Abstract
In the ELOQUENT-3 trial, the combination of elotuzumab, pomalidomide and dexamethasone (EloPd) proved to have a superior clinical benefit over pomalidomide and dexamethasone with a manageable toxicity profile, leading to its approval for the treatment of patients with relapsed/refractory multiple myeloma (RRMM) who have received at least two prior therapies, including lenalidomide and a proteasome inhibitor. We report here a real-world experience of 200 cases of RRMM treated with EloPd in 35 Italian centers outside of clinical trials. In our dataset, the median number of prior lines of therapy was two, with 51% of cases undergoing autologous stem cell transplant and 73% having been exposed to daratumumab. After a median follow-up of 9 months, 126 patients had stopped EloPd, most of them (88.9%) because of disease progression. The overall response rate was 55.4%, a finding in line with the pivotal trial results. Regarding adverse events, the toxicity profile in our cohort was similar to that in the ELOQUENT-3 trial, with no significant differences between younger (<70 years) and older patients. The median progression-free survival was 7 months, which was shorter than that observed in ELOQUENT-3, probably because of the different clinical characteristics of the two cohorts. Interestingly, International Staging System stage III disease was associated with worse progression-free survival (hazard ratio=2.55). Finally, the median overall survival of our series was shorter than that observed in the ELOQUENT-3 trial (17.5 vs. 29.8 months). In conclusion, our real-world study confirms that EloPd is a safe and possible therapeutic choice for patients with RRMM who have received at least two prior therapies, including lenalidomide and a proteasome inhibitor.
Introduction
The treatment landscape of multiple myeloma (MM) has changed dramatically over the years as the result of the introduction of several new drugs that have improved these patients’ survival.1,2 At present, proteasome inhibitors and immunomodulatory drugs are still the fundamental backbones of MM therapy. However, given the encouraging results from clinical trials, especially among double-refractory MM patients, monoclonal antibodies, a new class of drugs, are now being used with proteasome inhibitors and immunomodulatory drugs and are being incorporated in earlier lines of therapies.3 The use of triplet combinations in clinical practice allows for deeper and more sustained responses with an acceptable safety profile.3 Elotuzumab is a humanized immunoglobulin G1 immunostimulatory monoclonal antibody that is directed against signaling lymphocytic activation molecule F7 (SLAMF7).4 SLAMF7 is a glycoprotein expressed on myeloma cells and natural killer cells, which promote MM cell proliferation and survival.5 Thus, the mechanism of action of elotuzumab is the prevention of interactions that allow the growth and sustainment of neoplastic cells. Moreover, elotuzumab stimulates natural killer cells by strengthening their antibody-dependent cellular cytotoxicity.6,7 As found in in vitro models, this phenomenon is amplified when elotuzumab is combined with lenalidomide.8 It was hypothesized that a similar effect would be observed in patients with relapsed or refractory MM (RRMM). Indeed, based on the results from a phase III trial (ELOQUENT-2), elotuzumab was first approved by the Food and Drug Administration (FDA) in November 2015 and by the European Medicines Agency in January 2016 in combination with lenalidomide and dexamethasone for the treatment of MM patients who had received at least one prior line of therapy.9 Our group confirmed the safety and efficacy of this combination in a cohort of RRMM treated outside clinical trials.10-13
Like lenalidomide, pomalidomide is an immunomodulatory drug that directly determines MM cell death and has immune-enhancing effects via binding to cereblon.14 However, compared to lenalidomide, pomalidomide demonstrated a more potent antineoplastic activity towards lenalidomide-resistant MM cell lines in vitro and in preclinical in vivo studies. Moreover, it was shown that the combination of elotuzumab with pomalidomide exerts synergistic antimyeloma effects.15 These results laid the groundwork for an in vivo combination. ELOQUENT-3, a multicenter, randomized, controlled, open-label, phase II trial, investigated the efficacy and safety of elotuzumab in combination with pomalidomide and dexamethasone (EloPd) compared to pomalidomide and dexamethasone in the setting of patients with RRMM who had been previously treated with lenalidomide and a proteosome inhibitor.16 After a follow-up of 45 months, the study demonstrated that the triplet combination still improved progression-free survival (PFS) and overall survival (OS), with a lower rate of adverse events compared to that in the control arm.17
Here, we present the outcomes of 200 heavily pre-treated MM patients who received EloPd outside of clinical trials to evaluate the safety and efficacy (response, PFS, OS, time to next treatment) of this triple combination in a real-world setting.
Methods
Patients
Data from a retrospective cohort of RRMM patients treated with EloPd in 35 Italian centers were collected for the purpose of this retrospective analysis. The databases contained clinical information such as age, gender, date of diagnosis, laboratory parameters, treatment history, and date of last follow-up or death extracted from clinical records at the time of inclusion and updated on an ongoing basis. The 35 databases included 200 consecutive patients with RRMM who received at least one cycle of EloPd as salvage treatment between October 2020 and December 2022.
