Summary
Allergen-specific immunotherapy (AIT) has shown beneficial effects against atopic dermatitis (AD); however, the mechanisms and parameters underlying the efficacy of AIT remain unclear. Here, we report that the community structure and function of the oral and gut microbiota are changed in patients with AD undergoing AIT. Transplantation of fecal microbiota from patients who respond well to AIT improves AD-like dermatitis in mice. The abundance of Brevundimonas vesicularis in the gut of AD patients has been found to be positively correlated with disease severity and is decreased following AIT. Furthermore, we find that B. vesicularis from the oral cavity might ectopically colonize the gut of AD patients. In AD model mice, meanwhile, B. vesicularis promotes the skewing of the Treg/Th17 balance toward Th17 polarization and attenuates the efficacy of ovalbumin-specific immunotherapy. Our findings provide potential strategies for the optimization of AIT for AD via the modulation of the gut microbiota.
Keywords: atopic dermatitis, allergen-specific immunotherapy, microbiota, oral cavity, gut
Graphical abstract

Highlights
-
•
The oral and gut microbiota are changed in patients with AD undergoing AIT
-
•
B. vesicularis in the oral and gut microbiota is decreased following AIT
-
•
Oral B. vesicularis may ectopically colonizes the gut of patients with AD
-
•
B. vesicularis promotes Th17 polarization and attenuates the efficacy of AIT
Liu et al. reveal the alteration of oral and gut microbiota in patients with AD undergoing AIT and identify a specific microbe, Brevundimonas vesicularis, that acts as an aggravating factor in AD-induced inflammation and influences the efficacy of AIT.
Introduction
Atopic dermatitis (AD) is a chronic, inflammatory skin disease that affects 10%–30% of children and 3%–5% of adults worldwide.1,2 AD is characterized by a T helper 2 (Th2)-dominant inflammatory immune deviation with differential inflammation-related contributions from other Th-type cells, such as Th17, Th22, and Th1, and Asian AD patients are characterized by increased infiltration of Th17 inflammation,3,4,5 whereas the function of regulatory T cells (Tregs) is impaired in AD.6,7 Although the introduction of many novel drugs, such as biologics and JAK inhibitors, has greatly increased the efficacy of therapy, the treatment of this condition remains difficult, and patients often relapse if therapy is discontinued. Thus, strategies that can reverse the immune deviation and modulate the pathophysiology of AD are urgently needed. Allergen-specific immunotherapy (AIT) can change the progression of allergic diseases and has demonstrated therapeutic efficacy against AD in several clinical trials.8,9,10,11 The mechanism may involve the regulation of the balance between allergen-specific immunoglobulin E (IgE) and IgG4 and/or the induction of regulatory T cells (Tregs).12,13 However, the therapeutic efficacy of AIT against AD varies among patients, and the parameters influencing the efficacy of AIT need to be further explored.
It has been established that the gut microbiome is involved in the pathogenesis of AD, especially in children.14,15,16,17 Compared with healthy individuals, patients with AD have lower gut microbiota diversity18,19 but a greater abundance of several microbes, such as Escherichia coli, Staphylococcus, and Blautia.16,20,21 Probiotics have been used for the alleviation of AD for decades, with combinations of several strains of Lacticaseibacillus and/or Bifidobacterium having demonstrated both therapeutic and preventive effects.22,23 Gut and oral microbiota have been associated with the efficacy of AIT in several allergic diseases. Gut microbiota diversity is increased during sublingual immunotherapy (SLIT) in peanut-allergic adults,24 while a high ratio of the salivary bacterium Prevotella is associated with clinical remission in patients with pollinosis who undergo SLIT.25 The gut microbiota can modify the levels of short-chain fatty acids (SCFAs) in the gut and regulate Treg induction, thus participating in the pathogenesis of several inflammatory diseases.26,27 However, neither the precise role of the gut microbiome in AD and AIT nor the underlying mechanisms have been fully explored to date.
The ectopic colonization of the gut by oral microbiota is a common phenomenon in both physiological and pathophysiological conditions.28,29 Interactions between the oral and gut microbiota can reshape the microbial ecosystem of the intestine and finally modulate physiological functions, which is referred to as the “oral-gut axis.”30 It has been reported that the ectopic colonization of the intestine by Klebsiella spp. from the oral cavity contributes to the exacerbation of inflammatory bowel disease.31 Nevertheless, the associated mechanisms remain unclear, although the results of some studies have suggested that microbes from the oral cavity induce the production of pro-inflammatory cells and mediators, thus modulating the immune responses.31,32 We have previously demonstrated that the site specificity of the oral and gut microbiota is decreased in patients with AD compared with that in healthy individuals21; however, whether microbial translocation occurs between the oral cavity and the gut in these patients remains unknown.
In this study, we explored the involvement of the oral and gut microbiota in AIT for AD. We found that the community structure and function of the oral and gut microbiota were altered in AD patients undergoing AIT. Brevundimonas vesicularis in the gut was related to the disease severity and B. vesicularis from the oral cavity colonized the gut of patients with AD. In AD model mice, meanwhile, intragastric B. vesicularis administration exacerbated AD-like dermatitis and promoted the skewing of the Treg/Th17 balance toward Th17 polarization, consequently attenuating the efficacy of ovalbumin (OVA)-specific immunotherapy against AD.
Results
The improvement in AD following AIT is accompanied by alterations in the oral and gut microbiota
We first explored the effects of house dust mite (HDM)-specific AIT on AD (Figure 1A). Patient information at baseline and post treatment is presented in Table S1. After 6 months of treatment, the Scoring Atopic Dermatitis (SCORAD) index and Eczema Area and Severity Index (EASI) scores of patients with AD were significantly decreased compared with those at baseline, and the improvement persisted for 12 months of treatment. The pruritus index, sleep quality index, and Dermatology Life Quality Index (DLQI) scores were significantly reduced at both 6 and 12 months of treatment (nearly half of baseline at 6 months). The improvement was more pronounced at 6 months than at 12 months. The eosinophil count was significantly decreased after 12 months of AIT compared with that at baseline (Figure 1B and Table S1). Collectively, our results revealed that AIT exerted therapeutic effects among AD patients with HDM sensitization.
Figure 1.
The improvement in AD following AIT is accompanied by alterations in the oral and gut microbiota
(A) Study design. Forty-seven AD patients with HDM sensitization were recruited. All patients received repeated subcutaneous injections of dust-mite therapeutic vaccines. The initial buildup period lasted 4–5 months and comprised weekly vaccine injection at gradually increasing doses until the recommended maintenance dose was reached. The maintenance dose was administered at 4- to 6-week intervals. Peripheral blood, oral cavity, and stool samples were collected from each patient at baseline and at 6 and 12 months. Clinical assessment was performed by the same investigator.
(B) Clinical changes in AD patients undergoing AIT. Mean values of SCORAD index, EASI, pruritus index, sleep quality index, DLQI, and eosinophil count are shown. Baseline, n = 47; 6 months, n = 47; 12 months, n = 42 (for eosinophil count) or 46 (for other parameters). Paired Student’s t test: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. SCORAD, Scoring of Atopic Dermatitis; EASI, eczema area and severity index; DLQI, Dermatology Life Quality Index.
(C) A principal coordinates analyses based on unweighted UniFrac distances for the oral and gut microbiota is shown along the first two principal coordinate axes at baseline and at 6 and 12 months of treatment. Each point represents a single sample. Red, before AIT; yellow, 6 months of AIT; blue, 12 months of AIT.
(D) Boxplots indicate the Euclidean distance of samples among baseline and at 6 and 12 months of AIT as in (C). Wilcoxon rank-sum test; boxplot center represents median and box the interquartile range (IQR). Whiskers extend to the most extreme data point <1.5 IQR.
(E) Alpha-diversity of the microbiota in the oral cavity and gut at the ASV level. For the oral cavity, baseline, n = 46; 6 months, n = 42; 12 months, n = 27. For the gut, baseline, n = 39; 6 months, n = 36; 12 months, n = 22. Wilcoxon rank-sum test, boxplot center represents median and box the IQR. Whiskers extend to the most extreme data point <1.5 IQR. See also Figure S1.
The oral and fecal microbiota of the AD patients undergoing AIT were then analyzed using 16S rRNA sequencing (Figure 1A). The oral microbiota of the patients at 6 months of treatment diverged from that at baseline as observed in the unweighted UniFrac principal coordinates analysis; moreover, the difference between 12 months and 6 months of treatment was smaller than that between 6 months and baseline or between 12 months and baseline (Figures 1C and 1D). At 12 months of treatment, the gut microbiota of the patients clustered separately from that at baseline and 6 months of treatment, and the difference between the 6-month time point and baseline was smaller than that between the 12-month time point and baseline or between the 12-month and 6-month treatment time points (Figures 1C and 1D). Similarly, the β-diversity assessment of the oral microbiota based on unweighted UniFrac was significantly different among the three groups, and the gut microbiota of the patients at 12 months of treatment differed from that at baseline (Figure S1C). Analysis based on weighted UniFrac distances showed similar but weak segregation among the three groups for both the oral and gut microbiota (Figures S1A, S1B, and S1D). These data indicated that the pattern of change was different between the oral and gut microbiota. No significant difference in the α-diversity of the microbiota from either the oral or stool samples was observed before and after AIT, except for a significant increase at the amplicon sequence variant (ASV) level for the gut microbiota at 12 months of treatment compared with that at baseline and 6 months of treatment (Figure 1E). For functional prediction analysis, there were alterations in amino acid metabolism-related and lipid metabolism-related pathways in the oral microbiota at 6 months of treatment (Figure S1E). For example, the pathways involved in lysine degradation and fatty acid metabolism were enriched at baseline, but the pathways for arginine and proline metabolism were enriched at 6 months. A greater number of functional pathways were changed in the gut microbiota at 12 months of treatment compared with that at baseline. Specifically, there was an enrichment of pathways for tryptophan metabolism, butanoate metabolism, and propanoate metabolism (Figure S1E), suggesting that pathways associated with regulatory immune responses might be involved in AIT.
Collectively, the results indicated that AIT exerted therapeutic effects in patients with AD, concomitant with extensive alterations in the structure and function of both the oral and gut microbiota.
