Mantle cell lymphoma (MCL) is an aggressive, rare, B-cell non-Hodgkin lymphoma often characterized by recurrent relapses after initial therapy.1 Blastoid and pleomorphic variants, representing <20% of MCL cases, are considered high-risk and are associated with a high proliferation index and poor prognoses.2 Patients with these variants rarely achieve durable remission or prolonged clinical outcomes with standard chemotherapies.2 High-risk Mantle Cell Lymphoma International Prognostic Index (MIPI) score and Ki-67 index >30% are also poor prognostic factors in MCL.1 The 5-year overall survival (OS) rate for patients with high-risk MIPI score has been reported to be 35% compared with 81% and 63% for those with low- and intermediate-risk scores, respectively.3 Ibrutinib, a Bruton tyrosine kinase (BTK) inhibitor, demonstrated favorable responses in patients with relapsed/refractory (R/R) MCL, but has been associated with less activity in patients with blastoid morphology.4,5
Acalabrutinib is a next-generation, potent, highly selective BTK inhibitor approved for the treatment of patients with R/R MCL who have received ≥1 prior therapy. Previous updates on this study (ACE-LY-004; clinicaltrials gov. Identifier: NCT02213926) demonstrated that the safety and efficacy of acalabrutinib were maintained over time6-8 and using a data cutoff of February 24, 2020, median OS had not yet been reached.8 Here, we report the final efficacy results using a data cutoff of December 4, 2020, which includes updated OS data and long-term safety findings in patients with R/R MCL, including patients with blastoid/pleomorphic morphology, high-risk MIPI score, and Ki-67 index >50%. Notably, the median follow-up of 38.1 months was the same at the February 24, 2020, and December 4, 2020, data cutoff dates because the patients who remained in the study had time on study greater than the median; the median follow-up of patients (n=65) who were alive at the time of analysis was 54.7 months.
ACE-LY-004 was an open-label, multicenter, single-arm phase II study of acalabrutinib in patients with R/R MCL. Acalabrutinib 100 mg was administered orally twice daily until progressive disease or unacceptable toxicity. Patients ≥18 years with confirmed MCL who relapsed after, or were refractory to, one to five previous therapies were included. The primary endpoint was investigator-assessed overall response rate (ORR), defined as the proportion of patients who achieved partial response (PR) or complete response (CR) according to the Lugano response criteria for non-Hodgkin lymphoma.9 Secondary endpoints included investigator-assessed duration of response (DOR), progression-free survival (PFS), OS, and safety. More details on the study design have been previously published.6 A total of 124 patients were enrolled; the median age was 68 years. Many patients had high disease burden, including 37.1% of patients with bulky lymph nodes of ≥5 cm and 71.8% of patients with extranodal involvement. Other key risk factors indicative of poor prognosis were blastoid/pleomorphic morphology in 26 (21.0%) patients, high-risk MIPI score in 21 (16.9%) patients, and Ki-67 index ≥50% in 32 (25.5%) patients. About a quarter (26.6%) of patients had a history of cardiac disorders, including atrial fibrillation (6.5%). Almost half (45.2%) had a history of hypertension. Other patient characteristics were previously reported.7 The median number of prior therapies was two (range, 1–5); 59 (47.6%) patients had one prior therapy and 65 (52.4%) patients had ≥2 prior therapies.
Fifty-four (43.5%) patients received acalabrutinib for >24 months, including 14 (11.3%) patients who received treatment for >60 months. Eighteen (14.5%) patients remained on treatment; the median dose intensity for all patients was 98.6% (Online Supplementary Table S1).
