Abstract
While current MR-Linac (MRL) treatment workflows utilize a large table overlay during CT simulation to convert indexing between the two machines, we developed a look-up-table (LUT) as an alternative approach. After populating the LUT, index conversion factors were verified at three separate table locations. The resultant root-mean-square isocenter shifts on the MRL were 0.04/0.08 cm, 0.08/0.07 cm, and 0.09/0.08 cm with/without using the table overlay during simulation in the lateral, longitudinal, and vertical directions, respectively, which is within registration tolerance. Clinical implementation of the LUT has resulted in a more efficient MRL treatment workflow while maintaining accurate patient setup.
1. Introduction
Systematic errors in the delivery of radiation therapy lead to suboptimal treatment plan delivery and translational shifts in dose distribution [1], [2]. Thus, patient positioning and immobilization between the simulator and treatment machines must be consistent to avoid setup errors [3]. However, these systems may have unique indexing layouts, which makes consistent patient and immobilization device setup challenging. One common scenario in which this is encountered is with patients treated on an MR-Linac (MRL) who were simulated on a CT scanner [4], [5].
One solution to harmonize the two indexing systems is to use a commercial CT table overlay, which is a board with the same MRL table indexing that attaches to the top of the CT table during simulation (Fig. S1). Thus, patient positioning information during CT simulation can be recorded in direct relation to MRL table indexing. However, this requires the transportation and installation/removal of the CT table overlay for each patient when sharing the CT simulator with conventional linac patients or simulating MRL patients across multiple CT scanners. This is non-trivial due to the size and weight of the CT table overlay and adds a time and effort burden on the simulation team. For example, the Elekta CT table overlay is over 2 m long and weighs over 17 kg. Furthermore, repeated installation of the CT table overlay could lead to errors such as attaching to the wrong CT table index or not properly attaching the CT table overlay to the index bar on the CT table (allowing the CT table overlay to slide along the CT table). These errors can lead to safety issues as well as geometric shifts of marked isocenter relative to its expected position in the TPS.
To address these issues, we developed an alternative technique to ensure consistent setup between a CT simulator and MRL without using a CT table overlay. By analyzing CT table positions at different CT table overlay indices, a look-up-table (LUT) was created to convert CT table coordinates to MRL indices. The CT table overlay was only used once during commissioning of the LUT. Following LUT commissioning, all clinical CT simulations for MRL patients occurred on the native CT table since the CT coordinates could be converted to MRL indices. As a result, this procedure eliminated the unnecessary time, physical exertion, and potential errors associated with transporting and installing the CT table overlay for each MRL patient.
2. Materials and methods
The data supporting the findings of this study are openly available in Figshare (https://doi.org/10.6084/m9.figshare.24777396). Equipment model and manufacturer information used in this protocol is listed in Table S1.
2.1. Commissioning of table index LUT
Commissioning of the table index LUT included two steps. First, the LUT was created by installing the CT table overlay on the CT table and scanning a phantom at various MRL table indices while recording the corresponding CT table indices and CT longitudinal coordinates. After the LUT was populated, the LUT conversion factors were verified by creating test plans offline using the treatment planning system (TPS) with the phantom indexed at three locations on the CT scanner with and without the CT table overlay. At the MRL, the phantom was indexed on the table (according to the index from the CT table overlay or the index provided by the LUT) and driven to isocenter for the associated plan. The MR image was acquired and fused with the reference CT image. The isocenter shift due to phantom setup error is captured by the registration offset between the two images using the online TPS. These values were recorded and compared between setups with the CT table overlay vs. the LUT.
2.2. Clinical workflow during CT simulation for MRL patients utilizing the LUT
A clinical workflow document was drafted for the simulation team to follow for the clinical implementation of the LUT during the simulation of MRL patients. This included pertinent information for using of wing board and head-and-neck board immobilization devices. Troubleshooting scenarios are also included. This document was vetted by the simulation team to ensure its comprehensibility for future staff.
