].
It is not clear why age > 50 compared to 30–50 was associated with receipt of 2 HAV vaccines. It may be that older patients in our clinic may have been more likely to accept the vaccine or may have been more informed about the risk of HAV infection. Other studies that evaluated the effect of multiple patient factors on immunization recommendation adherence among PWH, did not find age to be a significant predictor [20], [23]. One study found that younger age (18–29 years, as opposed to other age groups) was associated with HAV vaccination among PWH who are MSM or who inject drugs [22]. We did not observe an association between age and time to hepatitis B vaccine immunization series completion in a similar study conducted in our clinics [25].
Delayed HAV vaccination among persons with active substance use disorders may be due to the substance use disorder affecting follow-up and socioeconomic barriers. Immunization rates among people with substance use disorders has been found to be lower than rates among the general population for multiple vaccines [26], [27], [28]. We observed that patients with an active substance use disorder history had a significantly longer time to completion of hepatitis B vaccination than those with no substance use history in a study conducted during the same timeframe in our clinics [25]. Strategies to increase immunization rates among people who inject drugs include factors of convenience, such as having immunizations available on site and incentives, such as payment for completing vaccinations [29].
Strengths of our study include a large and diverse patient population and the multivariable analysis of factors associated with vaccination. Prior studies evaluated HAV vaccine rates among persons with HIV who had other risk factors for HAV infection, apart from HIV. Our study is among the first to report HAV immunization rates among all PWH, regardless of other risk factors for HAV acquisition or severe infection. Another strength of our study was that we utilized Cox proportional hazards regression, allowing patients who were lost to follow-up to be included in the analysis.
Our study included several limitations. The retrospective design may have resulted in incomplete data capture. We were not able to include the risk factors of homelessness or chronic liver disease due to etiologies other than hepatitis B and C viral infections. Another limitation is that the study population included patients receiving care at two large county HIV clinics in Houston, Texas, and may not be generalizable to other PWH populations. Our study evaluated receipt of HAV vaccines, rather than providers’ vaccine orders. We evaluated receipt of at least 1 or 2 HAV vaccine doses, but we did not collect data on completion of the vaccine series. Persons who received combined HAV-HBV vaccines (52.6 % of patients in our study) need to complete three doses in order to have completed the full immunization series.
It is critical to understand the factors that have led to immunization gaps in the study population so we can find solutions to barriers and improve immunization rates. Provider barriers may include lack of knowledge of immunization recommendations, concerns about vaccine efficacy, inadequate time to address immunizations during clinic visits, and lack of strong provider recommendation during clinic visit [30], [31]. Patient barriers may include missed clinic visits [23], multiple vaccines needed at the same time, patient vaccine hesitancy related to lack of concern about vaccine-preventable diseases, fear of side effects, and inconvenience of vaccine visits. System barriers may include cumbersome EMR vaccine orders, lack of EMR vaccination prompts, lack of interoperability between state immunization registries and EMR, and clinic visits not coinciding with vaccination due dates. In some studies, standing order sets for vaccines [32], [33], [34], provider notification of recommended immunizations [34], and immunization recall and reminder systems have led to improvements in immunization rates [35], [36], [37]. A prior systematic review of 12 interventions recommended by the Community Preventive Services Task Force to increase vaccination coverage found that reminder systems, either for providers or clients, were among the lowest cost to implement and the most cost effective for increasing the number of persons being vaccinated [38]. We hypothesize that HAV vaccination is being overlooked in our clinics due to competing needs for other immunizations and other priorities at clinic visits. Vaccines are available in our clinics and immunizations can be given on site. Ensuring that an anti-HAV IgG test in checked at the time of entry to care and electronic record reminders for HAV vaccination all our patient with HIV who have a negative anti-HAV IgG may be a low-cost intervention to increase HAV vaccine rates among our clinics. Our study highlights an opportunity to study barriers to immunization and evaluate which strategies may work best to improve HAV immunization rates among our clinics.