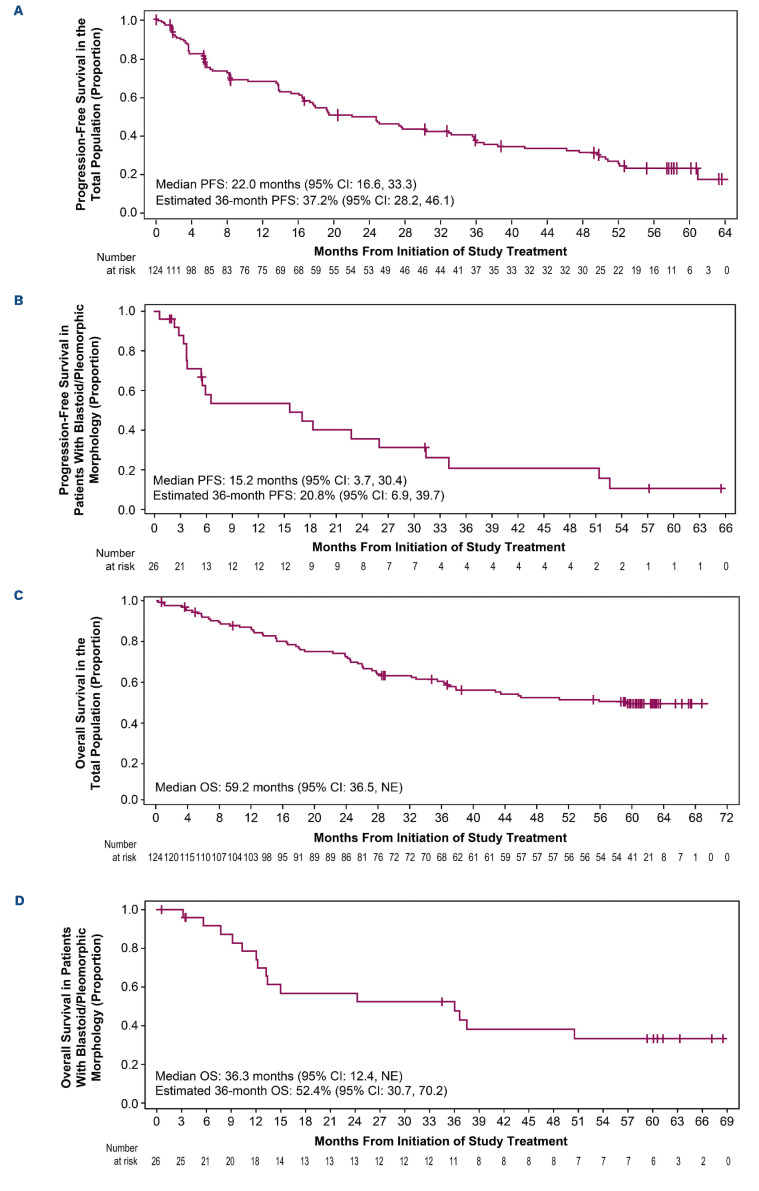
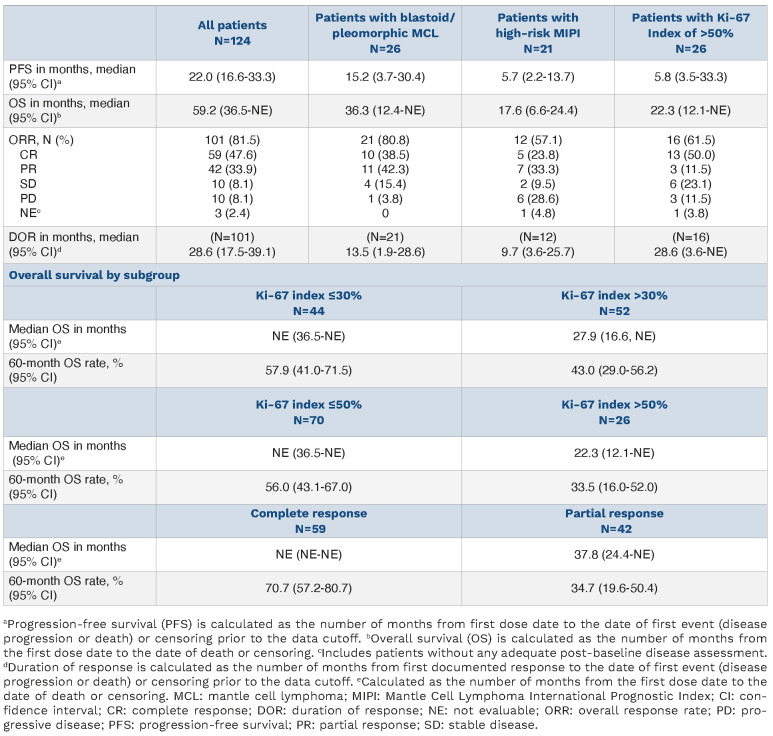
In the total population, ORR and CR rates were 81.5% (95% confidence interval [CI]: 73.5-87.9) and 47.6% (95% CI: 38.5-56.7), respectively. With a median follow-up of 38.1 months, median DOR (mDOR) and median PFS (mPFS) were 28.6 months (95% CI: 17.5-39.1) and 22.0 months (95% CI: 16.6-33.3), respectively (Figure 1A). The estimated median OS (mOS) was 59.2 months (95% CI: 36.5-not evaluable [NE]; Figure 1C; Table 1) and the estimated 5-year OS rate was 49.5% (95% CI: 40.1-58.2).
Efficacy outcomes by subgroups of patients with blastoid/pleomorphic morphology, high-risk MIPI score, Ki-67 index, and response were also reported (Table 1; Online Supplementary Figure S1). In the subgroup of 26 patients with blastoid/pleomorphic MCL, mPFS and mOS were 15.2 months (95% CI: 3.7-30.4; Figure 1B) and 36.3 months (95% CI: 12.4-NE; Figure 1D), respectively, with an ORR of 80.8% (95% CI: 60.6-93.4), similar to the overall population.
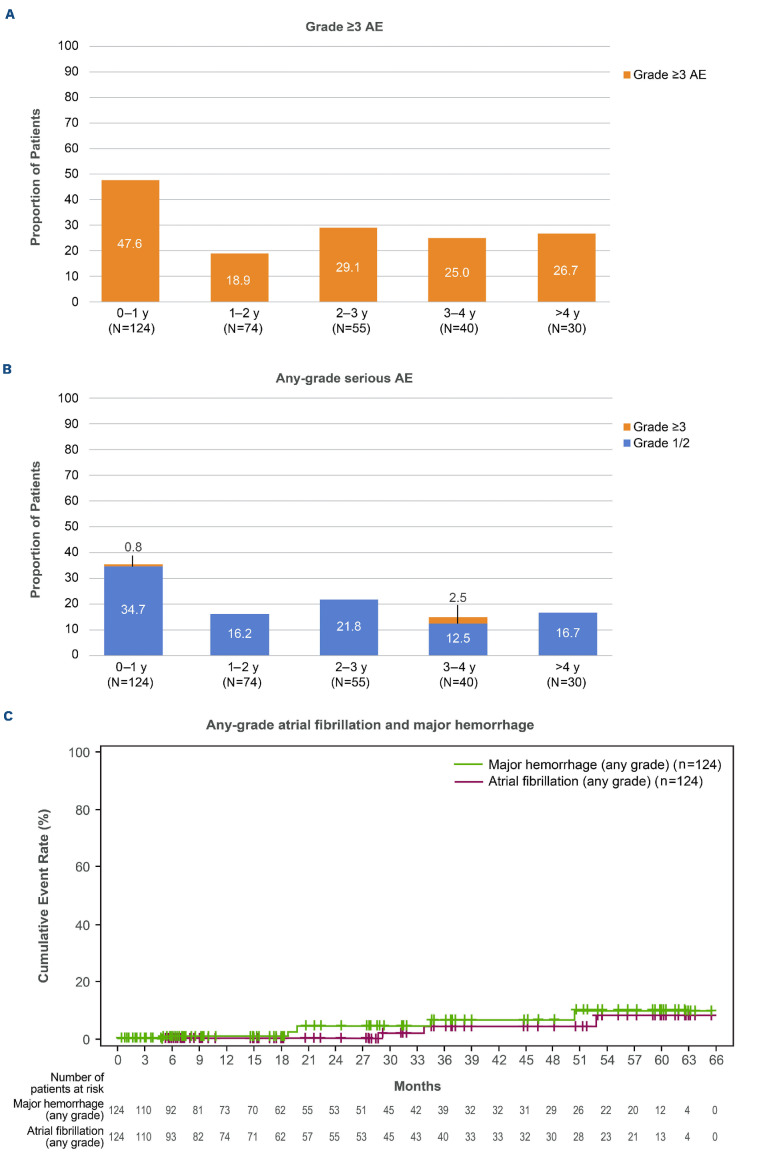