2.3. Workflow review by simulation staff
The LUT was implemented as part of our MRL simulation workflow in early 2020. Prior to this, we simulated 41 patients with the CT table overlay. Since, we simulated 265 patients using the LUT without the CT table overlay. We asked four simulation therapists, who had experience with both workflows, to answer a series of survey questions that reviewed the two workflows.
3. Results
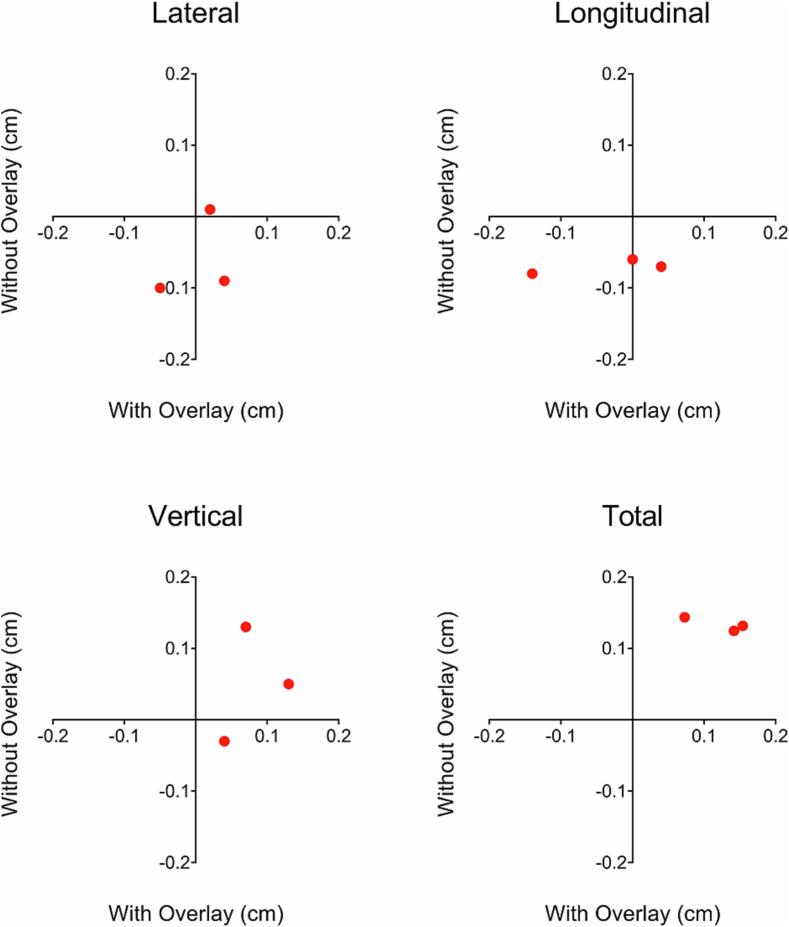
The matched lateral, longitudinal, vertical, and total displacement isocenter shifts between plans with and without use of the CT table overlay are illustrated in Fig. 1. The root-mean-square isocenter shifts were 0.04 ± 0.05 cm, 0.08 ± 0.09 cm and 0.09 ± 0.05 cm when using the CT table overlay and 0.08 ± 0.06 cm, 0.07 ± 0.01 cm, and 0.08 ± 0.08 cm when using the LUT without the CT table overlay in the lateral, longitudinal, and vertical directions, respectively. Instructions for each step of LUT commissioning and accompanying photos are provided in the Supplementary Materials in Appendix A and Fig. S2-8. The LUT for our CT simulator is presented in Appendix B. The clinical workflow document for conducting CT simulations on MRL patients without the CT table overlay is presented in Appendix C. In this workflow document, key differences between conventional simulations and MRL simulations with the LUT are highlighted. Namely, the requirement of zeroing the table at the appropriate index prior to the CT scan, placement of the marked isocenter such that the longitudinal CT coordinate is a multiple of 20 mm, and LUT offsets that are required when using immobilization devices are clarified.
