Abstract
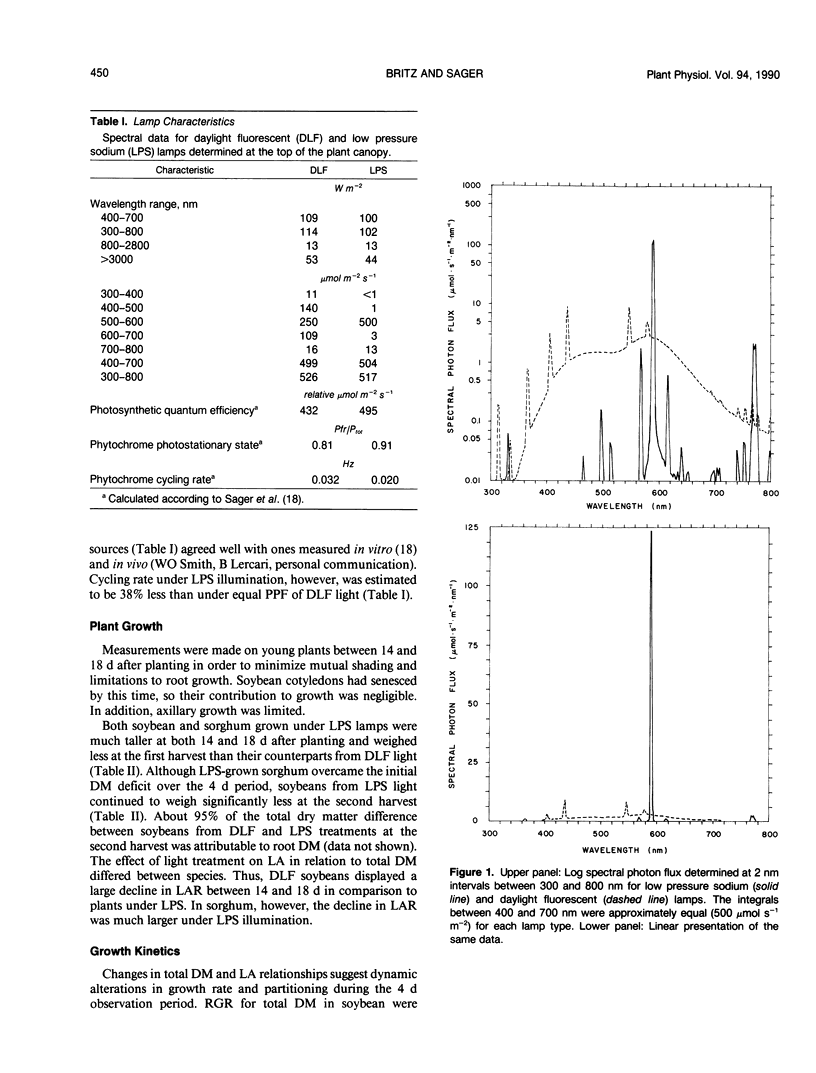
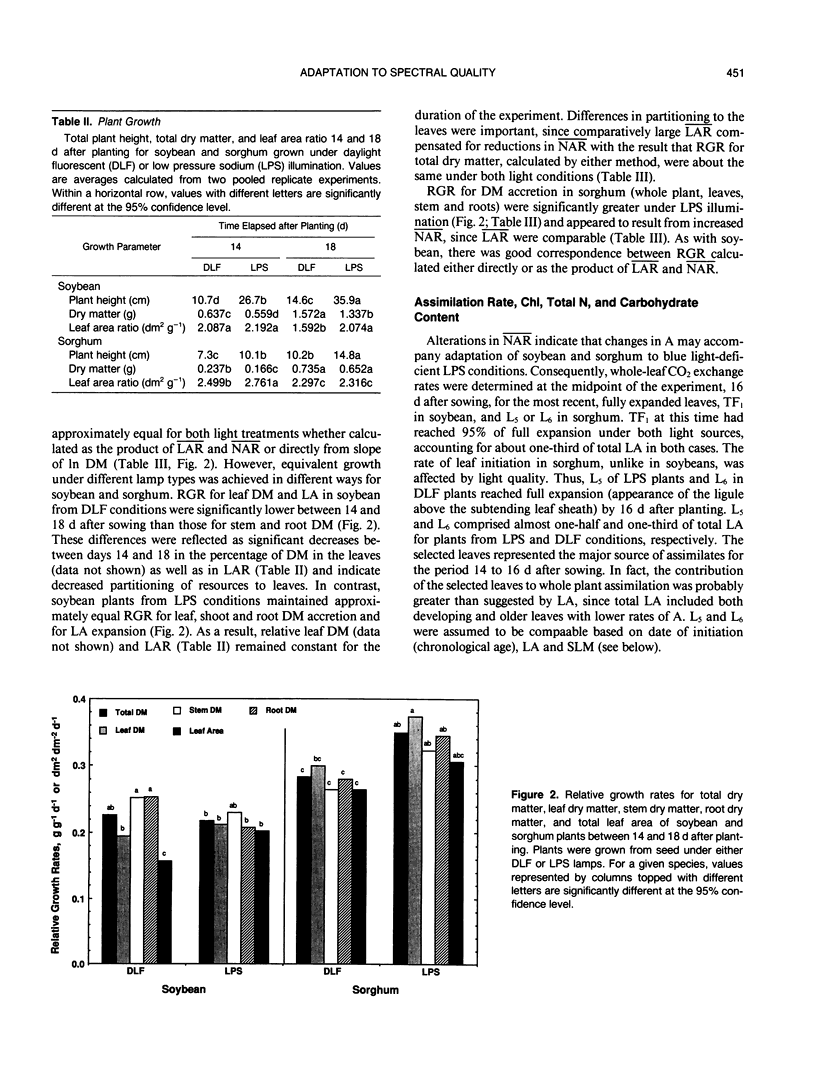
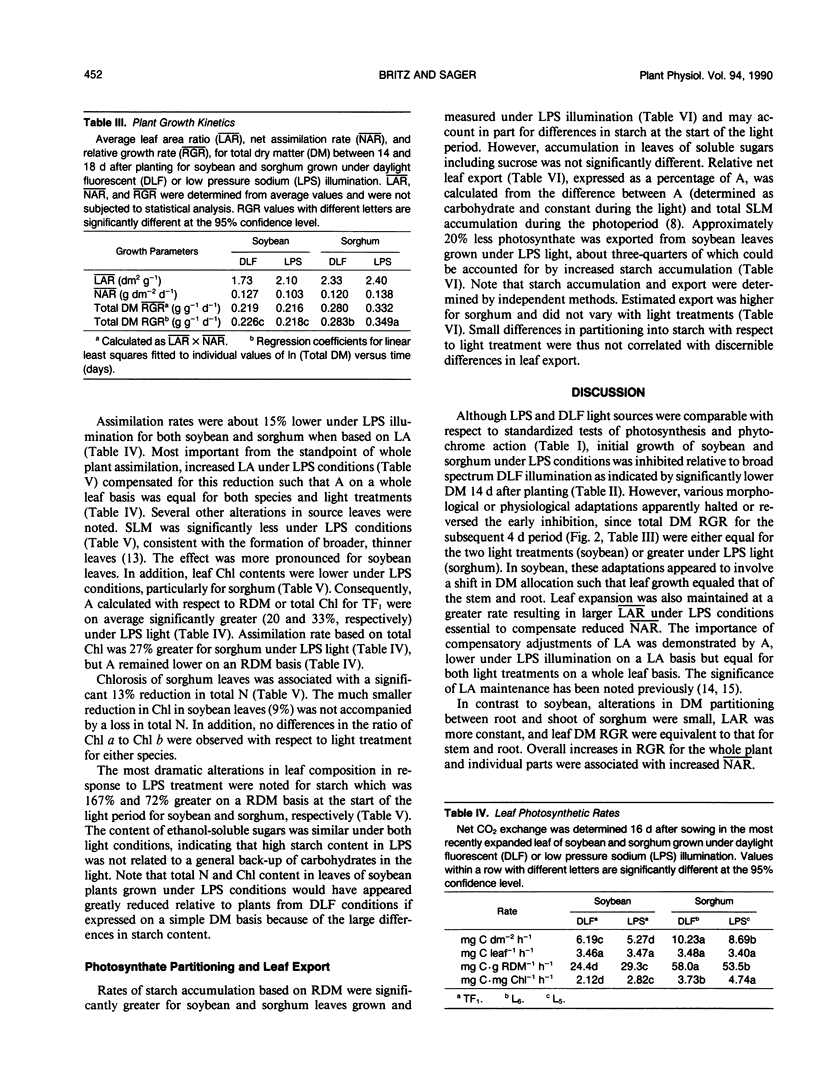
The role of blue light in plant growth and development was investigated in soybean (Glycine max [L.] Merr. cv Williams) and sorghum (Sorghum bicolor [L.] Moench. cv Rio) grown under equal photosynthetic photon fluxes (approximately 500 micromoles per square meter per second) from broad spectrum daylight fluorescent or blue-deficient, narrow-band (589 nanometers) low pressure sodium (LPS) lamps. Between 14 and 18 days after sowing, it was possible to relate adaptations in photosynthesis and leaf growth to dry matter accumulation. Soybean development under LPS light was similar in several respects to that of shaded plants, consistent with an important role for blue light photoreceptors in regulation of growth response to irradiance. Thus, soybeans from LPS conditions partitioned relatively more growth to leaves and maintained higher average leaf area ratios (mean LAR) that compensated lower net assimilation rates (mean NAR). Relative growth rates were therefore comparable to plants from daylight fluorescent lamps. Reductions in mean NAR were matched by lower rates of net photosynthesis (A) on an area basis in the major photosynthetic source (first