Abstract
Objective
The aim of the present study was to investigate trunk kinematics and spine muscle activation during walking after minimally invasive surgery in patients with L4-L5 degenerative spondylolisthesis suffering from lumbar instability (LI).
Methods
Eleven patients suffering from LI and 13 healthy controls (HC) were enrolled. Trunk kinematics and spine muscle activation patterns during walking were collected. Maximal trunk ranges of motion were also recorded from standing position. Assessments were performed pre-operatively (T0), 1 month (T1) and 3 months (T2) after MIS.
Results
We found significant improvement in spine muscle activation during walking at T2 compared to T0, mainly involving right/left symmetry at the operated level (L4-L5) and up-down synchronization from L3 to S1. Significant improvements in trunk rotation nearing to the HC group during walking were also found at T2 after surgery, though no changes were observed in the maximal range of motion of the trunk during standing. Furthermore, trunk rotation improvement correlated with a lower grade of residual disability.
Conclusions
Our findings indicate that trunk rotation improves after surgery, and impaired aspects of spine muscle activation can be improved with surgery. These biomechanical parameters could represent novel tools for monitoring the effect of surgery in LI and preventing impaired spine mobility and muscle activation.
Keywords: Low back pain, Spine muscle activation, Cross-correlation, Center of activity, Surface electromyography, Vertebral column, Surgical procedures
Graphical abstract
Abbreviations list
- LBP
low back pain
- LI
Lumbar Instabilities
- DDD
degenerative disk disease
- sEMG
surface electromyography
- CoA
Center of Activity
- FW
Full Width
- FWHM
Full Width Half Maximum
- swLI
subjects with lumbar instability
- FBSS
failed back surgery syndrome
- MIS
Minimally Invasive Surgery
- HC
healthy controls
- ODI
Oswestry Disability Index
Introduction
More than 80 % of the working adult population in the world suffers from low back pain (LBP), which is a major cause of disability,1 and is most commonly associated with degenerative disk disease (DDD).2,3,4 More than half of subjects with LBP present segmental spine instability5 which may increase the likelihood of nerve endings being damaged and causing pain.6,7,8 Particularly, subjects with DDD-related spinal instability complain of chronic disabling axial pain that is unresponsive to conservative treatments and requires surgery.9 MRI, CT, and X-rays, albeit necessary for diagnosis and classification, are not effective tools for investigating the spine during dynamic tasks. Conversely, 3-D motion analysis systems, may allow for full characterization of spine biomechanics during movements.10,11,12,13,14,15,16 Furthermore, when compared with subjects without LBP, subjects with LBP have significant differences in muscle activity as measured by surface electromyography (sEMG),17 with back muscles sEMG asymmetry representing a strong indicator of LBP.18 sEMG-derived indexes, such as the Center of Activity (CoA) and Full Width (FW), have shown to characterize differences in the amplitude and timing of EMG activity of subjects with pathological conditions.19,20,13,21,22 Additionally, measurements resulting from cross-correlation analysis are commonly used for investigating spatial and temporal relationships between time-varying signals and can provide further insight into muscle activation patterns muscle latencies or muscle recruitment.23
Recently, we compared the gait of subjects with lumbar instability (swLI) due to DDD and a group of healthy subjects through an optoelectronic motion analysis system integrated with sEMG.24 We found a series of sEMG abnormalities regarding spinal muscle activation in terms of left-right symmetry, top-down synchronization, and spatiotemporal modulation during walking. The sEMG abnormalities were strictly correlated with the painful lumbar area and occurred mainly in the segment involved in the instability. Furthermore, subjects with failed back surgery syndrome (FBSS) had a higher grade of impairment in spine muscle activation, with stronger involvement of the trunk motion and gait performance.24
Preserving the integrity of the spinal muscles has progressively been considered a major achievement in modern spine surgery, especially in elective surgeries for LBP. Iatrogenic deafferentation and denervation of the paraspinal muscles,25 which may occur in up to 15 % of subjects suffering from FBSS,26 may compromise trunk function in its role as a facilitator of energetically efficient gait pattern and dynamic balance modulator.27,28,29,30 In the last few decades, Minimally Invasive Surgery (MIS) has been proposed as an alternative to conventional surgery for many different conditions affecting the spine.27,31,29,30,32 MIS has been found to preserve the anatomical and functional integrity of spine muscles and peri-articular tendons, as well as reduce intra-operative blood loss, post-operative pain, and hospitalization time.8,33,34,27,35 Although MIS has been shown to improve quality of life and pain,36,37 its effects on gait behavior have only been investigated in terms of spatiotemporal and kinetic parameters of the lower limbs, with less compensation between lower limbs reported after surgery. However, trunk kinematics and sEMG parameters following surgery have never been studied.38
Therefore, the aims of the present study were to investigate the spine muscle activation in terms of left-right muscle symmetry and top-down muscle synchronization in swLI who underwent MIS. Furthermore, we analyzed the effects of MIS in terms of spine kinematics and spatio-temporal parameters. We hypothesized that some of the parameters differentiating swLI from healthy controls (HC) might modify and reflect the clinical improvements after surgery.
Material and methods
Study design
This study was designed as a prospective observational pilot study. Enrollment was carried out from July 2013 to December 2017 (30 months). For all included patients, clinical and instrumental (motion analysis) evaluations were performed pre-operatively (T0) and 1 month (T1), and 3 months (T2) after MIS. Ethical committee approval was obtained (ICOT-ASL2017 Latina, University of Rome, Polo Pontino). All patients provided specific informed consent according to institutional guidelines. The study conformed to the Helsinki declaration for studies involving human or animal subjects.
Patient selection
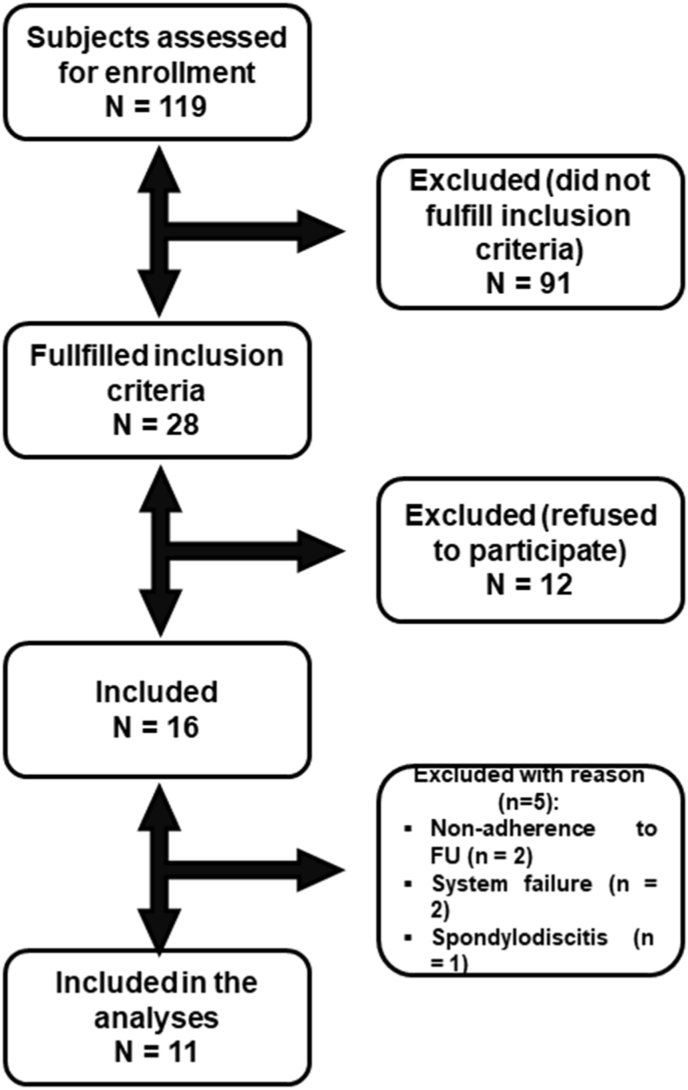
One hundred nineteen subjects referred to our Department of Neurosurgery during the enrollment period who were diagnosed with LI sustained by L4-L5 degenerative spondylolisthesis were considered for eligibility (Fig. 1). Eleven swLI aged 25–74 years were included in the analyses (Fig. 1). Demographic and clinical data are reported in Table 1. In all patients, the involved level was L4-L5. Thirteen healthy subjects constituted the HC group.
