Summary
Pre-existing anti-human leukocyte antigen (HLA) allo-antibodies constitute a major barrier to transplantation. Current desensitization approaches fail due to ineffective depletion of allo-specific memory B cells (Bmems) and long-lived plasma cells (LLPCs). We evaluate the efficacy of chimeric antigen receptor (CAR) T cells targeting CD19 and B cell maturation antigen (BCMA) to eliminate allo-antibodies in a skin pre-sensitized murine model of islet allo-transplantation. We find that treatment of allo-sensitized hosts with CAR T cells targeting Bmems and LLPCs eliminates donor-specific allo-antibodies (DSAs) and mitigates hyperacute rejection of subsequent islet allografts. We then assess the clinical efficacy of the CAR T therapy for desensitization in patients with multiple myeloma (MM) with pre-existing HLA allo-antibodies who were treated with the combination of CART-BCMA and CART-19 (ClinicalTrials.gov: NCT03549442) and observe clinically meaningful allo-antibody reduction. These findings provide logical rationale for clinical evaluation of CAR T-based immunotherapy in highly sensitized candidates to promote successful transplantation.
Keywords: transplantation, immunotherapy, allo-antibodies, desensitization, rejection, CAR T cells, long-lived plasma cells, CD19, BCMA, TACI
Graphical abstract

Highlights
-
•
CD19+ Bmems and CD19− LLPCs are cellular sources of pre-existing allo-antibodies
-
•
CART-19 + CART-BCMA eliminate Bmems, LLPCs, and allo-antibodies and prevent AMR
-
•
CART-19 + CART-BCMA eliminate allo-antibodies in humans
-
•
Immunotherapies targeting CD19 + BCMA represent promising desensitization strategies
Bmems and LLPCs maintain allo-antibodies that present barriers to transplantation. Zheng et al. demonstrate that CART-19 and CART-BCMA/TACI eliminate these cells and desensitize mice, mitigating transplant rejection. Preliminary results also suggest efficacy in humans. Their study supports clinical evaluation of immunotherapies targeting BCMA and CD19 to desensitize highly sensitized transplant candidates.
Introduction
Pre-existing anti-human leukocyte antigen (HLA) allo-antibodies due to prior pregnancy, blood transfusion, or transplantation deprive many patients of life-saving organ transplantation, leading to significant morbidity and mortality while on transplant waitlists.1,2 Current allo-antibody eradication (desensitization) protocols employing plasmapheresis, intravenous immunoglobulin G (IgG), and anti-CD20 (rituximab) are often ineffective. The lack of efficacy of rituximab may be explained by its limited ability to eradicate tissue-resident memory B cells and/or by its inability to target plasma cells directly.3,4,5 Several approaches directly targeting plasma cells (PCs) or PC-supportive factors including anti-CD38, proteasome inhibitors, anti-BlyS, anti-interleukin-6 (IL-6), and a CXCR4 antagonist have been tested. However, none of these has yet emerged as a standard-of-care for desensitization.6
Chimeric antigen receptor (CAR) T cells targeting CD19 and B cell maturation antigen (BCMA) are highly effective in patients with B cell-derived malignancies and multiple myeloma (MM), respectively. Here, we tested whether these CAR T cells could effectively eliminate physiologic memory B cells (Bmems) and long-lived PCs (LLPCs) to durably deplete allo-antibodies and enable successful transplantation.
Results
Allo-specific clones reside within the B cell and both CD19+ and CD19− PC compartments
Treatment with CD19-targeted CAR T cells (CART-19) is being developed as a treatment for autoimmune diseases and is proving effective at eradicating autoantibodies. However, we previously demonstrated that CART-19 does not deplete vaccine-induced protective antibodies as well as HLA allo-antibodies,7,8 and this has been confirmed by others.9,10,11 We tested this premise in a murine model of skin grafting that promotes robust allo-sensitization. C57BL/6J mice (B6) received two serial skin allografts from BALB/cJ donors (BALB/c). Two weeks after rejection of the second skin graft, B cells, CD19+ PCs, and CD19− PCs were sorted by flow cytometry, and allo-specific cells in each fraction were enumerated by ELISPOT (Figure 1A). As expected, allo-specific B cells were detected (Figures 1B and 1C). Importantly, allo-specific PCs were detected in both CD19+ and CD19− fractions. This finding was consistent with the aforementioned human studies that indicated the importance of a CD19− PC population.
Figure 1.
Allo-specific clones reside in the B cell and both CD19+ and CD19− PC compartments
B6 mice (n = 3/group) were sensitized two serial skin grafts from B6 (isograft control) or BALB/c (allograft) donors.
(A) Two weeks after rejection of the second skin graft, B cells, CD19+ PCs, and CD19− PCs were sorted.
(B) Sorted cells were analyzed by ELISPOT for secretion of IgG specific to BALB/c MHC (H2-Kd, H2-Dd, I-Ad). PCs were assessed directly after sorting, while B cells were activated for 4 days prior to ELISPOT. Representative wells are shown.
(C) Allo-specific cells in each cell compartment as a percentage of plated cells. Error bars indicate mean ± SD. ∗p < 0.05, ∗∗∗p < 0.005.
Validation of murine CAR T cells targeting B cells and PCs
We next developed CAR T cells to target B cells as well as CD19+ and CD19− PC compartments. We constructed a murine CD19-targeted CAR as previously described (CART-19) (Figures 2A and 2B).12 To target murine BCMA, we employed an MM CAR strategy incorporating the BCMA ligand APRIL as the extracellular domain (Figure 2A).13 We selected one construct, hereafter termed APRIL-CAR, that was highly sensitive to target cells with varying levels of BCMA expression (Figures 2A–2C and S1A–S1E). As expected, this CAR targeted mTACI, which is also expressed on PCs (Figure S2). CART-19 eliminated B cells and CD19+ PCs in vitro (Figures S1F–S1J). APRIL-CAR T cells had no impact on B cells but efficiently depleted all PC subsets (Figures S1F–S1J).
Figure 2.
Combination CAR T cells effectively deplete B cells and PCs
(A) Schematic of CARs targeting murine CD19 and BCMA.
(B) Expression of indicated CARs in murine T cells.
(C) CAR T cell-mediated cytotoxicity of K562 cells expressing murine CD19 (mCD19) or murine BCMA (mBCMA).
(D) Schematic of in vivo CAR T cell evaluation.
(E–G) Total B cells (E and F) and PCs (G) were measured 14 days after CAR T infusion (n = 3–5/group).
(H and I) Evaluation of PC subsets in spleen and bone marrow by gating on CD138+TACI+ PCs shown in (G) (n = 3–5/group).
(J) IgG-specific PCs were measured by ELISPOT 14 days after CAR T infusion (n = 6–9/group).
Error bars indicate mean ± SD. Statistical comparisons performed using Mann-Whitney U test, and only significant differences are indicated (∗p < 0.05). All experiments are representative of 3 or more independent replicates.
Next, we evaluated the efficacy of these constructs in vivo in naive B6 mice (Figure 2D). Because ongoing generation of new PCs from the B cell compartment could complicate the analysis of PC depletion, and because our goal was to purge allo-reactive cells from both Bmem and PC compartments, we combined APRIL-CART treatment with CART-19 (Combo-CART).
