Abstract
In a recent study, Wilton and colleagues link activation of the classical complement pathway with corticostriatal synapse loss and cognitive decline in Huntington’s disease.1
In a recent study, Wilton and colleagues link activation of the classical complement pathway with corticostriatal synapse loss and cognitive decline in Huntington’s disease.
Huntington’s disease (HD) is an autosomal-dominant neurodegenerative condition resulting from a CAG trinucleotide repeat expansion in exon 1 of the huntingtin gene. Progressive motor dysfunction manifests around 45 years of age, albeit with large variation, and can be preceded by cognitive and psychiatric disturbances.2 Despite ubiquitous expression of mutant huntingtin protein (mHTT) throughout the brain, HD is pathologically characterized by initial atrophy of the subcortical striatum. Corticostriatal inputs are particularly susceptible to degeneration, but the mechanism behind this selectivity is debated. A recent study in Nature Medicine1 emphasizes the key role of the classical complement pathway—an innate immune response that mediates synapse removal via microglial engulfment.3 Wilton and colleagues showed dysregulated complement expression in human striata and cerebrospinal fluid (CSF) and provide the first in vivo evidence that suppression of complement activity protects corticostriatal synapses and cognitive function in HD mice.
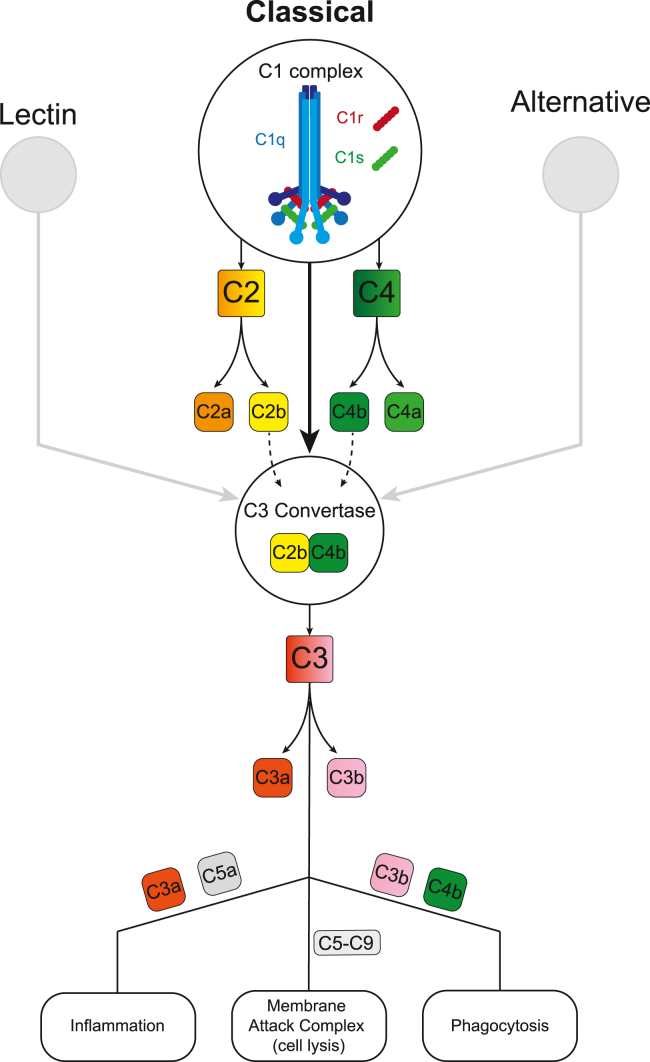
The pro-inflammatory complement pathway is a protease cascade mediated by three effector networks (classical, alternative, and lectin), converging on activation of a C3 convertase. C1q acts as the initiating protein within the classical pathway (Figure 1), which can ultimately eliminate synapses through phagocytosis via an interaction between C3b (or inactivated C3b [iC3b]) and the microglial-expressed C3 receptor (CR3).
Figure 1.