Transplantation of fecal microbiota from patients undergoing AIT improves AD-like dermatitis in mice
We next performed fecal microbiota transplantation (FMT) to confirm the involvement of the gut microbiota in the effects of AIT using a mouse model of MC903-induced AD. Mice were intragastrically administered with antibiotics before application of MC903. Fecal microbiota from healthy controls, untreated AD patients, or patients showing a marked improvement following AIT (those that achieved a 50% decrease in the EASI) was administered intragastrically to AD mice (Figure 2A). Intragastric PBS administration served as the control, which was subjected to the same paradigm as the FMT groups. The microbial communities in the mice after different FMT treatments clustered separately compared with pre-FMT (Figure S2D). There was a markedly increased proportion of gut microbiota from the donors at the genus level after FMT, indicating colonization of transferred microbiota after FMT (Figure S2E). Mice administered MC903 displayed erythema, edema, ear scaling, increased ear thickness, and increased levels of total IgE (Figures 2B, 2C, and 2E); however, these effects were markedly decreased after the transplantation of fecal microbiota from patients who showed a marked improvement following AIT (Figures 2B–2E). No significant difference in skin inflammation was detected between mice that received fecal microbiota from untreated AD patients and those treated with PBS. AD model mice that received fecal microbiota from healthy controls only showed a significant decrease in the thickness of the epidermis (Figures 2B–2E). The proportion of CD4+CD25+Foxp3+ Tregs in the spleen of AD mice was reduced after the transplantation of fecal microbiota from AD patients; in contrast, the proportion of Tregs was higher in the central lymph nodes (CLNs), mesenteric lymph nodes (MLNs), and spleens of AD mice receiving fecal microbiota from patients who showed a good response to AIT than in those of model mice receiving PBS or fecal microbiota from untreated patients (Figure 2F). Taken together, these results demonstrated that the transplantation of fecal microbiota from patients with AD who responded well to AIT alleviated AD-like dermatitis in mice and increased the Treg proportion.
Figure 2.
Transplantation of fecal microbiota from patients undergoing AIT improves AD-like dermatitis in mice
(A) Experimental design. Mice were intragastrically administered with antibiotic cocktail (ABX) daily for 3 days, then were topically treated with 2 nmol of MC903 daily for 12 days. Once the dermatitis was fully induced, the same dose of MC903 was applied every other day for 25 days to maintain the skin inflammation. At the same time, mice were gavaged with fecal microbiota from AD patients (AD group), healthy controls (HC group), and AIT-effective AD patients (AIT group) daily for 15 days, and intragastric PBS was used as control (NC group).
(B) Gross appearance and H&E staining of the ears on day 44. Scale bars, 250 μm.
(C) Dynamic changes of ear thickness at the indicated time points. n = 7 or 8.
(D) Ear thickness of the epidermis (EM) on day 44. n = 7 or 8.
(E) Levels of serum total IgE at day 19 and day 44 as measured by ELISA. n = 5–8.
(F) CD4+CD25+Foxp3+ Tregs from the CLNs, MLNs, and spleens, as measured by flow cytometry. Representative dotplots and aggregated statistics of biological replicates are shown. n = 5.
Data are shown as mean ± SEM (C–F). Unpaired two-tailed t test: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001; ns, not significant. See also Figure S2.
Abundance of B. vesicularis in the oral and gut microbiota is decreased after AIT
We next characterized the changes occurring in the composition of the oral and gut microbiota of patients with AD after AIT. The ten most abundant phyla are shown in Figure 3A. The composition of the oral microbiota changed after both 6 and 12 months of treatment; however, no significant difference in microbial composition was observed between the two time points. For example, the abundance of Actinobacteria and Fusobacteria increased after 6 months of treatment and remained at a similarly high level until 12 months of treatment (Figures 3A and S3A; Data S1). In contrast, the composition of the gut microbiota showed a progressive change with the continuation of therapy. The abundance of the most dominant phyla in the gut microbiota, Bacteroidetes and Firmicutes, was gradually decreased and increased, respectively. These results were consistent with the observed changes in the diversity of the oral and gut microbiota (Figures 1C and 1D). At the genus level, the patterns of microbial enrichment in the two habitats differed before and after treatment. Notably, the abundances of Stenotrophomonas and Brevundimonas were significantly reduced at both 6 and 12 months of treatment (Figure 3B and Data S1). To validate the characteristics of the oral and gut microbiota at the species level, we identified the discriminatory taxa at the ASV level. The results showed that B. vesicularis was the only species that displayed significantly decreased abundance after both 6 and 12 months of treatment in both the oral cavity and gut (Figures 3C and S3B; Data S1). Collectively, our results revealed the alterations occurring in the microbial composition and specific microbes of the oral and gut microbiota with AIT and further showed that the abundance of B. vesicularis was decreased in both habitats after immunotherapy.
Figure 3.
Abundance of B. vesicularis in the oral and gut microbiota is decreased after AIT
(A) Phylum abundance of the oral and gut microbiota at baseline and at 6 and 12 months of AIT. Legend indicates the color for each phylum of bacterium represented.
(B) Heatmap of the genus in the oral and gut microbiota at baseline and at 6 and 12 months of AIT. Paired Wilcoxon signed-rank test: ∗adjusted p < 0.05, ∗∗adjusted p < 0.01.
(C) Boxplot for relative abundance of Brevundimonas at the ASV level in the oral cavity and gut.
See also Figure S3.
B. vesicularis from the oral cavity colonizes the gut of patients with AD
We have previously reported that the oral and gut microbiota of patients with AD display decreased site specificity.21 Accordingly, we next compared the microbial communities of the two habitats in patients with AD after AIT. Analysis based on unweighted UniFrac distances showed that the distance between the oral and gut microbiota of AD patients was significantly increased after AIT (Figure 4A). Compared with baseline, the numbers of shared genera and ASVs in oral and stool samples from single individuals were significantly reduced at both 6 and 12 months of treatment (Figure 4B). These findings suggested that the oral and gut microbiota became more distinct after AIT. Because of the close distance between the gut and oral microbiota in untreated AD patients, we hypothesized that there might be ectopic colonization of oral microbes in the gut. To confirm this possibility, we analyzed the correlation between the oral and gut microbiota in patients with AD. The results showed that most of the genera were positively correlated with the microbes of their respective habitats. However, Brevundimonas in the stool was strongly positively correlated with the genera of the oral cavity, but not the gut, which suggested that Brevundimonas in the stool might have originated in the oral cavity. In contrast, Brevundimonas from the oral cavity was found to be positively correlated with other genera in the oral microbiota but negatively correlated with several gut-specific genera, such as Bifidobacterium, which has been reported to be one of the probiotics in AD (Figure 4C). We further analyzed the correlation of the abundance of B. vesicularis between the two habitats and identified a significant positive correlation between the two body sites at baseline (r = 0.582, p = 1.81e−05; Figure 4D). Collectively, our data suggested that B. vesicularis from the oral cavity might have ectopically colonized the gut of patients with AD and further implied that the ecological niches of the oral cavity and gut recovered after AIT.
Figure 4.
B. vesicularis from the oral cavity colonizes the gut of patients with AD
(A) Boxplots indicating the unweighted UniFrac distances of samples between oral and gut microbiota collected from the same individual at baseline (n = 38), at 6 months (n = 32), and at 12 months (n = 22). Wilcoxon rank-sum test.
(B) Boxplots indicating the number of shared genera and ASVs in the oral cavity and gut from the same individual.
(C) Network plot correlations between the oral and gut microbiota co-abundance groups identified. The connections between nodes represent significant Spearman correlations between genera. Red line, positive correlation; blue line, negative correlation. |R| > 0.45; adjusted p < 0.05.
(D) Correlation of B. vesicularis in the oral cavity and gut. n = 47.
See also Figure S4.
B. vesicularis is associated with AD-related clinical parameters
Next, to validate the involvement of B. vesicularis in AD, we analyzed the correlation between the abundance of this bacterium and AD-related clinical parameters using mixed-effects models. The results showed that AD severity, as evaluated by SCORAD index and EASI scores, was highly positively correlated with the abundance of B. vesicularis in both the oral cavity and gut, although the correlation was significant only in the gut. The abundance of B. vesicularis in the oral cavity and gut showed a significant positive correlation with the eosinophil count. Additionally, B. vesicularis was significantly positively correlated with the itch index and DLQI only in the gut. No significant correlation was detected between B. vesicularis and the sleep index (Figure S4). Combined, our results suggested that the abundance of B. vesicularis, especially in the gut, was positively correlated with the severity of AD.
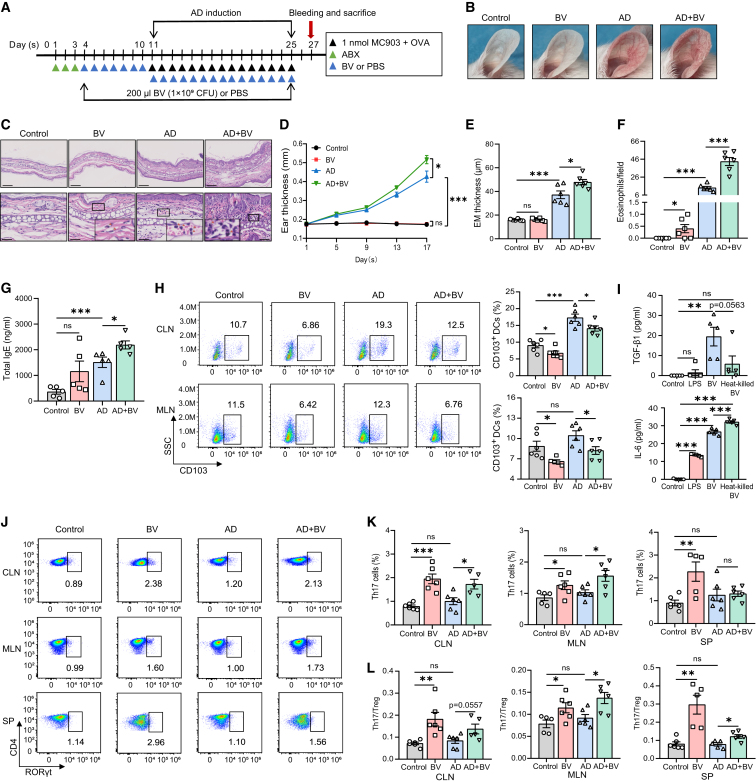
Intragastric B. vesicularis aggravates MC903-induced AD-like dermatitis in mice
To further explore the role of B. vesicularis in AD, B. vesicularis was intragastrically administered to antibiotic-pretreated mice with MC903 + OVA-induced AD (Figures 5A and S5A). The AD mice exhibited evident skin inflammation manifesting as erythema, edema, and ear scaling, accompanied by greater ear thickness (Figures 5B–5E), increased eosinophil infiltration, and elevated levels of total serum IgE (Figures 5F and 5G). These effects were exacerbated following the administration of B. vesicularis, while the expression of thymic stromal lymphopoietin in the skin lesions was also significantly increased (Figures 5B–5G and S5B). However, no significant difference in morphology or total IgE levels was observed in the model mice treated with B. vesicularis alone, although the level of eosinophil infiltration was slightly elevated (Figures 5B–5G).