Fig. 1.
Study flow-chart. The figure represents the patients' enrollment of the LI subjects undergoing MIS.
Table 1.
Clinical characteristics.
| MIS group | HC group | Time of assessment | |
|---|---|---|---|
| Age (mean ± SD) | 58.2 ± 14.36 | 46.58 ± 12.69 | |
| Gender (F:M) | 9:2 | 9:4 | |
| Height (m) (mean ± SD) | 1.58 ± 0.07 | 1.70 ± 0.08 | |
| BMI (mean ± SD) | 27.10 ± 4.09 | 27.40 ± 11.53 | |
| normal weight (n) | 3 | 2 | |
| overweight (n) | 3 | 4 | |
| class 1 obese (n) | 5 | 5 | |
| ODI % (mean ± SD) | 60.91 ± 12.41 | T0 | |
| 28.67 ± 10.25 | T1 | ||
| 12.33 ± 5.85 | T2 | ||
| SF-12 (physical component) | 26.71 ± 5.72 | T0 | |
| 40.25 ± 8.79 | T1 | ||
| 53.93 ± 2.20 | T2 | ||
| SF-12 (mental component) | 28.96 ± 10.42 | T0 | |
| 46.57 ± 7.05 | T1 | ||
| 54.15 ± 6.51 | T2 |
MIS, Minimally Invasive Surgery group; HC, Healthy Control group; BMI, Body Mass Index; ODI, Oswestry Disability index; SF-12, 12-item Short Form Questionnaire; T0, baseline assessment; T1, 1-month after surgery assessment; T2, 3-months after surgery assessment.
The diagnosis was based on the following clinical and radiological criteria: lumbar pain exacerbated by the axial load, worsening in flexion; MRI findings suggestive of DDD and segmental instability; and standard and dynamic X-rays reporting segmental spondylolisthesis with instability in flexion and/or extension. In this study, segmental instability was defined radiologically as > 3 mm dynamic sagittal translation in the sagittal plane.39,40,41,42
Age >80 years, previous spinal surgery, severe lumbar stenosis, or disc herniation with mono- or poly-radicular impairment, presence of leg pain, isthmic spondylolysis, two or more segments affected by DDD, and global sagittal imbalance43 were considered exclusion criteria.
Data from 13 healthy volunteers aged 27–69 years constituting the HC group were also collected for comparison.
Minimally invasive surgical procedure
All patients were in the prone position on a radiolucent surgical carbon bed. A c-arm was used throughout the procedure for intraoperative guidance. Surgical procedures were performed in a standardized manner as described in our previous investigation.44 The instrumentation system was the same for each case (Precept Modular and MAS-TLIF, Nuvasive, San Diego, CA, US). Pedicular screws were inserted using the pure percutaneous technique bilaterally on L4 and L5 under fluoroscopic guidance. On one side, the two incisions were connected, and the zygapophyseal joint reached, using the approach described by Wiltse et al45 The articular joint was removed using a high-speed drill and a rongeur, and the intervertebral disc was removed, and vertebral endplates carefully prepared. An intersomatic cage (TLIF anterior, Coroent, Nuvasive, San Diego, CA, US) was inserted and positioned in the discal space under fluoroscopic guidance. Lastly, a single titanium rod was placed on each side and a dynamometric locking device was used to lock the system after obtaining mild posterior compression to increase the segmental lordosis and disc angle,29 reducing the chances of intersomatic cage posterior pullout.
Biomechanical assessment
Procedures
Walking task
Subjects were required to walk while barefoot at their self-selected speed along a 10-m-long walkway approximately 10 m long. Six walking trials were acquired per subject. We rejected the first walking trial and considered the subsequent five trials. To prevent fatigue, trials were separated by a 1-min rest.
Stationary trunk movements
Subjects were required to perform three series of standing in the upright posture with their arms crossed over their chests and three series of maximal trunk flexion-extension, bending, and rotation.
Kinematic recordings
The kinematic parameters were acquired using the optoelectronic SMART-DX 500 motion analysis system (BTS, Milan, Italy) consisting of eight infrared cameras (with a sampling rate of 300 Hz). The system was used to acquire the motion of 22 passive spherical markers 15 mm in diameter during both the walking and standing trials. The markers were placed over anatomical landmarks according to the Davis model.46 Anthropometric data were collected for each subject.16 Kinematic data were normalized between the two consecutive heel strikes when reduced to 100 samples in the gait cycle using a polynomial procedure.
The following spatio – temporal gait parameters were considered for the statistical analysis: step length (cm) and width (cm), stance phase duration (%), swing phase duration (%), double support phase duration (%), cadence (steps/min), and speed (m/sec).
To assess trunk kinematics, we determined the trunk and pelvis joint centers of rotation and calculated the trunk range of motion in the sagittal (flexion-extension), frontal (lateral bending), and transverse planes (rotation) during the gait cycle and standing posture.47,48,49,50
sEMG recordings
The sEMG signals were recorded at a sampling rate of 1000 Hz using a 16-channel Wi-Fi transmission surface electromyography system (FreeEMG System, BTS, Milan, Italy). After skin preparation, circular (1 cm diameter), bipolar, Ag/AgCl surface electrodes (FIAB SpA, Florence, Italy) prepared with electro-conductive gel were placed over the right and left sides of the paraspinal muscles (longissimus) at the L3-L4, L4-L5, and L5-S1 levels and over the right and left sides of the lumbar iliocostalis at 2-cm intervals. Electrodes were placed on the center of the muscle belly in the direction of the muscle fibers according to the European Recommendations for Surface Electromyography.51
The sEMG data were digitally filtered using 10 and 500 Hz as the lower and upper cut-off frequencies of the Hamming filter, respectively. The acquired sEMG signals were processed by subtracting their average value and full-wave-rectified and filtered using a zero-lag fourth-order Butterworth with a low pass filter cut-off of 3 Hz.16
For each individual, the sEMG signal from each muscle was normalized to its peak value across all trials.52
2.4.1.5. Clinical assessment
The disability grade was assessed using the Oswestry Disability Index (ODI) score (0–100 %, where 0–20 % indicates minimal disability; 81–100 % complete disability)53 and the Physical and Mental Health scores of the 12-item Short Form Questionnaire (SF-12).54
Statistical analysis
Based on an effect size ranging from 1.03 to 1.21,40 a sample of at least 20 subjects (10 subjects with LI and 10 HCs) was calculated to identify significant differences in the spatiotemporal parameters between subjects with LI undergoing surgery and HCs at a significance level of 95 % and 80 % power.40
Statistical analyses were performed using SPSS software ver. 20.0 (IBM, Armonk, NY, USA). Given the small sample, we used non-parametric statistical methods.55 The Mann–Whitney test was used to evaluate differences swLI and HCs at T0 and T2. The Friedman test with post-hoc analysis (Wilcoxon test) was used to evaluate the effect of time from surgery on biomechanical features of swLI.