Author contributions
All authors attest they meet ICMJE criteria for authorship. Conception/design of work: E.T.C, K.A.S, W.A.K, H.M.E, J.A.W.; Acquisition, analysis and interpretation of data: E.T.C, K.A.S, J.C., A.R.O., F.B.H., W.A.K., R.L.A., H.M.E., J.A.W.; Drafting/revising for important intellectual content: E.T.C, K.A.S, J.C., A.R.O., F.B.H., W.A.K., R.L.A., H.M.E., J.A.W.
Funding
This research did not receive any specific funding from public, commercial, or not-for-profit agencies.
CRediT authorship contribution statement
Emily T. Ciocca: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. Kristen A. Staggers: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. Jennifer Carey: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Supervision, Writing – original draft, Writing – review & editing. Antone R. Opekun: . F. Blaine Hollinger: . Wendy A. Keitel: Formal analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. Robert L. Atmar: Formal analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. Hana M. El Sahly: Formal analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. Jennifer A. Whitaker: .
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Data were analyzed with the support of the Baylor College of Medicine Institute for Clinical and Translational Research (ICTR).
Data availability
The data that has been used is confidential.
References
- 1.Nelson N.P., Weng M.K., Hofmeister M.G., Moore K.L., Doshani M., Kamili S., et al. Prevention of Hepatitis A virus infection in the united states: Recommendations of the advisory committee on immunization practices, 2020. MMWR Recomm Rep. 2020;69:1–38. doi: 10.15585/mmwr.rr6905a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ida S., Tachikawa N., Nakajima A., Daikoku M., Yano M., Kikuchi Y., et al. Influence of human immunodeficiency virus type 1 infection on acute hepatitis A virus infection. Clin Infect Dis. 2002;34:379–385. doi: 10.1086/338152. [DOI] [PubMed] [Google Scholar]
- 3.Vento S., Garofano T., Renzini C., Cainelli F., Casali F., Ghironzi G., et al. Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. N Engl J Med. 1998;338:286–290. doi: 10.1056/NEJM199801293380503. [DOI] [PubMed] [Google Scholar]
- 4.Palella F.J., Jr., Baker R.K., Moorman A.C., Chmiel J.S., Wood K.C., Brooks J.T., et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 5.Smith C.J., Ryom L., Weber R., Morlat P., Pradier C., Reiss P., et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet. 2014;384:241–248. doi: 10.1016/S0140-6736(14)60604-8. [DOI] [PubMed] [Google Scholar]
- 6.Corey L., Holmes K.K. Sexual transmission of hepatitis A in homosexual men: incidence and mechanism. N Engl J Med. 1980;302:435–438. doi: 10.1056/NEJM198002213020804. [DOI] [PubMed] [Google Scholar]
- 7.Ochnio J.J., Patrick D., Ho M., Talling D.N., Dobson S.R. Past infection with hepatitis A virus among Vancouver street youth, injection drug users and men who have sex with men: implications for vaccination programs. CMAJ. 2001;165:293–297. [PMC free article] [PubMed] [Google Scholar]
- 8.Villano S.A., Nelson K.E., Vlahov D., Purcell R.H., Saah A.J., Thomas D.L. Hepatitis A among homosexual men and injection drug users: more evidence for vaccination. Clin Infect Dis. 1997;25:726–728. doi: 10.1086/513757. [DOI] [PubMed] [Google Scholar]
- 9.Lugoboni F., Pajusco B., Albiero A., Quaglio G. Hepatitis A Virus among Drug Users and the Role of Vaccination: A Review. Front Psychiatry. 2011;2:79. doi: 10.3389/fpsyt.2011.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster M.A., Hofmeister M.G., Kupronis B.A., Lin Y., Xia G.L., Yin S., et al. Increase in Hepatitis A Virus Infections - United States, 2013–2018. MMWR Morb Mortal Wkly Rep. 2019;68(413–5) doi: 10.15585/mmwr.mm6818a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster MA, Hofmeister MG, Albertson JP, Brown KB, Burakoff AW, Gandhi AP, et al. Hepatitis A Virus Infections Among Men Who Have Sex with Men - Eight U.S. States, 2017-2018. MMWR Morb Mortal Wkly Rep. 2021;70:875-8. 10.15585/mmwr.mm7024a2. [DOI] [PMC free article] [PubMed]