Figure 5.
Intragastric B. vesicularis aggravates MC903-induced AD-like dermatitis in mice
(A) Experimental design. Mice were intragastrically administered with antibiotic cocktail daily for 3 days, then gavaged with 200 μL of B. vesicularis (1 × 109 CFU) daily for 7 days. Intragastric PBS was used as control. Thereafter, gavage was continued, and mice were topically treated with 1 nmol of MC903 and 20 μg/μL of OVA daily for 15 days to produce the AD mouse model.
(B) Gross appearance of the ears on day 27.
(C) Representative H&E staining for the skin lesion (scale bars, 250 μm). Enlarged pictures show the infiltration of eosinophils (scale bars, 50 μm).
(D) Dynamic changes of ear thickness at the indicated time points. n = 6.
(E) Ear thickness of the epidermis (EM) on day 27. n = 6.
(F) Numbers of infiltrating eosinophils counted from H&E staining. n = 6.
(G) Levels of serum total IgE at baseline and day 27 as measured by ELISA. n = 5.
(H) CD11c+MHCII+CD103+ DCregs from the CLNs and MLNs, as measured by flow cytometry. Representative dotplots and aggregated statistics of biological replicates are shown. n = 5 or 6.
(I) Levels of TGF-β and IL-6 in the supernatants of CD11c+ DC culture, as measured by ELISA. n = 5.
(J and K) CD4+RORγt+ Th17 cells from the CLNs, MLNs, and spleens, as measured by flow cytometry. Representative dotplots show the frequency of gated RORγt+ Th17 cells among CD4+ T cells (J). Aggregated statistics of biological replicates are shown in (K). n = 5 or 6.
(L) Th17/Treg ratios from the CLNs, MLNs, and spleens, as measured by flow cytometry. n = 5 or 6.
Data are shown as mean ± SEM (D–I, K, and L). Unpaired two-tailed t test: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001; ns, not significant. See also Figures S5 and S6.
CD103+ dendritic cells (DCs) in the intestine have been shown to promote the expansion of Foxp3+ Tregs and are regarded as regulatory DCs (DCregs).33 Compared with PBS treatment, B. vesicularis administration significantly decreased the percentage of CD103+ DCs in both the CLNs and MLNs of mice, irrespective of whether MC903 + OVA was applied (Figure 5H). B. vesicularis moderately induced the expression of CD86 and major histocompatibility complex class II (MHCII) on CD11c+ DCs in the CLNs and MLNs of AD model mice (Figure S5C). In addition, the administration of B. vesicularis alone reduced the expression of programmed cell death-1 ligand (PD-L1) in the CLNs, similar to that seen with MC903 + OVA application (Figure S5C). CD11c+ DCs isolated from the spleens were stimulated with live or heat-killed B. vesicularis for 24 h in vitro. Live B. vesicularis administration resulted in significantly increased levels of transforming growth factor β1 (TGF-β1) and interleukin-6 (IL-6) in the supernatant of culture, while the heat-killed bacteria-treated DCs selectively secreted a great amount of IL-6 (Figure 5I). Collectively, these results suggested that B. vesicularis inhibited the tolerogenic capacity of DCregs by inducing the expression of co-stimulatory molecules and downregulating the level of PD-L1, as well as increasing the expression of pro-inflammatory factors.
We further assessed CD4+ Th cell polarization after B. vesicularis treatment. The proportion of CD4+CD25+Foxp3+ Tregs was decreased in the spleens of mice treated with B. vesicularis; however, no significant difference in the Treg proportion was seen in the CLNs and MLNs between B. vesicularis-treated mice and the controls (Figure S5D). Notably, compared with control mice, animals receiving B. vesicularis showed increased expansion of CD4+RORγt+ Th17 cells and an elevated Th17/Treg ratio in the CLNs, MLNs, and spleens in both the MC903 + OVA-treated and vehicle-treated groups (Figures 5J–5L and S5E), indicative of a skewing of the Treg/Th17 cell balance toward Th17 polarization. CD11c+ DCs isolated from the MLNs or spleens were stimulated with live or heat-killed B. vesicularis and then co-cultured with naive CD4+ T cells isolated from the spleens for 4 days. CD4+ T cells co-cultured with B. vesicularis-treated DCs differentiated into a greater number of Th17 cells than Tregs (Figures S5G and S5H), and the live B. vesicularis-treated co-culture system produced significantly more IL-17A than the control system (Figure S5I). Heat-killed B. vesicularis-treated DCs also promoted the induction of Th17 cells and inhibited the expansion of Tregs (Figures S4G and S4H). We did not find any difference in the frequencies of CD4+IFN-γ+ Th1 cells or CD4+IL-4+ Th2 cells between B. vesicularis-treated mice and the controls, except for a modest increase in Th1 cell proportions in AD mice receiving B. vesicularis compared with those receiving PBS (Figure S5F).
To verify that the effects of B. vesicularis on skin inflammation were specific rather than a generic bacterial response, we isolated three common gut commensal microbes, Bacteroides uniformis, Ruminococcus gnavus, and Prevotella copri, that were decreased in abundance after AIT from the patients’ stools. All three of the commensal bacteria-treated DCs were unable to increase the Th17/Treg ratio in the co-culture system (Figures S6A and S6B). Furthermore, we selected one of the bacterial strains, B. uniformis, and found that B. uniformis-treated AD mice did not exhibit more severe morphological appearance, greater ear thickness, and higher levels of total IgE (Figures S6C–S6F). Likewise, the decreased percentage of CD103+ DCs and the increased Th17/Treg ratio observed in B. vesicularis-treated AD mice were not found in the AD mice intragastrically administered with B. uniformis (Figures S6G–S6J).
Taken together, our findings showed that B. vesicularis exacerbated skin inflammation and specifically promoted the skewing of the Treg/Th17 cell balance toward Th17 polarization in AD mice.
B. vesicularis attenuates AIT efficacy in AD mice
To verify the pro-inflammatory effect of B. vesicularis in AIT, an OVA-specific immunotherapy mouse model was established by the subcutaneous administration of OVA and aluminum hydroxide to AD mice (Figure 6A). Redness, scaling, ear swelling, and eosinophil infiltration levels were significantly lower in AD mice subjected to OVA immunotherapy than in those treated with MC903 + OVA alone. A slight reduction in total IgE and OVA-specific IgE levels was found in the OVA immunotherapy group. When the AD mice undergoing immunotherapy were administered B. vesicularis intragastrically before and during treatment, the improvement in inflammation was significantly abrogated, indicating that B. vesicularis interfered with the therapeutic effect of OVA-specific immunotherapy (Figures 6B–6F). Consistent with our previous results, the intragastric application of B. vesicularis alone did not induce visible AD-like dermatitis in normal mice (Figures 6B–6F). Our results further showed that AIT significantly increased the percentage of CD103+ DCs in both the CLNs and MLNs in AD model mice. However, compared with the OVA immunotherapy group, AD mice subjected to AIT with the intragastric administration of B. vesicularis exhibited fewer CD103+ DCs in both the CLNs and MLNs (Figure 6G), accompanied by a decrease in PD-L1 expression in the CLNs and an increase in CD86 expression in the MLNs (Figure 6H). As expected, in mice receiving AIT, the intragastric administration of B. vesicularis markedly increased the percentage of Th17 cells and the Th17/Treg ratio in the CLNs, MLNs, and spleens, compared with that seen with intragastric PBS administration (Figures 6I, S7A, and S7B). Moreover, intragastric B. vesicularis administration reversed the AIT-induced expansion of CD4+CD25+Foxp3+ Tregs in the CLNs to levels comparable to those seen in the AD group (Figure S7A). We also found that intragastric B. vesicularis administration reversed the OVA immunotherapy-induced reduction in Th1 cell proportions in the spleen (Figure S7C), possibly due to the high production of interferon-γ (IFN-γ) induced by B. vesicularis (Figure S5I). These results indicated that B. vesicularis treatment strongly skewed the Th17/Treg ratio, thereby attenuating the efficacy of AIT in AD mice.
Figure 6.
B. vesicularis attenuates AIT efficacy in AD mice
(A) Experimental design. Mice were intragastrically administered with antibiotic cocktail daily for 3 days and then gavaged with 200 μL of B. vesicularis (1 × 109 CFU) daily for 7 days. PBS was used as control. Thereafter, gavage was continued, and mice were topically treated with 1 nmol of MC903 and 20 μg/μL of OVA daily for 14 days to establish the AD mouse model. Once the dermatitis was fully induced, the same dose of MC903 and OVA was applied every other day for 5 days to maintain the skin inflammation. Starting on day 10 of dermatitis induction, mice were injected subcutaneously with 200 μL of OVA solution once every 4 days for three consecutive times, using normal saline as control.
(B) Gross appearance of the ears and representative H&E staining with enlarged picture showing eosinophils on day 30. Scale bars, 50 μm.
(C) Dynamic changes of ear thickness at the indicated time points. n = 6.
(D) Ear thickness of the epidermis (EM) on day 30. n = 6.
(E) Numbers of infiltrating eosinophils counted from H&E staining. n = 5 or 6.
(F) Levels of serum total IgE and OVA-specific IgE at baseline and day 30 as measured by ELISA. n = 5 or 6.
(G) CD11c+MHCII+CD103+ DCregs from the CLNs and MLNs, as measured by flow cytometry. Representative dotplots and aggregated statistics of biological replicates are shown. n = 5 or 6.
(H) Mean fluorescence intensity (MFI) of PD-L1, CD86, or MHCII on CD11c+MHCII+ DCs from the CLNs and MLNs, as measured by flow cytometry. n = 5 or 6.
(I) Th17/Treg ratios from the CLNs, MLNs, and spleens, as measured by flow cytometry. n = 5 or 6.
Data are shown as mean ± SEM (C–I). Unpaired two-tailed t test: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001; ns, not significant. See also Figure S7.
Discussion
The gut microbiota has been strongly implicated in the pathogenesis of AD14,15,16,17,34; however, whether it is involved in the effect of AIT in AD remains unclear, as does the putative underlying mechanism. In this study, we revealed that the composition and function of the oral and gut microbiota were altered in patients with AD who underwent AIT. B. vesicularis, which might ectopically colonize the gut from the oral cavity, was positively correlated with AD severity and played a crucial role in the exacerbation of AD-induced inflammation. B. vesicularis also attenuated the efficacy of AIT in AD model mice by promoting the skewing of the Treg/Th17 balance toward Th17 polarization. We report that the oral and gut microbiota can modulate the responses to AIT against AD and identified a specific microbe, B. vesicularis, that acted as an aggravating factor in AD-induced inflammation and influenced the efficacy of AIT. Our results provided potential strategies for improving the efficacy of immunotherapy via the modulation of the gut microbiota.
