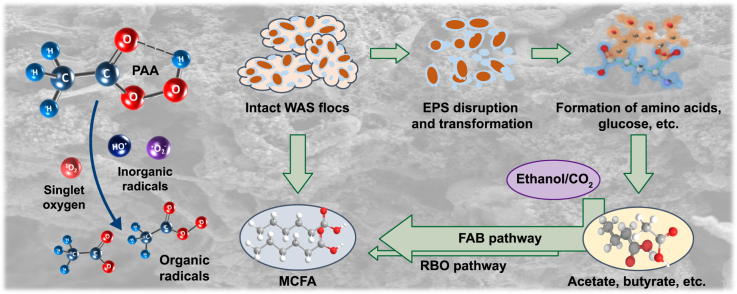
Abstract
Peracetic acid (PAA), known for its environmentally friendly properties as a oxidant and bactericide, is gaining prominence in decontamination and disinfection applications. The primary product of PAA oxidation is acetate that can serve as an electron acceptor (EA) for the biosynthesis of medium-chain fatty acids (MCFAs) via chain elongation (CE) reactions. Hence, PAA-based pretreatment is supposed to be beneficial for MCFAs production from anaerobic sludge fermentation, as it could enhance organic matter availability, suppress competing microorganisms and furnish EA by providing acetate. However, such a hypothesis has rarely been proved. Here we reveal that PAA-based pretreatment leads to significant exfoliation of extracellular polymeric substances (EPS) from sludge flocs and disruption of proteinic secondary structures, through inducing highly active free radicals and singlet oxygen. The production of MCFAs increases substantially to 11,265.6 mg COD L−1, while the undesired byproducts, specifically long-chain alcohols (LCAs), decrease to 723.5 mg COD L−1. Microbial activity tests further demonstrate that PAA pretreatment stimulates the CE process, attributed to the up-regulation of functional genes involved in fatty acid biosynthesis pathway. These comprehensive findings provide insights into the effectiveness and mechanisms behind enhanced MCFAs production through PAA-based technology, advancing our understanding of sustainable resource recovery from sewage sludge.
Keywords: Sewage sludge, Medium-chain fatty acids (MCFAs), Extracellular polymeric substances (EPS), Peracetic acid (PAA), Metabolic activity
Graphical abstract
Highlights
-
•
Peracetic acid (PAA) can boost sludge disruption and provide electron acceptors.
-
•
Medium-chain fatty acids (MCFAs) are promoted to 11,265.6 mg COD L−1 by PAA.
-
•
Free radicals and singlet oxygen are the reactive species for PAA oxidation.
-
•
PAA promotes protein-like substrate hydrolysis and chain elongation.
-
•
PAA induces profitable microbial communities and metabolic pathways.
1. Introduction
The recycling of sewage sludge, including primary sludge and waste activated sludge (WAS), which are inherent byproducts of biological wastewater treatment, is an important part of achieving carbon neutrality at wastewater treatment plants (WWTPs) [[1], [2], [3]]. As a low-cost and simple technology, anaerobic fermentation can effectively reduce the contamination properties of sludge and convert organic fractions into value-added products [4,5]. Compared to short-chain fatty acids (SCFAs, i.e., traditional fermentation products), medium-chain fatty acids (MCFAs) carry higher energy densities. They are more hydrophobic and can be easily separated from fermentation liquor [[6], [7], [8]]. MCFAs, denoting straight-chain monocarboxylates containing 6–12 carbon atoms, primarily encompass caproate, heptylate, and caprylate when produced through biotechnological means [9]. MCFAs can be employed in diverse industries, such as food additives and antimicrobial agents [6]. They are also precursors to manufacture industrial products, e.g., surfactants, lubricants, plasticizers, etc., or to synthesize jet fuel and diesel [10]. Consequently, MCFAs have a higher economic and practical value.
Sewage sludge is a suitable and cheap substrate for MCFAs production. On one hand, most organics in sludge can be biodegraded into acetate-dominated SCFAs. Chain elongation (CE) microorganisms prefer to initiate CE with acetate as the initial electron acceptor (EA) [11]. Meanwhile, sewage sludge is rich in microorganisms and extracellular hydrolytic enzymes, e.g., acid-producing bacteria, CE microorganisms, and protease, benefiting hydrolysis/acidification of substrates and SCFAs upgrading [12]. When electron donor (ED, commonly ethanol) is sufficient, CE microorganisms will likely be enriched to upgrade SCFAs to MCFAs. For example, it was reported that 6615 mg COD per L MCFAs were produced from anaerobic WAS fermentation due to the stimulation of 170 mM ethanol [13]. When WAS alkaline fermentation liquid was applied as EA for CE, with different concentrations of ethanol provided as ED, MCFAs production was elevated from 5570 to 7670 mg COD L−1 [6]. However, EA provided by sewage sludge is often limited since the organic components in hard cell walls and extracellular polymeric substance (EPS) are resistant to biological hydrolysis [12,14], resulting in hindered MCFAs production [15,16]. Meanwhile, potential competing microbes in sewage sludge allow the conversion of ED and EA to byproducts, e.g., long-chain alcohols (LCAs) and methane, leading to dispersive carbon fluxes and incomplete CE [10]. To accelerate sludge hydrolysis and eliminate competitive inhibiting processes, sludge pretreatment is essential before anaerobic fermentation. Yin et al. proved that Fenton-persulfate oxidation could promote sludge acidification and suppress microorganisms capable of producing LCAs, increasing MCFAs production from 2367.7 to 3445.9 mg COD L−1 [16]. Wang et al. indicated that after free ammonia (FA) pretreatment for 24 h, MCFAs production from WAS was promoted from 1400 to 8300 mg COD L−1 [15]. Nevertheless, adding persulfate may induce a sulfate-reducing process, and fermentation broth reflux would increase sludge volume. Therefore, finding an economical and eco-friendly pretreatment technology is urgently needed to scale up the application of MCFAs recovery from WAS.
Peracetic acid (PAA, E0 = 1.81 eV) is a wide-spectrum antimicrobial agent for many industries, e.g., medical and food, without byproducts generation. Also, the PAA-based advanced oxidation process (AOP) has exhibited excellent functions in chemical/microbiological micropollutant removal from water/wastewater [17]. Recently, the inhibition of PAA in the biological wastewater treatment process and its effect on WAS dissolution have attracted much attention. It was reported that PAA could inhibit the enzyme activity associated with nitrification and decrease nitrate production rates [18]. Appels et al. reported that PAA oxidation caused effective solubilization of sludge organics and increased methane production from anaerobic WAS digestion by 21% [19]. It was also unveiled that PAA could efficiently dissolve sludge cells and EPS into soluble components [20]. Additionally, Li et al. demonstrated that PAA suppressed non-functional microorganisms, i.e., hydrogen-consuming microorganisms, during WAS dark fermentation for hydrogen production, resulting in H2 cumulation increasing to 14.2 mL per g VSS with considerable SCFAs as liquid-phase products [21]. Although PAA-based oxidation has been demonstrated feasible for sludge solubilization and primary fermentation, its enhancement effect on MCFAs production from WAS and the related mechanisms have not been elucidated. Therefore, we propose employing PAA oxidation as a pretreatment strategy for sludge disintegration and MCFAs production. Our hypotheses are based on the following premises: (1) PAA can be activated in situ within sludge that is rich in iron [22] and accelerate organic components solubilization and conversion, providing more soluble materials as precursors for EA (i.e., SCFAs) formation. (2) The only byproduct of PAA oxidation is acetic acid, which can be consumed by CE microorganisms as EA to produce MCFAs. (3) PAA has bactericidal or bio-inhibitory effects that may inhibit the production of LCAs and increase electron transfer efficiency from ED to MCFAs.
This work aims to explore the feasibility and corresponding mechanism of PAA pretreatment in enhancing MCFAs production from WAS. Firstly, WAS disintegration performance based on PAA oxidation is elaborated from three aspects: soluble substrate production, changes in sludge properties, and extracellular organics transformation. Subsequently, the details of different doses (i.e., 0–25 mg per g TSS) of PAA pretreatment on MCFAs biosynthesis from sludge are assessed based on ED consumption, MCFAs accumulation, byproduct (i.e., LCAs) production, and product distributions. Meanwhile, community succession of MCFAs-forming bioreactors mediated by PAA pretreatment is elucidated by 16S rRNA sequencing. Furthermore, mechanisms of enhanced MCFAs production were clarified from three perspectives: reaction mechanisms of PAA oxidation for WAS disintegration, metabolic activities of key bioprocesses, and variations in microbial functions and functional gene expression. The findings observed in this work would fill the knowledge gap of PAA-based AOP for enhanced MCFAs production from WAS and expand the understanding of the role of PAA in sludge resource recovery.
2. Material and methods
2.1. Main parameters of WAS and PAA
WAS applied for the experiments was from a local WWTP in Tianjin, China. After impurities were removed and concentrated, WAS was stored at 4 °C. The key characteristics of the WAS were as follows: total suspended solids (TSS) of 26.34 ± 0.46 g L−1, volatile suspended solids (VSS) of 15.86 ± 0.28 g L−1, a total COD (TCOD) of 24540 ± 917 mg L−1, total carbohydrates of 2862 ± 123 mg COD L−1, and total proteins of 14756 ± 225 mg COD L−1. The inoculum was cultured by WAS with ethanol as ED. The key properties of this inoculum were characterized by total solids (TS) of 17.69 ± 0.13 g L−1, volatile solids (VS) of 9.79 ± 0.12 g L−1, a TCOD of 16800 ± 591 mg L−1, total carbohydrates of 1853 ± 81 mg COD L−1, and total proteins of 9401 ± 157 mg COD L−1. The commercial PAA solution was purchased from Tianjin Damao Chemical Reagent Factory, and the PAA content in the solution was 12%.
