Abstract
Background
Sepsis-associated acute kidney injury (SA-AKI) is a common complication of sepsis and greatly increases patient mortality. Recombinant human Klotho protein (Klotho) is a protective protein that can be secreted by the kidney. The aim of this study was to explore the protective effect of Klotho on SA-AKI and its molecular mechanism.
Methods
In vivo, a mouse SA-AKI model was constructed by cecum ligation perforation (CLP). In vitro, a human renal tubular cell epithelial cell line (HK2) was induced with lipopolysaccharide (LPS) in the SA-AKI model. Determine renal injury markers, inflammatory factors, oxidative stress and molecular proteins related to the ferroptosis signaling pathway.
Results
Klotho reduced the release of renal injury markers and inflammatory cytokines, decreased oxidative stress, improved renal histopathological changes, ameliorated mitochondrial damage in mouse renal tubular epithelial cells, increased HK2 cell viability and reduced reactive oxygen species (ROS) accumulation. Exogenous supplementation with Klotho increased the Klotho content in circulating blood, renal tissue and HK2 cells.
Conclusions
In the SA-AKI model, Klotho attenuated renal tissue injury, increased HK2 cell viability, decreased inflammatory factor expression and oxidative stress, restored tubular epithelial mitochondrial function, and increased its level in circulating blood, renal tissue and HK2 cells. Klotho probably exerts its protective effects by activating Nrf2 to inhibit the ferroptosis signaling pathway.
Keywords: Sepsis-associated acute kidney injury (SA-AKI), ferroptosis, Klotho, oxidative stress
Highlight box.
Key findings
• Klotho treatment suppresses ferroptosis and alleviates kidney injury.
What is known and what is new?
• The ferroptosis level was increased in the renal tissue of septic mice.
• The ferroptosis level was increased in the HK2 cells. Klotho increases the expression level of Klotho in cecum ligation perforation (CLP) mice and HK2 cells, and up-regulated Nrf2 in sepsis-associated acute kidney injury (SA-AKI), effects of ferroptosis inhibition.
What is the implication, and what should change now?
• It is quite conceivable that Klotho may be a unique protein which does not only serve as a potentially useful biomarker for kidney disease, but also functions as a protective protein to alleviate kidney injury, promote kidney regeneration, to arrest or slow down progression and to ameliorate complications. Research on ferroptosis in SA-AKI is still in its infancy, and pharmacologically targeting ferroptosis may be a promising therapeutic approach for sepsis and SA-AKI.
Introduction
Sepsis is defined as an inflammatory response syndrome caused by a dysregulated host response to infection, which can be life-threatening in severe cases (1). The kidney is one of the most commonly affected organs in sepsis (2). Sepsis and acute kidney injury (AKI) have long been critical illnesses independently associated with mortality, and the correlation between them is as high as 50% (3). Sepsis-associated AKI (SA-AKI) is defined as sepsis in the presence of AKI, AKI without other significant factors explaining AKI, or a disease characterized by the presence of both sepsis and AKI (4). Compared with other causes of AKI, the pathogenesis of SA-AKI patients is more complex, patients with SA-AKI have a significantly higher mortality rate and medical costs, and there are no specific drugs and methods available to date (5).
Apoptosis and necrosis are considered to be the main forms of cell death; however, an increasing number of studies have confirmed that nonapoptotic cell death is also a programmed form of cell death, also known as regulatory necrosis (6). Ferroptosis is an oxidative stress-dependent regulatory necrosis that differs significantly from other known forms of cell death, such as apoptosis, necrosis, and autophagy, in its biochemistry, histomorphology, and genetics (7). A previous study has shown that ferroptosis is a key mechanism for sequential tubular cell death after ischemic kidney injury and that inhibition of the ferroptosis signaling pathway can promote kidney recovery and reduce the severity of kidney injury (8). There are no relevant studies on ferroptosis and SA-AKI.
Klotho is a family of aging-related proteins that exist in vivo primarily as α-, β-, and γ-Klotho isoforms (9). Alpha-Klotho, also known as “soluble” Klotho protein, is the main functional form in the circulation and is highly expressed in the kidney (especially in the distal and proximal tubules) but also in the blood, urine, and cerebrospinal fluid (9-11). It has been shown that α-Klotho has better organ protective effects, including the heart, brain, and blood vessels, but its effects and mechanisms on septic kidneys are not clear (12-14). Therefore, in this study, α-Klotho (hereafter referred to as Klotho) was selected to explore its role in SA-AKI.