The mechanism underlying the effect of AIT is not fully understood. The skewing of allergen-specific effector T cells toward a regulatory phenotype is considered crucial for successful AIT.12 Allergen-specific Tregs and regulatory B cells orchestrate the following: a general immunoregulatory response, including the suppression of cytokine production by inflammatory DCs; a reduction in effector Th1, Th2, and Th17 cell numbers; a decrease in the allergen-specific IgE/IgG4 ratio; and suppression of the migration of mast cells, basophils, eosinophils, and effector T cells.12 Although the gut microbiota has been associated with the efficacy of AIT in patients with food allergies,24 the role and mechanism of the gut microbiota in AIT remains largely unknown. It has been reported that the transplantation of fecal microbiota from healthy mice to AD mice ameliorates AD-related allergic responses, results in the restoration of the gut microbiota to the donor state, regulates the Th1/Th2 balance via Treg signals, and leads to increased levels of SCFAs.35 The increased production of propionic acid by intestinal microbiota stabilizes IL-10 expression in B cells, which promotes the efficacy of SLIT in mice with allergic rhinitis.36 In our study, the transplantation of fecal microbiota from patients with AD who responded well to AIT alleviated AD-like symptoms in mice and induced the expansion of Tregs. The gut microbiota of AD patients undergoing AIT differed from that of untreated patients in both composition and function, while the pathways for tryptophan metabolism, butanoate metabolism, and propanoate metabolism were enriched in the gut microbiota after 1 year of AIT. SCFAs such as butyrate and propionate exert anti-inflammatory effects via Treg induction.37,38,39 Fang et al.40,41 reported that the oral administration of Bifidobacterium longum and Limosilactobacillus reuteri attenuated AD-related inflammation by remodeling gut microbial composition and inducing the production of tryptophan metabolites. In our study, the increase in tryptophan and SCFA metabolism might result in Treg production and the amelioration of AD-related inflammation, thus promoting the efficacy of AIT. Changes in microbial metabolites in the gut of patients with AD after AIT merits further investigation.
It has been shown that an increase in the abundance of several bacteria (i.e., Staphylococcus, E. coli, and Klebsiella pneumoniae) in the gut plays a part in the aggravation of AD symptoms.20,42,43 In the present study, B. vesicularis was identified as an aggravating factor for AD. B. vesicularis is a gram-negative rod bacterium reported to be a causal agent of nosocomial infections and is regarded as one of the emerging global opportunistic pathogens.44 It has been reported that a combination of three species, including B. vesicularis, is associated with the allergic or non-allergic asthma phenotype.45 B. vesicularis was the only species in the oral cavity and gut that was consistently and significantly decreased after 6 and 12 months of immunotherapy in our study and was closely correlated with the exacerbation of AD. The abundance of B. vesicularis in the gut microbiota was decreased after AIT, which was accompanied by the recovery of site specificity. We hypothesized that a proportion of B. vesicularis transmigrated from the oral cavity to the gut in patients with AD. Consistent with this hypothesis, we found that Brevundimonas in the stool was positively correlated with the genera in the oral cavity rather than with those in the gut, and a positive correlation with the abundance of B. vesicularis was identified between the two habitats. The findings of ectopic colonization of the intestine by B. vesicularis in the patients with AD remain to be further verified, as 16S rRNA sequencing analysis has its limitations in tracing the source of a certain bacterial taxa from one habitat to another. It has been reported that the unweighted UniFrac distance between the oral and gut microbiota of AD patients is decreased compared with that of healthy individuals,21 which suggests that microbiota may translocate between the oral cavity and the gut. Ectopic colonization is most commonly reported in inflammatory bowel disease30,31,32; however, the mechanisms underlying how the colonization of the gut by oral bacteria initiates intestinal inflammation remain unclear. Ectopic colonization by oral pathobionts may directly activate mucosal inflammatory responses or serve as cognate antigens for T cells that have transmigrated from the oral cavity,32 thereby exacerbating colitis. In the present study, the changes in the oral microbiota in AD may have disturbed the microecology of the oral cavity, leading to weakened physical and/or chemical compartmentalization between oral and intestinal mucosa; consequently, pro-inflammatory bacteria, such as B. vesicularis, could overcome the barriers between the oral cavity and the gut and translocate into the latter.
Our study further demonstrated that B. vesicularis promoted the skewing of the Treg/Th17 balance toward Th17 polarization, thereby exacerbating skin inflammation, which was alleviated by OVA-specific AIT in AD mice. CD103+ DCs in the gut might have played a decisive role in the B. vesicularis-induced immune response. CD103+ DCs of the intestinal mucosa are essential for tolerance to commensal bacteria. These cells originate in the lamina propria and migrate to the MLNs where they drive the differentiation of gut-homing Foxp3+ Tregs.46,47 A mixture of probiotics can modulate the structure of the gut microbiota and alleviate AD symptoms by promoting the expansion of intestinal CD103+ DCs and Treg differentiation,48 which is consistent with the effect of AIT in AD model mice seen in our study. The reduced abundance of B. vesicularis allows the expansion of CD103+ DCs in the MLNs and CLNs and their tolerogenic potential, which may contribute to the protective effect of immunotherapy. AD is a highly heterogeneous disease that is characterized by Th2-dominant inflammation, whereas Th17-type and Th22-type inflammation also differentially contribute to the pathogenesis of AD. It is noteworthy that Asian patients with AD have a substantial proportion of Th17-type inflammation.4 Thus, attenuation of Th17 polarization and impairment of Th17/Tregs would promote the efficacy of AIT for AD. OVA-induced AD inflammation in mice can be ameliorated by inhibiting IL-17 secretion from Th17 cells.49 IL-17A downregulates the expression of genes involved in epidermal barrier formation50 and serves as an inducer for Th2 immune responses in AD.51 There is a Th17/Treg imbalance in AD, and the Th17/Treg ratio is positively correlated with AD severity and serum IgE levels.52 Several studies have reported that the Th17/Treg ratio is decreased during AIT.53,54 IL-17 produced by Th17 cells reduces Treg concentrations, thus eliminating the therapeutic effects of oral tolerance promoted by AIT in mice.55 Consistent with previous studies, we found that the number of Tregs was increased in AD mice undergoing immunotherapy. B. vesicularis inhibited AIT-induced Treg expansion in the CLNs, induced the polarization of Th17 cells, and increased the Th17/Treg ratio, accompanied by the induction of Th1 cells, both in vivo and in vitro. The exact mechanism by which B. vesicularis regulates the Th17/Treg balance remains incompletely understood. Whether the effects of B. vesicularis are due to a transient or long-term colonization remains unclear. B. vesicularis promotes the secretion of TGF-β and IL-6 by DCs. It is known that TGF-β can upregulate the expression of RORγt in the presence of IL-6, therefore inducing the differentiation of CD4+ T cells toward Th17 polarization.56 Heat-killed B. vesicularis is sufficient to induce high levels of IL-6, which might also help in suppressing Treg expansion induced by TGF-β.56,57 Notably, although B. vesicularis alone altered the number and function of CD103+ DCs and affected the polarization of Th cells to various degrees in healthy mice, these effects were not enough to induce either dermatitis or systemic allergic inflammation or to induce Th17 cytokines (Figure S5E). These data indicated that there might be self-regulation in the local microenvironment or adjustment between B. vesicularis and other bacteria under physiological conditions.
In conclusion, our study revealed the specific changes occurring in the oral and gut microbiota of patients with AD undergoing AIT and provided evidence for the ectopic colonization of the gut by oral microbiota in AD. Future studies should explore the therapeutic potential by modulating the gut and/or oral microbiome through the “oral-gut-skin axis” in patients with AD who are receiving immunotherapy.