The adverse event (AE) profile remained consistent with the known acalabrutinib safety profile (Online Supplementary Table S2). Median treatment exposure was 17.5 months (range, 0.1–65.3). Grade ≥3 AE were reported in 82 (66.1%) patients. Serious AE were reported in 62 (50.0%) patients, with the most common being pneumonia (8 [6.5%]). Serious grade ≥3 AE were observed in 61 (49.2%) patients. The highest incidence of grade ≥3 AE and any-grade serious AE occurred in the first year of acalabrutinib therapy (Figure 2A, B).
Figure 1.
Progression-free survival and overall survival in the total population (A, C) and in a subgroup of patients with blastoid and pleomorphic disease (B, D). A total of 59 out of 124 patients died (47.6%); causes include disease progression (N=40), adverse event (N=6 [1 patient experienced treatment-related myelodysplastic syndrome and died from pneumonia]), other cause (N=6), or unknown cause (N=7). Deaths due to “other cause” include secondary acute myeloid leukemia (N=2), lung cancer (N=1), pneumonia (N=1), intestinal obstruction (N=1), and graft-versus-host disease (N=1). Deaths due to “unknown cause” include a patient who died at home (N=1) and who had been qualified to next line of therapy (N=1). PFS: progression-free survival; CI: confidence interval; NE: not estimable; OS: overall survival.
Selected AE of clinical interest were atrial fibrillation (any grade, n=3 [2.4%]; no grade 3/4), hypertension (any grade, n=5 [4.0%]; grade 3/4, n=2 [1.6%]), major hemorrhage (n=5 [4.0%], all grade 3/4), and infections (any grade, n=84 [67.7%]; grade 3/4, n=21 [16.9%]). One of the five patients reporting major hemorrhage had previously experienced treatment-related atrial fibrillation (grade 2, 1030 days after initiating acalabrutinib), for which anticoagulation was initiated; 16 days later the patient suffered a treatment-related subdural hematoma (grade 4, day 1,046), prompting discontinuation of acalabrutinib. Of the three patients experiencing atrial fibrillation, two had a history of hypertension; one received aspirin for cardiac prophylaxis, and the other patient is described above (received anticoagulants). The cumulative incidence of atrial fibrillation (any grade) and major hemorrhage (any grade) remained low over time (Figure 2C). Major hemorrhage (grade 2 hematuria) and infection (grade 2 sinusitis) led to dose reduction in one patient each. One additional patient had an acalabrutinib dose reduction due to an AE of fatigue.