All patients were treated with EloPd according to marketing approval as previously described.16,17 Specifically, elotuzumab was given at a dose of 10 mg/kg i.v. on days 1, 8, 15, and 22 during the first two cycles and at a dose of 20 mg/kg once daily on day 1 of each following cycle. The dose of pomalidomide was 4 mg orally once daily on days 1 to 21 of each cycle, whereas that of dexamethasone was 40 mg (or 20 mg in patients aged older than 75 years) once weekly, except on days of elotuzumab administration, when patients received both oral (28 mg [or 8 mg in patients aged older than 75 years]) and intravenous (8 mg) dexamethasone. All patients were given premedication with diphenhydramine (25 to 50 mg) or its equivalent, ranitidine (50 mg) or its equivalent, and acetaminophen (650 to 1,000 mg) or its equivalent 30 to 90 minutes before the elotuzumab infusions. All patients received antibacterial, antiviral, and antithrombotic prophylaxis during treatment. EloPd was administered in 28-day cycles until disease progression, unacceptable toxicity, or withdrawal of consent.
The time-to-event endpoints were PFS, OS, and time to next treatment. Safety profile and response were also evaluated for the purposes of the study. Response to treatment and disease progression were evaluated according to the International Myeloma Working Group (IMWG) criteria.18,19 Patients had to reach at least partial remission (PR) in order to be considered to have had a response.
The Institutional Ethics Committee of each of the participating hospitals approved the study, which was conducted according to the principles of the Declaration of Helsinki and Good Clinical Practice guidelines.
Statistical analysis
Statistical comparisons for categorical variables were performed using two-way tables for the Fisher exact test and multi-way tables for the Pearson χ2 test. Multivariable ordinal regression analysis was used to examine the effects of potential confounders on the association between the best response and several variables that were statistically significant on univariable analysis by the Pearson χ2 test or Fisher exact test. PFS, OS and time to next treatment were analyzed using the Kaplan-Meier method. PFS was measured from the initiation of EloPd treatment until death from any cause or progression or last follow-up. OS was measured from the initiation of EloPd treatment until death from any cause or last follow-up The time to next treatment was measured from the initiation of EloPd treatment to the earliest date of starting any subsequent therapy or last follow-up. The statistical significance of associations between individual variables and survival was calculated using the log-rank test. The prognostic impact of the outcome variable was investigated by univariable and multiple Cox regression analyses. Results are expressed as hazard ratios (HR) and 95% confidence intervals (95% CI). A P value <0.05 was considered statistically significant. STATA for Windows v.9 and SPSS Statistics v.21 were used to analyze the data.
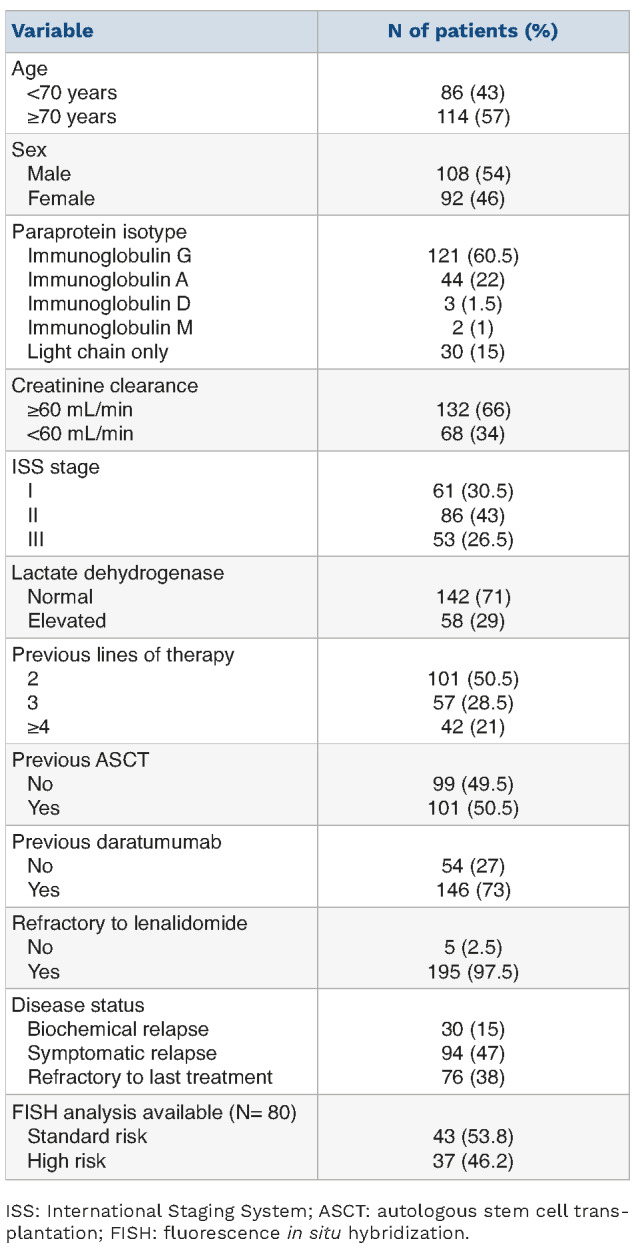
Table 1.
Main characteristics of the patients at baseline.