The Watson–William's test for circular data7 was used to investigate between- and within-group differences in EMG Center of Activity (CoA) activation parameters.56,57,24
To evaluate the correlations between improvements in kinetic, kinematic, and clinical parameters, we calculated Spearman's correlation coefficients (ρ). A p-value <0.05 was considered significant. Details on cross – correlation analysis, FWHM and CoA calculations are described in Appendix A.
Results
Clinical findings
The Friedman test revealed a significant within-group effect regarding all clinical measurements (ODI: p = 0.006; SF12 physical component: p = 0.030; SF12 mental component: p = 0.030). Post-hoc analysis revealed significant improvements in all clinical scales between T0 and T1 (ODI: p = 0.003; SF12 physical component: p = 0.003; SF12 mental component: p = 0.004). Significant improvements were also found between T0 and T2 (ODI: p = 0.004; SF12 physical component: p = 0.004) but not for the SF12 mental component (p = 0.050).
Gait parameters and trunk kinematics
The spatiotemporal parameters and trunk kinematic results are reported in Table 2. At T0, during walking, swLI had a significantly reduced step length (p = 0.034), gait speed (p = 0.047), stance duration (p = 0.023), trunk obliquity (p = 0.028), and trunk rotation (p = 0.034) and increased double support duration (p = 0.040) compared to HCs. During standing, swLI had significantly reduced trunk flexion-extension (p < 0.001), bending (p = 0.008), and rotation (p = 0.040) compared to HCs. The Friedman test revealed a significant effect of time from surgery on the trunk rotation during walking (p = 0.038), but no significant effect was revealed during standing. Post-hoc analysis also revealed a significant increase in trunk rotation during gait at T2 compared to T0 (p = 0.006; Table 2). At T2, Mann–Whitney test revealed no differences in trunk rotation between the LI group and the HC group, suggesting that this feature had improved to normative values.
Table 2.
Spatio-temporal and kinematic parameters.
| T0 |
T1 |
T2 |
HC |
|||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Gait task | ||||||||
| Stance duration [%] | 63.5 | 2.2 | 63.3 | 2.6 | 63.1 | 2.7 | 62.1 | 0.8 |
| Swing duration [%] | 36.5 | 2.2 | 36.7 | 2.6 | 36.9 | 2.7 | 37.9 | 0.8 |
| Double support duration [%] | 14.0 b | 3.0 | 14.2 | 3.6 | 13.7 | 2.9 | 12.0 | 1.4 |
| Cadence [step/min] | 100.1 | 9.0 | 100.0 | 5.1 | 101.3 | 15.6 | 99.8 | 9.7 |
| Step length [cm] | 47 b | 8.6 | 45.7 | 7.2 | 47.6 | 6.5 | 55.5 | 5.7 |
| Step width [cm] | 15.9 | 2.8 | 16.0 | 2.4 | 15.4 | 1.4 | 17.5 | 3.1 |
| Speed [m/s] | 0.84 b | 0.19 | 0.83 | 0.15 | 0.89 | 0.21 | 1.02 | 0.16 |
| Trunk Tilt (°) | 4.9 | 2.5 | 5.0 | 2.1 | 4.3 | 1.6 | 4.7 | 4.1 |
| Trunk obliquity (°) | 6.2 b | 3.2 | 6.3 | 3.9 | 6.7 | 3.0 | 8.8 | 2.3 |
| Trunk rotation (°) | 10.5 b | 4.1 | 12.2 | 2.8 | 12.5a | 4.6 | 14.0 | 3.2 |
| Stationary trunk movement tasks | ||||||||
| Maximal flexion (°) | 83.6 b | 7.9 | 79.0 | 21.9 | 85.1 | 12.9 | 104.1 | 9.6 |
| Maximal bending (°) | 55.5 b | 14.5 | 52.6 | 10.6 | 51.8 | 14.0 | 75.5 | 18.4 |
| Maximal rotation (°) | 44.76 b | 19.9 | 55.7 | 16.4 | 48.5 | 19.2 | 58.5 | 9.7 |
ap < 0.05 within patients at T2 and T0, identifying the modified parameters.
bp < 0.05 between patients and healthy controls at baseline.
sEMG findings
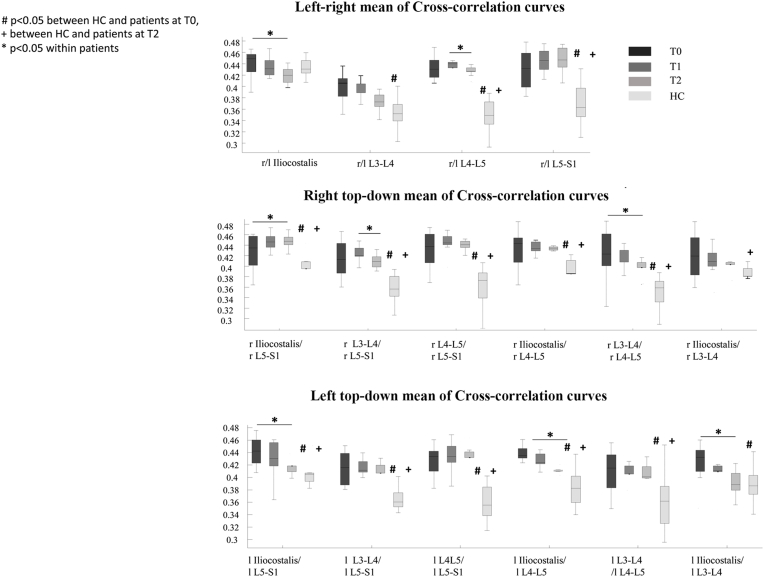
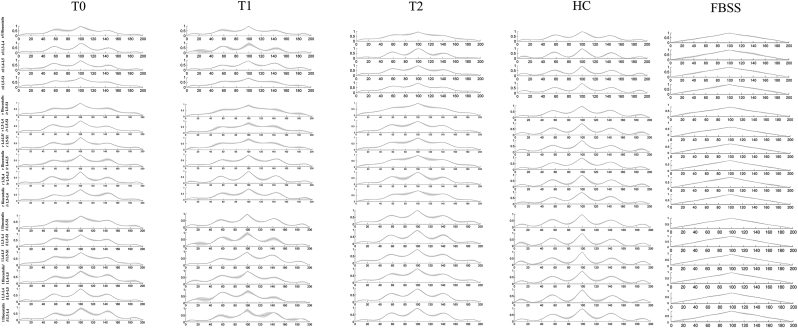
SwLI had significantly higher mean values in both the right/left and up-down cross-correlation curves at T0 compared with the HC group for almost all muscles (Fig. 2). The Friedman test revealed significant effect of time from surgery on the right/left cross-correlation curve values at L4-L5 and on the up-down cross-correlation curve values at the right iliocostalis/right L5-S1, right L3-L4/right L5-S1, right L3-L4/right L4-L5, left iliocostalis/left L5-S1, left iliocostalis/left L4-L5, and left iliocostalis/left L3-L4 (Fig. 3). Wilcoxon post-hoc analysis revealed a significant decrease in all of these right/left and up-down cross-correlation curve values at T2 compared to T0 (Fig. 2). At T2, Mann–Whitney test revealed no differences in the left iliocostalis/left L3-L4 cross-correlation curve values between swLI and HC, suggesting improvement of this feature up to normative values. Fig. 3 shows the left-right and up-down cross-correlation curves in a representative swLI before and after MIS, and in a representative HC.