2.2. WAS disintegration by PAA pretreatment
Effective sludge flocs disintegration is the essential precondition for the acidogenic fermentation of WAS. In this batch experiment, we employed five serum bottles as reactors, each containing 150 mL of WAS. Subsequently, a certain volume of PAA solution was dosed to these reactors to reach a concentration of 0, 10, 15, 20, or 25 mg PAA per g TSS, respectively. This pretreatment process was conducted over a 24-h period to ensure complete peroxidation [19,23]. These tests were conducted in triplicates. After pretreatment, we analyzed the contents of soluble organic components (such as proteins and carbohydrates). Furthermore, changes in extracellular substances and excitation-emission matrix (EEM) fluorescence intensities were determined to expound the impacts of PAA oxidation on WAS properties. Fourier transform infrared (FTIR) and X-ray photoelectron spectroscopy (XPS) were used to assess the structure variations of extracellular organic components induced by PAA oxidation.
2.3. Bioreactor operation for MCFAs production
To evaluate the performance of MCFAs production from PAA pretreated WAS, we employed five serum bottles (V = 300 mL) as anaerobic bioreactors. Briefly, 135 mL pretreated WAS was employed as substrate, and 15 mL inoculum was applied as inoculum. The procedures and PAA dosage for sludge pretreatment were the same as in Section 2.2. The inoculated sludge (i.e., inoculum) was acclimatized by fermentation of WAS with 50 mM 2-bromoethanesulfonate as methane inhibitor and 160 mM ethanol as ED [13]. The initial pH values for all reactors were 6.5 and adjusted to 6.0 on day 6 when the pH dropped to about 5.5. To trigger effective MCFAs production, 160 mM ethanol and 25 mM 2-bromoethanesulfonate were employed as ED and methanogen inhibitors, respectively. Before anaerobic fermentation, all reactors were flushed with N2, capped by butyl rubber stoppers, and wrapped with sealing films. All trials were performed in triplicates. These reactors were operated for 14 days under 35 °C, during which MCFAs, SCFAs, LCAs, and ethanol were determined every two days.
2.4. Influence of PAA pretreatment on individual steps associated with MCFAs production
Three key bio-steps, i.e., hydrolysis, acidification, and CE, are associated with MCFAs production when sewage sludge serves as substrate. PAA pretreatment could result in soluble substrates increment and cause microbe damage to some degree. Thus, how does PAA pretreatment affect microbial activities that determine MCFAs production? To clarify the potential impacts, several standard substances were used to simulate these processes and differentiate the response of each step to PAA pretreatment. Three batch tests were conducted, i.e., hydrolysis, acidification, and CE tests, and the sludge withdrawn from the above anaerobic reactors (described in section 2.3) was used as inoculum. Before experiments, the residual substances in the inocula were removed by washing with tap water. Each trial was performed in triplicates, as depicted in Supplementary Information (SI).
2.5. Microbial evolution and prediction of functional genes
Microbial evolution was characterized by 16S rRNA sequencing, with 338F and 806R as primers [6]. Functional gene prediction was performed according to research conducted by Huang et al. [24]. Sludge samples were taken from four bioreactors, including the control, 15, 20, and 25 mg PAA per g TSS, for microbial analysis and prediction of functional genes at the end of anaerobic fermentation (i.e., on day 14).
2.6. Analytic methods and statistical assessment
TS, VS, TSS, and VSS of sludge were detected by standard procedures [25]. COD, proteins, and carbohydrates were determined using HACH reagents, Lowry-Folin, and anthrone-sulfuric methods [22]. A meter (PHS-3E, Leici) was used to measure the oxidation reduction potential (ORP) of sludge. The zeta potential of sludge samples was detected by a zeta potential analyzer (Litesizer 500). A gas chromatography (GC2014, SHIMADZU), equipped with a flame ionization detector (FID), was employed to detect SCFAs, MCFAs, ethanol, and LCAs (i.e., butanol and hexanol) contents [26]. Protease and α-glucosidase activities were analyzed according to research conducted by Xie et al. [12]. The changes in the biodegradability of sewage sludge were assessed by quantities of biodegradable substances due to PAA pretreatment using EEM fluorescence technology. FTIR spectroscopy and XPS were determined according to Wang et al. [27]. Free radicals and singlet oxygen were analyzed by an electron spin resonance (ESR) spectrometer (JEOL JES-FA200) using DMPO (5,5-dimethyl-1-pyrroline-N-oxide) and TEMP (2,2,6,6-tetramethyl-4-piperidine) as trapping agents. Sludge morphology was detected by scanning electron microscopy (SEM), with element content determined by energy dispersive spectroscopy (EDS). Experimental data were analyzed using the SPSS software, with p < 0.05 statistically significant.
3. Results and discussion
3.1. WAS disintegration and organics transformation trigged by PAA oxidation
3.1.1. Soluble substrates production
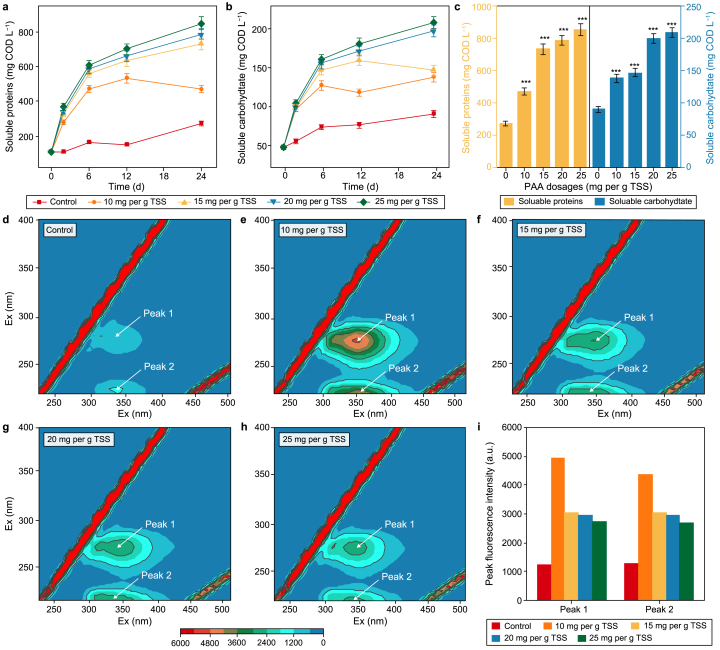
The forms of proteins and carbohydrates, as the primary organic fractions presented in WAS, are significantly influenced by PAA pretreatment (Fig. 1). Their contents in the supernatant gradually increase with time and PAA dose within 24 h of reaction (Fig. 1a and b). The release rates of proteins and carbohydrates are significantly greater in the first 6 h, indicating that PAA can rapidly oxidize sludge flocs and effectively convert particulate organic matter to soluble substrates. After pretreatment, soluble proteins and carbohydrates concentrations reach 850.2 and 208.1 mg COD L−1 for the 25 mg PAA per g TSS group (Fig. 1c), which are respectively elevated by 212.9% (p < 0.001) and 133.0% (p < 0.001). Similarly, Yang et al. reported that after pretreatment with 90 mg per g TSS peroxymonosulfate for 24 h, dissolved proteins and carbohydrates increased to about 630 and 160 mg COD L−1, respectively, and SCFAs were eventually accumulated to 4894 mg COD L−1 [28]. This suggests that PAA and PMS have similar oxidative and destructive abilities on sludge flocs, thus providing more precursors for SCFAs formation that can act as EA for MCFAs production.
Fig. 1.
a–b, Changes of soluble proteins (a) and carbohydrates (b) within 24 h of PAA oxidation. c, Soluble proteins and carbohydrates contents after pretreatment with significance test results. d–h, EEM spectra after pretreatment: Control (d), 10 (e), 15 (f), 20 (g), and 25 mg PAA per g TSS (h). i, Peak fluorescence intensity of EEM spectra. “∗” represents p < 0.05, “∗∗” represents p < 0.01, “∗∗∗” represents p < 0.001. Error bars represent standard deviations of triplicate experiments.
EEM spectra of pretreated WAS can further disclose the enhanced effect of PAA oxidation on WAS dissolution. As displayed in Fig. 1d–h, two fluorescence peaks, peak 1 and peak 2, appear on the spectrogram, and their color reflects the changes in organic matter content [29]. The fluorescence intensity is enhanced by PAA pretreatment at all doses compared to the control, implying more soluble material release (Fig. 1i). Peak 1 represents soluble microbial byproducts like organics, and they are widely considered biodegradable substrates, whose significant increase indicates the improvement of biochemical degradability [30]. Interestingly, the peak fluorescence intensity decreases when the PAA dose exceeds 10 mg per g TSS (Fig. 1f–h), inconsistent with elevated proteins and carbohydrates releases. It may be attributed to the higher level of strong oxidative species (such as ·OH) triggered by increased PAA dose, which could react with the dissolved organics in sludge and destroy their fluorescent groups.