Results
Patients
Overall, 200 RRMM patients treated with EloPd between October 2020 and December 2022 in 35 Italian centers entered this study. Their baseline characteristics are shown in Table 1. At the start of treatment with EloPd, 26.5% of patients had stage III disease according to the International Staging System (ISS), 30.5% were in ISS stage I, and 43% were in ISS stage II. Seventy-six cases (38%) had disease refractory to the previous line of therapy, a symptomatic relapse was observed in 94 patients (47%) and a biochemical relapse in 30 (15%); almost all cases (97.5%) were refractory to lenalidomide. Fifty-one patients (25.5%) showed mild renal impairment, while kidney function was severely compromised in 17 patients (8.5%). Before EloPd treatment, 101 patients (50.5%) had received two lines of therapy, approximately half of the patients (51%) had undergone autologous stem cell transplant (ASCT), while roughly three-quarters (73%) of patients had been exposed to daratumumab. All 146 patients who received daratumumab were refractory to this treatment. One hundred and eleven patients received EloPd immediately after a daratumumab-containing regimen, while 35 patients received other therapy schedules between the daratumumab-containing regimen and treatment with the EloPd regimen. Fluorescence in situ hybridization (FISH) data were available for 80 patients. Forty-three patients (53.8%) had favorable cytogenetic abnormalities, while 37 patients (46.2%) were categorized as being at high risk, because they harbored one of the following aberrations: t(4;14), t(14;16) and del(17p).
Response evaluation
At the last follow-up, 193 of the 200 enrolled patients were evaluable for response (7 cases had not yet completed the first cycle of therapy). Of these 193 patients, 107 (55.4%) reached at least partial remission (≥PR). More in detail, six (3.1%) achieved a complete remission (CR), 39 (20.2%) a very good partial response (VGPR), and 62 (32.1%) a PR. The median time to response was 1.8 months.
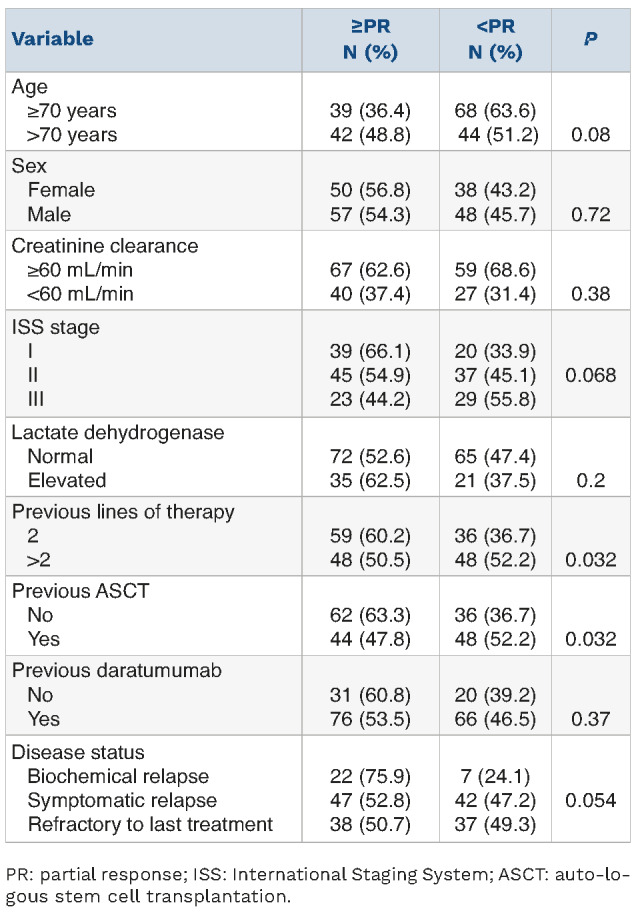
The overall response rate (ORR) was statistically higher in patients who did not undergo ASCT (63.3% vs. 47.8%; P=0.032) (Table 2), while a trend towards statistical significance was observed in cases with ISS stage I (stage I=66.1%, stage II=54.9%, and stage III=44.2%; P=0.068), in those treated at biochemical relapse (biochemical relapse=75.9%, symptomatic relapse=52.8%, refractory disease=50.7; P=0.054) and in older patients (>70 years=48.8%, ≤70 years =36.4%; P=0.08) (Table 2). Gender, creatinine clearance, lactate dehydrogenase concentration, number of prior lines of therapy, and previous exposure to daratumumab did not affect the probability of achieving a response to EloPd (Table 2). No differences in ORR were observed between patients receiving EloPd immediately after a daratumumab-containing regimen and those who received other schedules of therapy between a daratumumab-containing regimen and EloPd (ORR: 55% vs. 43%, respectively; P=0.42).