Fig. 2.
Box plot of mean values of the cross-correlation curves. The box plots reported in the upper panel show the mean values of the left/right cross-correlation comparisons. The box plots in the middle panel show the mean values of the right top-down cross-correlation comparisons. The box plots in the lower panel show the mean values of the left top-down cross-correlation comparisons. #p < 0.05 between HC and patients at T0,+between HC and patients at T2 *p < 0.05 within patients; l = left; r = right.
Fig. 3.
Cross-correlation curves. Cross-correlation curves at T0, T1, and T2 in a representative subject from MIS group and HC group are represented. The graphs reported in the upper panels show the cross-correlation curves between the left and right muscles. The graphs reported in the middle and lower panels show the up-down cross-correlation curves between pairs of muscles of the right and left sides, respectively. On the right side, a graph illustrating a representative subject with failed back open surgery syndrome retrieved from Miscusi et al. 2019(with permission) was added for visual comparison. It is possible to note that the sinusoidal form of the curve observed in healthy subjects is still preserved in the LI patients after surgery suggesting a muscle activation symmetry and synchronization more similar to that of healthy subjects than to that of subjects with failed back surgery.
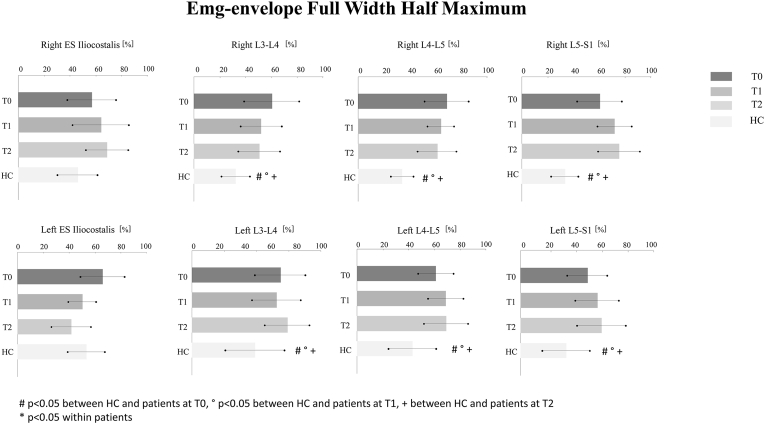
At T0, significant differences were found in the FWHM between swLI and HC for all muscles except the right and left erector spinae iliocostalis (Fig. 4). In the Watson–William's test, the LI group had significant differences in the CoA values compared to the HC at T0 for the right iliocostalis muscles, the right L4-L5, and L5-S1 (Table 3). The Friedman test did not reveal a significant effect of the time from surgery on the baseline FWHM or CoA.
Fig. 4.
FWHM values. Mean values and standard deviations of the FWHM of all muscles in LI at T0 (dark gray bars), T1 (gray bars), T2 (light gray bars) and HC (white bars) groups, respectively, are reported. #p < 0.05 between HC and patients at T0, ° p < 0.05 between HC and patients at T1,+between HC and patients at T2*p < 0.05 between patients at T2 compared to T0.
Table 3.
CoA findings.
| CoA | T0 | T1 | T2 | HC | ||||
| Mean (°) | AD | Mean (°) | AD | Mean (°) | AD | Mean (°) | AD | |
| r iliocostalis | 18.20# | 1.08 | 33.53# | 0.79 | 3.88# | 0.42 | 299.50 | 0.84 |
| r L3-L4 | 280.51 | 1.04 | 272.56 | 0.59 | 311.74 | 0.78 | 242.12 | 0.78 |
| r L4-L5 | 40.26# | 1.06 | 5.82 | 0.88 | 21.10 | 0.88 | 297.93 | 1.03 |
| r L5-S1 | 67.43# | 0.85 | 49.29 | 0.43 | 36.15 | 0.66 | 0.60 | 0.57 |
| l iliocostalis | 312.88*° | 0.89 | 354.43 | 0.64 | 296.50 | 0.97 | 298.88 | 0.88 |
| l L3-L4 | 259.45 | 0.83 | 266.40 | 0.88 | 190.00 | 0.72 | 239.58 | 0.82 |
| l L4-L5 | 294.33* | 1.21 | 29.57 | 1.02 | 11.32 | 1.03 | 324.44 | 0.83 |
| l L5-S1 | 38.78 | 0.79 | 69.38 | 0.85 | 47.49 | 0.77 | 0.18 | 0.70 |
*p < 0.05 within patients at T1 and T0; ° p < 0.05 between patients at T2 and T0; #p < 0.05 between patients and healthy controls; AD = angular deviation; l = left; r = right.
Correlation findings
The improvements in trunk rotation significantly correlated with the improvements in ODI (ρ = 0.556, p = 0.038), SF12 physical component (ρ = 0.586, p = 0.029), and mean cross-correlation curve values for the left iliocostalis/left L3-L4 (ρ = 0.555, p = 0.038). No significant correlations were found between the other clinical or biomechanical data.