Limitations of the study
The exact mechanism by which B. vesicularis regulates the Th17/Treg balance needs further exploration. The present study provided preliminary evidence for the ectopic colonization of the gut by oral microbiota in patients with AD merely using 16S rRNA sequencing analysis; however, further studies are needed to validate that B. vesicularis in the gut of AD patients originate from the oral cavity. We could not exactly validate the mechanical correlation between the alteration of B. vesicularis and the efficacy of AIT. The 16S rRNA sequencing analysis does not ensure accuracy of functional predictions and strain-level analysis. Shotgun metagenomic sequencing can be utilized regarding functional annotation and potential mechanisms by which oral and gut microbiota are involved in AD.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Purified Rat Anti-Mouse IgE (Clone R35-72) | BD Biosciences | Cat # 553413; RRID: AB_394846 |
| Purified Mouse IgE, κ Isotype Control Standard (Clone C48-2) | BD Biosciences | Cat # 557080; RRID: AB_396578 |
| Biotin Rat Anti-Mouse IgE (Clone R35-118) | BD Biosciences | Cat # 553419; RRID: AB_394850 |
| Mouse anti-Ovalbumin IgE | Bio-Rad | Cat # MCA2259; RRID: AB_2285753 |
| Anti-mouse CD8a PerCP/Cy5.5 (Clone 53-6.7) | BioLegend | Cat # 100734; RRID: AB_2075238 |
| Anti-mouse CD4 FITC (Clone GK1.5) | eBioscience | Cat # 11-0041-82; RRID: AB_464892 |
| Anti-mouse CD3e PE-CYN7 (Clone 145-2C11) | eBioscience | Cat # 25-0031-82; RRID: AB_469572 |
| Anti-mouse IFN-γ Brilliant Violet 510™ (Clone XMG1.2) | BioLegend | Cat # 505842; RRID: AB_2734494 |
| Anti-mouse IFN-γ PE (Clone XMG1.2) | eBioscience | Cat # 17-7311-82; RRID: AB_466193 |
| Anti-mouse IL-4 APC (Clone11B11) | eBioscience | Cat # 17-7041-82; RRID: AB_469494 |
| Anti-mouse IL-17A Brilliant Violet 605™ (Clone TC11-18H10.1) | BioLegend | Cat # 506927; RRID: AB_11126144 |
| Anti-mouse RORγt Brilliant Violet 421™ (Clone Q31-378) | BD Biosciences | Cat # 562894; RRID: AB_2687545 |
| Anti-mouse CD25 APC (Clone PC61.5) | eBioscience | Cat # 17-0251-82; RRID: AB_469366 |
| Anti-mouse FOXP3 PE (Clone FJK-16S) | eBioscience | Cat # 12-5773-82; RRID: AB_465936 |
| Anti-mouse CD274 (B7-H1, PD-L1) Brilliant Violet 605™ (Clone 10F.9G2) | BioLegend | Cat # 124321; RRID: AB_2563635 |
| Anti-mouse CD274 PE-CYN7 (Clone MIH5) | eBioscience | Cat # 25-5982-82; RRID: AB_2573509 |
| Anti-mouse I-A/I-E Pacific Blue™ (Clone M5/114.15.2) | BioLegend | Cat # 107620; RRID: AB_493527 |
| Anti-mouse I-A/I-E Alexa Fluor® 700 (Clone M5/114.15.2) | BioLegend | Cat # 107622; RRID: AB_493727 |
| Anti-mouse CD86 APC (Clone GL1) | eBioscience | Cat # 17-0862-82; RRID: AB_469419 |
| Anti-mouse CD86 FITC (Clone GL1) | eBioscience | Cat # 11-0862-82; RRID: AB_465148 |
| Anti-mouse CD11c PE (Clone N418) | BioLegend | Cat # 117308; RRID: AB_313777 |
| Anti-mouse CD103 FITC (Clone 2E7) | BioLegend | Cat # 121420; RRID: AB_10714791 |
| FcR Blocking Reagent, mouse | Miltenyi Biotec | Cat # 130-092-575 |
| Biological samples | ||
| Human peripheral blood | Hospital for Skin Diseases, Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College | N/A |
| Stool samples from AD patients | Hospital for Skin Diseases, Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College | N/A |
| Oral swabs from AD patients | Hospital for Skin Diseases, Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College | N/A |
| Bacterial and virus strains | ||
| Brevundimonas vesicularis strain | CGMCC | Cat # 1.3355 |
| Bacteroides uniformis strain | This paper | N/A |
| Ruminococcus gnavus strain | This paper | N/A |
| Prevotella copri strain | This paper | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Streptomycin sulfate | MedChemExpress | Cat # HY-B0472 |
| Ampicillin sodium | MedChemExpress | Cat # HY-B0522A |
| Calcipotriol (MC903) | LEO Pharma | Cat # H20150664 |
| Recombinant Mouse IL-2 | R&D Systems | Cat # 402-ML-020 |
| Cell Stimulation Cocktail (plus protein transport inhibitors) | Thermo Fisher Scientific | Cat # 00-4975-93 |
| Foxp3/Transcription Factor Staining Buffer Set | eBioscience | Cat # 00-5523-00 |
| Fixation/Permeabilization Concentrate | Thermo Fisher Scientific | Cat # 00-5123-43 |
| Fixation/Permeabilization Diluent | Thermo Fisher Scientific | Cat # 00-5223-56 |
| Ovalbumin | Sigma-Aldrich | Cat # A5503 |
| Ovalbumin (Biotin) | USBiological | Cat # O8075-01 |
| Streptavidin HRP | BD Biosciences | Cat # 554066; RRID: AB_2868972 |
| Anti-PE MicroBeads | Miltenyi Biotec | Cat # 130-048-801 |
| CD4 (L3T4) MicroBeads, mouse | Miltenyi Biotec | Cat # 130-117-043 |
| TRIzol™ Reagent | Thermo Fisher Scientific | Cat # 15596018 |
| Lipopolysaccharides from Escherichia coli O111:B4 | Sigma-Aldrich | Cat # L4391 |
| Critical commercial assays | ||
| FastPure Host Removal and Microbiome DNA Isolation Kit | Vazyme | Cat # DC501-01 |
| HiScript II Q RT SuperMix for qPCR | Vazyme | Cat # R222-01 |
| AceQ qPCR SYBR Green Master Mix | Vazyme | Cat # Q141-02 |
| Mouse TGF-β1 ELISA Kit | Fcmacs Biotech | Cat # FMS-ELM029 |
| Mouse IL-6 ELISA Kit | Fcmacs Biotech | Cat # FMS- ELM006 |
| Mouse IL-17A ELISA Kit | Fcmacs Biotech | Cat # FMS-ELM016 |
| Mouse IFN-γ ELISA Kit | Fcmacs Biotech | Cat # FMS-ELM027 |
| Deposited data | ||
| Oral swabs and stool 16S rRNA sequencing data | This paper | ID: PRJEB56808; https://www.ebi.ac.uk/ena/browser/view/ |
| Oligonucleotides | ||
| qPCR primer sequences | This paper | See Method Details |
| Software and algorithms | ||
| R v3.4.0 | R | https://www.r-project.org/ |
| QIIME 2 (2020.2) | QIIME 2 | https://qiime2.org/ |
| FlowJo version 10.4 | TreeStar | https://www.flowjo.com/solutions/flowjo |
| GraphPad Prism 8.0 version 8.0 | GraphPad Software | https://www.graphpad.com/scientific- software/prism; RRID: SCR_002798 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to, and will be fulfilled by, the lead contact, Xu Yao (dryao_xu@126.com).
Materials availability
This study did not generate new unique reagents.
Data and code availability
The 16S rRNA sequencing data reported in this paper have been deposited in the European Bioinformatics Institute (EMBL-EBI), and are publicly available as of the date of publication. Accession numbers are listed in the Key resources table. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and study participant details
Human studies
In total, AD 47 patients with HDM sensitization were recruited at the Department of Allergy and Rheumatology, Hospital for Skin Diseases, Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College from October 2017 to May 2020 (see Table S1). All the patients fulfilled the diagnostic criteria of Hanifin & Rajka for AD, with a history of clinical exacerbation after exposure to HDM and a value of >3.5 kU/L for HDM specific-IgE measured by ImmunoCap System (Phadia, Uppsala, Sweden). All the patients received repeated subcutaneous injections of dust-mite therapeutic vaccines (Alutard SQ, ALK, Denmark). The procedure had two stages: initial buildup period and maintenance period. The initial buildup period lasted 4 to 5 months, and it comprised weekly vaccine injection at gradually increasing doses until the recommended maintenance dose (1.0 mL, 100,000 SQ-U/mL) was reached. The maintenance dose was administered at 4 to 6 weeks intervals. The patients undergoing AIT were followed up at 6 and 12 months of treatment initiation. None of the subjects had oral or intestinal diseases other than AD at the time of sampling, and none of the subjects had systemic diseases or received antibiotics treatment within 6 months before sample collection. Samples of the oral cavity and stool were collected from each patient. The subjects were instructed to avoid brushing teeth and eating food for 2 h prior to oral sampling. Oral samples were collected by rubbing the buccal mucosa, keratinized gingiva, supragingival plaque, and tongue dorsum with a swab, as reported previously.21 Stool samples were self-collected, stored on ice, and sent to the laboratory within 1 h of defecation. Clinical assessment was performed by the same investigator throughout the study period, utilizing SCORAD index, EASI, pruritus index (from SCORAD index), sleep quality index (from SCORAD index), and Dermatology Life Quality Index (DLQI). Peripheral blood was obtained for preparation of serum and analysis of blood cell counts. For fecal transplantation of microbiota, stool from AD 5 patients, 5 patients showing a marked improvement following 12 months of AIT (achieved a 50% decrease in the EASI), and 5 healthy volunteers were collected and stored at −80°C (see Table S2). The study was approved by the ethics committee of the Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College [ethical approvals: 2016-KY-023], and the informed written consent was obtained.
Animal studies
Animal studies were approved by the Animal Welfare Ethics Review Committee of the Institute of Dermatology, Chinese Academy of Medical Sciences. Six to eight-week-old female BALB/c mice were purchased from the Laboratory Animal Center of the Nanjing Medical University (Nanjing, Jiangsu, China). All the mice were maintained with a 12-h light/dark cycle at 19°C–24°C under specific pathogen-free condition. A standard extruded pellet diet and water were supplied unlimited. All the mice were randomly assigned for experiments.
Method details
16S rRNA gene sequencing and analysis
DNA extraction and 16S rRNA sequencing
Total genome DNA from the oral cavity and stool samples was extracted by Novogene Bioinformatics Technology (Beijing, China) using a previously described method,21 and sequenced the V3 to V4 region of bacterial 16S rRNA genes in 2 × 250 bp paired-end (PE) mode on the Illumina HiSeq2500 platform (Illumina, USA) according to the manufacturer’s instructions. The V3–V4 region of the 16S rRNA genes was amplified using the specific primer pair 341F/806R with barcoding. Sequencing libraries were generated using the TruSeq DNA PCR-Free Sample Preparation Kit (Illumina) following the manufacturer’s recommendations, and index codes were added.
16S sequencing data analysis
After quality control and removal of chimeric sequences, the paired end sequences were joined by FLASH (v1.2.7). To eliminate the influence of sequencing depth fluctuations, we downsized the number of reads to 50000 for each sample. Three oral swab samples (6 months of AIT) were excluded from the subsequent analysis due to an insufficient number of reads. Paired sequences were input into QIIME 2 (2020.2). Sequences were quality filtered and denoised using Deblur (q2-deblur) with parameters ‘--p-trim-length 400’ to generate ASVs. All ASVs were aligned with mafft58 (via q2-alignment) and used to construct a phylogeny with fasttree59 (via q2-phylogeny). Taxonomy was assigned to ASVs using the q2-feature-classifier60 classify-sklearn naïve Bayes taxonomy classifier against the Greengenes database (13_8, OTUs with 99% similarity). The QIIME pipeline was also used to compute α- and β-diversity using the Shannon Index and weighted and unweighted UniFrac distances based on the ASVs relative abundance. Functional profile was performed by Picrust2 based on ASVs abundance using q2-picrust2.
Unless otherwise stated, statistical analyses were performed using R software. The differential diversity and abundance of genera, or ASVs between AD patients and after AIT were tested using the paired Wilcoxon signed-rank test. Only genera, or ASVs that were present in at least 20% of samples from each body site were included in the test. The p-values were adjusted by the Benjamini-Hochberg correction for multiple tests when required. Permutational multivariate analysis of variance based on weighted and unweighted UniFrac distances was performed with 999 permutations in R (3.4.0, vegan package) to assess the impact of AD on the microbiota at baseline and 6 and 12 months. The correlation coefficients of repeated observations between genus were calculated using the method described by Bland and Altman.61 The relationships between microbial data and clinical parameters were assessed using Linear Mixed-Effects Models with the lmerTest package in R.
Antibiotic treatment to remove microbiota
In the mouse model of Calcipotriol (MC903; LEO Pharma)-induced AD, adult mice (6-week-old) were intragastrically administrated with antibiotics containing 200 μL of ampicillin (1 g/kg; MedChemExpress, USA) and streptomycin (2 g/L; MedChemExpress) daily for 3 days as previously described.62,63 After the last dose of antibiotics, the mice were topically treated with MC903.