Progression-free survival
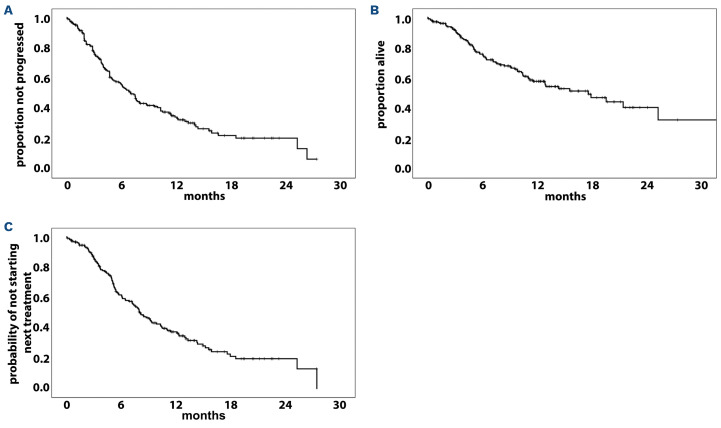
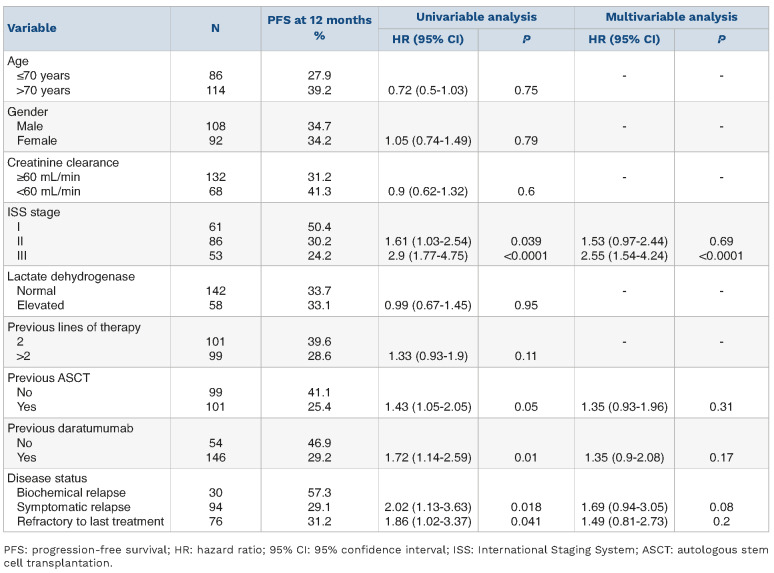
After a median follow-up of 9 months (range, 1-26), 121 patients (60.5%) out of 200 had experienced disease progression or died. The total number of deaths was 79 (39.5%). The median PFS was 7 months (95% CI: 5.8-8.2 months), and the 1-year probability of PFS was 33.6% (Figure 1A). Univariable analyses showed that ISS stage II (HR=1.61, 95% CI: 1.03-2.54; P=0.039), ISS stage III (HR=2.9, 95% CI: 1.77-4.75; P<0.0001), previous ASCT (HR=1.43, 95% CI: 1.05-2.05; P=0.05), previous daratumumab exposure (HR=1.72, 95% CI: 1.14-2.59; P=0.01) (Online Supplementary Figure S1A), symptomatic relapse (HR=2.02, 95% CI: 1.13-3.63; P=0.018) and refractory disease at the time of starting EloPd treatment (HR=1.86, 95% CI: 1.02-3.37; P=0.041) were associated with a significantly lower PFS rate (Table 3).
Table 2.
Association between overall response rate and main clinical-hematologic characteristics of multiple myeloma patients treated with elotuzumab plus pomaidomide and dexamethasone (N=193).
No differences in PFS were observed between patients receiving EloPd immediately after a daratumumab-containing regimen and those who received other schedules of therapy between a daratumumab-containing regimen and EloPd (HR=1.34, 95% CI: 0.83-2.16; P=0.23).
Notably, in the Cox multivariable analysis, only advanced ISS stage (III) maintained an independent prognostic impact on PFS (HR=2.55, 95% CI: 1.54-4.24; P<0.0001) (Table 3). Conversely, ISS stage II (HR=1.53, 95% CI: 0.97-2.44; P=0.69), previous ASCT (HR=1.35, 95% CI: 0.93-1.96; P=0.31), previous daratumumab exposure (HR=1.35, 95% CI: 0.9-2.08; P=0.17), symptomatic relapse (HR=1.69, 95% CI: 0.94-3.05; P=0.008) and refractory disease at the time of starting EloPd treatment (HR=1.49, 95% CI: 0.81-2.73; P=0.2) lost their independent predictive value on PFS.
Overall survival
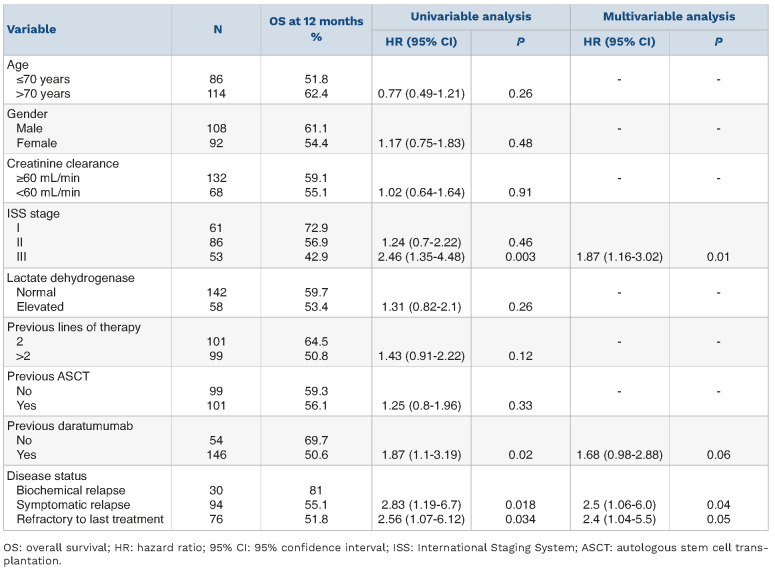
The median OS was 17.5 months (95% CI: 28-40.2), and the 1-year probability of OS was 57.9% (95% CI: 12-23.2 months) (Figure 1B). Univariable analyses showed that ISS stage III (HR=2.46, 95% CI: 1.35-4.48; P=0.003), previous daratumumab exposure (HR=1.87, 95% CI: 1.1-3.19; P=0.02) (Online Supplementary Figure S1B), symptomatic relapse (HR=2.83, 95% CI: 1.19-6.7; P=0.018) and refractory disease (HR=2.56, 95% CI: 1.07-6.12; P=0.034) at the start of EloPd treatment were associated with a significantly shorter OS (Table 4). No differences in OS were observed between patients receiving EloPd immediately after a daratumumab-containing regimen and those who received other schedules of therapy between a daratumumab-containing regimen and EloPd (HR=1.19, 95% CI: 0.7-2.01; P=0.53).