Discussion
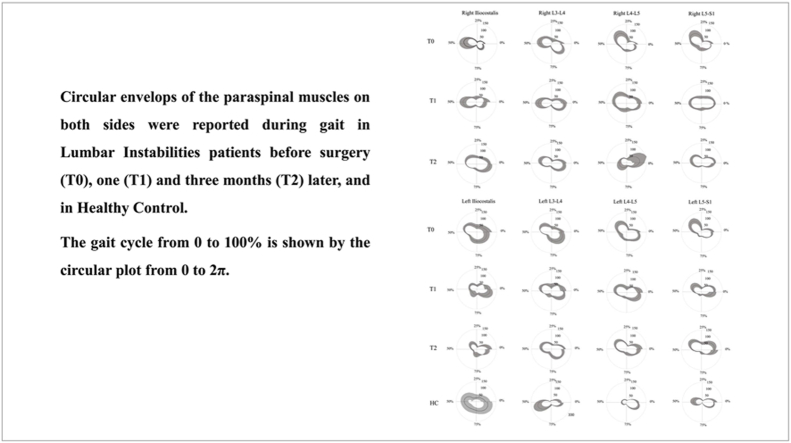
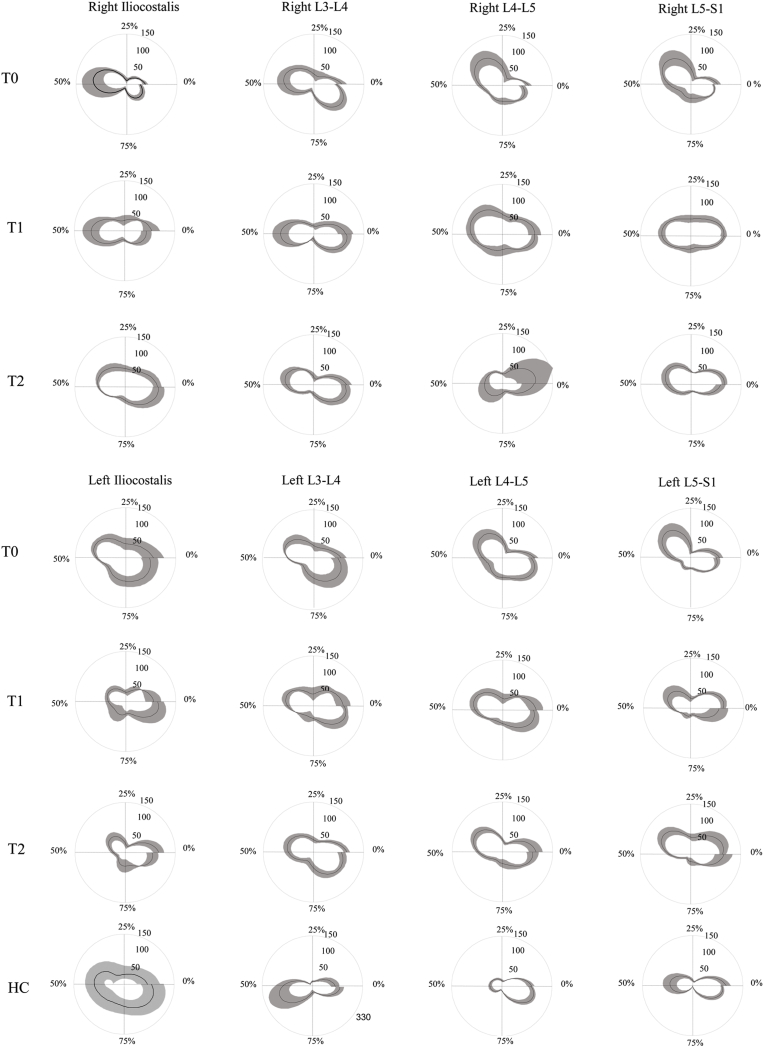
In this observational study, we evaluated whether spine kinematic and sEMG parameters modify after MIS for mono-segmental LI sustained by degenerative L4-L5 spondylolisthesis. This study extends our previous findings on spine biomechanics in a pre-surgical setting.24 In subjects suffering from LI, we previously reported24 a series of EMG abnormalities in spinal muscle activation in almost all muscles in terms of left-right symmetry, top-down synchronization, and spatio-temporal muscle activation modulation. Here, we found significant improvements 3 months after surgery compared to baseline (i.e., pre-surgery), mainly in terms of right/left symmetry and up-down synchronization. The reduced left/right asymmetry observed at L4-L5 segments was consistent with the radiological localization of the LI and suggests muscle symmetry abnormalities directly correlate with the affected lumbar level. The most significant changes were found in up-down synchronization, with a decrease in mean values in autocorrelation analysis curves for almost all spinal muscles from L3 to S1. Interestingly, the left iliocostalis/left L3-L4 modified to HCs values. Taken together, these findings suggest that some specific functional aspects of spinal muscle activation, such as left/right symmetry and up/down synchronization, can improve within 3 months after MIS, and that some parameters may even reach normative levels. Notably, the sinusoidal form of the curve, reflecting the similarity of the timing and shape of the EMG signals in healthy subjects, is still preserved in swLI after MIS and differs from that of subjects with failed back surgery (see the comparison in Fig. 3). Conversely, other functional aspects of spinal muscle activation, such as higher and longer activation, as well as anomalous modulation across the gait cycle as investigated by FWHM and CoA, appear unaffected by MIS (Fig. 5).
Fig. 5.
Circular envelope of the paraspinal muscles. Circular envelops of the paraspinal muscles of both sides, in LI at T0, T1 T2, and HC are reported. The circular plot from 0 to 2 π represents the 0–100 % of the gait cycle.
Although trunk rotation did not improve significantly in terms of maximum range of motion during the standing task, as expected after a one-level fusion surgical procedure, one of the most important findings of our study was that trunk rotation during walking, which requires a lower degree of motion than trunk rotation during the standing task (Table 2), improved to normative values 3 months after MIS. Trunk rotation has been reported to affect gait patterns, walking speed, and daily life activities58,59 by influencing balance, proprioceptive and vestibular functions, visual stabilization, and energy expenditure.11,12,23 In this study, we found a significant correlation between trunk rotation and both ODI and SF12 scores, reflecting the relationship between disability grade and trunk range of motion. These findings seem to suggest that improvements in trunk rotation range during gait could be a prognostic factor after MIS in terms of residual disability. This is also consistent with the results from previous investigations suggesting that trunk rotation is a predictor of gait recovery after rehabilitation in neurological disorders characterized by axial rigidity, such as Parkinson's disease.60,49 Therefore, rehabilitation should be focused on recovering trunk control and range of motion, eventually improving both gait and balance.61,62,63 Notably, improvements in trunk rotation also correlated with improvement in the left iliocostalis/left L3-L4 cross-correlation curves. The higher activity of the erector spinae iliocostalis with restriction in trunk motion has been proposed as a pain-response activity in patients suffering from chronic low back pain. This protective mechanism determines some grade of restriction in the relative motion between the pelvis and trunk, eventually resulting in lower coordination and compliance with perturbations.58 As a result, the increased trunk rotation during gait observed in our sample could be attributed to decreased paraspinal muscle activation following surgery, resulting in smoother trunk behavior. Although patients in our cohort had improved synergistic hyperactivation of the erector spinae, iliocostalis, and longissimus dorsi after surgery, allowing for wider and freer movement of the trunk during dynamic gait, MIS did not appear to be able to determine changes in the time-amplitude and spatial localization of the activation of the spinal muscles in swLI. These last findings suggest that surgery can create a substrate that could be enhanced by other interventions aimed at modifying motor activation and compensations that the subjects experienced over time as a result of pain and disability.64,65
Limitations
The present study has some limitations that should be considered when interpreting its results. Despite the use of sensitive quantitative measures of motion were used, the relatively small sample size is a limitation.
Furthermore, whereas surface EMG is simple to use on multiple muscles in both static and dynamic conditions and provides reliable information on general muscle activation and temporal events of muscular activation, it has some limitations, such as crosstalk and the need for appropriate methods to detect the physiological signal. Future research can focus on the use of surface electrode arrays that can detect a so-called sEMG “image,” or a time-varying electrical image which provides indirect information on muscle force, motor unit recruitment and de-recruitment strategies, the location of innervation zones and many other neurophysiological phenomena.66 Additionally, no comparative data on different surgical approaches, techniques, and instrumentation systems were available in this study. Therefore, our results should be verified in properly designed comparative clinical trials. Another limitation is represented by the lack of a age-matching procedure between subjects with LI and HS, which may have overrepresented the baseline differences between the groups while underrepresenting the effects of surgery in terms of normalization of the gait parameters. However, Furthermore, the follow-up time could be shorter than needed to evaluate the long-term effects of MIS on spinal muscles, their function, and any delayed gait impairment.6
Conclusions
In conclusion, our results seem to suggest that in patients with degenerative segmental spondylolisthesis, there is some grade of spine muscle impairment at the same level of the disease. Our results suggest that some biomechanical parameters, including muscle activation symmetry and synchronization, as well as trunk rotation during walking, may improve after MIS at early follow-up. MIS can reduce surgical injury to spinal structures, preventing loss of the ability to activate the spinal muscles symmetrically and synchronously during walking. However, the identification of relevant biomechanical parameters that are preserved or restored after surgery is a key point to be pursued in future studies. Notably, these novel data could also influence standard protocols on conservative management and rehabilitation after surgery for LI. We believe that the findings of this study can enhance understanding of the biomechanical effects of MIS, providing surgeons with additional measures to focus on as surgery outcomes. Furthermore, knowledge of the effects on biomechanics may help clinicians improve the prescription of post-surgery rehabilitation programs.