As expected, CART-19 resulted in robust depletion of total B cells in the spleen and bone marrow (BM) (Figures 2E, 2F, and S3). We then evaluated the depletion and re-emergence of B cells by analysis of spleen and BM at 2 and 6–7 weeks following CAR T cell infusion (Figure S4). CART-19 markedly depleted immature and mature B cell subsets except pre-pro-B cells, consistent with their lack of CD19 expression. 6–7 weeks later, most precursor/immature subsets in BM and spleen had returned to steady state. Levels of most mature subsets were still lower than in untreated mice but were significantly increased compared to the 2 week time point, consistent with deep but transient B cell depletion by CART-19.
Given the critical role of follicular T helper cells (Tfhs) in humoral immunity and the interaction between germinal center (GC) B cells and Tfhs, we also measured the levels of Tfhs following CART treatment (Figure S5A). 2 weeks following CART treatment, there was a trend toward lower Tfh numbers and frequency in mice that received CART-19, consistent with the depletion of GC B cells (Figure S4) and the role of GC B cells in the maintenance of Tfhs (Figure S5B).13,14 This appeared to be specific to Tfhs, as total CD4+ T cells and CD4+CD25+FoxP3+ regulatory T cells were similar between groups (Figures S5C and S5D). However, by week 6–7, as B cells began to repopulate, the number and frequency of Tfh were similar across groups (Figure S5B), and at this point, the GC B cell compartment had also normalized (Figure S4).
Within the PC compartment, CART-19 monotherapy depleted CD19+ PCs but did not eliminate CD19− LLPCs (Figures 2G–2I). The Combo-CART treatment, however, led to robust elimination of all PC subsets (Figures 2G–2J and S3). Together, these data demonstrated a robust pharmacodynamic profile of the Combo-CART treatment in vivo.
CAR T cells targeting CD19 and BCMA effectively eliminate donor-specific antibodies in allo-sensitized mice
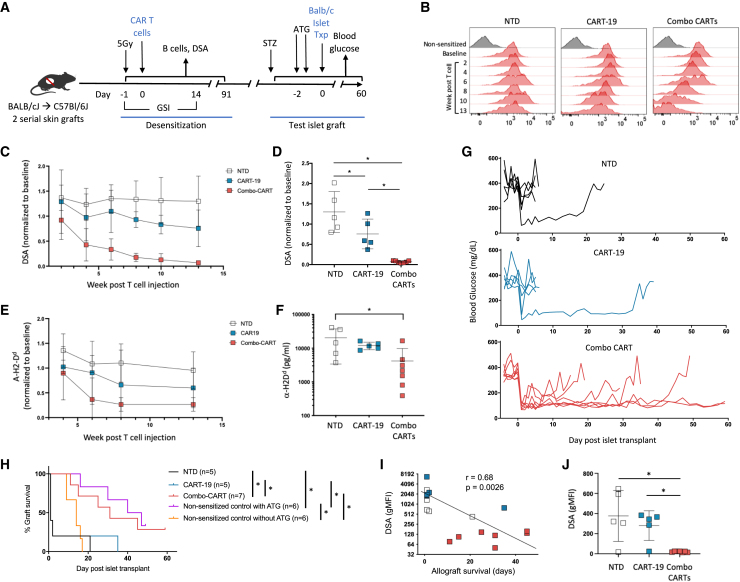
To test the efficacy of the Combo-CART as a desensitization approach, we allo-sensitized B6 mice by serial skin grafts from BALB/c donors (Figure 2A), which induced a durable (>13 weeks) donor-specific allo-antibody response as detected by flow-based T cell crossmatch (FCM) (Figures 3B–3D), B cell FCM (Figure S6), and ELISA (Figures 3E and 3F). After rejection of the second skin graft, mice were randomized to receive C57BL/6J non-CAR T cells (NTD), CART-19, or Combo-CART CAR T cells, and allo-antibodies were monitored over 13 weeks. NTD CAR T cells had no impact on allo-antibody levels (Figures 3B–3F and S6). While mice treated with CART-19 demonstrated a moderate decline in allo-antibodies, Combo-CART-treated mice experienced a robust decline to the level of nonsensitized mice by FCM and a drastic reduction as determined by ELISA (Figures 3B–3F and S6). The detection of some residual reactivity by ELISA could reflect a difference in sensitivity between the two methods and/or may reflect detection of some distinct antibodies due to differentially presented major histocompatibility complex (MHC) epitopes between the two assays. Interestingly, treatment with the APRIL-CART alone also had no significant impact on allo-antibody levels compared to NTD cell treatment (Figure S7), underscoring the need to target both B cell and PC compartments.
Figure 3.
Combination CAR T cells eliminate pre-existing DSAs and promote transplant
(A) Schematic of mouse model evaluating CAR T cells for desensitization (n = 5–7/group).
(B) Representative longitudinal flow crossmatch. Baseline represents the post-sensitization, pre-CART time point.
(C) DSAs by flow crossmatch based on T cell flow crossmatch (post-CART MFIs are normalized to the baseline pre-CART MFI).
(D) DSAs across groups at week 13.
(E and F) Longitudinal (E) and week 13 (F) ELISA-based measurement of serum H2-Dd-specific IgG. Results are representative of 3 replicates. Dotted line indicates nonsensitized mice.
(G) Individual blood glucose following test islet allograft (representative of 2 independent experiments).
(H) Kaplan-Meier analysis of islet allograft survival including control nonsensitized mice without T cell treatment and with and without ATG.
(I) Correlation of pre-transplant DSA and islet allograft survival. Spearman’s rank correlation is −0.68 (95% confidence interval [CI]: −0.51, −0.83) with a corresponding p = 0.0026.
(J) DSAs at time of islet allograft rejection or experimental endpoint (day 60).
All error bars indicate mean ± SD. Statistical comparisons were performed using ANOVA and Tukey’s test (C and E), Mann-Whitney U test (D, F, and J), and log-rank test using Benjamini-Hochberg correction (E), and only significant differences are indicated (∗p < 0.05). In (C), ∗p < 0.05 between Comb-CART vs. NTD (bottom) and Combo-CART vs. CART19.
Thus, we next sought to assess the functional impact of desensitization by Combo-CART. We challenged the recipients with a donor-strain test islet allograft, known to be exquisitely vulnerable to humoral allo-immunity (Figure 3A).15 Since the sensitized mice harbored allo-reactive T cells and we wished to assess the impact of humoral allo-immunity per se, we utilized anti-thymocyte globulin (ATG) as T cell immunosuppression.
The majority of NTD or CART-19 mice demonstrated prompt rejection (primary nonfunction) of islet allografts (Figures 3G and 3H). Combo-CART mice experienced prolonged graft function (median survival time [MST] = 31 days), which was similar to that of nonsensitized mice treated with ATG (MST = 30 days) (Figure 3H). Importantly, the kinetics of graft loss was consistent with acute antibody-mediated rejection as the level of pre-transplant donor-specific allo-antibodies (DSAs) inversely correlated with graft survival (Figure 3I). Allograft rejection in Combo-CART mice was presumably T cell mediated, as histologic analysis revealed robust intraislet T cell infiltration (Figure S8), and serum analysis at the time of rejection or the experimental endpoint (day 60) revealed no detectable DSAs (Figure 3J). Additionally, the variable time to rejection in the Combo-CART mice also suggests that rejection was driven by T cells.