3.1.2. Changes in sludge properties
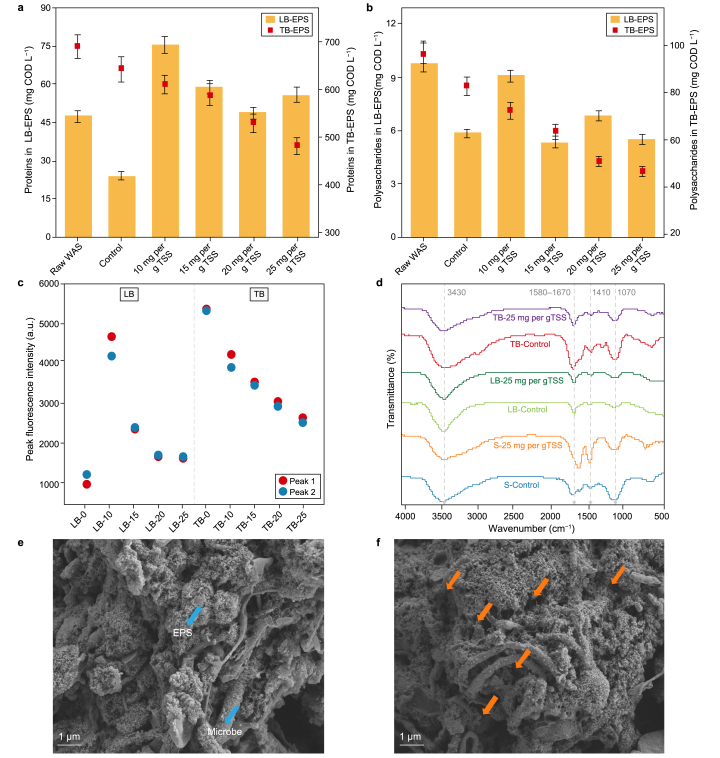
Previous studies have shown that PAA could cause cell inactivation and inhibit functional enzyme activities [17,31]. However, details of PAA pretreatment on EPS structure have been neglected [32]. As displayed in Fig. 2a and b, EPS is predominantly in the tightly bound (TB) state for the control, explaining the low rate of sludge solubilization. Proteins and polysaccharides contents in TB-EPS decrease gradually with the increase of PAA dose. For loosely bound (LB)-EPS, the control group has fewer proteins than raw WAS, while PAA groups all have higher proteinic organics than raw WAS. It implies that PAA oxidation leads to decreased EPS and migration of TB-EPS to LB-EPS [1,33]. Whereas the variation of polysaccharides is not in accord with proteins, which may be due to its low content in LB-EPS (i.e., 5.4–9.8 mg COD L−1). The EEM spectra further support the variation of protein contents in EPS (Fig. S1 and Fig. 2c). Apparently, the fluorescence peak intensity of TB fraction decreases significantly with PAA dose, while that of LB fraction in the control is lower than those of PAA groups.
Fig. 2.
a–b, Contents of proteins (a) and polysaccharides (b). c, Peak fluorescence intensity of extracted EPS. d, FTIR spectra of soluble and bound EPS fractions. e–f, changes of sludge structure reflected by SEM, i.e., the control (e) and PAA group (f).
Furthermore, Fig. 2d reflects the infrared spectra information of different EPS fractions extracted from the control and PAA-pretreated WAS. The infrared peak about 1580–1670 cm−1 or near 1410 cm−1 represents the stretching vibrations of C–N and C O, or C O symmetric stretching of –COOH groups in proteins, respectively [34]. The bond at 1070 cm−1 stands for the vibration of C–OH and C–O–C groups in organics (i.e., carbohydrates or polysaccharides) [34]. Generally, PAA pretreatment increases the infrared peak intensities of these organic component groups in LB fraction (e.g., C–OH and C–O–C) while significantly reducing their intensities in TB fraction (e.g., C–N, C O, C–OH, and C–O–C), consistent with Fig. 2a and b. It implies the disruption, migration, and dissolution of sludge EPS. As a response, some organic groups at bonds 1580–1670 cm−1 are increased in the soluble fraction after PAA pretreatment. In addition, the alteration of sludge structure by PAA can be visualized by SEM images (Fig. 2e and f). After pretreatment, sludge flocs become loose, and EPS is reduced, exposing more microorganisms. Accordingly, PAA destroys the protective barrier of microbial cells against extreme environments (i.e., oxidative stress) and facilitates the rupture of sludge cells. Chen and Pavlostathis [31] indicated that PAA affected the biological nitrogen removal system by causing cell lysis and enzyme inhibition. Ao et al. [17] reported that PAA disrupted the membrane and cell wall lipids and attacked the DNA and RNA in microorganisms. These results above illustrate that PAA disrupts the dense sludge floc structures by causing the disruption and migration of EPS fractions, thus boosting the dissolution of EPS and intracellular organics. EPS destruction is conducive to the contact of hydrolases and functional microorganisms with released organic components. Subsequently, the soluble organics produced by sludge disintegration would be rapidly and efficiently converted to SCFAs, supplying more directly accessible EA for CE bacteria.
3.1.3. Degradation and transformation of extracellular macromolecular organics
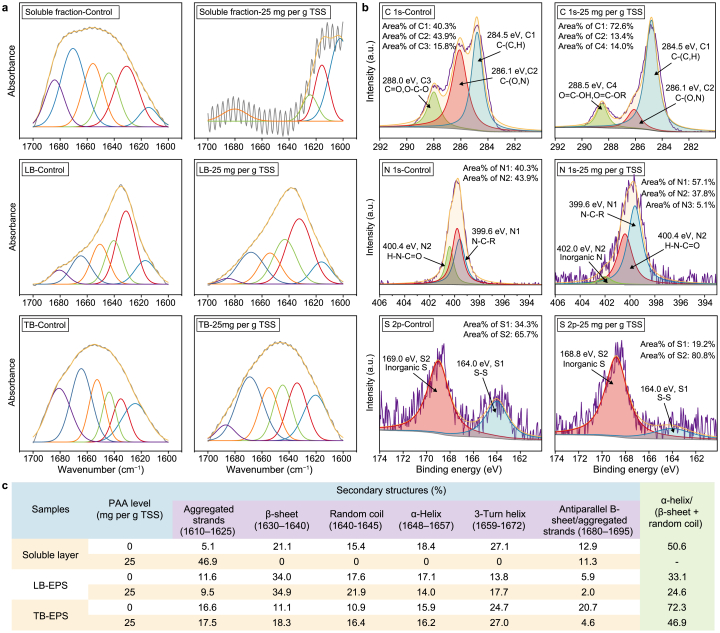
As the primary organic substrate in WAS, proteins occupy 60.1% of TCOD, whose extracellular transformation can be reflected by conformational structure alterations. Hence, for characterizing the change of proteinic conformation, FTIR spectra with wavelengths ranging from 1600 to 1700 cm−1 were subjected to deconvolution and curve-fitting analysis (Fig. 3a) [34]. The relative proportions of proteinic secondary structures in the soluble, LB, and TB fractions are significantly altered after PAA pretreatment. Particularly, the proteinic conformation in the soluble fraction is significantly disrupted by PAA oxidation, as only aggregated strands are found, i.e., the band at 1610–1625 or 1680–1695 cm−1. It has been indicated that proteinic stability is mostly determined by α-helix, β-sheet, and random coil, and a low ratio of α-helix/(β-sheet + random coil) could make the proteinic structure looser [35]. In Fig. 3c, the proportion of α-helix/(β-sheet + random coil) is largely reduced by PAA, with LB fraction from 33.1% to 24.6% and TB fraction from 72.3% to 46.9%. These observations demonstrate that PAA pretreatment changes the secondary structures of extracellular proteins, promoting their decomposition and transformation [36].
Fig. 3.
a, The second-derivative fitting curves of FTIR spectra from 1700 to 1600 cm−1, with samples being the soluble, LB, and TB fractions of control and PAA-pretreated WAS. b, XPS C 1s, N 1s, and S 2p analysis of aqueous phase obtained from the control and PAA-pretreated WAS. c, The area (%) of secondary structures determined by second-derivative fitting.
XPS C 1s spectra can be divided into four peaks, as shown in Fig. 3b. The peak at 284.8 eV (C1) is associated with C−(C,H), primarily ascribed to polysaccharides, lipids, and side chains of amino acids [36]. The ratio of C1 increases from 40.3% to 72.6% after PAA pretreatment, which proves the efficient WAS disintegration and particulate organics solubilization (Fig. 1). The peak at 286.1 eV (C2) belongs to C−(O,N) bonds from amines, amides, alcohols and ethers groups, while that at 288.0 eV (C3) is associated with O–C–O and C O primarily existed in acetal, hemiacetal, amide, carboxylic or carbonyl groups [37]. The apparent decrease of C2 (from 43.9% to 13.4%) and C3 (from 15.8% to 0) might be attributed to the effective decomposition of macromolecular substances or intermediates by PAA pretreatment. Accordingly, the peak at 288.5 eV (C4) increases from 0 to 14.0%, attributing to O C–OR and O C–OH in ester or carboxyl groups. It implies that PAA results in considerable substrate transformation and SCFAs accumulation during the pretreatment step [37]. N1 at 399.6 eV is closely related to the N–C−R bond in amino acids of proteins or peptidoglycans [38]. N2 at 400.4 eV reflects the presence of the H–N–C O bond, i.e., the pivotal structure in proteins and peptide chains [38]. The increase of N1 (from 35.7% to 57.1%) and N2 (from 22.0% to 37.8%) further supports the elevation of protein levels in the aqueous phase and the destruction of proteins and peptidoglycans induced by PAA pretreatment, concurrently (Fig. 3b). The peak at 402.0 eV represents the existence of inorganic N, and its increase from 0 to 5.1% intuitively indicates the promoted proteins and peptidoglycans degradation and the production of ammonia nitrogen [39]. Additionally, peaks at 164.0 eV (S1) and 169.0 eV (S2) in S 2p spectra, respectively, belong to the S–S bond and inorganic S (Fig. 3b) [36]. The decrease of the S–S bond and increase of inorganic S hints that PAA promotes the rupture of proteinic spatial structure and strengthens the degradation and transformation of extracellular macromolecular organics (e.g., proteins) in WAS [40].