Patients discontinued acalabrutinib mainly due to disease progression (62.1%) or AE (12.1%; Online Supplementary Table S1). Fifteen patients discontinued due to AE; in nine of these patients, AE were deemed related to acalabrutinib by the treating physician (diffuse large B-cell lymphoma [DLBCL], thrombocytopenia, myelodysplastic syndrome [MDS], leukocytosis, melanoma, cardiac arrythmia by atrial fibrillation and subdural hematoma, pulmonary fibrosis, hemorrhagic bullae and petechiae, and skin rash). All three patients with reported second primary malignancies related to acalabrutinib had received chemotherapy prior to study enrollment (R-CHOP [rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone] plus lenalidomide in the patient with DLBCL, hyper-CVAD [cyclophosphamide, vincristine, doxorubicin, and dexamethasone] therapy with alternating methotrexate/cytarabine in the patient with MDS, and bendamustine plus rituximab in the patient with melanoma). Death was reported in 59 patients; 40 (32.3%), six (4.8%), and 13 (10.5%) patients died due to disease progression, AE, and unknown or other causes, respectively. Five deaths due to AE were not considered to be related to acalabrutinib (MDS, aortic stenosis, pulmonary embolism, non-small cell lung cancer, and suicide), one of which occurred during the post-treatment follow-up period (MDS). The only treatment-related death in this study was in a patient who died of pneumonia. This patient discontinued acalabrutinib (day 937) due to treatment-related grade 4 MDS (mentioned above) and died 185 days (day 1,112) after the last dose of acalabrutinib.
There were 71 (57.3%) patients who received subsequent anticancer therapy post acalabrutinib treatment. Bendamustine with rituximab was used in 16 (12.9%) patients, radiotherapy was used in nine (7.3%) patients, and ibrutinib was used in eight (6.5%) patients.
The final results of this study demonstrated that efficacy and safety of acalabrutinib were maintained compared with data from a previous analysis (median follow-up of 26 months).7 Acalabrutinib also demonstrated efficacy in patients with blastoid/pleomorphic morphology, high-risk MIPI score, and Ki-67 index >30% and >50%, which are poor prognostic factors in MCL.
Table 1.
Progression-free survival, overall survival, response, and duration of response in the total population.
In our analysis of acalabrutinib treatment in 124 patients, mDOR, mPFS, and mOS were 28.6 months, 22.0 months, and 59.2 months, respectively, after a median follow-up of 38.1 months, which are longer than those reported in a pooled analysis of ibrutinib. In the analysis of ibrutinib, treatment in 370 patients with R/R MCL (median of 2 prior lines of therapy) resulted in mDOR, mPFS, and mOS of 21.8 months, 12.5 months, and 26.7 months, respectively, after a median follow-up of 41.4 months.5,10 Similarly, in a pooled analysis of zanubrutinib treatment in 112 patients with R/R MCL (median of 2 prior lines of therapy), mDOR, mPFS, and mOS were 24.9 months, 25.8 months, and 38.2 months, respectively, after a median follow-up of 24.9 months, with the majority of data from a study conducted in Chinese patients.11,12 Notably, the proportion of patients with blastoid and/or pleomorphic histology in the ibrutinib and zanubrutinib analyses compared with our analysis was 12% (n=44) and 13% (n=14), respectively, versus 21% (n=26), and the proportion of patients with high-risk MIPI scores was 32% and 21%, respectively, versus 17%. The patients with blastoid/pleomorphic morphology in this analysis of acalabrutinib achieved an ORR (80.8%) as high as that in the overall study population, with prolonged mPFS and mOS, a finding that was not observed in other high-risk subgroups such as those with high-risk MIPI scores or Ki-67 index >50%. The patients with blastoid morphology achieved an ORR of 50.0% in the ibrutinib analysis and 66.7% in the zanubrutinib study in Chinese patients.12 Although there were no reports of grade ≥3 atrial fibrillation (any grade, 2.4%) and a low incidence of grade ≥3 major hemorrhage (4.0%) in the present analysis, the ibrutinib analysis showed an overall incidence of grade ≥3 atrial fibrillation/flutter of 6.5% (any grade, 11.4%) and an incidence of grade ≥3 hemorrhage of 5.9%. In the zanubrutinib analysis, the incidence of any-grade atrial fibrillation/flutter was 1.8% (grade ≥3, 0.89%) and the incidence of any-grade major hemorrhage was 5.4%. Although these analyses all evaluated efficacy and safety of BTK inhibitors in R/R MCL, cross-trial comparisons are limited by differences in trial design, heterogeneity in patient populations, and the changing treatment landscape across the periods of enrollment for the various studies. The final results of this study with 38.1 months median follow-up support the use of acalabrutinib in patients with R/R MCL, including those with high-risk features such as blastoid/pleomorphic variants, high-risk MIPI score, and Ki-67 index >50%, and demonstrated a consistent safety profile. Ongoing studies are evaluating acalabrutinib in combination with other agents in this difficult-to-treat aggressive lymphoma.