In view of the effective elimination of DSAs by the Combo-CART treatment, it was important to assess the profile of the re-emerging B cells and total Ig in these recipients. Circulating B cells were undetectable at 4 weeks post-CAR T injection but began to return by 6 weeks in the Combo-CART group (Figure 4A). B cell repopulation in CART-19-treated mice was seen by week 8, the delay likely due to better engraftment in the absence of co-delivered APRIL-CAR T cells. By the time of islet graft rejection or experimental endpoint (day 60 post-islet transplant), B cells were detectable in all mice, and levels in the Combo-CART group mice were comparable to the NTD group (Figure 4B). As expected, total IgG declined with a nadir at 6 weeks post-CART injection followed by gradual increase (Figure 4C). At week 13, we measured the levels of all Ig isotypes and IgG subclasses. All Ig isotypes and subclasses were detectable but were still generally lower in Combo-CART mice compared to NTD-treated mice (Figure 4D). Collectively, these data indicating return of total Ig but not DSAs in the Combo-CART group suggest complete eradication of allo-specific Bmem and PC clones. The return of total Ig (lacking allo-specificity) is likely the product of newly emerged/emerging B cell/PC and Tfh clones following loss of CART activity.
Figure 4.
Reconstitution of the humoral immune compartment following CAR T treatment
(A and B) Total circulating B cells at the indicated time points post-CAR T injection (A) and at the time of islet rejection or experiment endpoint (B) from the experiment in Figure 2 (n = 5–7/group).
(C) Total serum IgG was measured at the indicated times relative to CAR T transfer from the experiment in Figure 2.
(D) Individual Ig isotypes and subclasses were measured 13 weeks post-CAR T transfer.
Error bars indicate mean ± SD. All statistical comparisons performed using ANOVA and Tukey’s test (A and B) and Mann-Whitney U test, and only significant differences are indicated (∗p < 0.05). In (A), ∗p < 0.05 between Comb-CART vs. NTD (bottom) and CART19 vs. NTD (top). In (C), ∗p < 0.05 between Comb-CART vs. NTD (bottom), CART19 vs. NTD (middle), and CART19 vs. Combo-CART (top). Results are representative of two independent experiments.
Combination of CAR T cells eliminates pre-existing anti-HLA allo-antibodies in patients
We next sought to assess the translational relevance of our observations in the murine model by evaluating patients with MM harboring pre-existing anti-HLA allo-antibodies who were treated with CART-BCMA and CART-19 following lymphodepleting chemotherapy (LDC) with cyclophosphamide and fludarabine. We used the clinical single-antigen bead assay to screen thirty subjects treated with CART-BCMA with or without CART-19 in a clinical trial16 and found five subjects with pre-existing allo-antibodies (Table S1), two of whom received CART-BCMA monotherapy (subjects 27 and 34) and three who received CART-BCMA and CART-19 (Combo-CART) (subjects 18, 21, and 26) (Figure 5A). All subjects exhibited durable control of MM (Figure 5B) and demonstrated CAR T cell expansion (Figure 5C). The three subjects treated with Combo-CART experienced B cell aplasia ranging from 56 to at least 347 days post-infusion (Figure 5D). Of the two subjects treated with CART-BCMA monotherapy, subject 27 did not experience B cell aplasia and subject 34 did until day 30 (likely due to LDC). It was difficult to interpret PC levels in BM, likely due to technical sampling challenges (Figure S9). Hence, serum soluble BCMA (sBCMA), shed from PC membranes and easily quantified by ELISA, has been used as an indirect measure of PC levels.17 Accordingly, sBCMA initially declined after CAR T cell infusion in all subjects except for subject 27, which may be explained by a ∼10-fold lower expansion of CART-BCMA in this individual (Figure 4E).
Figure 5.
Clinical desensitization after treatment with B cell- and PC-targeted CAR T cells
(A) Schematic of clinical study and CAR T assignment (CART-BCMA and/or CART-19) for the indicated five subjects.
(B) Swimmer’s plot of multiple myeloma response.
(C–F) Longitudinal measurement of (C) CAR T cells, (D) B cells, and (E) soluble BCMA (sBCMA) (F) allo-antibodies. Dotted lines in (F) indicate the laboratory threshold for assignment of unacceptable antigens. PD, progressive disease; MRD, minimal residual disease; PR, partial response; VGPR, very good partial response; CR, complete response; sCR, stringent complete response.
In the three subjects who received Combo-CARTs, HLA allo-antibodies declined by 47%–97% over the course of 12 months following CAR T cell infusion. A subset of representative antibodies are shown in Figure 4F, and values from all detected antibodies are presented in Table S2. Notably, this included allo-antibodies of high baseline mean fluorescence intensity (MFI) levels. In the two subjects (27 and 34) who received CART-BCMA monotherapy, only two antibody specificities with relatively low baseline levels showed moderate decline, while the higher-level allo-antibodies showed no MFI diminution. The decline in all allo-antibodies, including ones with high baseline levels, in subjects treated with the Combo-CART provides preliminary clinical evidence that such an approach may be effective at achieving clinically meaningful desensitization, enabling transplantation. The decline in allo-antibodies in subjects 21 and 26 accompanied a decline in total IgM, IgA, and IgG (Figure S10). We also measured protective IgG against tetanus toxoid, measles, mumps, and rubella and found that these generally mirrored the effect on HLA allo-antibodies (Figure S11), indicating, as expected, that antibody eradication is not antigen specific. All four IgGs declined in subjects 21 and 26 but were stable in subjects 27 and 34. Interpretation of endogenous total and protective IgG levels was not possible in subject 18 due to administration of IVIG starting 3 weeks after CAR T cell infusion.
Discussion
The success of CAR T cell therapy in oncology has spurred the development of engineered T cells for the treatment of autoimmunity, infectious diseases, and organ transplantation. Pre-clinical and clinical evidence of CART-19 cells used to treat lupus strongly support the efficacy, safety, and feasibility of CAR T cells beyond oncology.10,18 We previously showed that CART-19 monotherapy is insufficient to eradicate established antibodies including allo-antibodies in patients.7,8 Interestingly, DiLillo et al. previously reported desensitization in allo-sensitized human CD19 transgenic mice using an anti-CD19 monoclonal antibody (mAb).19 By 10 weeks post-treatment, allo-specific antibody levels were reduced by 59%, comparable to the antibody decline we observed with CART-19 monotherapy in mice. This is consistent with the notion that a population of CD19− LLPCs is able to maintain allo-antibody production.
A study of the fate of HLA allo-antibodies following CART-19 and CART-BCMA monotherapies was recently reported by Hill et al.9 Consistent with our previous report and our data from mice, CART-19 had no effect on antibodies in 4/4 evaluable subjects. However, assessment of the fate of HLA antibodies after CART-BCMA monotherapy was not feasible. Among 28 patients screened, only one subject was found to have pre-existing “low-level” anti-HLA antibodies but had no follow-up samples. In our cohort, there was no significant depletion of allo-antibodies in the two subjects treated with CART-BCMA monotherapy, although conclusive interpretation is limited by the small number of subjects in this cohort. Indeed, effective treatment with CAR T cells targeting BCMA has been shown to deplete protective vaccine- or pathogen-induced antibodies.20 However, the limited effect on B cells by CART-BCMA alone may permit PC repopulation by the Bmem compartment.