The classical complement pathway
C1q of the C1 complex binds and coats cell membranes, activating Cr and Cs serine proteases. This initiates cleavage of C2 and C4 into peptides (C2b and C4b) that recombine to form the C3 convertase complex. C3 convertase catalyses C3 into C3a and C3b, the latter of which tags synapses for phagocytic elimination through interaction with CR3. A more extensive overview can be found in Nature Reviews Immunology.4
The authors initially demonstrated that corticostriatal synapse loss coincides with a localized upregulation of complement proteins (C1q, C3, iC3b, and CR3) in striata from human postmortem tissue and in 3-month-old mHTT knock-in zQ175 mice. Importantly, this occurs prior to measurable cognitive decline in this model. Striatal CR3 expression also co-localized with Iba1+ microglia in zQ175 mice, which were shown to preferentially engulf fluorescently labelled cortical projections. The pathogenic trigger for this process is unclear; however, genetic ablation of mHTT in the striatum or cortex of BACHD mice was sufficient to prevent pathology, namely the C1q deposition, synaptic loss, and microglial engulfment. As mHTT inclusions are widespread by 3 months in the zQ175 striatum and cortex,5 it will be interesting to probe whether these protein conformers potentiate complement pathology. Furthermore, it seems pertinent to determine whether complement activity initially localizes to synapses associated with D2-expressing medium spiny neurons of the indirect pathway, which are the first to degenerate in HD.
Complement activity was associated with cognitive dysfunction in the zQ175 model, supporting C3 and C1q as potential targets for HD therapeutics. In a visual discrimination task, 4-month-old HD mice exhibited slower learning and reduced cognitive flexibility. Genetic knockout of CR3 entirely rescued these deficits, while also increasing the density of corticostriatal synapses by 60%. Cognitive flexibility was recently identified as one of the first functions to decline in a premanifest HD gene carrier cohort ∼24 years prior to predicted motor symptom onset.6 This indicates that complement-targeted therapeutics are particularly relevant as an early intervention method. Intraperitoneal administration of ANX-M1 (a C1q function-blocking antibody) at 4 months similarly produced a 70% increase in corticostriatal synapses, a 60% reduction in C3 deposition, and enhanced excitatory striatal input, although no effects on cognitive behavior were reported.
An open-label phase 2a clinical trial has recently concluded which investigated the use of an anti-C1q therapy (ANX005) in patients with HD (ClinVar: NCT04514367).7 ANX005 was well tolerated, demonstrated significant target engagement, and appeared to slow clinical progression in patients with high baseline complement activity. A small number of patients were in the trial which had no placebo arm, but the data support further development of this clinical program. In agreement with the mechanism proposed by the current study,1 ANX005 reduced neuroinflammation and a trend of decreased CSF YKL-40 levels was suggestive of lower microglial activity—a marker that was elevated in the above early premanifest HD gene carrier cohort.8
Of translational relevance, Wilton et al. identified a positive correlation between C3/iC3b levels in human CSF and CAP score (a disease burden metric9) in premanifest HD patients. Although this did not survive correction for age-related effects, levels of iC3b remained elevated in the late premanifest HD group compared to early premanifest individuals. iC3b expression may thus increase throughout the disease trajectory and could represent a novel biomarker of HD progression. Intriguingly, C1q, the most upstream factor of the classical pathway (Figure 1), showed no significant changes. In line with these data, an HD CSF biomarker exploration study showed unchanged expression of C1q subunits (C1qB and C1qC) across early-mid HD cohorts.10 However, C1qB levels were substantially reduced in late-stage HD, which may reflect extreme loss of C1q-expressing neurons via phagocytosis as highlighted in the current study.
Wilton and colleagues raise intriguing questions regarding the complement pathway as a neural substrate for cognitive flexibility and provide preclinical support for complement-related therapeutics and biomarker development in HD. The mechanism relies on mHTT expression, which is relevant to current HTT-lowering therapeutic approaches, but fails to address why corticostriatal inputs are particularly vulnerable. This necessitates further work to determine whether selectivity is associated with circuit-specific electrical properties or localized protective mechanisms in other pathways. It would also be interesting to establish whether mHTT expression in microglia potentiates the pathogenic mechanism. Nonetheless, neuroinflammation does not appear secondary to neuronal damage and thus supports complement inhibition as an early intervention in HD to safeguard neuronal circuitry and preserve cognitive function.