3.2. MCFAs production from PAA-pretreated sludge through anaerobic fermentation
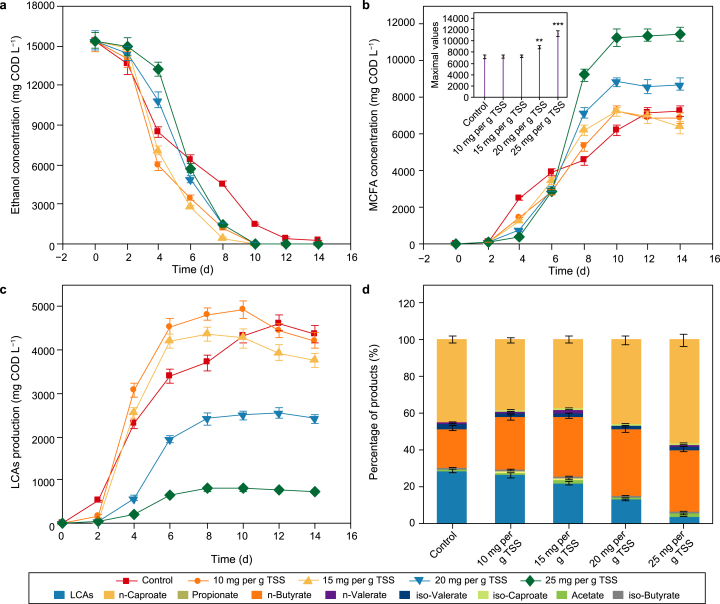
Fig. 4 displays ethanol consumption results and product generation from sewage sludge. Ethanol (i.e., ED) is completely utilized by CE microorganisms (Fig. 4a), and accumulative MCFAs stop rising at the end (Fig. 4b), suggesting the completion of the CE process. Caproate is the only MCFAs for all experiments, similar to previous studies using sewage sludge to produce MCFAs [11,13]. The highest MCFAs production is 7173.1 mg COD L−1 for the control, similar to a previous study in which MCFAs production was 6115 mg COD L−1 with sewage sludge as substrates and 170 mM ethanol as ED [13]. For experimental groups, MCFAs peak values are dependent on PAA dosage. PAA pretreatment with a dosage from 10 to 15 mg per g TSS only shortens the optimal fermentation time rather than further improves the maximal MCFAs production (p > 0.05). In contrast, the maximal MCFAs concentration is boosted from 8859.8 to 11265.6 mg COD L−1 when the PAA dose increases from 20 to 25 mg per g TSS, respectively increasing by 23.5% (p = 0.002) and 57.1% (p = 0) than the control. The maximal MCFAs production attained in this work is higher than that of pretreated sludge with free ammonia (8300 mg of COD L−1) [15]. Besides, the optimal time required for MCFAs accumulation in the PAA-pretreated groups is shortened from 12 to 10 days. These facts prove that PAA pretreatment promotes MCFAs production and accelerates MCFAs generation rate.
Fig. 4.
Concentrations evolution of ethanol (a), MCFAs (b), LCAs (c), and product distribution (d) in the control and PAA-pretreated reactors during anaerobic sewage sludge fermentation with ethanol as ED. “∗” represents p < 0.05, “∗∗” represents p < 0.01, “∗∗∗” represents p < 0.001. Error bars represent standard deviations of triplicate experiments.
LCAs, as the main byproducts of CE, usually occur with MCFAs formation. The maximum concentration of LCAs (including butanol and hexanol) is 4629.0 mg COD L−1 for the control, similar to that of 10 or 15 mg PAA per g TSS reactors (Fig. 4c). Whereas, PAA pretreatment at 20 or 25 mg per g TSS significantly decreases LCAs production (p = 0). Similarly, Yin and Wang reported that LCAs production from sewage sludge was repressed by AOP pretreatment [16]. Wang et al. found that FNA pretreatment largely promoted MCFAs production while decreasing LCAs accumulation in the sludge fermentation system supplemented with ethanol [41]. In contrast, Fe3O4 addition was revealed to concurrently promote MCFAs and LCAs generation [8]. Thereby, it could be speculated that PAA pretreatment suppresses the microbes that produce LCAs due to its microbiocidal effect, reducing the conversion of organic carbons to byproducts. Due to PAA pretreatment, MCFAs proportion increases from 44.4% to 56.9%, while LCAs proportion reduces from 28.6% to 4.0% (Fig. 4d). This indicates that PAA pretreatment promotes the carbon specificity towards MCFAs, and thus greatly avoids electron equivalents loss of ED.
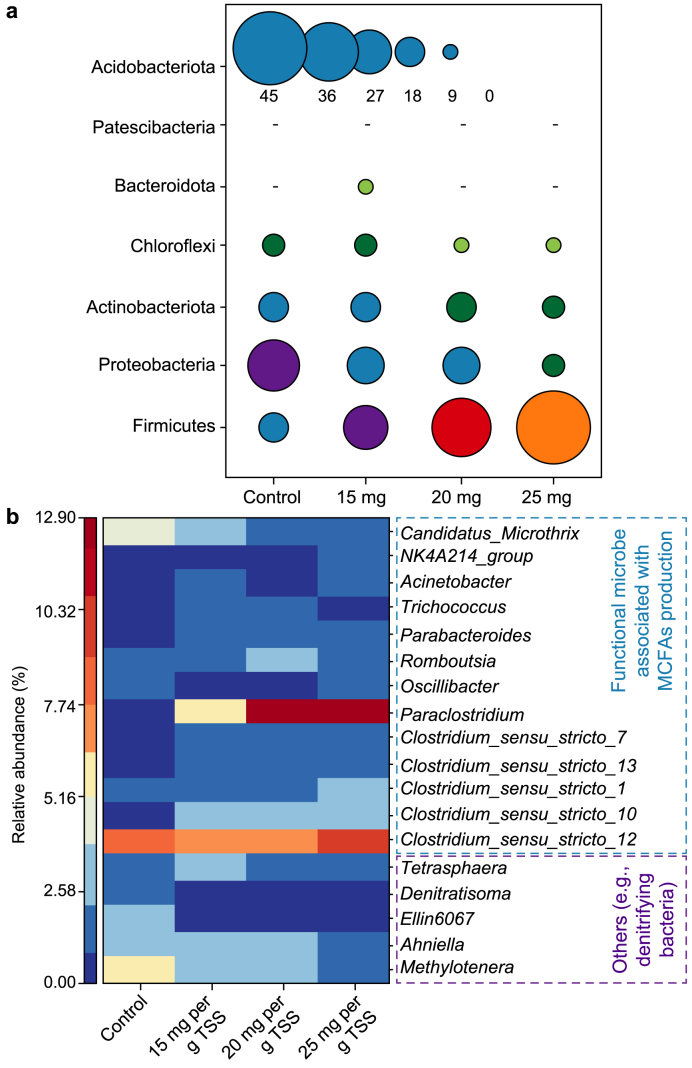
3.3. Microbial community evolutions
Fig. 5 elucidates the community succession of MCFAs-forming bioreactors mediated by PAA pretreatment. Rarefaction curves indicate the reliability of sequencing results (Fig. S2). Alpha diversity analysis suggests that PAA pretreatment has a negligible effect on community richness but increases community diversity (Table S1). As displayed in Fig. 5a, Firmicutes is the predominant phylum in experimental groups, whose abundance is positively associated with PAA dose, increasing from 20.3% (i.e., the control) to 45.0%. Firmicutes can hydrolyze and convert complex macromolecular organics into small molecules, thus promoting substrate transformation and providing EA for CE [12]. Firmicutes were also enriched in anaerobic systems for MCFAs generation [11].
Fig. 5.
Microbial community evolutions of the experimental (i.e., 15, 20, and 25 mg PAA per g TSS) and control reactors. a, Microbial abundance analysis at a phylum level. b, Heatmap of functional microorganisms and typical non-functional bacteria based on a genus level.