Other CAR-based therapies have been developed to modulate alloimmune responses in the setting of transplantation.21 Regulatory T cells (Tregs) expressing CARs specific to allo-MHC have been shown to provide immunosuppression following transplant in pre-clinical models,22,23 but such an approach was ineffective for desensitization.24 Recently two groups reported the use of HLA-based CARs, termed CHARs, to specifically target allo-reactive B cells.25,26 While CHAR T cells were able to lyse allo-specific B cell hybridomas, this approach, on its own, may not be effective given the lack of surface Ig expression in human LLPCs. In this regard, a combination of CHAR-T cells with additional PC-targeted therapy may provide a more selective depletion than the present Combo-CART strategy.
Limitations of the study
In the present article, we have not compared CAR T cells to other PC-depletion interventions. Clinical trials of desensitization have utilized several modalities including proteasome inhibitors (PIs) and the antibody-cleaving enzyme IdeS, as well as a number of biological agents. Future work warrants comparison of these modalities such as engineered T cells, mAbs (e.g., anti-CD38), bispecific T cell engagers (e.g., anti-BCMA x anti-CD3), PIs and IdeS, or combinations thereof. Each has distinct advantages and limitations. Whether CAR T cell platforms provide any advantage over others need to be evaluated in pre-clinical and clinical studies.
Another limitation of our study is that we have not tracked the allo-specific clones following desensitization and then after transplant. Based on the deep depletion of B cells and PCs, we infer that the Combo-CART therapy completely eradicated the allo-specific clones from both B and PC compartments. If this is the case, there may also be an opportunity for induction of humoral immune tolerance to prevent new allo-reactive clones from developing.
Lastly, we have not reported the effect of our CAR T regimens on other allo-antibodies in the mouse model. It is possible that memory to different types of antibody responses (e.g., anti-MHC class I, anti-MHC class II, anti-drug antibodies, vaccines, infections) occupies distinct cellular compartments within the B cell/PC pool and thus would respond differently to monotherapy vs. combination CAR T cell therapy.
The present study establishes a rationale for evaluating immunotherapies targeting BCMA ± CD19 in carefully designed desensitization trials in transplant candidates. Indeed, the efficacy and safety of CAR T cells as well as bispecific T cell-engaging antibodies will be evaluated in clinical trials for desensitization of very highly sensitized patients who face high mortality risk while on the kidney transplant waitlist (ClinicalTrials.gov: NCT05092347 and NCT06056102).
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-mouse CD138 BV421 | Biolegend | Cat#142507; RRID: AB_11204257 |
| Anti-mouse B220 PerCP-Cy5.5 | Thermofisher | Cat#45-0452-80; RRID: AB_906234 |
| Anti-mouse B220 APC | Biolegend | Cat#103212; RRID: AB_312997 |
| Anti-mouse B220 BV605 | Biolegend | Cat#103244; RRID: AB_2563312 |
| Anti-mouse TACI PE | Thermofisher | Cat#12-5942-81; RRID: AB_837121 |
| Anti-mouse CD19 APC-Cy7 | BD Biosciences | Cat#557655; RRID: AB_396770 |
| Anti-mouse CD19 APC | BioLegend | Cat#152410; RRID: AB_2629839 |
| Anti-mouse CD8 PacBlue | Biolegend | Cat#100725; RRID: AB_493425 |
| Anti-mouse CD4 BV785 | Biolegend | Cat#100552; RRID: AB_2563053 |
| Anti-mouse CD3 PE-Cy7 | Biolegend | Cat#100320; RRID: AB_312685 |
| Anti-mouse CD3 FITC | BioLegend | Cat#100204; RRID: AB_312661 |
| Anti-mouse IgD PerCP-Cy5.5 | Biolegend | Cat#405710; RRID: AB_1575113 |
| Anti-mouse CD21/35 BV421 | BD Biosciences | Cat#562756; RRID: AB_2737772 |
| Anti-mouse CD23 BV605 | Biolegend | Cat#101637; RRID: AB_2832279 |
| Anti-mouse CD93-PE | Biolegend | Cat#136504; RRID: AB_1967125 |
| Anti-mouse IgM PE-Cy7 | Thermofisher | Cat#25-5790-82; RRID: AB_469655 |
| Anti-mouse Igκ PE-Cy7 | BD Biosciences | Cat#560667; RRID: AB_1727535 |
| Goat anti-mouse IgM | Jackson | Cat#115-006-020; RRID: AB_2338469 |
| Rat anti-mouse IgM Biotin | Biolegend | Cat#406504; RRID: AB_315054 |
| Goat anti-mouse Ig | SouthernBiotech | Cat#1010-01; RRID: AB_2794121 |
| Goat anti-mouse IgA Biotin | SouthernBiotech | Cat#1040-08; RRID: AB_2794374 |
| Goat anti-mouse IgG1 Biotin | SouthernBiotech | Cat#1070-08; RRID: AB_2794413 |
| Goat anti-mouse IgG2B Biotin | SouthernBiotech | Cat#1090-08; RRID: AB_2794296 |
| Goat anti-mouse IgG2C Biotin | SouthernBiotech | Cat#1079-08; RRID: AB_2794467 |
| Goat anti-mouse IgG3 Biotin | SouthernBiotech | Cat#1100-08; RRID: AB_2794575 |
| Goat anti-mouse Kappa Biotin | SouthernBiotech | Cat#1050-08; RRID: AB_2794383 |
| Goat anti-mouse Lambda Biotin | SouthernBiotech | Cat#1060-08; RRID: AB_2794395 |
| Polyclonal Guinea Pig Anti-Insulin | DAKO | Cat#A0564; RRID:AB_2617169 |
| mouse anti-glucagon | Santa Cruz Biotechnology | Cat#sc-514592; RRID:AB_2629431 |
| donkey anti- guinea pig Cy3 | Jackson | Cat#706-165-148; RRID: AB_2340460 |
| donkey anti-mouse Cy2 | Jackson | Cat#715-225-151; RRID: AB_2340827 |
| Purified anti-mouse H-2Ld/H-2Db Antibody | Biolegend | Cat#114502; RRID: AB_313583 |
| Purified anti-mouse I-Ad Antibody | Biolegend | Cat#115002; RRID: AB_313617 |
| Purified anti-mouse H-2Dd Antibody | Biolegend | Cat#110602; RRID: AB_313483 |
| Purified anti-CD16/32 | Biolegend | Cat# 101302 (also 101301), RRID:AB_312801 |
| Anti-mouse CXCR5 Biotin | eBioScience | Cat#13-7185-82; RRID: AB_2572800 |
| Anti-mouse CD62L BUV395 | BD Biosciences | Cat#740218; RRID: AB_2739966 |
| Anti-mouse CD44 BV605 | Biolegend | Cat#103047; RRID: AB_2562451 |
| Anti-mouse/human CD45R/B220 BV650 | Biolegend | Cat#103241; RRID: AB_11204069 |
| Anti-mouse CD25 AF488 | Thermo Fisher Scientific | Cat# 53-0251-82, RRID:AB_763472 |
| Anti-mouse CD4 PerCP-Cy5.5 | Biolegend | Cat#100540; RRID: AB_893326 |
| Anti-mouse PD-1 PE | Biolegend | Cat#109103, RRID:AB_313420 |
| Anti-mouse FoxP3 PE-Cy7 | Thermo Fisher Scientific | Cat#25-5773-82, RRID:AB_891552 |
| Anti-mouse Bcl-6 AF 647 | BD Biosciences | Cat#561525; RRID: AB_10898007 |
| Bacterial and virus strains | ||
| pTRPE-GFP-T2A-mBCMA | This manuscript | N/A |
| pTRPE-muCD19 | Laboratory of Carl June (UPenn) | N/A |
| MSGV-1D328z(1–3) T2A mCherry | This manuscript | N/A |
| MSGV -mAPRIL28z(1)GFP | This manuscript | N/A |
| MSGV- mAPRIL28z(3)GFP | This manuscript | N/A |
| MSGV- mAPRIL28z(1–3)GFP | This manuscript | N/A |