CRediT authorship contribution statement
Massimo Miscusi: Investigation, Supervision. Mariano Serrao: Conceptualization, Data curation, Supervision, Writing – original draft, Writing – review & editing. Luca Ricciardi: Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. Carmela Conte: Data curation, Formal analysis, Writing – original draft, Visualization, Writing – review & editing. Stefano Filippo Castiglia: Conceptualization, Data curation, Supervision, Writing – original draft, Writing – review & editing. Giorgio Ippolito: Conceptualization, Investigation, Supervision, Writing – original draft, Writing – review & editing. Gianluca Coppola: Conceptualization, Investigation, Supervision, Writing – original draft, Writing – review & editing. Stefano Forcato: Data curation, Investigation, Visualization, Writing – original draft, Writing – review & editing. Alba Scerrati: Investigation, Methodology, Writing – original draft, Writing – review & editing. Antonino Raco: Conceptualization, Data curation, Investigation, Supervision, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Cross-correlation, full width half maximum (FWHM), and center of activity analyses
| Cross-Correlation analysis | To obtain information on the left-right activation symmetry and up-down synchronization, we evaluated the similarity of timing and shape of the EMG signals by using the cross-correlation analysis. The cross-correlation allows for a stationary first signal, x, while the second signal, y, is time-shifted incrementally forwards for a range of time. The signal x and y are the EMG envelope of two pairs of trunk muscles. A normalized cross-correlation function Rxy at each shift of time was calculated using the Nelson-Wong et al formula: Where N is the number of data points in the input signal records, τis the discrete temporal phase shift, and fs is the frequency at which the original signals were sampled. Rxy revealed the shape similarity between the two signals as a scalar between 0 and 1. Specifically, to compare the shape of the right and left muscle signals, the Rxy curves were calculated between the right and left sides of the iliocostalis and the L3-L4, L4-L5, and L5-S1 paraspinal muscles (longissimus). The mean of the Rxy curves, for each comparison, was used as an index of muscle synchronization (Matz, et al, 2016). Using the Rxy curves, the synchronization of the trunk muscles was calculated between the following two pairs of trunk muscles for each side: Erector spinae iliocostalis vs L5-S1; L3-L4 vs L5-S1; L4-L5 vs L5-S1; Erector spinae iliocostalis vs L4-L5; L3-L4 vs L4-L5; Erector spinae iliocostalis vs L3-L4 |
| Full-Width-Half-Maximum percentages | To obtain information on the time-amplitude features of the EMG signals across the gait cycle, we used the full width half maximum (FWHM) method (Martino et al, 2015). We calculated the FWHM as the sum of the duration of the intervals in which the paraspinal EMG activity exceeded 50 % (FW50) of its maximum (Matz et al, 2016). |
| Center of Activity | To obtain information on the spatial localization of the EMG signals according to the gait cycle subphases, we used the CoA calculated using circular statistics (Miscusi et al, 2019; Berens, 2009; Prince et al, 1994) and plotting in polar coordinates (with angle ϑ that varies from 0 to 360°). The CoA of the EMG waveform was calculated using the following formula: Where i is the i-time point within the gait cycle. |
References
- 1.Rubin D.I. 2007. Epidemiology and Risk Factors for Spine Pain. [DOI] [PubMed] [Google Scholar]
- 2.Cheung K.M.C., Karppinen J., Chan D., et al. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine. 2009;34:934–940. doi: 10.1097/BRS.0b013e3181a01b3f. [DOI] [PubMed] [Google Scholar]
- 3.Luoma K., Riihimäki H., Luukkonen R., Raininko R., Viikari-Juntura E., Lamminen A. Low back pain in relation to lumbar disc degeneration. Spine. 2000;25:487–492. doi: 10.1097/00007632-200002150-00016. [DOI] [PubMed] [Google Scholar]
- 4.Yang G., Liao W., Shen M., Mei H. 2018. Insight into Neural Mechanisms Underlying Discogenic Back Pain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panjabi M.M. Clinical spinal instability and low back pain. J Electromyogr Kinesiol. 2003;13:371–379. doi: 10.1016/s1050-6411(03)00044-0. [DOI] [PubMed] [Google Scholar]
- 6.Ayling O.G.S., Ailon T., McIntosh G., et al. Clinical outcomes research in spine surgery: what are appropriate follow-up times? J Neurosurg Spine. 2018;30:397–404. doi: 10.3171/2018.8.SPINE18715. [DOI] [PubMed] [Google Scholar]
- 7.Berens P. CircStat. A MATLAB toolbox for circular statistics. J Stat Software. 2009;31(1–21) [Google Scholar]
- 8.Bonaldi G., Brembilla C., Cianfoni A. Minimally-invasive posterior lumbar stabilization for degenerative low back pain and sciatica. A review. Eur. J. Radiol. 2015;84:789–798. doi: 10.1016/j.ejrad.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Madera M., Brady J., Deily S., et al. 2017. The Role of Physical Therapy and Rehabilitation after Lumbar Fusion Surgery for Degenerative Disease: A Systematic Review. [DOI] [PubMed] [Google Scholar]
- 10.Chung M.J., Wang M.J.J. The change of gait parameters during walking at different percentage of preferred walking speed for healthy adults aged 20-60 years. Gait Posture. 2010;31:131–135. doi: 10.1016/j.gaitpost.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Grossman G.E., Leigh R.J., Abel L.A., Lanska D.J., Thurston S.E. Frequency and velocity of rotational head perturbations during locomotion. Exp Brain Res. 1988;70:470–476. doi: 10.1007/BF00247595. [DOI] [PubMed] [Google Scholar]
- 12.Hirasaki E., Moore S.T., Raphan T., Cohen B. Effects of walking velocity on vertical head and body movements during locomotion. Exp Brain Res. 1999;127:117–130. doi: 10.1007/s002210050781. [DOI] [PubMed] [Google Scholar]
- 13.Martino G., Ivanenko Y.P., d'Avella A., et al. Neuromuscular adjustments of gait associated with unstable conditions. J Neurophysiol. 2015;114:2867–2882. doi: 10.1152/jn.00029.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miscusi M., Di Bartolomeo A., Scafa A., et al. The dynamic sagittal balance: definition of dynamic spino-pelvic parameters using a method based on gait analysis. World Neurosurg. X. 2023;100165 doi: 10.1016/j.wnsx.2023.100165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saunders J.B., Inman V.T., Eberhart H.D. The major determinants in normal and pathological gait. J. Bone Joint Surg. Am. 1953;35 A:543–558. [PubMed] [Google Scholar]
- 16.Winter D.A., MacKinnon C.D., Ruder G.K., Wieman C. An integrated EMG/biomechanical model of upper body balance and posture during human gait. Prog Brain Res. 1993;97:359–367. doi: 10.1016/s0079-6123(08)62295-5. [DOI] [PubMed] [Google Scholar]