Fecal transplantation of microbiota
Mice were intragastrically administered with antibiotic cocktail (ABX) daily for 3 days as mentioned above, and then were topically treated with 2 nmol of MC903 for daily 12 days. Once the dermatitis was fully induced, 2 nmol of MC903 was applied every other day for 25 days to maintain the skin inflammation. At the same time, mice were gavaged with gut microbiota from patients with AD (AD group), healthy controls (HC group), and AD patients markedly improved by AIT (AIT group) daily for 15 days. Intragastrical phosphate buffered saline (PBS) was used as control (NC group). To prepare the gut microbiota for gavage, 5 samples from each group were randomly selected and mixed with equal amount. One gram of the mixed stool was then suspended in 10 mL PBS. The stool was mixed with vortex for 2 min to homogenate. Then, undigested food and small particulate matter was successively removed through 2.0, 1.0, 0.5, and 0.25 mm stainless steel screens. An aliquot of 200 μL suspension for each mouse was used for gavage.
Bacteria strains, isolation, and preparation
B. vesicularis strain (CGMCC, #1.3355) was purchased from CGMCC and cultured aerobically in sterilized chopped meat medium (Bowei Biotech, China) under aerobic conditions at 37°C. Cultures were collected by centrifugation at 4000 rpm for 10 min, resuspended in sterile PBS solution to 5 × 109 colony-forming units (CFUs)/mL, and preserved in −80°C condition. For the isolation of Bacteroides uniformis, Ruminococcus gnavus, and Prevotella copri, human fecal samples were collected and resuspended in sterile cold PBS. The bacterial suspensions were spread onto Brain Heart Infusion broth containing 5% sheep blood agar or DSMZ 104 medium and incubated for 2 to 7 days under anaerobic conditions at 37°C. Bacterial DNA was extracted from each single colony and the identity of individual isolates was verified by Sanger sequencing of the V1-V9 regions of 16S rRNA genes.
Mouse model
Mice were intragastrically administered with ABX daily for 3 days and then were gavaged with 200 μL of B. vesicularis or B. uniformis (1 × 109 CFU) daily for 7 days. Intragastrical PBS was used as control. Thereafter, gavage was continued while mice were topically treated with 1 nmol of MC903 and 25 μL of OVA (20 μg/μL; Sigma-Aldrich, USA) daily for 15 days to produce the AD mouse model. For AIT experiment, mice were intragastrically administered with ABX daily for 3 days, and then were gavaged with 200 μL of B. vesicularis (1 × 109 CFU) daily for 7 days. Intragastrical PBS was used as control. Thereafter, gavage was continued while mice were topically treated with 1 nmol of MC903 and 25 μL of OVA (20 μg/μL) daily for 14 days to produce the AD mouse model. Once the dermatitis was fully induced, 2 nmol of MC903 was applied every other day for 5 days to maintain the skin inflammation. Starting on day 10 of dermatitis induction, mice were injected subcutaneously with 200 μL of OVA solution (100 μg OVA and 1 mg aluminum hydroxide dissolved in normal saline) once every 4 days for 3 consecutive times, using normal saline as a control. Fresh feces were collected in sterile plastic tubes using a clean technique at the time points indicated, then immediately stored at −80°C until used for 16S rRNA sequencing and quantitative real-time PCR analysis. At the end of the treatment, mice were euthanized using CO2. Skin tissue was either fixed in formalin for histopathological analysis or stored at −80°C for mRNA expression detection. Blood samples were collected for ELISA. The CLNs, MLNs, and spleens were harvested for further flow cytometry analysis and cell culture.
Skin inflammation assessment and histopathology
At the time points indicated, full thickness of the ears was measured with dial calipers. The ear was cut into pieces, fixed in 4% formalin, and 6 μm sections were prepared and stained with hematoxylin and eosin (H&E) for evaluation of the ear thickness and inflammation. Slides were imaged using NanoZoomer digital pathology microscope (Hamamatsu Photonics, Hamamatsu City, Japan).
ELISA
For analysis of serum total IgE and OVA-specific IgE, 96-well microplate was precoated with 100 μL/well of purified rat ant-mouse IgE (1 μg/mL) overnight at 4°C, then blocked with 200 μL/well of 10% heat-inactivated fetal calf serum (FBS; Gibco, USA) at room temperature for 30 min, followed by incubation with diluted sera at room temperature for 2 h. Biotin-labeled rat anti-mouse IgE (2 μg/mL) and Biotin-labeled OVA (2.5 μg/mL) was added afterward. Then, after washing the plate was incubated with 100 μL/well of streptavidin-HRP. After incubation for 40 min at room temperature, tetramethylbenzidine (TMB, Beyotime, China) was added and the reaction was stopped by adding 50 μL/well of 2M H2SO4. The absorbance was measured at wavelength 450 nm. The levels of TGF-β1, IL-6, IL-17A, and IFN-γ in the supernatants were measured using the ELISA kits (Fcmacs Biotech, China) according to the manufacturer’s instructions.
Flow cytometry
Single-cell suspension (106 cells/tube) was incubated with FcR Blocking Reagent (Miltenyi Biotec, Germany) to block Fc receptors for 15 min at 4°C. For surface staining, the cells were stained with antibodies or matched isotype control for 20 min at 4°C in the dark. The antibodies included anti-mouse CD3e-PECYN7, CD4-FITC, CD25-APC, CD86-APC, PD-L1-PECYN7 (eBioscience, USA), CD8-Percpcy5.5, CD11c-PE, MHCII-Alexa Flour 700, MHCII-pacific blue, PD-L1-brilliant violet 510 and CD103-FITC antibodies (Biolegend, USA). For intracellular staining, cells were cultured in the presence of 2 μL/mL cell stimulation cocktail (eBioscience) for 6 h. The cells were fixed and permeabilized using fixation and permeabilization solutions, then stained with anti-mouse Foxp3-PE, IL-4-APC, IFN-γ-PE antibodies (eBioscience), IFN-γ-Brilliant Violet 510, IL-17a-Brilliant Violet 605 (Biolegend), RORγt-Brilliant Violet 421 (BD Biosciences, USA) or isotype control for 30 min at 4°C in the dark. The cells were detected using BD FACSVerse (BD Biosciences) or Aurora (Cytek, Fremont, CA, USA), and data were analyzed using the FlowJo 10.0.7 software (Tree Star, Ashland, OR, USA). For flow cytometry analysis, cells in single live CD3+CD4+CD8− cells gates were identified as Th cells, then Th1 cells were gated as IFN-γ+, Th2 cells were gated as IL-4+, Tregs were gated as CD25+Foxp3+, and Th17 cells were gated as RORγt+.64
Quantitative real-time PCR (qRT-PCR)
Total DNA was extracted from 100 mg of fecal samples per mouse using a FastPure Host Removal and Microbiome DNA Isolation Kit (Vazyme, China). The relative abundance of total bacteria (16S rRNA) or B. vesicularis was determined by qRT-PCR. For other targeted gene expression analysis, total RNA was extracted from the ear skin using TRIzol (Invitrogen, Thermo Fisher Scientific, USA) according to standard protocol. Complementary DNA was prepared with HiScript II Q RT SuperMix, and qRT-PCR was carried out using AceQ qPCR SYBR Green Master Mix (Vazyme) according to the manufacturer’s instructions. Gene expression was analyzed by real-time PCR (LC-480, Roche). Each gene was normalized to β-actin and evaluated using the ΔΔCT method to calculate the fold change. The following primers were used for qRT-PCR:
16S rRNA forward primer 5′-AAGCGGTGGATGATGTGGAT-3’
16S rRNA reverse primer 5′-GCGTAGCAACTAAGGACAAGG-3’
B. vesicularis forward primer 5′-TTGCCTTTGATACTGGGTGTCTTG-3’
B. vesicularis reverse primer 5′-CTTCGCCACTGGTGTTCTTCC-3’
Tslp forward primer 5′-ACGGATGGGGCTAACTTACAA-3’
Tslp reverse primer 5′-AGTCCTCGATTTGCTCGAACT-3’
Il17a forward primer 5′-TCAGCGTGTCCAAACACTGAG-3’
Il17a reverse primer 5′-CGCCAAGGGAGTTAAAGACTT-3’
Il22 forward primer 5′-ATGAGTTTTTCCCTTATGGGGAC-3’
Il22 reverse primer 5′-GCTGGAAGTTGGACACCTCAA-3’
Rorγt forward primer 5′-GACCCACACCTCACAAATTGA-3’
Rorγt reverse primer 5′-AGTAGGCCACATTACACTGCT-3’
Il6 forward primer 5′-TAGTCCTTCCTACCCCAATTTCC-3’
Il6 reverse primer 5′-TTGGTCCTTAGCCACTCCTTC-3’
β-Actin forward primer 5′-GTGACGTTGACATCCGTAAAGA-3’
β- Actin reverse primer 5′-GCCGGACTCATCGTACTCC-3’
DC-T cell co-culture
MLNs and spleens were harvested and grinded with syringe pistons, and then were filtered through a 200-mesh strainer to get single-cell suspension for cell culture. For isolation of splenocytes, red blood cells were removed by RBC lysis buffer (Miltenyi Biotec). For experiments of CD4+ T cells and CD11c+ DCs co-culture, CD4+ T cells were sorted from splenocytes of wild-type BALB/c mice by CD4 (L3T4) MicroBeads (Miltenyi Biotec), and CD11c+ cells were sorted from MLNs or splenocytes of wild-type BALB/c mice by CD11c-PE antibody (eBioscience) and Anti-PE MicroBeads (Miltenyi Biotec). CD11c+ DCs (5 × 105 cells/ml) were then washed and co-cultured with live or heat-killed B. vesicularis, or commensal bacterial strains (2.5 × 107 CFU/mL), or LPS (1 μg/mL) for 24 h, in the presence of LPS (0.5 μg/mL; Sigma-Aldrich) in RPMI-1640 culture medium supplemented with 10% heat-inactivated FBS. For the analysis of pre-inflammatory cytokines, the supernatants were harvested and examined for TGF-β1 and IL-6 using ELISA. For DC-T cell co-culture, the pre-stimulated CD11c+ DCs (5 × 105 cells/ml) were co-cultured with CD4+ naive T cells (2 × 106 cells/ml) for 4 days, in the presence of IL-2 (10 ng/mL; R&D Systems, USA) in RPMI-1640 culture medium supplemented with 10% heat-inactivated FBS, 100 U/ml penicillin and 100 μg/mL streptomycin. Then the supernatants (from splenic DC-CD4+ T cell co-culture system) were harvested and examined for IL-17A and IFN-γ using ELISA, and the cells (from MLN DC-CD4+ T cell co-culture system) were analyzed for polarization of CD4+ Th cells analysis by flow cytometry.
