Abstract
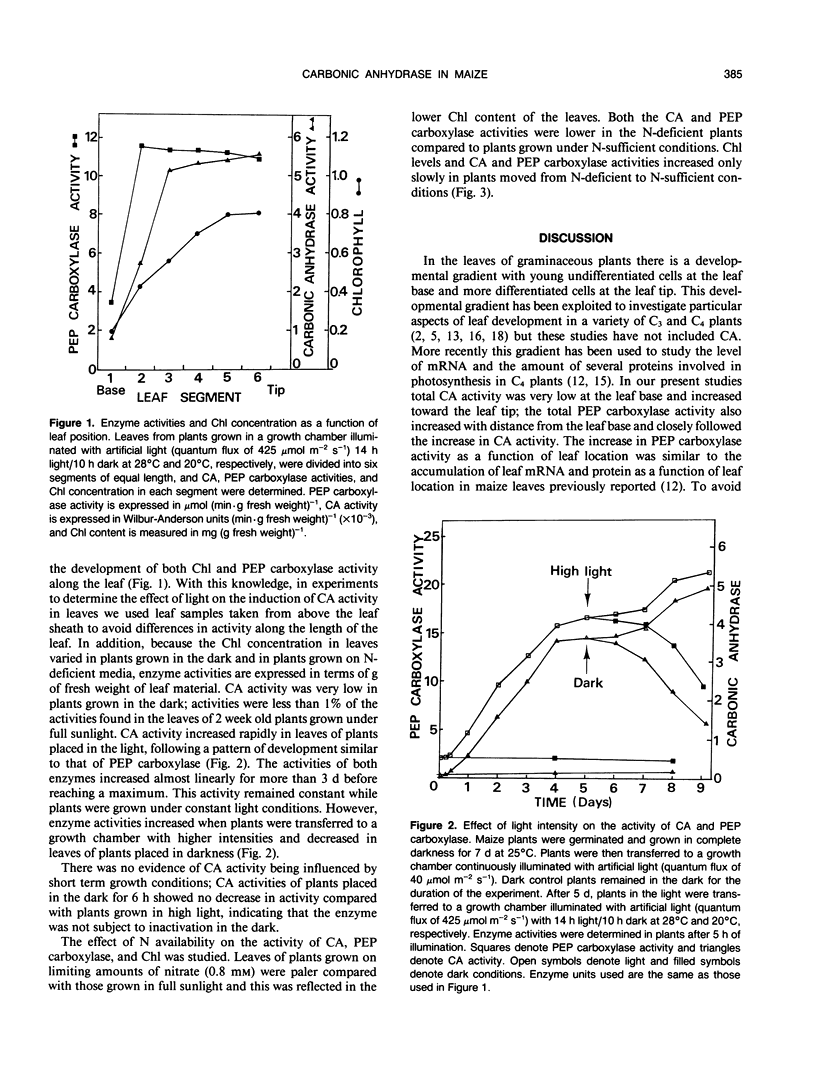
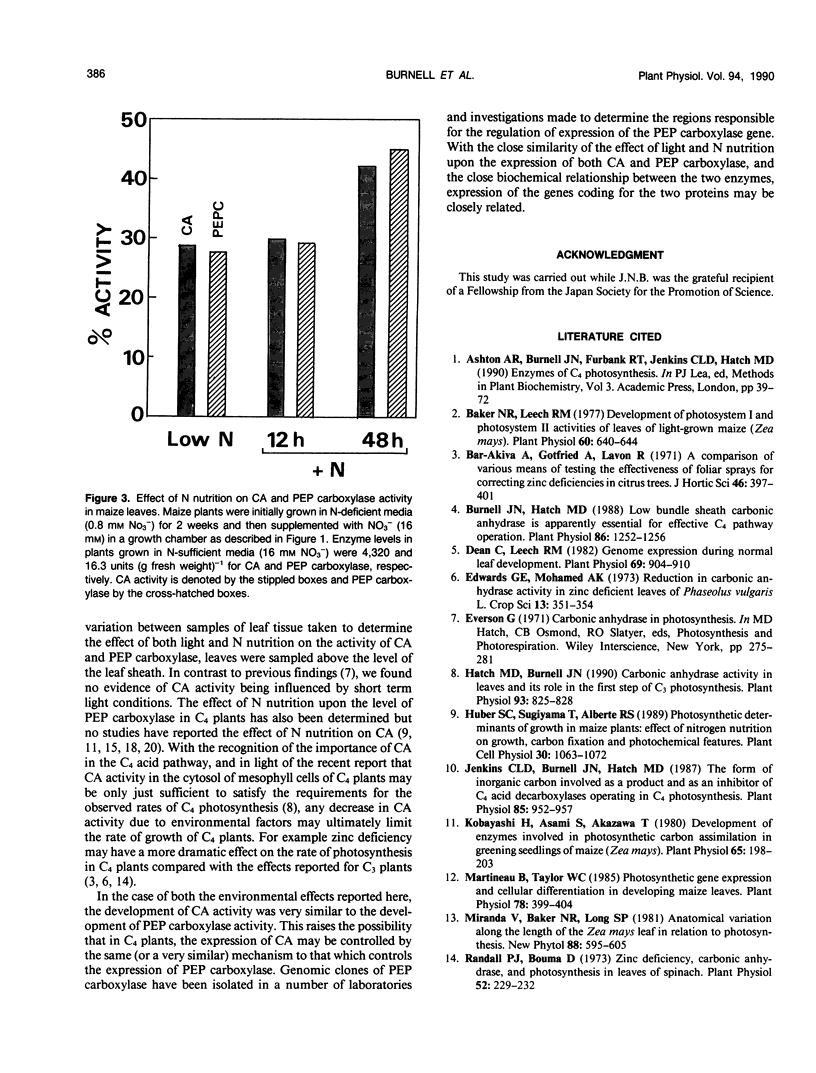
The regulation of carbonic anhydrase (CA) activity in maize (Zea mays L.) leaves by light and nitrogen nutrition was determined. CA activity increased by more than 100-fold in illuminated leaves and decreased in leaves placed in the dark; low levels of CA activity were observed in leaves illuminated with low light intensities. CA activity was reduced in plants grown under nitrogen deficiency and recovered only slowly when supplemented with nitrate. Parallel studies were conducted to follow the levels of phosphoenolpyruvate carboxylase. Experiments indicate that the level of CA and phosphoenolpyruvate carboxylase present in leaves may be controlled by similar mechanisms.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker N. R., Leech R. M. Development of Photosystem I and Photosystem II Activities in Leaves of Light-grown Maize (Zea mays). Plant Physiol. 1977 Oct;60(4):640–644. doi: 10.1104/pp.60.4.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnell J. N., Hatch M. D. Low bundle sheath carbonic anhydrase is apparently essential for effective c(4) pathway operation. Plant Physiol. 1988 Apr;86(4):1252–1256. doi: 10.1104/pp.86.4.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C., Leech R. M. Genome Expression during Normal Leaf Development : I. CELLULAR AND CHLOROPLAST NUMBERS AND DNA, RNA, AND PROTEIN LEVELS IN TISSUES OF DIFFERENT AGES WITHIN A SEVEN-DAY-OLD WHEAT LEAF. Plant Physiol. 