Notably, in the Cox multivariable analysis, advanced ISS stage (i.e., stage III) (HR=1.87, 95% CI: 1.16-3.02; P=0.01), symptomatic relapse (HR=2.5, 95% CI: 1.06-6.0; P=0.04) and refractory disease at the start of EloPd treatment (HR=2.4, 95% CI: 1.04-5.5; P=0.05) maintained an independent prognostic impact on the survival outcome (Table 4). In contrast, the effect of previous daratumumab treatment lost its independent prognostic significance on OS (HR=1.68, 95% CI: 0.98-2.88; P=0.06).
Time to next treatment and subsequent therapy
After discontinuation of EloPd therapy, 71 patients (35.5%) received subsequent treatment. The median time to the next treatment was 8.1 months (95% CI: 6.7-9.4), with a 1-year re-treating probability of 37.5% (Figure 1C). The type of subsequent treatment is shown in Table 5. Overall, 20 different salvage therapy regimens were used after discontinuation or failure of EloPd. Roughly one-third of patients (24 cases) received belantamab alone (23 cases) or in combination with isatuximab (1 patient), 24 patients (33.8%) were given a proteasome inhibitor-containing regimen (carfilzomib-based in 14 cases, bortezomib-based in 6 cases and ixazomib-based in 4 cases), while 13 patients (18.3%) received an anti-CD38-containing regimen (daratumumab-based in 10 cases and isatuximab-based in 3 cases). Finally, ten patients (14.1%) received a subsequent chemotherapeutic regimen (6 cases were treated with a cyclophosphamide-based regimen, 3 with bendamustine, and 1 with melphalan).-
Figure 1.
Kaplan-Meier curves for all 200 patients with relapsed or refractory multiple myeloma treated with the elotuzumab, pomalidomide and dexamethasone triple regimen. (A) Kaplan-Meier curve of progression-free survival. (B) Kaplan-Meier curve of overall survival. (C) Kaplan-Meier curve of time to next treatment.
Safety
At the last database update, the median number of EloPd courses administered was five (range, 1-20). EloPd treatment had been withdrawn from a total of 126 (62.5%) patients by the cutoff date, mainly due to disease progression (112 cases). Of the remaining cases, nine patients discontinued therapy because of toxicity (6 infections and 3 cases of pomalidomide-related severe skin rash) and five patients died of causes unrelated to the therapy. Infusion reactions occurred at first administration of elotuzumab in 11 patients (5.5%, all grade 1 or 2) and were promptly resolved in all patients (no discontinuation of treatment reported). Major adverse events are presented in Table 6 and include grade 3 or 4 neutropenia (21.5%), anemia (11%), lymphocytopenia (9.5%), and thrombocytopenia (9.5%), while infection rates and pneumonia were roughly 14% and 6.5%, respectively. The rate of adverse events was not significantly different between patients aged less or more than 70 years (data not shown).
Outcome analysis by cytogenetic risk
Data on cytogenetic abnormalities were available for only 40% of cases (80/200). However, the analytical weight of this biomarker for prognosis, also emphasized by the Revised ISS (R-ISS),20 prompted us to carry out an ancillary analysis, conscious that the relatively low incidence of accessible cases could bias the statistical accuracy. When comparing the main characteristics of the patients in each group, the patients for whom cytogenetic information was available differed from the remaining cases only for a smaller proportion of patients with creatinine clearance <60 mL/min (Online Supplementary Table S1). No difference in ORR was observed between the high-risk and the standard-risk groups (54.1 vs. 53.7%, respectively; P=0.97). The two subgroups showed a non-statistically different PFS (1-year PFS; high-risk group vs. standard-risk: 28.4% vs. 44.7%, respectively; HR=1.34, 95% CI: 0.77-2.34; P=0.29) (Online Supplementary Figure S2A), while a trend towards statistical significance in terms of OS was observed in standard-risk patients (1-year OS; high-risk group vs. standard-risk: 50.1 vs. 75.1%; HR=2, 95% CI: 0.94-4.29; P=0.07) (Online Supplementary Figure S2B).
Table 3.
Univariable and multivariable analyses of progression-free survival.
Discussion
Elotuzumab, as monotherapy, was first evaluated in a phase 1I dose-finding study, which demonstrated the safety and tolerability of the drug at either 10 mg/kg or 20 mg/kg, but, at the same time, the absence of response, especially in the setting of heavily pre-treated patients.21 Given the enhanced antimyeloma activity in combination with other drugs within preclinical studies, elotuzumab was tested in association with lenalidomide in a phase II study which showed better efficacy of the triplet regimen in the setting of relapsed-refractory patients.22 Those results were subsequently confirmed by the phase III ELOQUENT-2 trial23 and remain robust at a follow-up of 70 months.24
Recently, data from the ELOQUENT-3 trial showed that the addition of elotuzumab to pomalidomide and dexamethasone provided a significant clinical improvement, in terms of PFS and OS, over pomalidomide and dexamethasone, with a manageable toxicity profile in the treatment of RRMM patients who had received at least two prior therapies, including lenalidomide and a proteasome inhibitor.16,17 Furthermore, the addition of elotuzumab to pomalidomide and dexamethasone did not have a negative impact on health-related quality of life of MM patients.25 Based on the results of these trials, the FDA approved EloPd for this setting of MM patients.