- 12.Zimmermann R., Faber M., Dudareva S., Ingiliz P., Jessen H., Koch J., et al. Hepatitis A outbreak among MSM in Berlin due to low vaccination coverage: Epidemiology, management, and successful interventions. Int J Infect Dis. 2021;103:146–153. doi: 10.1016/j.ijid.2020.11.133. [DOI] [PubMed] [Google Scholar]
- 13.Latash J., Dorsinville M., Del Rosso P., Antwi M., Reddy V., Waechter H., et al. Notes from the Field: Increase in Reported Hepatitis A Infections Among Men Who Have Sex with Men - New York City, January-August 2017. MMWR Morb Mortal Wkly Rep. 2017;66(999–1000) doi: 10.15585/mmwr.mm6637a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boucher A., Meybeck A., Alidjinou K., Huleux T., Viget N., Baclet V., et al. Clinical and virological features of acute hepatitis A during an ongoing outbreak among men who have sex with men in the North of France. Sex Transm Infect. 2019;95:75–77. doi: 10.1136/sextrans-2017-053395. [DOI] [PubMed] [Google Scholar]
- 15.Ndumbi P., Freidl G.S., Williams C.J., Mardh O., Varela C., Avellon A., et al. to May 2017. Euro Surveill. 2016;2018:23. doi: 10.2807/1560-7917.ES.2018.23.33.1700641. [DOI] [Google Scholar]
- 16.Raczynska A., Wickramasuriya N.N., Kalinowska-Nowak A., Garlicki A., Bociaga-Jasik M. Acute Hepatitis A outbreak among men who have sex with men in krakow, poland; February 2017-February 2018. Am J Mens Health. 2019;13 doi: 10.1177/1557988319895141. 1557988319895141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng C.Y., Wu H.H., Zou H., Lo Y.C. Epidemiological characteristics and associated factors of acute hepatitis A outbreak among HIV-coinfected men who have sex with men in Taiwan, June 2015-December 2016. J Viral Hepat. 2018;25:1208–1215. doi: 10.1111/jvh.12926. [DOI] [PubMed] [Google Scholar]
- 18.Martin A., Meddeb L., Lagier J.C., Colson P., Menard A. Hepatitis A outbreak in HIV-infected patients in Southeastern France: questions and responses? Epidemiol Infect. 2020;148:e79. doi: 10.1017/S0950268820000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Advisory Committee on Immunization P., Fiore A.E., Wasley A., Bell B.P. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55:1–23. [PubMed] [Google Scholar]
- 20.Tedaldi E.M., Baker R.K., Moorman A.C., Wood K.C., Fuhrer J., McCabe R.E., et al. Hepatitis A and B vaccination practices for ambulatory patients infected with HIV. Clin Infect Dis. 2004;38:1478–1484. doi: 10.1086/420740. [DOI] [PubMed] [Google Scholar]
- 21.Hoover K.W., Butler M., Workowski K.A., Follansbee S., Gratzer B., Hare C.B., et al. Low rates of hepatitis screening and vaccination of HIV-infected MSM in HIV clinics. Sex Transm Dis. 2012;39:349–353. doi: 10.1097/OLQ.0b013e318244a923. [DOI] [PubMed] [Google Scholar]
- 22.DeGroote N.P., Mattson C.L., Tie Y., Brooks J.T., Garg S., Weiser J. Hepatitis A virus immunity and vaccination among at-risk persons receiving HIV medical care. Prev Med Rep. 2018;11:139–144. doi: 10.1016/j.pmedr.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson T.M., Klepser D.G., Bares S.H., Scarsi K.K. Predictors of vaccination rates in people living with HIV followed at a specialty care clinic. Hum Vaccin Immunother. 2021;17:791–796. doi: 10.1080/21645515.2020.1802163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aberg J.A., Kaplan J.E., Libman H., Emmanuel P., Anderson J.R., Stone V.E., et al. Primary care guidelines for the management of persons infected with human immunodeficiency virus: 2009 update by the HIV medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:651–681. doi: 10.1086/605292. [DOI] [PubMed] [Google Scholar]