Quantification and statistical analysis
Data are expressed as means ± SD or SEM. Statistical comparisons between 2 groups were analyzed with paired or unpaired two-tailed Student’s t test and multi-group data were performed with ANOVA using GraphPad Prism 9.0 (GraphPad Software Inc., San Diego, CA, USA). A p value <0.05 was considered statistically significant.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (82073446, 82103735, 81972939, 81803144, and 82273542), the National Key R&D Program of China (2022YFC3601800), the Key Project of Social Development in Jiangsu Province (BE2020632), the CAMS Innovation Fund for Medical Sciences (2021-I2M-1-059), Nanjing Incubation Program for National Clinical Research Center (2019060001), and the Key Project of the Innovation Program of Shanghai Municipal Education Commission (2021-01-07-00-07-E00078). The experiments were conducted in Jiangsu Key Laboratory of Molecular Biology for Skin Diseases and STIs.
Author contributions
X.Y. and W.L. designed the experiments, analyzed the data, and wrote the manuscript. S.X.L. coordinated the research and provided input on experimental design. X.L., B.X., X.X., and Z.W. performed the experiments, analyzed the data, and wrote the manuscript. Y.L., Y.G., S.L., A.W., Y.Z., and X.W. performed the experiments.
Declaration of interests
The authors declare no competing interests.
Published: December 19, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2023.101340.
Contributor Information
Sean Xiao Leng, Email: sleng1@jhu.edu.
Wei Li, Email: liweiderma@fudan.edu.cn.
Xu Yao, Email: dryao_xu@126.com.
Supplemental information
References
- 1.Odhiambo J.A., Williams H.C., Clayton T.O., Robertson C.F., Asher M.I., ISAAC Phase Three Study Group Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J. Allergy Clin. Immunol. 2009;124:1251–1258.e23. doi: 10.1016/j.jaci.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Barbarot S., Auziere S., Gadkari A., Girolomoni G., Puig L., Simpson E.L., Margolis D.J., de Bruin-Weller M., Eckert L. Epidemiology of atopic dermatitis in adults: Results from an international survey. Allergy. 2018;73:1284–1293. doi: 10.1111/all.13401. [DOI] [PubMed] [Google Scholar]
- 3.Gittler J.K., Shemer A., Suárez-Fariñas M., Fuentes-Duculan J., Gulewicz K.J., Wang C.Q.F., Mitsui H., Cardinale I., de Guzman Strong C., Krueger J.G., Guttman-Yassky E. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J. Allergy Clin. Immunol. 2012;130:1344–1354. doi: 10.1016/j.jaci.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noda S., Suárez-Fariñas M., Ungar B., Kim S.J., de Guzman Strong C., Xu H., Peng X., Estrada Y.D., Nakajima S., Honda T., et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J. Allergy Clin. Immunol. 2015;136:1254–1264. doi: 10.1016/j.jaci.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 5.Wang S., Zhu R., Gu C., Zou Y., Yin H., Xu J., Li W. Distinct clinical features and serum cytokine pattern of elderly atopic dermatitis in China. J. Eur. Acad. Dermatol. Venereol. 2020;34:2346–2352. doi: 10.1111/jdv.16346. [DOI] [PubMed] [Google Scholar]
- 6.Zhang D.J., Hao F., Qian T., Cheng H.X. Expression of Helper and Regulatory T Cells in Atopic Dermatitis: A Meta-Analysis. Front. Pediatr. 2022;10 doi: 10.3389/fped.2022.777992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stelmaszczyk-Emmel A., Zawadzka-Krajewska A., Szypowska A., Kulus M., Demkow U. Frequency and activation of CD4+CD25 FoxP3+ regulatory T cells in peripheral blood from children with atopic allergy. Int. Arch. Allergy Immunol. 2013;162:16–24. doi: 10.1159/000350769. [DOI] [PubMed] [Google Scholar]
- 8.Lee J., Park C.O., Lee K.H. Specific immunotherapy in atopic dermatitis. Allergy Asthma Immunol. Res. 2015;7:221–229. doi: 10.4168/aair.2015.7.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou J., Chen S., Song Z. Analysis of the long-term efficacy and safety of subcutaneous immunotherapy for atopic dermatitis. Allergy Asthma Proc. 2021;42:e47–e54. doi: 10.2500/aap.2021.42.200126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langer S.S., Cardili R.N., Melo J.M.L., Ferriani M.P.L., Moreno A.S., Dias M.M., Bueno-Filho R., Pocente R.H.C., Roxo-Junior P., Silva J., et al. Efficacy of House Dust Mite Sublingual Immunotherapy in Patients with Atopic Dermatitis: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Allergy Clin. Immunol. Pract. 2022;10:539–549.e7. doi: 10.1016/j.jaip.2021.10.060. [DOI] [PubMed] [Google Scholar]
- 11.Bae J.M., Choi Y.Y., Park C.O., Chung K.Y., Lee K.H. Efficacy of allergen-specific immunotherapy for atopic dermatitis: a systematic review and meta-analysis of randomized controlled trials. J. Allergy Clin. Immunol. 2013;132:110–117. doi: 10.1016/j.jaci.2013.02.044. [DOI] [PubMed] [Google Scholar]
- 12.Akdis M., Akdis C.A. Mechanisms of allergen-specific immunotherapy: multiple suppressor factors at work in immune tolerance to allergens. J. Allergy Clin. Immunol. 2014;133:621–631. doi: 10.1016/j.jaci.2013.12.1088. [DOI] [PubMed] [Google Scholar]
- 13.Berings M., Karaaslan C., Altunbulakli C., Gevaert P., Akdis M., Bachert C., Akdis C.A. Advances and highlights in allergen immunotherapy: On the way to sustained clinical and immunologic tolerance. J. Allergy Clin. Immunol. 2017;140:1250–1267. doi: 10.1016/j.jaci.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 14.Lee S.Y., Lee E., Park Y.M., Hong S.J. Microbiome in the Gut-Skin Axis in Atopic Dermatitis. Allergy Asthma Immunol. Res. 2018;10:354–362. doi: 10.4168/aair.2018.10.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Los-Rycharska E., Golebiewski M., Sikora M., Grzybowski T., Gorzkiewicz M., Popielarz M., Gawryjolek J., Krogulska A. A Combined Analysis of Gut and Skin Microbiota in Infants with Food Allergy and Atopic Dermatitis: A Pilot Study. Nutrients. 2021;13:1682. doi: 10.3390/nu13051682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ta L.D.H., Chan J.C.Y., Yap G.C., Purbojati R.W., Drautz-Moses D.I., Koh Y.M., Tay C.J.X., Huang C.H., Kioh D.Y.Q., Woon J.Y., et al. A compromised developmental trajectory of the infant gut microbiome and metabolome in atopic eczema. Gut Microb. 2020;12:1–22. doi: 10.1080/19490976.2020.1801964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galazzo G., van Best N., Bervoets L., Dapaah I.O., Savelkoul P.H., Hornef M.W., GI-MDH consortium. Lau S., Hamelmann E., Penders J. Development of the Microbiota and Associations With Birth Mode, Diet, and Atopic Disorders in a Longitudinal Analysis of Stool Samples, Collected From Infancy Through Early Childhood. Gastroenterology. 2020;158:1584–1596. doi: 10.1053/j.gastro.2020.01.024. [DOI] [PubMed] [Google Scholar]
- 18.Ye S., Yan F., Wang H., Mo X., Liu J., Zhang Y., Li H., Chen D. Diversity analysis of gut microbiota between healthy controls and those with atopic dermatitis in a Chinese population. J. Dermatol. 2021;48:158–167. doi: 10.1111/1346-8138.15530. [DOI] [PubMed] [Google Scholar]
- 19.Abrahamsson T.R., Jakobsson H.E., Andersson A.F., Björkstén B., Engstrand L., Jenmalm M.C. Low diversity of the gut microbiota in infants with atopic eczema. J. Allergy Clin. Immunol. 2012;129:434-40–440.e1-2. doi: 10.1016/j.jaci.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe S., Narisawa Y., Arase S., Okamatsu H., Ikenaga T., Tajiri Y., Kumemura M. Differences in fecal microflora between patients with atopic dermatitis and healthy control subjects. J. Allergy Clin. Immunol. 2003;111:587–591. doi: 10.1067/mai.2003.105. [DOI] [PubMed] [Google Scholar]
- 21.Li W., Xu X., Wen H., Wang Z., Ding C., Liu X., Gao Y., Su H., Zhang J., Han Y., et al. Inverse Association Between the Skin and Oral Microbiota in Atopic Dermatitis. J. Invest. Dermatol. 2019;139:1779–1787.e12. doi: 10.1016/j.jid.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Climent E., Martinez-Blanch J.F., Llobregat L., Ruzafa-Costas B., Carrión-Gutiérrez M.Á., Ramírez-Boscá A., Prieto-Merino D., Genovés S., Codoñer F.M., Ramón D., et al. Changes in Gut Microbiota Correlates with Response to Treatment with Probiotics in Patients with Atopic Dermatitis. A Post Hoc Analysis of a Clinical Trial. Microorganisms. 2021;9 doi: 10.3390/microorganisms9040854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li L., Han Z., Niu X., Zhang G., Jia Y., Zhang S., He C. Probiotic Supplementation for Prevention of Atopic Dermatitis in Infants and Children: A Systematic Review and Meta-analysis. Am. J. Clin. Dermatol. 2019;20:367–377. doi: 10.1007/s40257-018-0404-3. [DOI] [PubMed] [Google Scholar]
- 24.He Z., Vadali V.G., Szabady R.L., Zhang W., Norman J.M., Roberts B., Tibshirani R., Desai M., Chinthrajah R.S., Galli S.J., et al. Increased diversity of gut microbiota during active oral immunotherapy in peanut-allergic adults. Allergy. 2021;76:927–930. doi: 10.1111/all.14540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oka A., Kidoguchi M., Kariya S., Fujiwara T., Yuta A., Miyashita H., Higaki T., Ogawa Y., Kanai K., Makihara S.I., et al. Role of salivary microbiome in IL-10 production and efficacy of sublingual immunotherapy. Allergy. 2021;76:2617–2620. doi: 10.1111/all.14858. [DOI] [PubMed] [Google Scholar]