- 17.Geisser M.E., Ranavaya M., Haig A.J., et al. A meta-analytic review of surface electromyography among persons with low back pain and normal, healthy controls. J Pain. 2005;6:711–726. doi: 10.1016/j.jpain.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Wolf S.L., Nacht M., Kelly J.L. EMG feedback training during dynamic movement for low back pain patients. Behav Ther. 1982;13:395–406. [Google Scholar]
- 19.Fiori L., Ranavolo A., Varrecchia T., et al. Impairment of global lower limb muscle coactivation during walking in cerebellar ataxias. Cerebellum Lond. Engl. 2020;19:583–596. doi: 10.1007/s12311-020-01142-6. [DOI] [PubMed] [Google Scholar]
- 20.Ippolito G., Serrao M., Conte C., et al. Direct anterior approach for total hip arthroplasty: hip biomechanics and muscle activation during three walking tasks. Clin. Biomech. Bristol Avon. 2021;89 doi: 10.1016/j.clinbiomech.2021.105454. [DOI] [PubMed] [Google Scholar]
- 21.Tatarelli A., Serrao M., Varrecchia T., et al. Global muscle coactivation of the sound limb in gait of people with transfemoral and transtibial amputation. Sensors. 2020;20:2543. doi: 10.3390/s20092543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varrecchia T., De Marchis C., Rinaldi M., et al. Lifting activity assessment using surface electromyographic features and neural networks. Int J Ind Ergon. 2018;66(1–9) [Google Scholar]
- 23.Prince F., Winter D., Stergiou P., Walt S. Anticipatory control of upper body balance during human locomotion. Gait Posture. 1994;2:19–25. [Google Scholar]
- 24.Miscusi M., Serrao M., Conte C., et al. Spatial and temporal characteristics of the spine muscles activation during walking in patients with lumbar instability due to degenerative lumbar disk disease: evaluation in pre-surgical setting. Hum Mov Sci. 2019;66:371–382. doi: 10.1016/j.humov.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Ghiasi M.S., Arjmand N., Shirazi-Adl A., et al. Cross-sectional area of human trunk paraspinal muscles before and after posterior lumbar surgery using magnetic resonance imaging. Eur Spine J. 2016;25:774–782. doi: 10.1007/s00586-015-4014-y. [DOI] [PubMed] [Google Scholar]
- 26.North R.B., Ewend M.G., Lawton M.T., Kidd D.H., Piantadosi S. Failed back surgery syndrome: 5-year follow-up after spinal cord stimulator implantation. Neurosurgery. 1991;28:692–699. [PubMed] [Google Scholar]
- 27.Miscusi M., Polli F.M., Forcato S., et al. Comparison of minimally invasive surgery with standard open surgery for vertebral thoracic metastases causing acute myelopathy in patients with short- or mid-term life expectancy: surgical technique and early clinical results. J Neurosurg Spine. 2015;22:518–525. doi: 10.3171/2014.10.SPINE131201. [DOI] [PubMed] [Google Scholar]
- 28.Ricciardi L., Montano N., D'Onofrio G.F., et al. X-ray exposure in odontoid screwing for Anderson type II fracture: comparison between O-arm and C-arm-assisted procedures. Acta Neurochir (Wien) 2020;162:713–718. doi: 10.1007/s00701-019-04108-8. [DOI] [PubMed] [Google Scholar]
- 29.Ricciardi L., Stifano V., Proietti L., et al. Intraoperative and postoperative segmental lordosis mismatch: analysis of 3 fusion techniques. World Neurosurg. 2018;115:e659–e663. doi: 10.1016/j.wneu.2018.04.126. [DOI] [PubMed] [Google Scholar]
- 30.Ricciardi L., Stifano V., Sturiale C.L., D’onofrio G.F., Olivi A., Montano N. clinical and radiological outcomes; 2020. Minimally Invasive Decompression with Posterior Elements Preservation versus Laminectomy and Fusion for Lumbar Degenerative Spondylolisthesis: A Systematic Review and Meta-Analysis of Surgical. [PubMed] [Google Scholar]
- 31.Montano N., Ricciardi L., Olivi A. 2019. Comparison of Anterior Cervical Decompression and Fusion versus Laminoplasty in the Treatment of Multilevel Cervical Spondylotic Myelopathy: A Meta-Analysis of Clinical and Radiological Outcomes. [DOI] [PubMed] [Google Scholar]
- 32.Ricciardi L., Trungu S., Scerrati A., et al. Odontoid screw placement for Anderson type II odontoid fractures: how do duration from injury to surgery and clinical and radiological factors influence the union rate? A multicenter retrospective study. J Neurosurg Spine. 2021;34:27–31. doi: 10.3171/2020.6.SPINE20318. [DOI] [PubMed] [Google Scholar]
- 33.Joseph J.R., Smith B.W., La Marca F., Park P. 2015. Comparison of Complication Rates of Minimally Invasive Transforaminal Lumbar Interbody Fusion and Lateral Lumbar Interbody Fusion: A Systematic Review of the Literature. [DOI] [PubMed] [Google Scholar]
- 34.Keorochana G., Setrkraising K., Woratanarat P., Arirachakaran A., Kongtharvonskul J. 2018. Clinical Outcomes after Minimally Invasive Transforaminal Lumbar Interbody Fusion and Lateral Lumbar Interbody Fusion for Treatment of Degenerative Lumbar Disease: A Systematic Review and Meta-Analysis. [DOI] [PubMed] [Google Scholar]
- 35.Uribe J.S., Beckman J., Mummaneni P.V., et al. Does MIS surgery allow for shorter constructs in the surgical treatment of adult spinal deformity? Neurosurgery. 2017;80:489–497. doi: 10.1093/neuros/nyw072. [DOI] [PubMed] [Google Scholar]
- 36.Heemskerk J.L., Oluwadara Akinduro O., Clifton W., Quiñones-Hinojosa A., Abode-Iyamah K.O. Long-term clinical outcome of minimally invasive versus open single-level transforaminal lumbar interbody fusion for degenerative lumbar diseases: a meta-analysis. Spine J. Off. J. North Am. Spine Soc. 2018;21:2021. doi: 10.1016/j.spinee.2021.07.006. –2065. [DOI] [PubMed] [Google Scholar]
- 37.Kanno H., Aizawa T., Hahimoto K., Itoi E. Minimally invasive discectomy for lumbar disc herniation: current concepts, surgical techniques, and outcomes. Int Orthop. 2019;43:917–922. doi: 10.1007/s00264-018-4256-5. [DOI] [PubMed] [Google Scholar]
- 38.Wang J., Zou Q., Li S., et al. Gait asymmetry of lower extremities reduced immediately after minimally invasive surgery among patients with lumbar disc herniation. Clin. Biomech. Bristol Avon. 2022;98 doi: 10.1016/j.clinbiomech.2022.105720. [DOI] [PubMed] [Google Scholar]
- 39.Elmose S.F., Andersen G.O., Carreon L.Y., Sigmundsson F.G., Andersen M.O. Radiological definitions of sagittal plane segmental instability in the degenerative lumbar spine - a systematic review. Global Spine J. 2023;13:523–533. doi: 10.1177/21925682221099854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matz P.G., Meagher R.J., Lamer T., et al. 2016. Guideline Summary Review: An Evidence-Based Clinical Guideline for the Diagnosis and Treatment of Degenerative Lumbar Spondylolisthesis. [DOI] [PubMed] [Google Scholar]