Microbial composition at a genus level was further analyzed (Fig. 5b). PAA pretreatment significantly enriches functional microorganisms, i.e., those associated with hydrolysis, acidification, and CE, with their abundances rising from 18.3% to 39.7%. Paraclostridium, positively associated with substrate hydrolysis and MCFAs production [16,42], dominates in experimental groups, i.e., 12.2% for 20 mg PAA per g TSS and 12.9% for 25 mg PAA per g TSS, versus 0.5% in the control. Clostridium is a common SCFAs producer and a typical CE microorganism [43], whose abundance significantly increases with PAA dose, i.e., from 10.0% to 17.9%. Besides, NK4A214_group is related to butyric acid production [44], and Romboutsia is positive to SCFAs generation [11]. Their abundances are also enhanced in experimental groups. On the contrary, some non-functional bacteria (e.g., denitrifying bacteria) are significantly reduced. The enrichment of functional microorganisms is mainly attributed to PAA oxidation, which leads to the removal/inhibition of non-functional microbes and stimulates the metabolism of functional microorganisms by providing more soluble organics. Specifically, sludge floc is fragmented, inhibiting initial microbial activity due to PAA pretreatment. Meanwhile, substantial amounts of dissolved organics are directly available to anaerobes, triggering the revival and enrichment of hydrolytic and SCFAs-producing bacteria that are usually more tolerant to extreme environments [45,46]. Driven by ethanol, EA from sludge and PAA decomposition are elongated to MCFAs by CE microorganisms. Consequently, functional microorganisms are enriched by PAA pretreatment, and more substrates are converted to MCFAs.
3.4. Mechanisms of enhanced MCFAs production from WAS by PAA pretreatment
3.4.1. Reaction mechanisms of PAA oxidation for WAS disintegration
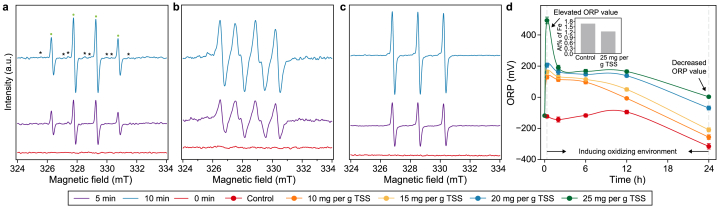
Previous studies reported that PAA led to pollutants degradation mainly by inducing the generation of inorganic radicals (i.e., •OH, •O2−), organic radicals (R–O• (i.e., CH3COO•, CH3•, CH3C(O)O•), and CH3C(O)OO•) and singlet oxygen (1O2), which are strongly oxidative to WAS flocs [47,48]. In Fig. 6a–c, the signal peaks of three adducts, i.e., DMPO−OH, PMPO−O2−, and TEMP−1O2, are found, with intensities detected at 10 min stronger than those at 5 min of reaction. This implies that •OH, •O2−, and 1O2 play important roles in the PAA-based sludge pretreatment system [1,33]. It was indicated the DMPOX signal could be accompanied by DMPO−OH, which might be formed via DMPO oxidation by R–O• and thus closely correlated with the presence of R–O• [49]. Since CH3OO• and CH3• are significantly weaker than CH3C(O)O• and CH3C(O)OO• in terms of oxidation capacity, the former is usually considered to be negligible for PAA-induced AOP [50]. It was recorded that DMPO could be oxidized by R–O• to generate a DMPOX signal [51]. As shown in Fig. 6a, weak DMPOX signals coexist with DMPO−OH adduct, consistent with previous studies, which proves the presence of CH3C(O)O• and CH3C(O)OO• during the pretreatment stage [49]. Fig. 6d reflects the dynamic variations of the redox state in the sludge pretreatment system. The ORP is largely enhanced by PAA-based AOP in the first 20 min, e.g., 493 mV at 25 mg per g TSS, illustrating the strong oxidative capacity of PAA. Then, it gradually decreases and finally approaches 0 or below after 24 h of pretreatment, implying the completion of oxidation reactions. Hence, the introduced PAA may not harm the subsequent fermentation stage.
Fig. 6.
a–c, ESR spectra detected from the PAA group (i.e., 25 mg per g TSS), including DMPO-OH (a), DMPO-O2− (b), and TEMP-1O2 (c). Note: ∗ and • stand for DMPOX and DMPO-OH adducts. d, ORP variations for the control and PAA groups during pretreatment, with the inner bar chart representing Fe element content detected by EDS.
How does PAA lead to active species production without external iron addition? Iron is the commonly existing transition metal in sewage sludge, ranging from 2.9 to 39.7 mg Fe per g TS [22]. It was demonstrated that Fe2+ could activate PAA to promote the degradation of naproxen, bisphenol-A, and methylene blue [52]. EDS results illustrate iron as the dominant transition metal in the control and PAA-pretreated sludge (Table S2). Iron content on the surface of PAA-induced WAS is lower than that of the control (Fig. 6d), attributing to the fact that PAA-induced AOP causes Fe2+ release from EPS and its redistribution in sludge flocs through disrupting sludge structure [53]. Thus, Fe2+ is likely to be the vital substance for PAA activation by WAS. The zeta potential results further indicate that PAA pretreatment increases the positive charge of the centrate liquid (Fig. S3). It can be further inferred that after PAA enters sludge, it is activated by Fe2+ in sludge, inducing a sequence of chemical reactions and strong oxidizing materials. Thereby, relative reaction mechanisms for Fe2+/PAA AOP could be proposed in equations (1)–(7) [48,49,52].
| CH3C(O)OOH + Fe2+ → Fe(III) + •OH + CH3C(O)O− | (1) |
| CH3C(O)OOH + Fe2+ → Fe(III) + OH− + CH3C(O)O• | (2) |
| CH3C(O)OOH + CH3C(O)O• → CH3C(O)OH + CH3C(O)OO• | (3) |
| CH3C(O)OOH + CH3C(O)OO− → CH3C(O)O− + CH3C(O)OH + 1O2 | (4) |
| CH3C(O)OOH + •OH → CH3C(O)OO• + H2O | (5) |
| CH3C(O)OOH + •OH → CH3C(O)OH + HO2• | (6) |
| HO2• → •O2− + H+ | (7) |
Accordingly, these reactive species cause oxidative stress to microorganisms in sludge and lead to the leakage of intracellular organic components [4]. Meanwhile, they disintegrate sludge structure and cause the decomposition and transformation of EPS, which provides abundant soluble substrates directly accessible to anaerobic bacteria and creates looser EPS for further microbial metabolism, facilitating rapid EA (i.e., SCFAs) generation for CE process in the subsequent fermentation stage [15].
3.4.2. Variation in metabolic activities of key bioprocesses
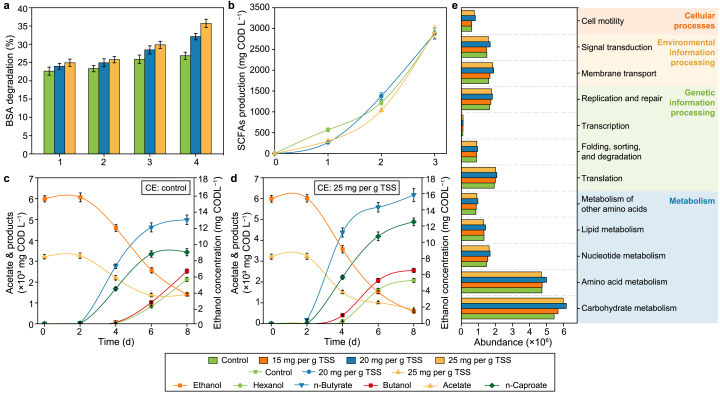
The organic matter released from sludge disintegration undergo hydrolysis, acidification, and CE processes to achieve carbon chain upgrading [8]. Is the microbial metabolic activity of these key processes affected by PAA pretreatment? Thus, the results of synthetic wastewater experiments reveal the potential impact at a macroscopic level (Fig. 7).
Fig. 7.
a, Degradation of BSA during a four-day fermentation. b, Accumulative SCFAs production during a three-day fermentation. c–d, MCFAs production with ethanol and acetate as substrates during an eight-day fermentation for the control (c) and experimental (d) groups. e, Predictions of microbial functions in the control and PAA systems. Error bars represent standard deviations of triplicate experiments.
After four days’ fermentation, the degradation extent of BSA is respectively 32.1% (p = 0.002) and 35.7% (p = 0.001) in the experimental groups of 20 and 25 mg PAA per g TSS, largely greater than the control (i.e., 26.9%) (Fig. 7a). Dextran is almost completely degraded for all groups on day 4, while PAA groups exhibit a lower degradation extent during the initial two-day fermentation (Fig. S4a). These results can be supported by protease and α-glucosidase activities in anaerobic reactors (Fig. S4b). It is implied that PAA might stimulate the metabolic activity of protein-like substrates and inhibit the degradation of polysaccharide-like substrates but generally behave as a facilitator of hydrolytic processes. Although PAA inhibits substrate acidification on day 1, glucose and amino acids of all groups could be completely metabolized after the three-day fermentation (Fig. 7b), indicating the slight effect of PAA pretreatment on the microbial activity of acidification [21].
In contrast, PAA pretreatment promotes ethanol and acetate consumption while increasing butyrate and caproate production (Fig. 7c and d). On day 8, butyrate and caproate are respectively 4984.2 and 3432.1 mg COD L−1 for the control, which are significantly lower than the 25 mg per g TSS group, i.e., 6176.2 (p = 0.006) and 4886.8 (p = 0.001) mg COD L−1. Meanwhile, the yield of LCAs in the experimental group is deducted, suggesting a reduced conversion to byproducts and an improved carbon specificity towards MCFAs [54].