Fig. 1.
Matched isocenter shifts at three table indexing positions in the lateral, longitudinal, and vertical directions as well as the total 3-dimensional displacement for adaptive plans that used the CT table overlay vs. plans that used the LUT and no CT table overlay during CT simulation.
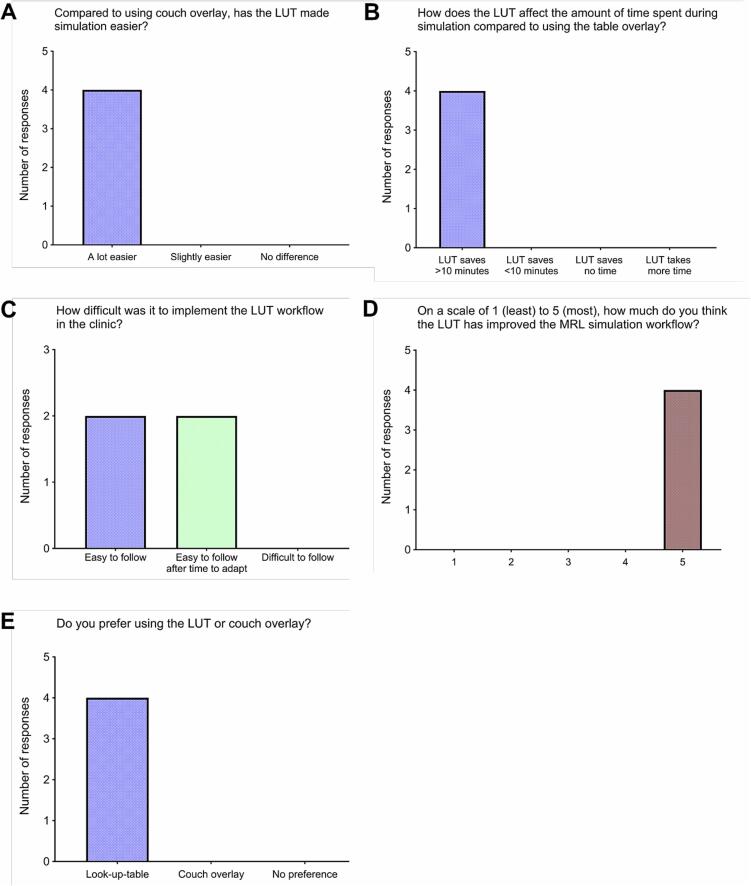
The responses to the workflow survey are provided in Appendix D and in visualized in Fig. 2. Each simulation therapist that was asked about implementing the LUT in the clinic preferred its use over the table overlay because it made the simulation easier and saved more than 10 min in simulation time. Though each therapist found the workflow easy to follow, half noted that it took some time to adapt to the new protocol. Each therapist who provided additional feedback noted that the CT table overlay was cumbersome to install due to its weight, which contributed to the preference for the LUT workflow.
Fig. 2.
Pooled responses to survey questions answered by simulation therapists who used both the CT table overlay and LUT in simulation workflows.
4. Discussion
In this manuscript, we demonstrated the proof-of-concept of simulating MRL patients on a CT simulator without requiring the use of the CT table overlay. To achieve this, we commissioned a LUT to convert between CT table indices and longitudinal coordinates to MRL table indices for patient setup and target localization. The minimal isocenter shift resulting from the LUT workflow compared to the table overlay workflow indicated that there was a clinically acceptable consistency between using LUT and a CT table overlay. These deviations are approximately the size of one pixel in the MR images and can be expected in rigid registration algorithms [6]. This protocol was quickly adopted by the simulation team at our institution, who consistently expressed definitive positive feedback to the change.