Acknowledgments
Declaration of interests
Through the offices of UCL Consultants Ltd, a wholly owned subsidiary of University College London, S.J.T. has undertaken consultancy services for Annexon, Inc.
References
- 1.Wilton D.K., Mastro K., Heller M.D., Gergits F.W., Willing C.R., Fahey J.B., Frouin A., Daggett A., Gu X., Kim Y.A., et al. Microglia and complement mediate early corticostriatal synapse loss and cognitive dysfunction in Huntington's disease. Nat. Med. 2023;29:2866–2884. doi: 10.1038/s41591-023-02566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bates G.P., Dorsey R., Gusella J.F., Hayden M.R., Kay C., Leavitt B.R., Nance M., Ross C.A., Scahill R.I., Wetzel R., et al. Huntington disease. Nat. Rev. Dis. Primers. 2015;1 doi: 10.1038/nrdp.2015.5. [DOI] [PubMed] [Google Scholar]
- 3.Stevens B., Allen N.J., Vazquez L.E., Howell G.R., Christopherson K.S., Nouri N., Micheva K.D., Mehalow A.K., Huberman A.D., Stafford B., et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 4.Reis E.S., Mastellos D.C., Hajishengallis G., Lambris J.D. New insights into the immune functions of complement. Nat. Rev. Immunol. 2019;19:503–516. doi: 10.1038/s41577-019-0168-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith E.J., Sathasivam K., Landles C., Osborne G.F., Mason M.A., Gomez-Paredes C., Taxy B.A., Milton R.E., Ast A., Schindler F., et al. Early detection of exon 1 huntingtin aggregation in zQ175 brains by molecular and histological approaches. Brain Commun. 2023;5 doi: 10.1093/braincomms/fcad010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langley C., Gregory S., Osborne-Crowley K., O'Callaghan C., Zeun P., Lowe J., Johnson E.B., Papoutsi M., Scahill R.I., Rees G., et al. Fronto-striatal circuits for cognitive flexibility in far from onset Huntington's disease: evidence from the Young Adult Study. J. Neurol. Neurosurg. Psychiatry. 2021;92:143–149. doi: 10.1136/jnnp-2020-324104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar R., Claassen D., Mongan A., Hoehn B., Lin P., Cahir-Mcfarland E., Guo C., Volman V., Taylor L., Chandra P., et al. A Phase 2 Open-Label Study to Assess the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Intravenous ANX005 in Patients with, or at Risk of, Manifest Huntington’s Disease (HD) (S32.009) Neurology. 2023;100:3366. [Google Scholar]
- 8.Scahill R.I., Zeun P., Osborne-Crowley K., Johnson E.B., Gregory S., Parker C., Lowe J., Nair A., O'Callaghan C., Langley C., et al. Biological and clinical characteristics of gene carriers far from predicted onset in the Huntington's disease Young Adult Study (HD-YAS): a cross-sectional analysis. Lancet Neurol. 2020;19:502–512. doi: 10.1016/S1474-4422(20)30143-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y., Long J.D., Mills J.A., Warner J.H., Lu W., Paulsen J.S., PREDICT-HD Investigators and Coordinators of the Huntington Study Group. PREDICT-HD Investigators and Coordinators of the Huntington Study Group Indexing disease progression at study entry with individuals at-risk for Huntington disease. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2011;156B:751–763. doi: 10.1002/ajmg.b.31232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caron N.S., Haqqani A.S., Sandhu A., Aly A.E., Findlay Black H., Bone J.N., McBride J.L., Abulrob A., Stanimirovic D., Leavitt B.R., Hayden M.R. Cerebrospinal fluid biomarkers for assessing Huntington disease onset and severity. Brain Commun. 2022;4:fcac309. doi: 10.1093/braincomms/fcac309. [DOI] [PMC free article] [PubMed] [Google Scholar]