Nuclear factor erythroid-2 related factor 2 (Nrf2), the core transcription factor of the endogenous cellular antioxidant stress system, can translocate to the nucleus and bind to specific gene loci, thereby attenuating the cellular oxidative stress response and protecting cellular function (15). In addition, studies have confirmed that increasing Nrf2 levels in various oxidative stress models can effectively reduce the related organ damage (16,17). However, the relationship between Nrf2 and ferroptosis-related signaling pathways in AKI in mice with sepsis and in human renal tubular epithelial (HK2) cells has not been clarified. In this study, by constructing SA-AKI mouse and cellular models, we elucidated whether the ferroptosis signaling pathway is involved in the development of SA-AKI. We examined whether Klotho protein inhibits ferroptosis by activating the ferroptosis signaling pathway and plays a role in HK2 cell protection in SA-AKI. We present this article in accordance with the ARRIVE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-23-573/rc).
Methods
Animal model preparation and grouping
Eight- to ten-week-old C57BL/6J male mice (weight: 20–25 g) were purchased from Beijing Spelford Laboratory Animal Technology Co., Ltd. (China). A cecal ligation perforation operation was used to prepare an animal model of sepsis. C57BL/6J mice were randomly divided into five groups: (I) sham-operated group (sham group, n=8); (II) cecum ligation perforation group (CLP group, n=8); (III) CLP + liprestatin-1 (Abcam, Cambridge, UK) group (CLP + Lip-1 group, n=8); (IV) CLP + recombinant human Klotho protein (Abcam) group (CLP + Klotho group, n=8); and (V) CLP + Klotho + Nrf2-IN-1 (GlpBio, Montclair, CA, USA) group (n=8).
Mice were injected with Lip-1 (10 mg/kg) and Klotho protein (10 mg/kg) intraperitoneally 1 h before the construction of the CLP model; Nrf2-IN-1 (10 mg/kg) was injected intraperitoneally 12 h before the construction of the CLP model. Mice were anesthetized with isoflurane (induction concentration 3.5%, maintenance concentration 3%). Experiments were performed under a project license (No. 2022006) granted by Animal Experiment Center of the Fourth Hospital of Hebei Medical University, in compliance with the National Institutes of Health (USA) Guidelines for the care and use of animals (NIH Publication No. 85-23, revised 1985: http://grants1.nih.gov/grants/olaw/references/phspol.htm). A protocol was prepared before the study without registration.
Cell cultures
HK2 cells were purchased from Pronosai Life Sciences (Wuhan, China). HK2 cells in a good growth state were inoculated into culture dishes after passaging and randomly divided into five groups: (I) blank control group (control group); (II) lipopolysaccharide (Beijing, China) group (LPS group); (III) LPS + Lip-1 group; (IV) LPS + Klotho group; and (V) LPS + Klotho + Nrf2-IN-1 group.
Except for the control group, the four groups of cells were cultured with medium containing 40 µg/mL LPS for 6 h. After that, the cells were cultured with new medium containing liproxstatin-1 (0.5 µM), klotho (100 µg/mL), or klotho (100 µg/mL) + Nrf2-IN-1 (20 µM) for 24 h.
Serum and tissue marker measurements
The serum levels of the oxidative factor markers tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), malondialdehyde (MDA), glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) were measured by enzyme-linked immunosorbent assay (ELISA) kits (Elabscience Biotechnology, Wuhan, China). Mouse kidney cortex was taken for tissue homogenization, and the levels of Fe2+ in the tissues were determined strictly and consistently according to the instructions provided in the assay kits (Elabscience Biotechnology). Serum creatinine (Scr), blood urea nitrogen (BUN) and the new markers of kidney injury, Kim-1, neutrophil gelatinase-associated lipocalin (NGAL) and Klotho, were measured using the appropriate kits (Jiancheng, Nanjing, China).
Histopathology
The mice were euthanized 24 h after successful construction of the CLP model, and the left kidney tissue was removed and fixed in 4% paraformaldehyde solution and 2.5% glutaraldehyde fixative for HE staining and electron microscopic observation (HT7800/HT7700, Hitachi Ltd., Tokyo, Japan).
Renal tubular injury was assessed independently by two pathologists and exhibited cellular degeneration and vacuolization, reduced brush border epithelium, tubular obstruction, and cast formation. Renal tubular injury was graded as follows: 0, normal; 1, tubular injury area ≤25%; 2, 25%< tubular injury area ≤50%; 3, 50%< tubular injury area ≤75%; and 4, 75%< tubular injury area ≤100%.