3.4.3. Variation in microbial functions and functional gene expression
The microbial potential functions were predicted by PICRUSt software (Fig. 7e), further elaborating on the changes in microbial metabolism at the genetic level. Generally, 11 of the selected 2-level KEGG pathways are stimulated by PAA pretreatment. The potential functions related to substrate degradation, i.e., carbohydrates and amino acids, are upregulated in PAA-pretreated systems. The higher abundances of carbohydrate and amino acid metabolisms indicate efficient conversion of organics to EA [43]. Additionally, functions of cell motility, membrane transport, and replication and repair affiliated to cellular processes, environmental information, and genetic information are enhanced in PAA reactors.
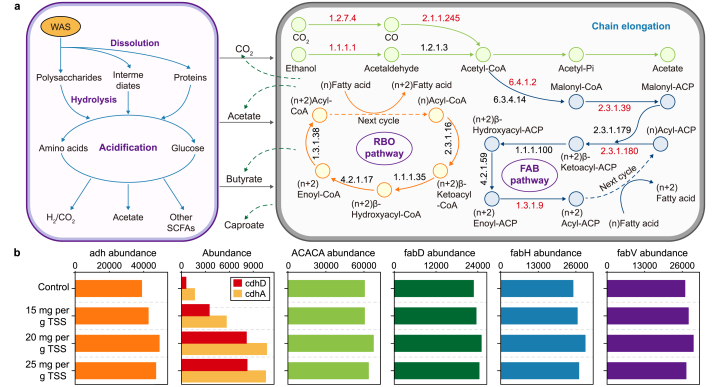
Upgrading of EA (i.e., SCFAs) is achieved by CE microorganisms via two cyclic processes, i.e., fatty acid biosynthesis (FAB) and reverse β-oxidation (RBO) (Fig. 8a) [10]. RBO is started by acetyl-CoA directly, while FAB is initiated by malonyl-CoA, derived from acetyl-CoA carboxylation with ATP expense [16]. The generation of acetyl-CoA and the entire CE pathways involve the participation of functional enzymes [7]. Generally, the genes encoding functional enzymes in the FAB pathway are strengthened in experimental groups and higher than in the RBO pathway (Table S3). Fig. 8b presents the main predictions of functional gene abundances encoding specific enzymes. Acetyl-CoA might be generated from ethanol and H2/CO2, and the former is the main pathway due to its higher abundance in functional enzymes. EC 1.1.1.1 (adh) is significantly enriched, indicating increased ethanol consumption in experimental groups, consistent with the synthetic wastewater experiment (Fig. 7d). Besides, the abundances of EC 1.2.7.4 (cdhA) and EC 2.1.1.245 (cdhD) are increased, and they are associated with acetyl-CoA production from H2/CO2. It implies more endogenous electron donors likely to be produced due to PAA pretreatment. Specifically, there is an increase in EC 6.4.1.2 (ACACA), EC 2.3.1.39 (fabD), EC 2.3.1.80 (fabH), and EC 1.3.1.9 (fabV), which are respectively functional enzymes involved in the generation of malonyl-ACP, and initiation/termination of CE in FAB pathway. It is hinted that PAA facilitates SCFAs upgrading through stimulating ethanol transformation and FAB pathway. These facts expound the positive influence of PAA pretreatment on microbiological metabolism in sludge fermentation systems, further revealing the mechanism of enhanced MCFAs production at a microscopic level.
Fig. 8.
a, Proposed metabolic pathways for WAS degradation and CE with ethanol as ED, with functional enzyme numbers listed and upgrading functional enzymes marked in red. b, Predicted functional enzyme abundances of CE microorganisms from the control, 15, 20, and 25 mg PAA per g TSS groups.
3.5. Implications
Since MCFAs have longer carbon chains, they possess higher energy density and are more hydrophobic compared to SCFAs [6], thus suitable for large-scale industrial production [26]. Accordingly, CE has recently become a growingly attractive anaerobic technology to convert primary fermentation products of WAS, i.e., SCFAs, into MCFAs with ethanol as ED [8]. However, the organic matter of WAS is well protected by EPS and hard cell walls [15]. Thus, pretreatment techniques are usually required. As a green oxidizer and disinfectant, PAA is widely available and easily accessible. PAA-based AOP has been proven powerful for WAS cracking, accumulation of SCFAs, methane, and hydrogen from sludge, sludge dewatering, and pollutants degradation [17,[19], [20], [21],23]. Nevertheless, these studies only focused on the traditional fermentation model or chemical oxidation-based PAA and neglected the underlying mechanisms. This study aims to fill the knowledge gap and broaden the understanding and applicability of PAA-based AOP in CE.
Results show that PAA significantly promotes sludge disruption and increases dissolved material quantities. Further characterization of WAS structure and property illustrates that PAA oxidation causes substantial EPS exfoliation from microbiological indications, disrupts the secondary structure of EPS and solution layer proteins, and enhances the degradation and conversion of macromolecular organic matter (e.g., proteins and polysaccharides). It is confirmed that PAA pretreatment creates a looser EPS and provides abundant accessible substrates for subsequent sludge fermentation. Consequently, MCFAs production is promoted to 11265.6 mg COD L−1, while the byproducts, i.e., LCAs, are decreased to 723.5 mg COD L−1. Mechanistic studies show that the production of free radicals (i.e., •OH, •O2−, CH3C(O)O•, and CH3C(O)OO•) and singlet oxygen (1O2) are the oxidation mechanisms for WAS disruption. However, the oxidative stress induced by PAA largely disappears after 24 h, rather than causing a negative influence on the subsequent bioprocesses. Further, hydrolytic enzyme tests and synthetic wastewater experiments demonstrate at a macroscopic level that PAA stimulates protease activity and enhances the metabolic activity of protein-like substrate degradation and CE process while it causes a slight effect on that of acidification. Additionally, KEGG pathways and functional gene analysis microscopically illustrate that PAA pretreatment up-regulates the functional genes related to substrate degradation and cellular activity, and increases the functional enzyme abundances in ethanol conversion and FAB pathway. These findings provide a systematic and comprehensive understanding of the effectiveness and mechanism for enhanced MCFAs generation by PAA-based AOP.
In this work, PAA is activated by Fe2+ contained in WAS without extra activator addition, and relative reactions occur under mild pH ranges, avoiding the increase of iron-bearing sludge at WWTPs (Fig. S5). The main product of PAA oxidation is acetic acid, which could serve as EA for MCFAs formation. This indicates that PAA has the dual role of cracking sludge and replenishing EA, posing no environmental risk. In contrast, other oxidant-induced AOP, such as calcium peroxide and persulfate [16,55], might cause great risks to the equipment and final sludge disposal due to the detrimental byproduct formation. It could be speculated that PAA would probably contribute to killing potential pathogenic bacteria and degrading micropollutants in WAS due to its powerful oxidizing property, further reducing the environmental risk of sludge. Besides, LCAs concentration is reduced to 723.5 mg COD L−1, clearly inferior to the current reported values [15,27]. It might be attributed to the potential inhibitions of PAA to LCAs microorganisms [16]. Inversely, functional microorganisms are enriched, the metabolic activity of key processes is heightened, and functional enzyme abundances in CE are enhanced. This suggests that the antimicrobial properties of PAA could selectively inhibit LCAs microorganisms, decreasing electron flow to byproducts but improving the conversion of EA and ED to MCFAs. In comparison, MCFAs production triggered by the PAA technique (i.e., 70.4 mg COD per mM ED) is significantly higher than that of other strategies, e.g., Fe2O3 (i.e., 53.9 mg COD per mM ED) [26], Fe3O4 (i.e., 46.8 mg COD per mM ED) [11], combined alkaline fermentation with CE (i.e., 20.2 mg COD per mM ED) [6], free ammonia (i.e., 59.3 mg COD per mM ED) [15], and ferrate pretreatment (i.e., 47.7 mg COD per mM ED) [27]. Despite the multiple advantages of PAA-based technology, this study is currently a proof of concept. Therefore, endeavors should be dedicated to driving the engineering application of this technology, such as combining it with other technologies, e.g., ultrasound radiation, thermal pretreatment, or pH adjustment, to enhance sludge solubilization and reduce PAA dosage [30,48]. The effectiveness of PAA-based AOP for inactivating pathogens and degrading contaminants in fermented sludge also warrants future investigations.
4. Conclusion
This work discloses the positive functions of PAA-based AOP on MCFAs production from sewage sludge. Results exhibit that PAA pretreatment largely disrupts sludge structure and promotes EPS decomposition. Consequently, this leads to a substantial increase in MCFAs production, accompanied by a notable reduction in the formation of undesired byproducts, such as LCAs. Microbial analysis proves functional microbe enrichment. Mechanistic investigations illustrate that sludge disruption is closely related to the generation of free radicals (i.e., •OH, •O2−, CH3C(O)OO•, and CH3C(O)O•) and singlet oxygen (1O2) through PAA activation by Fe2+ in WAS. Furthermore, microbial activity tests demonstrate the positive impacts of PAA pretreatment on protein-like substrate degradation and the CE process. Additionally, CE improvement might be ascribed to functional gene up-regulation in ethanol transformation and FAB pathway.