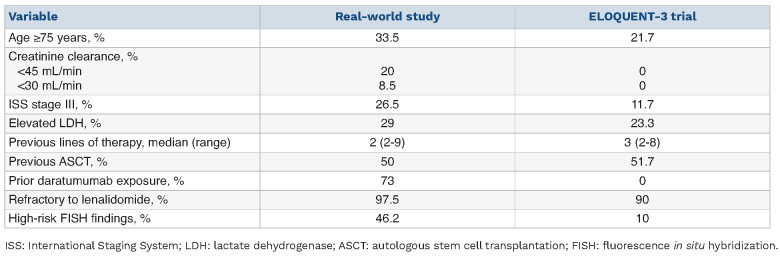
Here we have described an Italian real-world experience of the use of EloPd. To the best of our knowledge, our survey is the first real-world EloPd series. Real-world profiles are rarely fully represented in randomized clinical trials, a factor that can complicate treatment decision-making. In this regard, aging is a critical problem in MM patients’ management because of its association with frailty, increased comorbidities, poor tolerability of treatment, and higher risk of complications.26 In our series, approximately one-third of patients were ≥75 years old and 8.5% had severe renal impairment (creatinine clearance <30 mL/min). In comparison, in the registration trial, 21.7% of the patients were elderly and a creatinine clearance <45 mL/min was an exclusion criterion.
Table 4.
Univariable and multivariable analyses of overall survival.
The ELOQUENT-3 trial,16 and its update,17 showed the safety of the triplet EloPd drug regimen. Although some caution should be exercised because of the retrospective nature of the present study, similar adverse event profiles were documented in our real-world cohort, except for a slightly higher incidence of neutropenia, possibly due to the aforementioned differences in age and prevalence of severe renal impairment. Nevertheless, the incidence of infections was comparable.16 Of note, no significant differences were documented in the incidences of adverse events between younger (<70 years) and older patients.
The ORR in our real-world cohort was comparable to that in the ELOQUENT-3 trial (55.4% vs. 53%), with a similar number of patients reaching good quality responses,16 although the different clinical features of patients included in the two series should be taken into consideration (e.g., the median number of previous lines of therapies was 3 in the ELOQUENT-3 trial and 2 in our retrospective series) (Table 7). Interestingly, the only patients who showed a significantly lower response rate were those who had previously undergone ASCT. Conversely, there was a trend to a statistically significant higher response rate in patients with a low ISS stage and in those treated in biochemical relapse. These findings should be taken into consideration when choosing EloPd treatment.
The median time to achieve the best response was similar in our study and in the ELOQUENT-3 trial,16 being 1.8 months and 2 months, respectively.
PFS predictors should also be considered to reduce the chance of progression. In our series, the estimated median PFS was 7 months, shorter than the 10.3 months observed in the ELOQUENT-3 trial.16 This relatively poorer clinical outcome is possibly due to differences in baseline characteristics of patients in our real-world cohort and those in clinical trials (Table 7). Specifically, our cohort included a higher proportion of patients with advanced ISS stage (stage III) (26.5% vs. 11.7%) and a not negligible rate of patients with high-risk cytogenetics (46.2% vs. 10%) (Table 7), both categories having a poor prognosis. A multivariable model revealed that only ISS stage III was an independent predictor of shorter PFS.
In our series, the median OS was shorter than that observed in the ELOQUENT-3 trial (17.5 vs. 29.8 months).17 Nevertheless, OS results should be considered somewhat immature because of the relatively short follow-up. The differences in baseline characteristics between patients in our real-world cohort and those in the clinical trial could also have a negative impact on survival (Table 7). Again, at multivariable analysis, advanced ISS stage (stage III) showed an independent prognostic impact on the OS together with disease status at the start of EloPd therapy. In our cohort, PFS and OS were similar in both age groups (i.e., <70 years and ≥70 years), and EloPd showed a good safety profile even when used in the elderly (57% of our EloPd cohort), whose treatment is challenging because such patients are often frail, and have increased comorbidities, poor tolerability, and a higher risk of complications.26
Table 5.
Salvage therapy regimens after the elotuzumab, pomalidome and dexamethasone triple regimen.