Measurement of cell viability and intracellular reactive oxygen species (ROS)
Cell Counting Kit 8 (CCK-8) assays were performed according to the manufacturer’s manual (Beyotime, Shanghai, China). Briefly, 2,000 cells in 100 µL culture were added into each well of a 96-well plate for 0 and 24 h. At each time point, 10 µL of sterile CCK-8 was added to each well and incubated for another hour at 37 ℃. The absorbance at 450 nm was determined using a microplate reader.
The intracellular ROS levels in vitro and in vivo were detected by the fluorescent probe DCFH-DA according to the manufacturer’s instructions (Solarbio, Beijing, China). Briefly, samples were incubated in 10 µmol/L reagent for another 20 min at 37 ℃. Then, the cells were collected for fluorescence intensity detection.
Immunohistochemistry and fluorescence
Paraformaldehyde-fixed mouse kidney tissues were dehydrated in gradient alcohol, paraffin-embedded, sectioned, dewaxed, antigen-repaired in EDTA antigen repair buffer (pH 8.0), and blocked in bovine serum albumin (BSA) for 30 min. The sections were incubated with primary antibody at 4 ℃ overnight and secondary antibody at room temperature for 40 min. 4,6-diamino-2-phenyl indole (DAPI) was used to restrain the nuclei, and the sections were shaken dry, sealed with anti-fluorescence quenching sealer, observed and acquired under a fluorescence microscope (DM3000 LED, Leica, Wetzlar, Germany). Images were collected. The results were analyzed using ImageJ software.
The treated cells were fixed in 4% paraformaldehyde, rinsed in phosphate buffered saline (PBS), placed in Triton X-100 (mass fraction 0.5%) for 10 min and blocked in BAS for 1 h. The primary antibody was incubated overnight at 4 ℃ and rinsed in PBS; the secondary antibody was incubated at room temperature for 2 h and rinsed in PBS, dried and observed under a fluorescence microscope, and images were acquired. The results were analyzed using ImageJ software.
Western blot (WB)
Mouse kidney tissues and HK2 cells were added to RIPA lysis buffer at a fixed ratio (Sunncell, Wuhan, China). The supernatant was removed for protein quantification using a bicinchoninic acid (BCA) kit (Solarbio). After electrophoresis on 6–12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels, the proteins were transferred to polyvinylidene fluoride (PVDF) membranes. After being blocked in 5% skim milk powder for 1.5–2 h at room temperature, PVDF membranes were incubated overnight at 4 ℃ with the following primary antibodies: glutathione peroxidase 4 (GPX4) (1:1,000, Abcam), Nrf2 (1:1,000, Proteintech, Wuhan, China), Klotho (1:1,000, Abcam), and β actin (1:1,000, Servicebio, Wuhan, China), after which the PVDF membrane was immersed in secondary antibody (1:10,000, Abbkine, Wuhan, China) and incubated at room temperature for 1 h. Klotho requires a special secondary antibody, which is incubated with 5 µg/mL of recombinant Klotho and then conjugated to a chemiluminescent triple antibody for visualization. Finally, images were developed with chemiluminescent solution, and the grayscale values of the target bands were processed and measured using ImageJ software.
Statistical analysis
In this study, GraphPad Prism 9 software was used to plot the experimental data and SPSS 27 software was used to analyze the data statistically.
Results
Klotho ameliorates AKI in CLP mice
A total of 40 mice were included in this study after excluding dead and uninjured mice. Lip-1 is a specific ferroptosis inhibitor, and a study has shown that exogenous supplementation of Lip-1 improves lung tissue damage induced by CLP and LPS in acute lung injury (7). But as a chemical synthesis, there is currently no clinical research or evidence for its use. Clinical signs of sepsis were observed in the CLP group of mice, including reduced viability, decreased body weight, hypothermia, ocular pus, vertical hair, ascites, pus stool and pus urine.