- 26.Smith P.M., Howitt M.R., Panikov N., Michaud M., Gallini C.A., Bohlooly-Y M., Glickman J.N., Garrett W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun M., Wu W., Liu Z., Cong Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 2017;52:1–8. doi: 10.1007/s00535-016-1242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitamoto S., Nagao-Kitamoto H., Hein R., Schmidt T.M., Kamada N. The Bacterial Connection between the Oral Cavity and the Gut Diseases. J. Dent. Res. 2020;99:1021–1029. doi: 10.1177/0022034520924633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J., Jia Z., Zhang B., Peng L., Zhao F. Tracing the accumulation of in vivo human oral microbiota elucidates microbial community dynamics at the gateway to the GI tract. Gut. 2020;69:1355–1356. doi: 10.1136/gutjnl-2019-318977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park S.Y., Hwang B.O., Lim M., Ok S.H., Lee S.K., Chun K.S., Park K.K., Hu Y., Chung W.Y., Song N.Y. Oral-Gut Microbiome Axis in Gastrointestinal Disease and Cancer. Cancers. 2021;13 doi: 10.3390/cancers13092124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atarashi K., Suda W., Luo C., Kawaguchi T., Motoo I., Narushima S., Kiguchi Y., Yasuma K., Watanabe E., Tanoue T., et al. Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation. Science. 2017;358:359–365. doi: 10.1126/science.aan4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitamoto S., Nagao-Kitamoto H., Jiao Y., Gillilland M.G., 3rd, Hayashi A., Imai J., Sugihara K., Miyoshi M., Brazil J.C., Kuffa P., et al. The Intermucosal Connection between the Mouth and Gut in Commensal Pathobiont-Driven Colitis. Cell. 2020;182:447–462.e14. doi: 10.1016/j.cell.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coombes J.L., Siddiqui K.R.R., Arancibia-Cárcamo C.V., Hall J., Sun C.M., Belkaid Y., Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zuo W., Sun C.-H. The Role of Intestinal Microbiota in Atopic Dermatitis. Int. J. Dermatol. Venereol. 2021;5:155–159. [Google Scholar]
- 35.Kim J.H., Kim K., Kim W. Gut microbiota restoration through fecal microbiota transplantation: a new atopic dermatitis therapy. Exp. Mol. Med. 2021;53:907–916. doi: 10.1038/s12276-021-00627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou C.J., Xie B.L., Han H.Y., Wang Y., Wang Y.H., Hong J.Y., Wei Y.X., Liu Z.G., Feng Y., Yang G., Yang P.C. Short-Chain Fatty Acids Promote Immunotherapy by Modulating Immune Regulatory Property in B Cells. J. Immunol. Res. 2021;2021 doi: 10.1155/2021/2684361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanford J.A., Zhang L.J., Williams M.R., Gangoiti J.A., Huang C.M., Gallo R.L. Inhibition of HDAC8 and HDAC9 by microbial short-chain fatty acids breaks immune tolerance of the epidermis to TLR ligands. Sci. Immunol. 2016;1 doi: 10.1126/sciimmunol.aah4609. [DOI] [PubMed] [Google Scholar]
- 38.Schwarz A., Bruhs A., Schwarz T. The Short-Chain Fatty Acid Sodium Butyrate Functions as a Regulator of the Skin Immune System. J. Invest. Dermatol. 2017;137:855–864. doi: 10.1016/j.jid.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 39.Keshari S., Balasubramaniam A., Myagmardoloonjin B., Herr D.R., Negari I.P., Huang C.M. Butyric Acid from Probiotic Staphylococcus epidermidis in the Skin Microbiome Down-Regulates the Ultraviolet-Induced Pro-Inflammatory IL-6 Cytokine via Short-Chain Fatty Acid Receptor. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20184477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fang Z., Pan T., Li L., Wang H., Zhu J., Zhang H., Zhao J., Chen W., Lu W. Bifidobacterium longum mediated tryptophan metabolism to improve atopic dermatitis via the gut-skin axis. Gut Microb. 2022;14 doi: 10.1080/19490976.2022.2044723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fang Z., Pan T., Wang H., Zhu J., Zhang H., Zhao J., Chen W., Lu W. Limosilactobacillus reuteri Attenuates Atopic Dermatitis via Changes in Gut Bacteria and Indole Derivatives from Tryptophan Metabolism. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms23147735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Penders J., Thijs C., van den Brandt P.A., Kummeling I., Snijders B., Stelma F., Adams H., van Ree R., Stobberingh E.E. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study. Gut. 2007;56:661–667. doi: 10.1136/gut.2006.100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kirjavainen P.V., Arvola T., Salminen S.J., Isolauri E. Aberrant composition of gut microbiota of allergic infants: a target of bifidobacterial therapy at weaning? Gut. 2002;51:51–55. doi: 10.1136/gut.51.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryan M.P., Pembroke J.T. Brevundimonas spp: Emerging global opportunistic pathogens. Virulence. 2018;9:480–493. doi: 10.1080/21505594.2017.1419116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zou X.L., Wu J.J., Ye H.X., Feng D.Y., Meng P., Yang H.L., Wu W.B., Li H.T., He Z., Zhang T.T. Associations Between Gut Microbiota and Asthma Endotypes: A Cross-Sectional Study in South China Based on Patients with Newly Diagnosed Asthma. J. Asthma Allergy. 2021;14:981–992. doi: 10.2147/JAA.S320088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johansson-Lindbom B., Svensson M., Pabst O., Palmqvist C., Marquez G., Förster R., Agace W.W. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J. Exp. Med. 2005;202:1063–1073. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scott C.L., Aumeunier A.M., Mowat A.M. Intestinal CD103+ dendritic cells: master regulators of tolerance? Trends Immunol. 2011;32:412–419. doi: 10.1016/j.it.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Xu B., Ling S., Xu X., Liu X., Wang A., Zhou Y., Luo Y., Li W., Yao X. A New Formulation of Probiotics Attenuates Calcipotriol-Induced Dermatitis by Inducing Regulatory Dendritic Cells. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.775018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guan J., Li Y., Lu F., Feng J. Adipose-derived stem cells ameliorate atopic dermatitis by suppressing the IL-17 expression of Th17 cells in an ovalbumin-induced mouse model. Stem Cell Res. Ther. 2022;13:98. doi: 10.1186/s13287-022-02774-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gutowska-Owsiak D., Schaupp A.L., Salimi M., Selvakumar T.A., McPherson T., Taylor S., Ogg G.S. IL-17 downregulates filaggrin and affects keratinocyte expression of genes associated with cellular adhesion. Exp. Dermatol. 2012;21:104–110. doi: 10.1111/j.1600-0625.2011.01412.x. [DOI] [PubMed] [Google Scholar]
- 51.Nakajima S., Kitoh A., Egawa G., Natsuaki Y., Nakamizo S., Moniaga C.S., Otsuka A., Honda T., Hanakawa S., Amano W., et al. IL-17A as an inducer for Th2 immune responses in murine atopic dermatitis models. J. Invest. Dermatol. 2014;134:2122–2130. doi: 10.1038/jid.2014.51. [DOI] [PubMed] [Google Scholar]
- 52.Ma L., Xue H.B., Guan X.H., Shu C.M., Wang F., Zhang J.H., An R.Z. The Imbalance of Th17 cells and CD4(+) CD25(high) Foxp3(+) Treg cells in patients with atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2014;28:1079–1086. doi: 10.1111/jdv.12288. [DOI] [PubMed] [Google Scholar]
- 53.Hu Q., Jin L., Zeng J., Wang J., Zhong S., Fan W., Liao W. Tryptophan metabolite-regulated Treg responses contribute to attenuation of airway inflammation during specific immunotherapy in a mouse asthma model. Hum. Vaccin. Immunother. 2020;16:1891–1899. doi: 10.1080/21645515.2019.1698900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He Y.T., Zhou Y., Shao Q., Gan C., Chen M., Bao Y.L., Gu H.Y., Zhang S.L., Cui Y., Tian M. Immunoregulatory Effects of Subcutaneous Immunotherapy on Lymphocyte Subgroups and Cytokines in Children with Asthma. J. Immunol. Res. 2019;2019 doi: 10.1155/2019/7024905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kawakami H., Koya T., Kagamu H., Kimura Y., Sakamoto H., Yamabayashi C., Furukawa T., Sakagami T., Miyabayashi T., Hasegawa T., et al. IL-17 eliminates therapeutic effects of oral tolerance in murine airway allergic inflammation. Clin. Exp. Allergy. 2012;42:946–957. doi: 10.1111/j.1365-2222.2012.04006.x. [DOI] [PubMed] [Google Scholar]
- 56.Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M., Weiner H.L., Kuchroo V.K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 57.Veldhoen M., Hocking R.J., Atkins C.J., Locksley R.M., Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 58.Katoh K., Misawa K., Kuma K.i., Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Price M.N., Dehal P.S., Arkin A.P. FastTree 2--approximately maximum-likelihood trees for large alignments. PLoS One. 2010;5 doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bokulich N.A., Kaehler B.D., Rideout J.R., Dillon M., Bolyen E., Knight R., Huttley G.A., Gregory Caporaso J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2's q2-feature-classifier plugin. Microbiome. 2018;6:90. doi: 10.1186/s40168-018-0470-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bland J.M., Altman D.G. Calculating correlation coefficients with repeated observations: Part 1--Correlation within subjects. BMJ. 1995;310:446. doi: 10.1136/bmj.310.6977.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bonomo R.R., Cook T.M., Gavini C.K., White C.R., Jones J.R., Bovo E., Zima A.V., Brown I.A., Dugas L.R., Zakharian E., et al. Fecal transplantation and butyrate improve neuropathic pain, modify immune cell profile, and gene expression in the PNS of obese mice. Proc. Natl. Acad. Sci. USA. 2020;117:26482–26493. doi: 10.1073/pnas.2006065117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou D., Pan Q., Shen F., Cao H.X., Ding W.J., Chen Y.W., Fan J.G. Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci. Rep. 2017;7:1529. doi: 10.1038/s41598-017-01751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee J.Y., Hall J.A., Kroehling L., Wu L., Najar T., Nguyen H.H., Lin W.Y., Yeung S.T., Silva H.M., Li D., et al. Serum Amyloid A Proteins Induce Pathogenic Th17 Cells and Promote Inflammatory Disease. Cell. 2020;180:79–91.16. doi: 10.1016/j.cell.2019.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The 16S rRNA sequencing data reported in this paper have been deposited in the European Bioinformatics Institute (EMBL-EBI), and are publicly available as of the date of publication. Accession numbers are listed in the Key resources table. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.