- 25.Sheikh D., Staggers K.A., Carey J., Keitel W.A., Atmar R.L., El Sahly H.M., et al. Delays in hepatitis B immunization series completion in people with human immuunodeficiency virus. OFID. 2023;10:ofad543. doi: 10.1093/ofid/ofad543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quaglio G., Lugoboni F., Mezzelani P., Des Jarlais D.C., Lechi A. Hepatitis vaccination among drug users. Vaccine. 2006;24:2702–2709. doi: 10.1016/j.vaccine.2005.12.045. [DOI] [PubMed] [Google Scholar]
- 27.Iversen J., Wand H., Kemp R., Bevan J., Briggs M., Patten K., et al. Uptake of COVID-19 vaccination among people who inject drugs. Harm Reduct J. 2022;19:59. doi: 10.1186/s12954-022-00643-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koepke R., Sill D.N., Akhtar W.Z., Mitchell K.P., Guilfoyle S.M., Westergaard R.P., et al. Hepatitis A and Hepatitis B vaccination coverage among persons who inject drugs and have evidence of hepatitis C infection. Public Health Rep. 2019;134:651–659. doi: 10.1177/0033354919874088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campbell J.V., Garfein R.S., Thiede H., Hagan H., Ouellet L.J., Golub E.T., et al. Convenience is the key to hepatitis A and B vaccination uptake among young adult injection drug users. Drug Alcohol Depend. 2007;91(Suppl 1):S64–S72. doi: 10.1016/j.drugalcdep.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 30.Hurley LP, Bridges CB, Harpaz R, Allison MA, ST OL, Crane LA, et al. Physician Attitudes Toward Adult Vaccines and Other Preventive Practices, United States, 2012. Public Health Rep. 2016;131:320-30. 10.1177/003335491613100216. [DOI] [PMC free article] [PubMed]
- 31.Aziz M., Kessler H., Huhn G. Providers' lack of knowledge about herpes zoster in HIV-infected patients is among barriers to herpes zoster vaccination. Int J STD AIDS. 2013;24:433–439. doi: 10.1177/0956462412472461. [DOI] [PubMed] [Google Scholar]
- 32.Tan L.J., VanOss R., Ofstead C.L., Wetzler H.P. Maximizing the impact of, and sustaining standing orders protocols for adult immunization in outpatient clinics. Am J Infect Control. 2020;48:290–296. doi: 10.1016/j.ajic.2019.07.023. [DOI] [PubMed] [Google Scholar]
- 33.Goebel L.J., Neitch S.M., Mufson M.A. Standing orders in an ambulatory setting increases influenza vaccine usage in older people. J Am Geriatr Soc. 2005;53:1008–1010. doi: 10.1111/j.1532-5415.2005.53320.x. [DOI] [PubMed] [Google Scholar]
- 34.Erlandson K.M., Streifel A., Novin A.R., Hawkins K.L., Foster C., Langness J., et al. Low rates of vaccination for herpes zoster in older people living with HIV. AIDS Res Hum Retroviruses. 2018;34:603–606. doi: 10.1089/AID.2017.0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacobson Vann J.C., Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005:CD003941. doi: 10.1002/14651858.CD003941.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Humiston S.G., Bennett N.M., Long C., Eberly S., Arvelo L., Stankaitis J., et al. Increasing inner-city adult influenza vaccination rates: a randomized controlled trial. Public Health Rep. 2011;126(Suppl 2):39–47. doi: 10.1177/00333549111260S206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hurley L.P., Beaty B., Lockhart S., Gurfinkel D., Breslin K., Dickinson M., et al. RCT of centralized vaccine reminder/recall for adults. Am J Prev Med. 2018;55:231–239. doi: 10.1016/j.amepre.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 38.Jacob V., Chattopadhyay S.K., Hopkins D.P., Murphy Morgan J., Pitan A.A., Clymer J.M., et al. Increasing coverage of appropriate vaccinations: A community guide systematic economic review. Am J Prev Med. 2016;50:797–808. doi: 10.1016/j.amepre.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.