| MSGV- mAPRIL28zz(1–3)GFP | This manuscript | N/A |
| Biological samples | ||
| Patient sera | Translational and Correlative Studies Laboratory (Center for Cellular Immunotherapies) at UPenn; from NCT03549442 | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Penicillin-Streptomycin | Gibco | Cat#15140122 |
| GlutaMAX | Gibco | Cat#35050061 |
| HEPES | Gibco | Cat#15630080 |
| RPMI 1640 | Gibco | Cat#11875085 |
| NaPyruvate | Gibco | Cat#11360070 |
| Nonessential amino acid | Gibco | Cat#11140035 |
| Recombinant mouse interleukin-2 | R&D Systems | Cat#402-ML |
| Recombinant mouse interleukin-7 | Peprotech Inc. | Cat#217-17 |
| H-2D(d) RGPGRAFVTI Monomer | NIH Tetramer Core Facility at Emory University | N/A |
| I-A(d) RGHNVNGVTAACSHE Monomer | NIH Tetramer Core Facility at Emory University | N/A |
| Streptozotocin | Pharmacia & Upjohn | N/A |
| Anti-thymocyte globulin | Accurate Chemical & Scientific Corporation | Cat#ACL2305 |
| Collagenase P | Roche Diagnostics | Cat#11213873001 |
| Streptavidin-BV421 | Biolegend | Cat#405225 |
| ACK Lysing Buffer | Lonza | Cat#10-548E |
| Critical commercial assays | ||
| mouse CD8+ T cell Isolation Kit | Stemcell technologies | Cat#19853 |
| mouse CD138 Positive Selection Kit | Stemcell technologies | Cat#18957 |
| mouse IgG ELISpot basic kit | Mabtech | Cat#3825-2H |
| CellTrace Violet | Molecular Probes | Cat#C34557 |
| CountBright Absolute Counting Beads | Invitrogen | Cat#C36950 |
| Lipofectamine 2000 | Invitrogen | Cat#11668027 |
| LIVE/DEAD Fixable Aqua Dead Cell Stain Kit | Invitrogen | Cat#L34957 |
| Dynabeads™ Mouse T-Activator CD3/CD28 for T cell Expansion and Activation | Gibco | Cat#11453D |
| 2-Mercaptoethanol | Gibco | Cat#21985023 |
| Fixation/Permeabilization Solution Kit | BD Biosciences | Cat#554714 |
| Foxp3/Transcription Factor Staining Buffer Set | eBioscience | Cat#50-112-8857 |
| Zombie NIR™ Fixable Viability Kit | Biolegend | Cat#423106 |
| Experimental models: Cell lines | ||
| HEK 293T | ATCC | Cat#CRL-3216 |
| K-562 | ATCC | Cat#CCL-243 |
| Platinum-E | Cell Biolabs, Inc. | RV-101 |
| Experimental models: Organisms/strains | ||
| Mouse: C57BL/6J | The Jackson Laboratory | JAX:000664 |
| Mouse: BALB/cJ | The Jackson Laboratory | JAX:000651 |
| Software and algorithms | ||
| FlowJo v10 | FlowJo, LLC | https://www.flowjo.com |
| GraphPad Prism v9.1.0 | GraphPad | https://www.graphpad.com |
| R Foundation for Statistical Computing version 4.0.3 | R project | https://www.r-project.org/ |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Vijay Bhoj (vbhoj@pennmedicine.upenn.edu).
Materials availability
This study generated one lentiviral and four retroviral transfer plasmids encoding mouse BCMA protein and CARs recognizing mouse BCMA with mutated ITAMS. All are available from the lead contact with a completed Materials Transfer Agreement.
Data and code availability
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This study does not report original code.
-
•
Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request.
Experimental model and study participant details
Human subjects
The present study included 5 subjects from a trial at the University of Pennsylvania (ClinicalTrials.gov # NCT03549442). Female and male adult subjects with multiple myeloma treated with CAR T cell therapies were included in this study. Additional demographic information including age, gender, and prior therapies for each subject is provided in Table S1. Written informed consent for participation was obtained from patients according to the Declaration of Helsinki and protocols were approved by the institutional review board at the University of Pennsylvania.
Cell lines
Human embryonic kidney 293T (HEK293T) and K562 cell line was purchased from the American Type Culture Collection. K562 cells were transduced with lentiviruses encoding click-beetle green luciferase followed by lentiviruses encoding mCD19 or mBCMA and were sorted for antigen-positive cells by fluorescence-activated cell sorting (FACS). HEK293T and K562 cells were maintained in complete RPMI 1640 medium (Gibco) + 10% GlutaMAX (Gibco) + 10% fetal bovine serum (FBS; Sigma-Aldrich) + 1% HEPES (Gibco). SF1.1.1 hybridoma (reactive to H-2Kd) and M5 hybridoma (reactive to I-Ab, I-Ad, I-Aq, I-Ed, and I-Ek) were purchased from American Type Culture Collection (ATCC, Manassas, Virginia), cultured according to ATCC recommendations, and used to validate ELISPOT assays.
Mouse model studies
Male and female BALB/cJ and C57BL/6J mice (Jackson Laboratory) between the ages of 8–12 weeks were used for animal studies. Mice were maintained in the animal facility of the University of Pennsylvania in specific pathogen-free rooms and housed in groups under a 12h:12h light:dark cycle, with food and water available ad libitum. The protocol and all animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) of The University of Pennsylvania and performed in accordance with the Guide for the Care and Use of Laboratory Animals prepared by the US National Institutes of Health (NIH).
Method details
CAR T cell production
Spleens were harvested from 8 to 12 weeks donor mice and then mashed through a 40um filter. The filter was then washed with T cell media (TCM, RPMI 1640 medium (Gibco) with 1% GlutaMAX (Gibco), 10% fetal bovine serum (Sigma-Aldrich), 1% HEPES (Gibco), 1% NaPyruvate (Thermo Fisher Scientific), 1% non-essential amino acid (Thermo Fisher Scientific), and 1% 55mM 2-Mercaptoethanol (Gibco)). Red blood cells (RBCs) were lysed using ACK lysis buffer (Gibco). CD8+ T cells were enriched using a mouse CD8+ T cell Isolation Kit (Stemcell technologies) following the manufacturer’s instructions. The T cells were cultured at 1 million/ml in TCM with recombinant mouse interleukin-2 (IL-2, 100 IU/mL, R&D Systems) and interleukin-7 (IL-7, 1 ng/mL, Peprotech Inc.) and CD28/CD3 beads (Thermo Fisher Scientific) that were added at a 1:1 ratio with the CD8+ T cells. Two days later, cells were transduced with retroviral vectors by spinoculation. Retrovirus supernatants were seeded into wells pre-coated with retronectin according to the manufacturer’s instructions (Takara Bio) and centrifuged for 1 h at 2000 rpm. T cells were seeded onto the wells and centrifuged briefly. The next day, cells were counted and passed to a concentration of 0.8 x 106/mL with the addition of IL-2 and IL-7 and some cells were taken for analysis of CAR expression by flow cytometry. The next day, cells were used for in vitro and/or in vivo experiments.