CRediT authorship contribution statement
Yufen Wang: Investigation, Methodology, Data Curation, Writing - Original Draft, Software, Writing - Review & Editing. Haixiao Guo: Formal Analysis, Investigation, Visualization. Xuecheng Li: Investigation, Methodology, Formal Analysis. Xueming Chen: Conceptualization, Validation. Lai Peng: Writing - Review & Editing, Validation. Tingting Zhu: Methodology, Supervision, Validation. Peizhe Sun: Conceptualization, Supervision, Methodology. Yiwen Liu: Conceptualization, Resources, Writing - Review & Editing, Supervision, Project Administration, Funding Acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This study was partially funded by the National Natural Science Foundation of China through project 52000135. The first author is funded by the Shanghai Tongji Gao Tingyao Environmental Science & Technology Development Foundation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ese.2023.100355.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Guo H., Tian L., Wang Y., Zheng K., Hou J., Zhao Y., Zhu T., Liu Y. Enhanced anaerobic digestion of waste activated sludge with periodate-based pretreatment. Environmental Science and Ecotechnology. 2023;13 doi: 10.1016/j.ese.2022.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Z., Zheng M., Duan H., Yuan Z., Hu S. A 20-year journey of partial nitritation and anammox (PN/A): from sidestream toward mainstream. Environ. Sci. Technol. 2022;56(12):7522–7531. doi: 10.1021/acs.est.1c06107. [DOI] [PubMed] [Google Scholar]
- 3.Zheng M., Li H., Duan H., Liu T., Wang Z., Zhao J., Hu Z., Watts S., Meng J., Liu P., Rattier M., Larsen E., Guo J., Dwyer J., Akker B.V.D., Lloyd J., Hu S., Yuan Z. One-year stable pilot-scale operation demonstrates high flexibility of mainstream anammox application. Water Res. X. 2023;19 doi: 10.1016/j.wroa.2023.100166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y., Zheng K., Guo H., Tian L., He Y., Wang X., Zhu T., Sun P., Liu Y. Potassium permanganate-based advanced oxidation processes for wastewater decontamination and sludge treatment: a review. Chem. Eng. J. 2023;452 [Google Scholar]
- 5.Paulinetti A.P., Augusto I.M.G., Batista L.P.P., Tavares A.G.B., Albanez R., Ratusznei S.M., Lovato G., Rodrigues J.A.D. Anaerobic digestion as a core process for sustainable energy production in the soybean biorefinery: a techno-economic assessment. Sustainable Horizons. 2022;3 [Google Scholar]
- 6.Wu S.-L., Wei W., Sun J., Xu Q., Dai X., Ni B.-J. Medium-Chain fatty acids and long-chain alcohols production from waste activated sludge via two-stage anaerobic fermentation. Water Res. 2020;186 doi: 10.1016/j.watres.2020.116381. [DOI] [PubMed] [Google Scholar]
- 7.He J., Shi Z., Luo T., Zhang S., Liu Y., Luo G. Phenol promoted caproate production via two-stage batch anaerobic fermentation of organic substance with ethanol as electron donor for chain elongation. Water Res. 2021;204 doi: 10.1016/j.watres.2021.117601. [DOI] [PubMed] [Google Scholar]
- 8.Luo T., Xu Q., Wei W., Sun J., Dai X., Ni B.-J. Performance and mechanism of Fe3O4 improving biotransformation of waste activated sludge into liquid high-value products. Environ. Sci. Technol. 2022;56(6):3658–3668. doi: 10.1021/acs.est.1c05960. [DOI] [PubMed] [Google Scholar]
- 9.Wu L., Wei W., Chen Z., Ni B.-J. Medium-chain carboxylate productions through open-culture fermentation of organic wastes. J. Clean. Prod. 2022;373 [Google Scholar]
- 10.Wang J., Yin Y. Biological production of medium-chain carboxylates through chain elongation: an overview. Biotechnol. Adv. 2022;55 doi: 10.1016/j.biotechadv.2021.107882. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y., Hou J., Guo H., Zhu T., Zhang Y., Liu Y. New insight into mechanisms of ferroferric oxide enhancing medium-chain fatty acids production from waste activated sludge through anaerobic fermentation. Bioresour. Technol. 2022;360 doi: 10.1016/j.biortech.2022.127629. [DOI] [PubMed] [Google Scholar]
- 12.Xie J., Xin X., Ai X., Hong J., Wen Z., Li W., Lv S. Synergic role of ferrate and nitrite for triggering waste activated sludge solubilisation and acidogenic fermentation: effectiveness evaluation and mechanism elucidation. Water Res. 2022;226 doi: 10.1016/j.watres.2022.119287. [DOI] [PubMed] [Google Scholar]
- 13.Wu S.-L., Luo G., Sun J., Wei W., Song L., Ni B.-J. Medium chain fatty acids production from anaerobic fermentation of waste activated sludge. J. Clean. Prod. 2021;279 [Google Scholar]
- 14.Tian L., Guo H., Wang Y., Wang X., Zhu T., Liu Y. Urine source separation-based pretreatment: a sustainable strategy for improving methane production from anaerobic digestion of waste activated sludge. Sustainable Horizons. 2022;4 [Google Scholar]
- 15.Wang Y., Wei W., Wu W., Sun J., Xu Q., Wang D., Song L., Ni B.-J. Improving medium-chain fatty acid production from anaerobic fermentation of waste activated sludge using free ammonia. ACS ES&T Engineering. 2021;1(3):478–489. [Google Scholar]
- 16.Yin Y., Wang J. Production of medium-chain carboxylic acids using sewage sludge pretreated by combined Fenton and persulfate oxidation. J. Clean. Prod. 2022;369 [Google Scholar]
- 17.Ao X.-w., Eloranta J., Huang C.-H., Santoro D., Sun W.-j., Lu Z.-d., Li C. Peracetic acid-based advanced oxidation processes for decontamination and disinfection of water: a review. Water Res. 2021;188 doi: 10.1016/j.watres.2020.116479. [DOI] [PubMed] [Google Scholar]
- 18.Chen J., Song T., Long S., Zhu K.J., Pavlostathis S.G. Effect of peracetic acid solution on a nitrifying culture: kinetics, inhibition, cellular and transcriptional responses. Water Res. 2022;219 doi: 10.1016/j.watres.2022.118543. [DOI] [PubMed] [Google Scholar]
- 19.Appels L., Assche A.V., Willems K., Degrève J., Impe J.V., Dewil R. Peracetic acid oxidation as an alternative pre-treatment for the anaerobic digestion of waste activated sludge. Bioresour. Technol. 2011;102(5):4124–4130. doi: 10.1016/j.biortech.2010.12.070. [DOI] [PubMed] [Google Scholar]
- 20.Zhang W., Cao B., Wang D., Ma T., Xia H., Yu D. Influence of wastewater sludge treatment using combined peroxyacetic acid oxidation and inorganic coagulants re-flocculation on characteristics of extracellular polymeric substances (EPS) Water Res. 2016;88:728–739. doi: 10.1016/j.watres.2015.10.049. [DOI] [PubMed] [Google Scholar]
- 21.Li C., Liu X., Du M., Yang J., Lu Q., Fu Q., He D., Zhao J., Wang D. Peracetic acid promotes biohydrogen production from anaerobic dark fermentation of waste activated sludge. Sci. Total Environ. 2022;844 doi: 10.1016/j.scitotenv.2022.156991. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y., Wang X., Zheng K., Guo H., Tian L., Zhu T., Liu Y. Ultrasound-sodium percarbonate effectively promotes short-chain carboxylic acids production from sewage sludge through anaerobic fermentation. Bioresour. Technol. 2022;364 doi: 10.1016/j.biortech.2022.128024. [DOI] [PubMed] [Google Scholar]
- 23.Liu Z., Zhou A., Duan Y., Wang S., Gao Y., Chen X., Cui Z., Guo Z., Yue X. Unraveling the behavior of nitrite on promoting short-chain fatty acids accumulation from waste activated sludge by peracetic acid pretreatment: extracellular polymeric substance decomposition and underlying mechanism. Sci. Total Environ. 2022;841 doi: 10.1016/j.scitotenv.2022.156793. [DOI] [PubMed] [Google Scholar]
- 24.Huang Y., Zhang H., Liu X., Ma B., Huang T. Iron-activated carbon systems to enhance aboriginal aerobic denitrifying bacterial consortium for improved treatment of micro-polluted reservoir water: performances, mechanisms, and implications. Environ. Sci. Technol. 2022;56(6):3407–3418. doi: 10.1021/acs.est.1c05254. [DOI] [PubMed] [Google Scholar]
- 25.APHA . American Public Health Association; Washington, DC: 2005. Standard Methods for the Examination of Water and Wastewater. [Google Scholar]
- 26.Wang Y., He Y., Zheng K., Wei W., Ngo H.H., Guo W., Ni B.-J., Zhu T., Horn H., Liu Y. Ferric oxide stimulates medium-chain carboxylic acids synthesis from waste activated sludge via ethanol-driven chain elongation: mechanisms and implications. J. Clean. Prod. 2023;389 [Google Scholar]