For sites that have a Unity MRL and CT simulator with analogous table indexing, the LUT and associated operating procedures should be immediately applicable. However, the presented commissioning process and clinical utilization of a LUT is also applicable for alternative simulation/treatment table index conversions, such as for alternative MRL systems or MR simulators. While this is made easier with a corresponding simulator table overlay, one isn’t necessarily required for commissioning. An analogous in-house device could be used, or one could directly mark the CT table with the MRL index locations. Alternatively, one could measure the relative distances between each table’s indices, map each table index to the machine’s longitudinal coordinate, and then create a virtual transformation between the two tables. Regardless of the methodology behind the generation of the LUT, end-to-end validation is required prior to clinical use.
Within the clinical workflow document, we highlighted a few points that deviated from standard CT simulation procedures. One such is the requirement for zeroing the CT table at the appropriate index (in this case H5) prior to the simulation. This is necessary so that the LUT can correctly link the CT table coordinates to the MRL table indices. We noticed that, in our clinic, this was one of the most error-prone steps, so a few troubleshooting scenarios are presented in the clinical workflow document described in Appendix C.
A longitudinal CT coordinate that is a multiple of 20 mm corresponds to the precise location of an index position on the MRL table, which ultimately simplifies patient setup. During planning, the Monaco TPS requests an MRL index position as a localization point, which is then used to calculate the shifts to final isocenter position. If the localization point can be placed at the same location as markings on the patient or immobilization devices, it minimizes additional setup error that needs to be accounted for during the adaptive positioning step of MRL treatment. This is important because rather than shifting the table, the Unity reoptimizes the plan to account for isocenter displacement. Thus, large fields that occupy most of the available 22 cm opening within the MRL bore may not be able to adequately reoptimize to account for large isocenter displacements [7]. Note that the use of external transverse setup lasers in the MRL treatment room would also allow for precise patient setup, negating the requirement for the localization point to be a multiple of 20 mm.
The use of immobilization devices during simulation may require an additional LUT offset. This is because the head of the MRL table contains electronics and ports that may prevent the immobilization devices from being attached properly. For example, the wing board used in many thoracic and abdominal treatments at our institution is normally indexed at H5 on the CT table. The corresponding MRL table index from the LUT is 1.0, but the wing board will not properly index on the MRL couch in this location. As a result, the wing board is shifted inferiorly 10 MRL index positions, which corresponds to a + 200 mm offset. Thus, when using the LUT to convert the longitudinal CT coordinate of marked isocenter in these patients, the MRL table index that is used in the TPS should correspond to the longitudinal CT coordinate plus 200 mm. Another example is the use of a head-and-neck board with thermoplastic masks, which requires a −80 mm offset for LUT conversion. The simulation team should include this information in the record-and-verify system (templates that are used at our institution for cases with and without offsets are available in Appendix C). It should be noted that while the indexing of one immobilization device is always possible, the indexing of two or more immobilization devices may be limited or not possible if the index spacing of the CT table and the CT table overlay/MRL table are not fully compatible (i.e., there is not a corresponding MRL index at the same relative position of the CT table index). Our LUT in Appendix B, demonstrates that each CT table index does have a corresponding MRL table index, so it is possible to index any number of immobilization devices. However, this won’t necessarily be the case for all simulator/treatment table combinations.
Our clinic’s experience with substituting the CT table overlay with the LUT has been very positive. Training the simulation team to utilize the LUT was straightforward, and CT simulations of MRL patients have become more streamlined without the need of using the CT table overlay. Commissioning of the LUT and verification of its geometric accuracy was simple. This process can easily be applied to alternate flat-table simulation/treatment machine combinations, including those with varying indexing increments, as long as end-to-end verification is performed to address any compatibility issues, such as those discussed with multiple immobilization devices.