Isolation of CD138+ plasma cells
Spleens were harvested from 8 to 12 weeks donor C57BL/6J (Jackson Laboratory) mice and then mashed through a 40um filter which was washed with TCM. CD138+ plasma cells were enriched using a mouse CD138 Positive Selection Kit (Stemcell technologies), following the manufacturer’s instructions. The yield of CD138+ T cells was about 1x106 per spleen, and ∼70% of the isolated cells expressed CD138. The cells were then used for flow cytometry based in vitro killing assays.
Enzyme-linked Immunosorbent assay (ELISA)
96-well microplate was coated with goat anti-mouse IgM (Jackson ImmunoResearch) or goat anti-mouse Ig (Southern Biotech) and blocked with 2% BSA. Mouse sera was added in 2-fold serial dilutions and incubated for 1 h at room temperature. Detection was achieved with biotinylated rat anti-mouse IgM (Biolegend, RMM-1), goat anti-mouse IgA, goat anti-mouse IgG1, goat anti-mouse IgG2b, goat anti-mouse IgG2c, goat anti-mouse IgG3, goat anti-mouse Kappa/Lambda (all Southern Biotech) or H-2D(d) monomer (folded with RGPGRAFVTI peptide, NIH Tetramer Facility). Biotinylated antibodies were then detected with streptavidin-horseradish peroxidase reagent (BD Biosciences), followed by development with TMB substrate. The developing reaction was stopped with 1M phosphoric acid and read at 450 nM.
Human protective antibodies were measured using the following kits according to the manufacturer’s instructions: anti-measles IgG (Serion Immunodiagnostica GmbH), anti-mumps IgG (Calbiotech), anti-rubella IgG (Phoenix Pharmaceuticals Inc), anti-tetanus toxoid IgG (Serion Immunodiagnostica GmbH). Total IgM, IgA, and IgG were measured by nephelometry by the clinical laboratory at the Hospital of the University of Pennsylvania.
Enzyme-linked immune absorbent spot (ELISpot)
Quantification of total IgG-producing B cells/PCs was performed with mouse IgG ELISpot basic kit (Mabtech). After red cell lysis, 1x105 splenocytes or bone marrow cells were seeded in ethanol pre-blocked PVDF plates. The plates were incubated for 16 h at 37°C before being developed in accordance with the manufacturer’s instructions. Antibody secreting cells (ASC) in each experiment were counted using a CTL ImmunoSpot S6 Ultra M2 analyzer (CTL Analyzers LLC). For B cell activation, B-poly-S (Cellular Technologies, Shaker Heights, OH). diluted 1:1000 in R10 was used to culture the cells for 4 days at 37°C in 5% CO2.
Allo-specific B/PC ELISPOT was performed as follows. On the day of the assay, plates were washed twice with PBS and blocked with complete culture media for at least 1 h at room temperature. The blocking solution was then removed and cells were added at 500 to 500,000 per well depending on the optimal cell number defined empirically for each antigen. The plates were incubated for 16–24 h at 37°C in 5% CO2. Plates were developed by first washing twice with sterile PBS, then twice with PBST(0.05% Tween 20 in PBS). To measure total IgG, a biotinylated anti-IgG antibody (Cellular Technology) was added at 1 μg/ml diluted in PBS/0.5% FBS. To measure allo-antigen-specific responses, a cocktail of biotinylated MHC proteins (H-2Dd, H-2Kd, I-Ad) was added at 2 μg/ml/protein. The plates were then incubated at room temperature for 2 h, washed twice with PBST before the addition of streptavidin-CTL-Red (Cellular technology limited) diluted 1:1000 with PBS/0.5% FBS. The plates were incubated for 1 h at room temperature, then washed twice with sterile water. The plates were allowed to air dry before being counted on the CTL ImmunoSpot Analyzer (Cellular Technologies).
Flow crossmatch
1x106 donor splenocytes were incubated with recipient serum, diluted 1/16, for 45 min on ice. After incubation, splenocytes were washed 3 times with FACS buffer and incubated with anti-CD16/32 antibody (BioLegend) for 5min. After Fc receptor blocking, samples were stained with antibodies to CD3, B220, and Igκ and incubated on ice for 20min. The MFI was assessed on CD3+B220- T cells and CD3-B220 + B cells was analyzed to represent DSA levels. Normalization was performed by dividing MFI of each post-CART sample by the MFI of pre-CART FCM (sample obtained 1 day prior to TBI), which is post-sensitization. All samples across timepoint from each mouse were run together as batch-testing.
Cytotoxicity assays
Flow based cytotoxicity assays were used with primary plasma cells and splenocytes. Target cells were incubated with T cells at an effector to target ratio of 10:1 for 18–24 h. Cells were then stained with a viability dye and antibodies against CD138, TACI, B220, CD19. Counting beads were added before analysis by flow cytometry to quantify the number of live PCs and B cells remaining in cultures. Luciferase-based cytotoxicity assays using K562 cells were performed by coculture of luciferase expressing target cells with differing amounts of transduced T cells. After 4-6h of incubation at 37°C, luciferin was added to each well including wells with target cells only and plates were read using a Synergy H4 plate reader (Agilent). The percent specific lysis was calculated using the formula: % specific lysis = 100 × (1 - experimental/no T cell control). All data are presented as a mean ± standard deviation of triplicate wells.
HLA alloantibody measurement
Pre-CART baseline neat (undiluted) serum was screened for the presence of anti-HLA alloantibodies using the LABScreen Single Antigen HLA Class I and Class II kits from One Lambda Inc. (Thermo Fisher, West Hills, CA). Five subjects were identified with pre-existing anti-HLA alloantibodies and, thereafter, baseline and post-CAR T serum samples were tested neat using the relevant class-I or class-II single antigen bead assay. Samples from subject 18 who received monthly IVIG were spaced at least 2 weeks away from last IVIG dose.
Skin sensitization
12-15-week BALB/cJ female mice (Jackson Laboratory) were used as skin graft donors for C57BL/6J recipients. The mice were euthanized and shaved prior to skin harvest. The harvested skin was cut into pieces around 1 cm2 and kept in normal saline on ice. The thorax and back area of the mice were shaved and sterilized with iodine, followed by an alcohol wipe. The grafts were secured to C57BL/6J recipient anesthetized recipient mice with four absorbable sutures and tissue glue after the removal of recipient skin. All allografts were monitored daily by visual and manual inspection.
Induction of diabetes and islet transplantation
Immune-competent 8–12 weeks old female BALB/cJ and C57BL/6J (Jackson Laboratory) were used as islet donors and recipients, respectively. Recipients C57BL/6J mice were rendered diabetic by intraperitoneal injection of streptozotocin (STZ) (Tera Parenteral Medicines, Inc.) at a dose of 200 mg/kg. Five days after STZ administration, animals with three consecutive (daily) non-fasting blood glucose levels >350 mg/dL were used as islet recipients. At day three and day one prior to islet transplant, recipient mice received two doses of 0.3mL anti-thymocyte globulin (ATG) (Accurate Chemical & Scientific Corp) intraperitoneal injection.