Figure 2.
Incidence of adverse events (AE) over time for grade ≥3 AE (A), any-grade serious AE (B), and any-grade select AE of interest (C; atrial fibrillation and major hemorrhage). A patient with multiple severity grades for a given AE was counted only once under the maximum severity. Multiple onsets of the same AE within a specific yearly interval were counted once, and the same AE term continuing across several yearly intervals was counted in each of the intervals; y: years.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Helen Yang of AstraZeneca for biostatistics support.
Funding Statement
Funding: This work was funded by AstraZeneca. Writing and editorial assistance were provided by Tracy Diaz, PhD, and Sarah Huh, PharmD, of Peloton Advantage, an OPEN Health company, and funded by AstraZeneca.
References
- 1.Jain P, Wang ML. Mantle cell lymphoma in 2022 - a comprehensive update on molecular pathogenesis, risk stratification, clinical approach, and current and novel treatments. Am J Hematol. 2022;97(5):638-656. [DOI] [PubMed] [Google Scholar]
- 2.Jain P, Wang M. Blastoid mantle cell lymphoma. Hematol Oncol Clin North Am. 2020;34(5):941-956. [DOI] [PubMed] [Google Scholar]
- 3.Hoster E, Klapper W, Hermine O, et al. Confirmation of the mantle-cell lymphoma International Prognostic Index in randomized trials of the European Mantle-Cell Lymphoma Network. J Clin Oncol. 2014;32(13):1338-1346. [DOI] [PubMed] [Google Scholar]
- 4.Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369(6):507-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rule S, Dreyling M, Goy A, et al. Outcomes in 370 patients with mantle cell lymphoma treated with ibrutinib: a pooled analysis from three open-label studies. Br J Haematol. 2017;179(3):430-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang M, Rule S, Zinzani PL, et al. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): a single-arm, multicentre, phase 2 trial. Lancet. 2018;391(10121):659-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang M, Rule S, Zinzani PL, et al. Durable response with single-agent acalabrutinib in patients with relapsed or refractory mantle cell lymphoma. Leukemia. 2019;33(11):2762-2766. [DOI] [PubMed] [Google Scholar]
- 8.Wang M, Rule S, Zinzani PL, et al. Acalabrutinib monotherapy in patients with relapsed/refractory mantle cell lymphoma: longterm efficacy and safety results from a phase 2 study. Blood. 2020;(Suppl 1):S38-39. [Google Scholar]
- 9.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rule S, Dreyling M, Goy A, et al. Ibrutinib for the treatment of relapsed/refractory mantle cell lymphoma: extended 3.5-year follow up from a pooled analysis. Haematologica. 2019;104(5):e211-e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou K, Zou D, Zhou J, et al. Zanubrutinib monotherapy in relapsed/refractory mantle cell lymphoma: a pooled analysis of two clinical trials. J Hematol Oncol. 2021;14(1):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song Y, Zhou K, Zou D, et al. Zanubrutinib in relapsed/refractory mantle cell lymphoma: long-term efficacy and safety results from a phase 2 study. Blood. 2022;139(21):3148-3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.