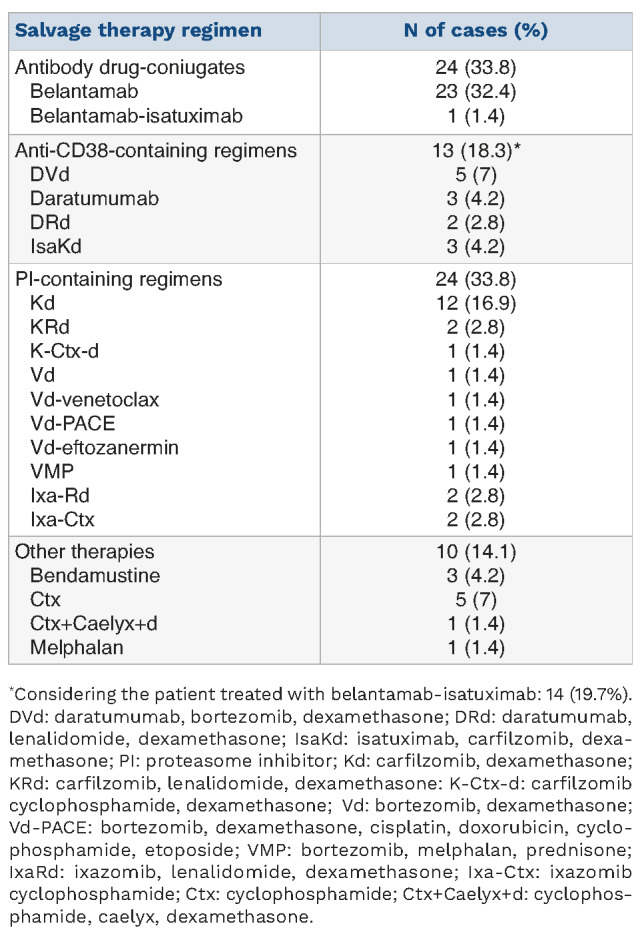
Table 6.
Incidence of serious adverse events among the patients treated with the elotuzumab, pomalidomide and dexamethasone triple regimen (N=200).
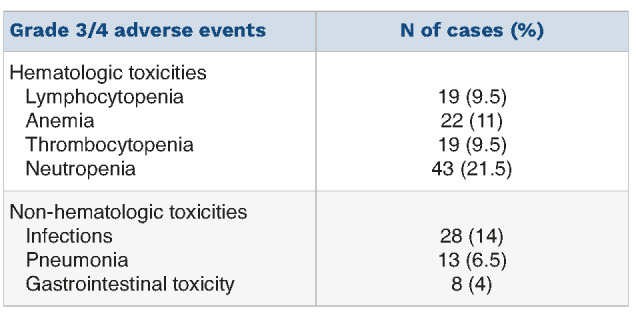
Table 7.
Comparison of the characteristics at baseline between the cohort of patients treated with the elotuzumab, pomalidomide and dexamethasone triple regimen in the real-world setting and those enrolled in the ELOQUENT-3 clinical trial.
There are two key reasons why the information on patients exposed to daratumumab is interesting. First of all, the data are lacking in the ELOQUENT-3 trial. Secondly, daratumumab-based therapy is currently the standard of care for most MM patients, both in the first- and in the second-line, enabling the evaluation of the impact of previous daratumumab treatment on the efficacy of EloPd in the real-world setting. In fact, in our experience, prior daratumumab treatment did not affect either the probability of achieving a response or outcome indicators in RRMM patients treated with EloPd.
The IMWG consensus recommends using ISS stage and cytogenetic abnormalities to analyze OS risk stratification.27 Unfortunately, cytogenetic analysis is rarely performed in a real-world setting. Although we were conscious that the relatively low number of accessible cases (approximately 40%) might lead to incorrect statistical interpretations, the prognostic importance of FISH information, highlighted by the R-ISS,20 motivated us to conduct an additional investigation. In this respect, high-risk patients, defined as those with poor cytogenetics (t[4;14], t[14;16], or del[17p]), did not show a significantly shorter PFS or OS, although the low number of cases did not allow assessment of the independent prognostic value of this parameter in multivariable analysis. Among the study’s strengths, we highlight that the number of patients enrolled in our real-world study is more than three times greater than that of the cohort of patients enrolled in the EloPd arm (n=60) of the ELOQUENT-3 trial.
Furthermore, taking into account the growing number of patients receiving anti-CD38 monoclonal antibody in the early phase of treatment, data on the efficacy of EloPd in patients previously exposed to daratumumab represent an additional value coming from our retrospective observation, since this information has not yet been provided by a randomized clinical trial. Conversely, among the weaknesses, the follow-up time is relatively short to draw definitive conclusions about OS and the well-known biases associated with the retrospective nature of the study must be mentioned.
In conclusion, our real-world data confirm the results obtained in the ELOQUENT-3 controlled clinical trial.16,17 EloPd is a safe and possible therapeutic choice for RRMM patients who have received at least two prior therapies, including lenalomide and a proteasome inhibitor. Notably, prior treatment with daratumumab did not have a negative impact of the efficacy of the EloPd triplet regimen. Several clinical trials are currently exploring the efficacy of elotuzumab in association with other antimyeloma drugs, such as iberdomide (CC-220),28 isatuximab29 and belantamab,30 in the setting of RRMM patients.
Supplementary Material
Funding Statement
Funding: This study was partially supported by the Italian Ministry of Health- Ricerca Corrente annual program 2024.