trifoliolate) leaf. Lower A in soybean resulted from reduced leaf dry matter per unit leaf area, but lower A under LPS conditions in sorghum correlated with leaf chlorosis and reduced total nitrogen (not observed in soybean). In spite of a lower A, mean NAR was larger in sorghum from LPS conditions, resulting in significantly greater relative growth rates (mean LAR was approximately equal for both light conditions). Leaf starch accumulation rate was higher for both species and starch content at the end of the dark period was elevated two- and three-fold for sorghum and soybean, respectively, under LPS conditions. Possible relations between starch accumulation, leaf export, and plant growth in response to spectral quality were considered.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Britz S. J., Hungerford W. E., Lee D. R. Photoperiodic Regulation of Photosynthate Partitioning in Leaves of Digitaria decumbens Stent. Plant Physiol. 1985 Aug;78(4):710–714. doi: 10.1104/pp.78.4.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwell R. D., Cataldo A. M., Salcman M. Cytochemical localization of glucose-6-phosphatase activity in cerebral endothelial cells. J Histochem Cytochem. 1983 Jun;31(6):818–822. doi: 10.1177/31.6.6302165. [DOI] [PubMed] [Google Scholar]
- Chatterton N. J., Silvius J. E. Photosynthate Partitioning into Starch in Soybean Leaves: I. Effects of Photoperiod versus Photosynthetic Period Duration. Plant Physiol. 1979 Nov;64(5):749–753. doi: 10.1104/pp.64.5.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong T. Y., Goodchild D. J., Anderson J. M. Effect of Light Quality on the Composition, Function, and Structure of Photosynthetic Thylakoid Membranes of Asplenium australasicum (Sm.) Hook. Plant Physiol. 1985 Jul;78(3):561–567. doi: 10.1104/pp.78.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirecki R. M., Teramura A. H. Effects of Ultraviolet-B Irradiance on Soybean : V. The Dependence of Plant Sensitivity on the Photosynthetic Photon Flux Density during and after Leaf Expansion. Plant Physiol. 1984 Mar;74(3):475–480. doi: 10.1104/pp.74.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter J. R. Leaf area partitioning as an important factor in growth. Plant Physiol. 1977 Jan;59(1):10–14. doi: 10.1104/pp.59.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry N. Limiting Factors in Photosynthesis: IV. Iron Stress-Mediated Changes in Light-Harvesting and Electron Transport Capacity and its Effects on Photosynthesis in Vivo. Plant Physiol. 1983 Apr;71(4):855–860. doi: 10.1104/pp.71.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]