Mouse pancreatic islet isolation was performed by collagenase P (Roche Diagnostics) digestion and density gradient separation. Islets were maintained in a suspension of RPMI-1640 medium containing 10% FBS and 2% pen/strep. Recipient mice were anesthetized by inhalation of 2–5% isoflurane (Isoflurane USP, Clipper Distribution). After swabbing shaved skin area with Betadine and Alcohol, a small skin and muscle incision (1.0–1.5 cm) was performed over the left flank of the mouse to access the left kidney. Hand-picked 500 islets were injected with a 27-gauge butterfly needle underneath the left renal capsule. The incision was closed with 4-0 absorbable suture and 1mL normal saline was administrated subcutaneously after surgery. Blood glucose levels were monitored daily for 2 weeks post-operation and then every other day until euthanasia or experimental endpoint.
Immunohistochemistry, and immunofluorescence
Tissue samples were fixed in 10% formalin solution, and paraffin blocks were cut into 5-mm sections. For immunofluorescence staining, sections were prepared and stained using guinea pig anti-insulin (DAKO, Carpinteria, CA) and mouse anti-glucagon (Santa Cruz Biotechnology, Dallas, Texas). Detection was performed with the secondary antibody donkey anti-guinea pig Cy3 and donkey anti-mouse Cy2 (Jackson, West Grove, PA) for 20 min at room temperature. Slides were mounted using ProLong Gold reagent with DAPI. Immunohistochemistry for CD4, CD8, CD19 (Abcam, Cambridge, MA) and CD138 (BD Biosciences, San Jose, CA), staining was carried out using the Vector ImmPRESS Polymer System, Peroxidase (HRP) Substrate After de-paraffinization, antigen retrieval was carried out by boiling the slides in tris-based, pH 9 (Vector, Burlingame, CA) for 20 min. All antibodies at optimal dilution were incubated overnight at 4°C. Slides were then incubated with anti-rabbit or Rat HRP polymer for 30 min at room temperature followed by DAB+ substrate-chromogen solution for 5 min at room temperature. Slides were counterstained with hematoxylin and mounted.
Quantification and statistical analysis
Graphs were constructed using R (R Foundation for Statistical Computing, version 4.0.3). Statistical methods used are indicated in figure legends. Where applicable, data with error bars indicate mean ± SD. Values of p < 0.05 were considered statistically significant. Some figures were created using BioRender.com.
Acknowledgments
The authors thank Mary Kaminski and Drs. Avery Posey, Don L. Siegel, Binod Kumar, and Qian Zhang for advice and helpful discussion. Z.Z. hopes that through this article, he can pay deepest tribute to the late Dr. Jianfeng Zhou and express gratitude for his unwavering support. The authors also thank the following funding sources: the National Blood Foundation (V.G.B.), the Burroughs Wellcome Fund (V.G.B.), NIH/NHLBI (R01 HL137335-01A1; V.G.B., B.S.-J., M.C.M.), and NIH/NIAID (U01 AI163087-01; V.G.B., A.N., A.L.G., M.C.M.).
Author contributions
Conceptualization, V.G.B., A.N., M.C.M., B.S.-J., and A.L.G.; methodology, Z.Z., C.M., M.Y., S.R., J.J.K., K.P., W.W., C.L., V.G.B., A.N., M.C.M., B.S.-J., and A.L.G.; validation, Z.Z., C.M., V.G.B., and A.N.; formal analysis, A.N., V.G.B., Z.Z., and D.A.; investigation, V.G.B., Z.Z., C.M., M.Y., S.R., W.W., C.L., K.P., X.X., H.Z., E.L., J.J.K., T.O., V.G.B., B.M., J.H., and R.K.; resources, J.S.; writing – original draft, Z.Z., A.N., and V.G.B.; writing – review & editing, M.Y., D.A., J.J.K., J.A.F., B.S.-J., D.M., A.L.G., Z.Z., A.N., and V.G.B.; visualization, Z.Z., C.M., W.W., C.L., A.L.G., D.A., A.N., and V.G.B.; supervision, D.A., J.A.F., B.S.-J., M.C.M., D.M., A.N., and V.G.B.
Declaration of interests
J.A.F. is an inventor on patents in the field of T cell therapy for cancer, from which he has received royalties. J.A.F. is also a member of the scientific advisory boards of Cartography Bio. and Shennon Biotechnologies, Inc. M.C.M. is an inventor on patents pending or granted related to CAR T cell technology that are assigned to the University of Pennsylvania and licensed to Novartis Ag, from which he receives royalties; these include patents on the CAR T cell therapies described in this study (PCT/US2011/064191, PCT/US2018/061239, PCT/US2018/063255). M.C.M. is also a founder, stockholder, and co-chair of the scientific advisory boards for Cabaletta Bio and Verismo Therapeutics. A.L.G. has received grants from Novartis, grants from National Institutes of Health, and grants from the Leukemia & Lymphoma Society while this study was conducted, grants and personal fees from Janssen, personal fees from GlaxoSmithKline, personal fees from Legend Biotech, grants from CRISPR Therapeutics, grants from Tmunity Therapeutics, personal fees from Amgen, grants from the Leukemia & Lymphoma Society, and grants from National Institutes of Health outside the submitted work; in addition, A.L.G. has a patent for US15/757,123 pending and licensed to Novartis, a patent for US16/764,459 pending, and a patent for US16/768,260 pending and stock ownership in Cabaletta Bio. V.G.B. is an inventor on patents pending or granted related to CAR T cell technology that are assigned to the University of Pennsylvania and licensed to Cabaletta Bio and Tmunity, from which he receives royalties.
Published: December 19, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2023.101336.
Contributor Information
Ali Naji, Email: ali.naji@pennmedicine.upenn.edu.
Vijay G. Bhoj, Email: vbhoj@pennmedicine.upenn.edu.