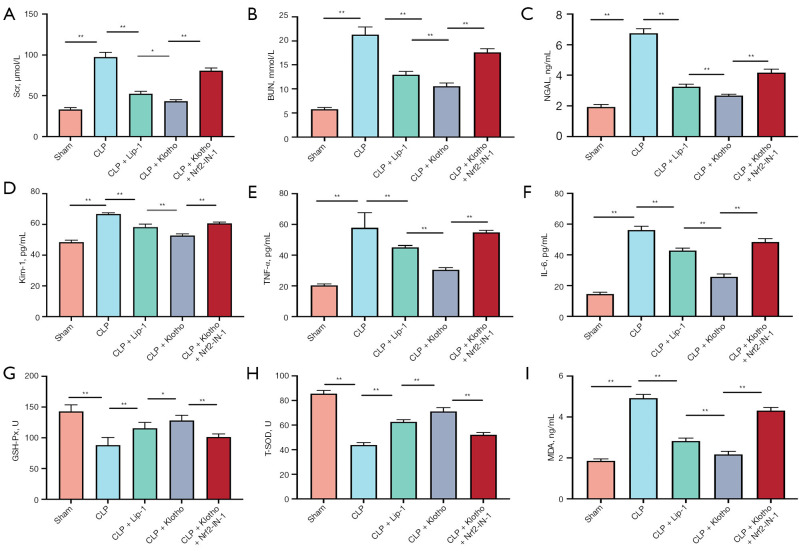
In addition, the markers of renal injury Scr, BUN, NGAL and Kim-1 were significantly elevated in CLP mice, and Lip-1 and Klotho interventions were able to reduce the levels of Scr, BUN, NGAL and Kim-1, while this protective effect of Lip-1 and Klotho was diminished after Nrf2-IN-1 treatment (Figure 1A-1D). At 24 h of CLP model preparation, the values of Scr and BUN in the CLP group were significantly increased, which were significantly different from those in the sham group, and the mean serum Scr and BUN in the CLP group could be increased to 96 µmol/L and 21.3 mmol/L, respectively, and the levels of serum NGAL and Kim-1 were also dramatically increased. The concentrations of Scr, BUN, NGAL and Kim-1 varied with different interventions.
Figure 1.
Effect of Klotho on renal function, inflammatory factors, oxidative stress and pathological kidney injury. (A,B) Levels of Scr (A) and BUN (B) in serum. n=6. (C,D) Levels of NGAL (C) and Kim-1 (D) in serum. n=6. (E,F) Levels of TNF-α (E) and IL-6 (F) in serum. n=6. (G-I) Levels of GSH-Px (G), T-SOD (H) and MDA (I) in serum. n=6. The results are expressed as the means ± standard deviation. *, P<0.05; **, P<0.01. CLP, cecal ligation and puncture; Lip-1, liprostatin-1; Klotho, recombinant human Klotho protein; Scr, serum creatinine; BUN, blood urea nitrogen; NGAL, neutrophil gelatinase-associated lipocalin; Kim-1, kidney injury molecule 1; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; GSH-Px, glutathione peroxidase; SOD, superoxide dismutase; MDA, malondialdehyde.
Klotho reduces inflammatory factors and oxidative stress in CLP mice
Both serum TNF-α and IL-6 levels were significantly increased in the CLP group, and Klotho treatment reduced both levels (Figure 1E,1F). In addition, GSH-Px levels and SOD activity were significantly lower in the CLP group than in the sham group mice, while MDA levels were dramatically increased. Treatment with Lip-1 and Klotho increased renal GSH-Px and SOD levels and decreased MDA levels, while the effect of Lip-1 and Klotho was diminished with the use of Nrf2-IN-1 (Figure 1G-1I).
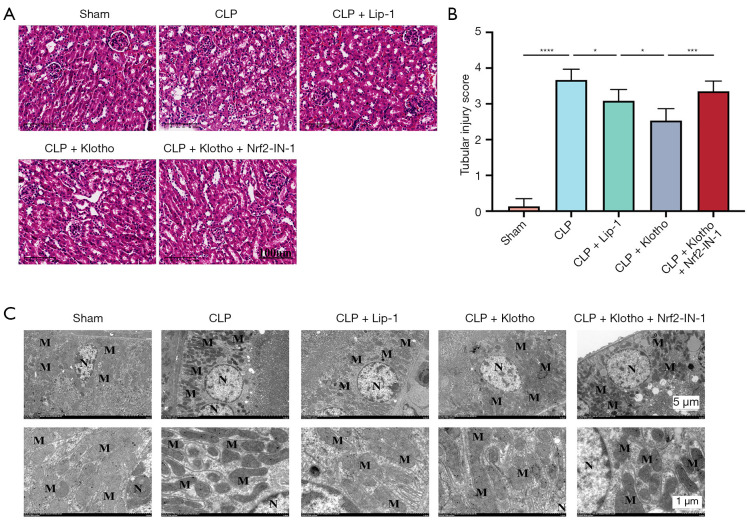
The results of HE staining showed that the CLP group had necrosis, cytolysis, fragmentation, inflammatory cell infiltration, vascular filling, interstitial edema, and increased neutrophil infiltration in the kidney tissue compared with the sham group. While the application of Lip-1 and Klotho significantly reduced the degree of renal tissue injury and decreased inflammatory cell infiltration, the protective effect of Lip-1 and Klotho was diminished after Nrf2-IN-1 intervention (Figure 2A,2B).