- 41.Miscusi M., Carnevali C., Ricciardi L., et al. Histomorphology and immunohistochemical patterns in degenerative disc disease and clinical-radiological correlations: a prospective study. Eur Spine J. 2020;29:1410–1415. doi: 10.1007/s00586-020-06412-9. [DOI] [PubMed] [Google Scholar]
- 42.Urrutia J., Besa P., Campos M., et al. The Pfirrmann classification of lumbar intervertebral disc degeneration: an independent inter- and intra-observer agreement assessment. Eur Spine J. 2016;25:2728–2733. doi: 10.1007/s00586-016-4438-z. [DOI] [PubMed] [Google Scholar]
- 43.Mummaneni P.V., Park P., Shaffrey C.I., et al. The MISDEF2 algorithm: an updated algorithm for patient selection in minimally invasive deformity surgery. J Neurosurg Spine. 2020;32:221–228. doi: 10.3171/2019.7.SPINE181104. [DOI] [PubMed] [Google Scholar]
- 44.Ricciardi L., Stifano V., Rivera Perla K.M., et al. One center's experience with hybrid technique for lumbar spine instrumentated surgeries: evaluation of different instrumentation systems and their management. World Neurosurg. 2018;120:153–158. doi: 10.1016/j.wneu.2018.08.204. [DOI] [PubMed] [Google Scholar]
- 45.Wiltse L.L., Man N., Macnab I. Classification of spondylolisis and spondylolisthesis. Clin Orthop. 1976;117:23–29. PMID: 1277669. [PubMed] [Google Scholar]
- 46.Davis R.B., Õunpuu S., Tyburski D., Gage J.R. A gait analysis data collection and reduction technique. Hum Mov Sci. 1991;10:575–587. [Google Scholar]
- 47.Caliandro P., Iacovelli C., Conte C., et al. Trunk-lower limb coordination pattern during gait in patients with ataxia. Gait Posture. 2017;57:252–257. doi: 10.1016/j.gaitpost.2017.06.267. [DOI] [PubMed] [Google Scholar]
- 48.Conte, C., F. Pierelli, C. Casali, A. Ranavolo, F. Draicchio, G. Martino, M. Harfoush, L. Padua, Coppola, Gianluca, Sandrini, Giorgio, and Serrao, Mariano. Upper body kinematics in patients with cerebellar ataxia - PubMedat <https://pubmed.ncbi.nlm.nih.gov/25063003/>. [DOI] [PubMed]
- 49.Serrao M., Chini G., Caramanico G., et al. Prediction of responsiveness of gait variables to rehabilitation training in Parkinson's disease. Front Neurol. 2019;10 doi: 10.3389/fneur.2019.00826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Serrao M., Pierelli F., Sinibaldi E., et al. Progressive modular rebalancing system and visual cueing for gait rehabilitation in Parkinson's disease: a pilot, randomized, controlled trial with crossover. Front Neurol. 2019;10 doi: 10.3389/fneur.2019.00902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hermens H.J., Freriks B., Disselhorst-Klug C., Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. 2000 doi: 10.1016/S1050-6411(00)00027-4. [DOI] [PubMed] [Google Scholar]
- 52.Burden A. How should we normalize electromyograms obtained from healthy participants? What we have learned from over 25 years of research. J. Electromyogr. Kinesiol. Off. J. Int. Soc. Electrophysiol. Kinesiol. 2010;20:1023–1035. doi: 10.1016/j.jelekin.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 53.Monticone M., Baiardi P., Ferrari S., et al. Development of the Italian version of the oswestry disability index (ODI-I): a cross-cultural adaptation, reliability, and validity study. Spine. 2009;34:2090–2095. doi: 10.1097/BRS.0b013e3181aa1e6b. [DOI] [PubMed] [Google Scholar]
- 54.Kodraliu G., Mosconi P., Groth N., et al. Subjective health status assessment: evaluation of the Italian version of the SF-12 health survey. Results from the MiOS project. J Epidemiol Biostat. 2001;6:305–316. doi: 10.1080/135952201317080715. [DOI] [PubMed] [Google Scholar]
- 55.Dwivedi A.K., Mallawaarachchi I., Alvarado L.A. Analysis of small sample size studies using nonparametric bootstrap test with pooled resampling method. Stat Med. 2017;36:2187–2205. doi: 10.1002/sim.7263. [DOI] [PubMed] [Google Scholar]
- 56.Mari S., Serrao M., Casali C., et al. Lower limb antagonist muscle co-activation and its relationship with gait parameters in cerebellar ataxia. Cerebellum Lond. Engl. 2014;13:226–236. doi: 10.1007/s12311-013-0533-4. [DOI] [PubMed] [Google Scholar]
- 57.Martino G., Ivanenko Y.P., Serrao M., et al. Locomotor patterns in cerebellar ataxia. J Neurophysiol. 2014;112:2810–2821. doi: 10.1152/jn.00275.2014. [DOI] [PubMed] [Google Scholar]
- 58.Lamoth C.J.C., Meijer O.G., Daffertshofer A., Wuisman P.I.J.M., Beek P.J. Effects of chronic low back pain on trunk coordination and back muscle activity during walking: changes in motor control. Eur Spine J. 2006;15:23–40. doi: 10.1007/s00586-004-0825-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park J.H., Hyun S.J., Roh S.W., Rhim S.C. A comparison of unilateral laminectomy with bilateral decompression and fusion surgery in the treatment of grade I lumbar degenerative spondylolisthesis. Acta Neurochir (Wien) 2012;154:1205–1212. doi: 10.1007/s00701-012-1394-1. [DOI] [PubMed] [Google Scholar]
- 60.Castiglia S.F., Trabassi D., De Icco R., et al. Harmonic ratio is the most responsive trunk-acceleration derived gait index to rehabilitation in people with Parkinson's disease at moderate disease stages. Gait Posture. 2022;97:152–158. doi: 10.1016/j.gaitpost.2022.07.235. [DOI] [PubMed] [Google Scholar]
- 61.Bagheri R., Parhampour B., Pourahmadi M., et al. The effect of core stabilization exercises on trunk-pelvis three-dimensional kinematics during gait in non-specific chronic low back pain. Spine. 2019;44:927–936. doi: 10.1097/BRS.0000000000002981. [DOI] [PubMed] [Google Scholar]
- 62.Coulombe B.J., Games K.E., Neil E.R., Eberman L.E. Core stability exercise versus general exercise for chronic low back pain. J Athl Train. 2017;52:71–72. doi: 10.4085/1062-6050-51.11.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuligowski T., Cieślik B., Kuciel N., Dębiec-Bak A., Skrzek A. Effect of core stabilizing training on young individuals presenting different stages of degenerative disc disease—preliminary report. Int J Environ Res Publ Health. 2021;18 doi: 10.3390/ijerph18073499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moseley G.L., Butler D.S. 2015. Fifteen Years of Explaining Pain: The Past, Present, and Future. [DOI] [PubMed] [Google Scholar]
- 65.O'Sullivan P.B., Caneiro J.P., O'Keeffe M., et al. Cognitive functional therapy: an integrated behavioral approach for the targeted management of disabling low back pain. Phys Ther. 2018;98:408–423. doi: 10.1093/ptj/pzy022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Campanini I., Disselhorst-Klug C., Rymer W.Z., Merletti R. Surface EMG in clinical assessment and neurorehabilitation: barriers limiting its use. Front Neurol. 2020;11:934. doi: 10.3389/fneur.2020.00934. [DOI] [PMC free article] [PubMed] [Google Scholar]