CRediT authorship contribution statement
Neil Hughes: Conceptualization, Methodology, Data curation, Validation, Writing – original draft. Travis C. Salzillo: Methodology, Data curation, Validation, Writing – original draft. Sastry Vedam: Conceptualization, Methodology, Writing – review & editing. Tze Yee Lim: Data curation, Validation, Writing – review & editing. Xin Wang: Data curation, Validation, Writing – review & editing. He Catherine Wang: Data curation, Validation, Writing – review & editing. Mustefa Mohammedsaid: Data curation, Validation, Writing – review & editing. Clifton D. Fuller: Validation, Writing – review & editing. Jihong Wang: Validation, Writing – review & editing. Jinzhong Yang: Conceptualization, Methodology, Data curation, Validation, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported in part by the National Institutes of Health (NIH) through Cancer Center Support Grant P30CA016672. TCS was supported by a training fellowship from The University of Texas Health Science Center at Houston Center for Clinical and Translational Sciences TL1 Program (TL1TR003169). C.D.F. received/receives funding and salary support during the period of study execution directly related to this effort from: NIDCR Academic Industrial Partnership Grant (R01DE028290). CDF received/receives funding and salary support during the period of study execution directly unrelated to this effort from: the NIH/NIBIB Research Education Programs for Residents and Clinical Fellows Grant (R25EB025787-01); an NCI institutional training award (T32CA261856); an NIH/NCI Cancer Center Support Grant (CCSG) Pilot Research Program Award from the UT MD Anderson CCSG Radiation Oncology and Cancer Imaging Program Seed Grant (P30CA016672); and an NSF Division of Civil, Mechanical, and Manufacturing Innovation (CMMI) grant (NSF 1933369). CDF has received direct industry grant support, honoraria, and travel funding from Elekta AB unrelated to this project. Direct infrastructure support is provided to CDF by the multidisciplinary the Radiation Oncology/Cancer Imaging Program (P30CA016672-44) of the MD Anderson Cancer Center Support Grant (P30CA016672) and the MD Anderson Program in Image-guided Cancer Therapy.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.phro.2023.100524.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.van Herk M. Errors and margins in radiotherapy. Semin Radiat Oncol. 2004;14:52–64. doi: 10.1053/j.semradonc.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Hector C.L., Webb S., Evans P.M. The dosimetric consequences of inter-fractional patient movement on conventional and intensity-modulated breast radiotherapy treatments. Radiother Oncol. 2000;54:57–64. doi: 10.1016/S0167-8140(99)00167-X. [DOI] [PubMed] [Google Scholar]
- 3.Cuccia F., Alongi F., Belka C., Boldrini L., Hörner-Rieber J., McNair H., et al. Patient positioning and immobilization procedures for hybrid MR-Linac systems. Radiat Oncol. 2021;16:183. doi: 10.1186/s13014-021-01910-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonald B.A., Vedam S., Yang J., Wang J., Castillo P., Lee B., et al. Initial feasibility and clinical implementation of daily mr-guided adaptive head and neck cancer radiation therapy on a 1.5t mr-linac system: prospective r-ideal 2a/2b systematic clinical evaluation of technical innovation. Int J Radiat Oncol. 2021;109:1606–1618. doi: 10.1016/j.ijrobp.2020.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg S.A., Henke L.E., Shaverdian N., Mittauer K., Wojcieszynski A.P., Hullett C.R., et al. A multi-institutional experience of mr-guided liver stereotactic body radiation therapy. Adv Radiat Oncol. 2019;4:142–149. doi: 10.1016/j.adro.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brock KK, Mutic S, McNutt TR, Li H, Kessler ML. Use of image registration and fusion algorithms and techniques in radiotherapy: Report of the AAPM Radiation Therapy Committee Task Group No. 132. Med Phys 2017;44:e43–76. https://doi.org/10.1002/mp.12256. [DOI] [PubMed]
- 7.Roberts D.A., Sandin C., Vesanen P.T., Lee H., Hanson I.M., Nill S., et al. Machine QA for the elekta unity system: a report from the elekta MR-linac consortium. Med Phys. 2021;48 doi: 10.1002/mp.14764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.