- 27.Wang Y., Wang X., Wang D., Zhu T., Zhang Y., Horn H., Liu Y. Ferrate pretreatment-anaerobic fermentation enhances medium-chain fatty acids production from waste activated sludge: performance and mechanisms. Water Res. 2023;229 doi: 10.1016/j.watres.2022.119457. [DOI] [PubMed] [Google Scholar]
- 28.Yang J., Liu X., Wang D., Xu Q., Yang Q., Zeng G., Li X., Liu Y., Gong J., Ye J., Li H. Mechanisms of peroxymonosulfate pretreatment enhancing production of short-chain fatty acids from waste activated sludge. Water Res. 2019;148:239–249. doi: 10.1016/j.watres.2018.10.060. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y., Zheng K., Guo H., Tong Y., Zhu T., Liu Y. Unveiling the mechanisms of how vivianite affects anaerobic digestion of waste activated sludge. Bioresour. Technol. 2022;343 doi: 10.1016/j.biortech.2021.126045. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y., Zheng K., Ding J., Guo H., Chen X., Zhu T., Sun P., Liu Y. Ultrasonic radiation enhances percarbonate oxidation for improving anaerobic digestion of waste activated sludge. Chem. Eng. J. 2023;457 [Google Scholar]
- 31.Chen J., Pavlostathis S.G. Evaluation of the effect of peracetic acid solution on the performance of a continuous-flow biological nitrogen removal (BNR) system. Chem. Eng. J. 2022;431 [Google Scholar]
- 32.Wang J., Shang K., Guo X., Xia X., Da L. A novel and effective pretreatment to stimulate short-chain fatty acid production from waste activated sludge anaerobic fermentation by ferrous iron catalyzed peracetic acid. J. Chem. Technol. Biotechnol. 2020;95(3):567–576. [Google Scholar]
- 33.Wang Y., Sun P., Guo H., Wang D., Zhu T., Liu Y. Enhancing methane production from anaerobic digestion of waste activated sludge through a novel sodium percarbonate (SPC) pretreatment: reaction kinetics and mechanisms. ACS ES&T Engineering. 2022;2(7):1326–1340. [Google Scholar]
- 34.Yin C., Meng F., Chen G.-H. Spectroscopic characterization of extracellular polymeric substances from a mixed culture dominated by ammonia-oxidizing bacteria. Water Res. 2015;68:740–749. doi: 10.1016/j.watres.2014.10.046. [DOI] [PubMed] [Google Scholar]
- 35.Li Y., Wang D., Yang G., Yuan X., Xu Q., Yang Q., Liu Y., Wang Q., Ni B.-J., Tang W., Jiang L. Enhanced dewaterability of anaerobically digested sludge by in-situ free nitrous acid treatment. Water Res. 2020;169 doi: 10.1016/j.watres.2019.115264. [DOI] [PubMed] [Google Scholar]
- 36.Li Y., Wang D., Yang G., Yuan X., Yuan L., Li Z., Xu Q., Liu X., Yang Q., Tang W., Jiang L., Li H., Wang Q., Ni B. In-depth research on percarbonate expediting zero-valent iron corrosion for conditioning anaerobically digested sludge. J. Hazard Mater. 2021;419 doi: 10.1016/j.jhazmat.2021.126389. [DOI] [PubMed] [Google Scholar]
- 37.Liu Z., Zhou A., Liu H., Wang S., Liu W., Wang A., Yue X. Extracellular polymeric substance decomposition linked to hydrogen recovery from waste activated sludge: role of peracetic acid and free nitrous acid co-pretreatment in a prefermentation-bioelectrolysis cascading system. Water Res. 2020;176 doi: 10.1016/j.watres.2020.115724. [DOI] [PubMed] [Google Scholar]
- 38.Yu Q., Jin X., Zhang Y. Sequential pretreatment for cell disintegration of municipal sludge in a neutral Bio-electro-Fenton system. Water Res. 2018;135:44–56. doi: 10.1016/j.watres.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 39.Li Y., Yang F., Miao S., Wang D., Li Z., Yuan X., Yuan L., Liu Q. Achieved deep-dewatering of dredged sediments by Fe(II) activating persulfate pretreatment: filtrating performance and mechanistic insights. Chem. Eng. J. 2021;405 [Google Scholar]
- 40.Wu B., Ni B.-J., Horvat K., Song L., Chai X., Dai X., Mahajan D. Occurrence state and molecular structure analysis of extracellular proteins with implications on the dewaterability of waste-activated sludge. Environ. Sci. Technol. 2017;51(16):9235–9243. doi: 10.1021/acs.est.7b02861. [DOI] [PubMed] [Google Scholar]
- 41.Wang C., Wang Y., Chen Z., Wei W., Chen X., Mannina G., Ni B.-J. A novel strategy for efficiently transforming waste activated sludge into medium-chain fatty acid using free nitrous acid. Sci. Total Environ. 2023;862 doi: 10.1016/j.scitotenv.2022.160826. [DOI] [PubMed] [Google Scholar]
- 42.Silva Rabelo C.A.B., Camargo F.P., Sakamoto I.K., Varesche M.B.A. Metataxonomic characterization of an autochthonous and allochthonous microbial consortium involved in a two-stage anaerobic batch reactor applied to hydrogen and methane production from sugarcane bagasse. Enzym. Microb. Technol. 2023;162 doi: 10.1016/j.enzmictec.2022.110119. [DOI] [PubMed] [Google Scholar]
- 43.Wu S.-L., Wei W., Xu Q., Huang X., Sun J., Dai X., Ni B.-J. Revealing the mechanism of biochar enhancing the production of medium chain fatty acids from waste activated sludge alkaline fermentation liquor. ACS ES&T Water. 2021;1(4):1014–1024. [Google Scholar]
- 44.Weinert-Nelson J.R., Biddle A.S., Williams C.A. Fecal microbiome of horses transitioning between warm-season and cool-season grass pasture within integrated rotational grazing systems. Animal Microbiome. 2022;4(1):41. doi: 10.1186/s42523-022-00192-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo L., Lu M., Li Q., Zhang J., She Z. A comparison of different pretreatments on hydrogen fermentation from waste sludge by fluorescence excitation-emission matrix with regional integration analysis. Int. J. Hydrogen Energy. 2015;40(1):197–208. [Google Scholar]
- 46.Wang Y., Sun P., Guo H., Zheng K., Zhu T., Liu Y. Performance and mechanism of sodium percarbonate (SPC) enhancing short-chain fatty acids production from anaerobic waste activated sludge fermentation. J. Environ. Manag. 2022;313 doi: 10.1016/j.jenvman.2022.115025. [DOI] [PubMed] [Google Scholar]
- 47.Zhang P., Zhang X., Zhao X., Jing G., Zhou Z. Activation of peracetic acid with zero-valent iron for tetracycline abatement: the role of Fe(II) complexation with tetracycline. J. Hazard Mater. 2022;424 doi: 10.1016/j.jhazmat.2021.127653. [DOI] [PubMed] [Google Scholar]
- 48.Wang J., Wan Y., Ding J., Wang Z., Ma J., Xie P., Wiesner M.R. Thermal activation of peracetic acid in aquatic solution: the mechanism and application to degrade sulfamethoxazole. Environ. Sci. Technol. 2020;54(22):14635–14645. doi: 10.1021/acs.est.0c02061. [DOI] [PubMed] [Google Scholar]
- 49.Lin J., Zou J., Cai H., Huang Y., Li J., Xiao J., Yuan B., Ma J. Hydroxylamine enhanced Fe(II)-activated peracetic acid process for diclofenac degradation: efficiency, mechanism and effects of various parameters. Water Res. 2021;207 doi: 10.1016/j.watres.2021.117796. [DOI] [PubMed] [Google Scholar]
- 50.Dai C., Li S., Duan Y., Leong K.H., Liu S., Zhang Y., Zhou L., Tu Y. Mechanisms and product toxicity of activated carbon/peracetic acid for degradation of sulfamethoxazole: implications for groundwater remediation. Water Res. 2022;216 doi: 10.1016/j.watres.2022.118347. [DOI] [PubMed] [Google Scholar]
- 51.Wang J., Xiong B., Miao L., Wang S., Xie P., Wang Z., Ma J. Applying a novel advanced oxidation process of activated peracetic acid by CoFe2O4 to efficiently degrade sulfamethoxazole. Appl. Catal. B Environ. 2021;280 [Google Scholar]
- 52.Kim J., Zhang T., Liu W., Du P., Dobson J.T., Huang C.-H. Advanced oxidation process with peracetic acid and Fe(II) for contaminant degradation. Environ. Sci. Technol. 2019;53(22):13312–13322. doi: 10.1021/acs.est.9b02991. [DOI] [PubMed] [Google Scholar]
- 53.Guo H., Tian L., Liu S., Wang Y., Hou J., Zhu T., Liu Y. The potent effects of polyoxometalates (POMs) on controlling sulfide and methane production from sewers. Chem. Eng. J. 2023;453 [Google Scholar]
- 54.Contreras-Dávila C.A., Carrión V.J., Vonk V.R., Buisman C.N.J., Strik D.P.B.T.B. Consecutive lactate formation and chain elongation to reduce exogenous chemicals input in repeated-batch food waste fermentation. Water Res. 2020;169 doi: 10.1016/j.watres.2019.115215. [DOI] [PubMed] [Google Scholar]
- 55.Xu Q., Huang Q.-S., Wei W., Sun J., Dai X., Ni B.-J. Improving the treatment of waste activated sludge using calcium peroxide. Water Res. 2020;187 doi: 10.1016/j.watres.2020.116440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.