1982 Apr;69(4):904–910. doi: 10.1104/pp.69.4.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D., Burnell J. N. Carbonic anhydrase activity in leaves and its role in the first step of c(4) photosynthesis. Plant Physiol. 1990 Jun;93(2):825–828. doi: 10.1104/pp.93.2.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins C. L., Burnell J. N., Hatch M. D. Form of inorganic carbon involved as a product and as an inhibitor of c(4) Acid decarboxylases operating in c(4) photosynthesis. Plant Physiol. 1987 Dec;85(4):952–957. doi: 10.1104/pp.85.4.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H., Asami S., Akazawa T. Development of Enzymes Involved in Photosynthetic Carbon Assimilation in Greening Seedlings of Maize (Zea mays). Plant Physiol. 1980 Feb;65(2):198–203. doi: 10.1104/pp.65.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau B., Taylor W. C. Photosynthetic gene expression and cellular differentiation in developing maize leaves. Plant Physiol. 1985 Jun;78(2):399–404. doi: 10.1104/pp.78.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall P. J., Bouma D. Zinc deficiency, carbonic anhydrase, and photosynthesis in leaves of spinach. Plant Physiol. 1973 Sep;52(3):229–232. doi: 10.1104/pp.52.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiharto B., Miyata K., Nakamoto H., Sasakawa H., Sugiyama T. Regulation of expression of carbon-assimilating enzymes by nitrogen in maize leaf. Plant Physiol. 1990 Apr;92(4):963–969. doi: 10.1104/pp.92.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]