References
- 1.Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28(5):1122-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111(5):2516-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nooka AK, Kaufman JL, Hofmeister CC, et al. Daratumumab in multiple myeloma. Cancer. 2019;125(14):2364-2382. [DOI] [PubMed] [Google Scholar]
- 4.Bruzzese A, Martino EA, Vigna E, et al. Elotuzumab in multiple myeloma. Expert Opin Biol Ther. 2023;23(1):7-10. [DOI] [PubMed] [Google Scholar]
- 5.Durer C, Durer S, Lee S, et al. Treatment of relapsed multiple myeloma: evidence-based recommendations. Blood Rev. 2020;39:100616. [DOI] [PubMed] [Google Scholar]
- 6.Collins SM, Bakan CE, Swartzel GD, et al. Elotuzumab directly enhances NK cell cytotoxicity against myeloma via CS1 ligation: evidence for augmented NK cell function complementing ADCC. Cancer Immunol Immunother. 2013;62(12):1841-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsi ED, Steinle R, Balasa B, et al. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin Cancer Res. 2008;14(9):2775-2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balasa B, Yun R, Belmar NA, et al. Elotuzumab enhances natural killer cell activation and myeloma cell killing through interleukin-2 and TNF-α pathways. Cancer Immunol Immunother. 2015;64(1):61-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Medicines. Agency Empliciti, INN-elotuzumab. https://www.ema.europa.eu/en/documents/product-information/emplicitiepar-product-information_it.pdf Accessed on 6 June, 2023. [Google Scholar]
- 10.Gentile M, Specchia G, Derudas D, et al. Elotuzumab, lenalidomide, and dexamethasone as salvage therapy for patients with multiple myeloma: Italian, multicenter, retrospective clinical experience with 300 cases outside of controlled clinical trials. Haematologica. 2021;106(1):291-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruzzese A, Derudas D, Galli M, et al. Elotuzumab plus lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: extended 3-year follow-up of a multicenter, retrospective clinical experience with 319 cases outside of controlled clinical trials. Hematol Oncol. 2022;40(4):704-715. [DOI] [PubMed] [Google Scholar]
- 12.Morabito F, Zamagni E, Conticello C, et al. Adjusted comparison between elotuzumab and carfilzomib in combination with lenalidomide and dexamethasone as salvage therapy for multiple myeloma patients. Eur J Haematol. 2022;108(3):178-189. [DOI] [PubMed] [Google Scholar]
- 13.Morabito F, Zamagni E, Conticello C, et al. Survival risk scores for real-life relapsed/refractory multiple myeloma patients receiving elotuzumab or carfilzomib in combination with lenalidomide and dexamethasone as salvage therapy: analysis of 919 cases outside clinical trials. Front Oncol. 2022;12:890376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uccello G, Petrungaro A, Mazzone C, et al. Pomalidomide in multiple myeloma. Expert Opin Pharmacother. 2017;18(2):133-137. [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Girona A, Mendy D, Ito T, et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia. 2012; 26(11):2326-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dimopoulos MA, Dytfeld D, Grosicki S, et al. Elotuzumab plus pomalidomide and dexamethasone for multiple myeloma. N Engl J Med 2018;379(19):1811-1822. [DOI] [PubMed] [Google Scholar]
- 17.Dimopoulos MA, Dytfeld D, Grosicki S, et al. Elotuzumab plus pomalidomide and dexamethasone for relapsed/refractory multiple myeloma: final overall survival analysis from the randomized phase II ELOQUENT-3 trial. J Clin Oncol. 2023;41(3):568-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9):1467-1473. [DOI] [PubMed] [Google Scholar]
- 19.Rajkumar SV, Harousseau JL, Durie B, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117(18):4691-4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised International Staging System for multiple meloma: a report from International Myeloma Working Group. J Clin Oncol. 2015;33(26):2863-2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zonder JA, Mohrbacher AF, Singhal S, et al. A phase 1, multicenter, open-label, dose esclation study of elotuzumab in patients with advanced multiple myeloma. Blood. 2012; 120(3):552-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richardson PG, Jagannath S, Moreau P, et al.; 1703 study investigators. Elotuzumab in combination with lenalidomide and dexamethasone in patients with relapsed multiple myeloma: final phase 2 results from the randomised, open-label, phase 1b-2 dose-escalation study. Lancet Haematol. 2015;2(12):e516-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lonial S, Dimopoulos M, Palumbo A, et al. ; ELOQUENT-2 investigators. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373(7):621-631. [DOI] [PubMed] [Google Scholar]
- 24.Dimopoulos MA, Lonial S, White D, et al. Elotuzumab, lenalidomide, and dexamethasone in RRMM: final overall survival results from the phase 3 randomized ELOQUENT-2 study. Blood Cancer J. 2020;10(9):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weisel K, Dimopoulos MA, San-Miguel J, et al. Impact of elotuzumab plus pomalidomide/dexamethasone on health-related quality of life for patients with relapsed/refractory multiple myeloma: final data from the phase 2 ELOQUENT-3 trial. Hemasphere. 2023;7(3):e843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diamond E, Lahoud OB, Landau H. Managing multiple myeloma in elderly patients. Leuk Lymphoma. 2018;59(6):1300-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chng WJ, Dispenzieri A, Chim CS, et al. IMWG consensus on risk stratification in multiple myeloma. Leukemia. 2014;28(2):269-277. [DOI] [PubMed] [Google Scholar]
- 28.A study of iberdomide (CC-220) in combination with elotuzumab and dexamethasone for relapsed/refractory multiple myeloma (CC-220). ClinicalTrials.gov identifier: NCT 05560399. [Google Scholar]
- 29.Isatuximab pomalidomide elotuzumab and dexamethasone in relapsed and/or refractory multiple myeloma (IMPEDE). ClinicalTrials.gov identifier: NCT 04835129. [Google Scholar]
- 30.Novel combination of belantamab mafodotin and elotuzumab to enhance therapeutic efficacy in multiple myeloma. ClinicalTrials.gov identifier: NCT 05002816. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.