Supplemental information
References
- 1.Colvin M.M., Cook J.L., Chang P.P., Hsu D.T., Kiernan M.S., Kobashigawa J.A., Lindenfeld J., Masri S.C., Miller D.V., Rodriguez E.R., et al. Sensitization in Heart Transplantation: Emerging Knowledge: A Scientific Statement From the American Heart Association. Circulation. 2019;139:e553–e578. doi: 10.1161/CIR.0000000000000598. [DOI] [PubMed] [Google Scholar]
- 2.Schinstock C.A., Smith B.H., Montgomery R.A., Jordan S.C., Bentall A.J., Mai M., Khamash H.A., Stegall M.D. Managing highly sensitized renal transplant candidates in the era of kidney paired donation and the new kidney allocation system: Is there still a role for desensitization? Clin. Transplant. 2019;33 doi: 10.1111/ctr.13751. [DOI] [PubMed] [Google Scholar]
- 3.Audia S., Samson M., Guy J., Janikashvili N., Fraszczak J., Trad M., Ciudad M., Leguy V., Berthier S., Petrella T., et al. Immunologic effects of rituximab on the human spleen in immune thrombocytopenia. Blood. 2011;118:4394–4400. doi: 10.1182/blood-2011-03-344051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crickx E., Chappert P., Sokal A., Weller S., Azzaoui I., Vandenberghe A., Bonnard G., Rossi G., Fadeev T., Storck S., et al. Rituximab-resistant splenic memory B cells and newly engaged naive B cells fuel relapses in patients with immune thrombocytopenia. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abc3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su H., Zhang C.Y., Lin J.H., Hammes H.P., Zhang C. The Role of Long-Lived Plasma Cells in Antibody-Mediated Rejection of Kidney Transplantation: An Update. Kidney Dis. 2019;5:211–219. doi: 10.1159/000501460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwal D., Allman D., Naji A. Novel therapeutic opportunities afforded by plasma cell biology in transplantation. Am. J. Transplant. 2020;20:1984–1991. doi: 10.1111/ajt.15813. [DOI] [PubMed] [Google Scholar]
- 7.Bhoj V.G., Arhontoulis D., Wertheim G., Capobianchi J., Callahan C.A., Ellebrecht C.T., Obstfeld A.E., Lacey S.F., Melenhorst J.J., Nazimuddin F., et al. Persistence of long-lived plasma cells and humoral immunity in individuals responding to CD19-directed CAR T-cell therapy. Blood. 2016;128:360–370. doi: 10.1182/blood-2016-01-694356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Z., Schuster S.J., Lacey S.F., Milone M.C., Monos D., Bhoj V.G. Stable HLA antibodies following sustained CD19+ cell depletion implicate a long-lived plasma cell source. Blood Adv. 2020;4:4292–4295. doi: 10.1182/bloodadvances.2020002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill J.A., Kiem E.S., Bhatti A., Liu W., Keane-Candib J., Fitzpatrick K.S., Boonyaratanakornkit J., Gardner R.A., Green D.J., Maloney D.G., et al. Anti-HLA antibodies in recipients of CD19 versus BCMA-targeted CAR T-cell therapy. Am. J. Transplant. 2023;23:416–422. doi: 10.1016/j.ajt.2022.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mackensen A., Müller F., Mougiakakos D., Böltz S., Wilhelm A., Aigner M., Völkl S., Simon D., Kleyer A., Munoz L., et al. Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nat. Med. 2022;28:2124–2132. doi: 10.1038/s41591-022-02017-5. [DOI] [PubMed] [Google Scholar]
- 11.Walti C.S., Krantz E.M., Maalouf J., Boonyaratanakornkit J., Keane-Candib J., Joncas-Schronce L., Stevens-Ayers T., Dasgupta S., Taylor J.J., Hirayama A.V., et al. Antibodies against vaccine-preventable infections after CAR-T cell therapy for B cell malignancies. JCI Insight. 2021;6 doi: 10.1172/jci.insight.146743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kochenderfer J.N., Yu Z., Frasheri D., Restifo N.P., Rosenberg S.A. Adoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cells. Blood. 2010;116:3875–3886. doi: 10.1182/blood-2010-01-265041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baumjohann D., Preite S., Reboldi A., Ronchi F., Ansel K.M., Lanzavecchia A., Sallusto F. Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity. 2013;38:596–605. doi: 10.1016/j.immuni.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 14.Yusuf I., Stern J., McCaughtry T.M., Gallagher S., Sun H., Gao C., Tedder T., Carlesso G., Carter L., Herbst R., Wang Y. Germinal center B cell depletion diminishes CD4+ follicular T helper cells in autoimmune mice. PLoS One. 2014;9 doi: 10.1371/journal.pone.0102791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naji A., Barker C.F., Silvers W.K. Relative vulnerability of isolated pancreatic islets, parathyroid, and skin allografts to cellular and humoral immunity. Transplant. Proc. 1979;11:560–562. [PubMed] [Google Scholar]
- 16.Garfall A.L., Cohen A.D., Susanibar-Adaniya S.P., Hwang W.-T., Vogl D.T., Waxman A.J., Lacey S.F., Gonzalez V.E., Fraietta J.A., Gupta M., et al. Anti-BCMA/CD19 CAR T Cells with Early Immunomodulatory Maintenance for Multiple Myeloma Responding to Initial or Later-Line Therapy. Blood Cancer Discov. 2023;4:118–133. doi: 10.1158/2643-3230.BCD-22-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seipel K., Porret N., Wiedemann G., Jeker B., Bacher V.U., Pabst T. sBCMA Plasma Level Dynamics and Anti-BCMA CAR-T-Cell Treatment in Relapsed Multiple Myeloma. Curr. Issues Mol. Biol. 2022;44:1463–1471. doi: 10.3390/cimb44040098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kansal R., Richardson N., Neeli I., Khawaja S., Chamberlain D., Ghani M., Ghani Q.U.A., Balazs L., Beranova-Giorgianni S., Giorgianni F., et al. Sustained B cell depletion by CD19-targeted CAR T cells is a highly effective treatment for murine lupus. Sci. Transl. Med. 2019;11 doi: 10.1126/scitranslmed.aav1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DiLillo D.J., Griffiths R., Seshan S.V., Magro C.M., Ruiz P., Coffman T.M., Tedder T.F. B lymphocytes differentially influence acute and chronic allograft rejection in mice. J. Immunol. 2011;186:2643–2654. doi: 10.4049/jimmunol.1002983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y., Li C., Xia J., Li P., Cao J., Pan B., Tan X., Li H., Qi K., Wang X., et al. Humoral immune reconstitution after anti-BCMA CAR T-cell therapy in relapsed/refractory multiple myeloma. Blood Adv. 2021;5:5290–5299. doi: 10.1182/bloodadvances.2021004603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muralidharan K., Agarwal D., Naji A., Bhoj V.G. Therapeutic Opportunities for Immunoreceptor-Engineered T Cell Therapy for Modulation of Alloimmunity. J. Immunol. 2022;209:1811–1816. doi: 10.4049/jimmunol.2200542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dawson N.A., Lamarche C., Hoeppli R.E., Bergqvist P., Fung V.C., McIver E., Huang Q., Gillies J., Speck M., Orban P.C., et al. Systematic testing and specificity mapping of alloantigen-specific chimeric antigen receptors in regulatory T cells. JCI Insight. 2019;4 doi: 10.1172/jci.insight.123672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacDonald K.G., Hoeppli R.E., Huang Q., Gillies J., Luciani D.S., Orban P.C., Broady R., Levings M.K. Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J. Clin. Invest. 2016;126:1413–1424. doi: 10.1172/JCI82771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sicard A., Lamarche C., Speck M., Wong M., Rosado-Sánchez I., Blois M., Glaichenhaus N., Mojibian M., Levings M.K. Donor-specific chimeric antigen receptor Tregs limit rejection in naive but not sensitized allograft recipients. Am. J. Transplant. 2020;20:1562–1573. doi: 10.1111/ajt.15787. [DOI] [PubMed] [Google Scholar]
- 25.Betriu S., Rovira J., Arana C., García-Busquets A., Matilla-Martinez M., Ramirez-Bajo M.J., Bañon-Maneus E., Lazo-Rodriguez M., Bartoló-Ibars A., Claas F.H.J., et al. Chimeric HLA antibody receptor T cells for targeted therapy of antibody-mediated rejection in transplantation. HLA. 2023;102:449–463. doi: 10.1111/tan.15156. [DOI] [PubMed] [Google Scholar]
- 26.Gille I., Hagedoorn R.S., van der Meer-Prins E.M.W., Heemskerk M.H.M., Heidt S. Chimeric HLA antibody receptor T cells to target HLA-specific B cells in solid organ transplantation. HLA. 2023;102:436–448. doi: 10.1111/tan.15146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This study does not report original code.
-
•
Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request.