Figure 2.
Effect of Klotho on pathological kidney injury. (A) HE staining scanning of renal tissue. (B) Histopathological scores. n=5. (C) Morphological observation of the kidney under an electron microscope. The results are expressed as the means ± standard deviation. *, P<0.05; ***, P<0.001; ****, P<0.0001. HE, hematoxylin-eosin; CLP, cecal ligation and puncture; Lip-1, liprostatin-1; Klotho, recombinant human Klotho protein; M, mitochondrion; N, nucleus.
Klotho ameliorates renal mitochondrial damage in CLP mice
Transmission electron microscopy (TEM; HT7800/HT7700, Hitachi Ltd., Tokyo, Japan) showed that the mitochondria in the sham group were relatively normal in structure, round or rod-shaped with normal size and high number. The most obvious alteration caused by CLP was the destruction of mitochondrial structure with a significant decrease in number. The application of Lip-1 and Klotho improved CLP-induced mitochondrial damage, while the protective effect of Lip-1 and Klotho was diminished after the administration of Nrf2-IN-1(Figure 2C).
Klotho exerts renoprotective effects by activating Nrf-2 to inhibit the ferroptosis signaling pathway
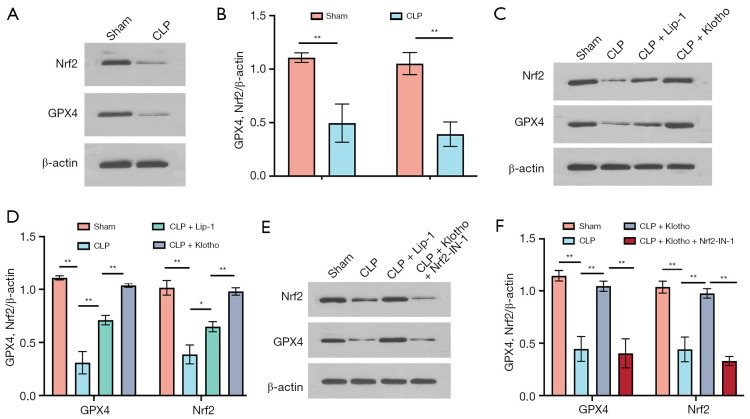
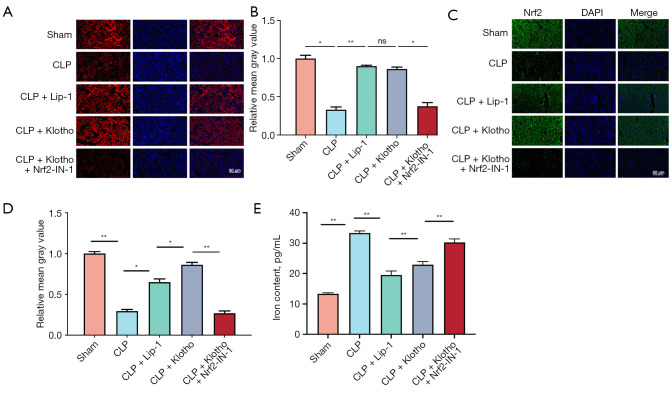
The results of this study showed that CLP significantly decreased the expression of GPX4 and Nrf2 proteins (Figure 3A,3B), and the inhibitors of the ferroptosis pathway Lip-1 and Klotho significantly attenuated the reduction in GPX4 and Nrf2 expression (Figure 3C,3D). In contrast, the expression level of GPX4 was significantly reduced after treatment with Nrf2-IN-1 (Figure 3E,3F). The above results were consistent with the results of the GPX4 and Nrf2 immunofluorescence assay in mouse kidney tissues (Figure 4A-4D). This study further clarified the temporal expression of GPX4 and Nrf2 in renal tissue 24 hours after CLP model preparation using immunofluorescence method, which is similar to the results of WB; and clarified the localization of GPX4 and Nrf2 in renal tissue: mainly expressed in renal tubules. The iron content of kidney tissues was measured, and the free divalent iron content was significantly higher in the CLP group (Figure 4E). In addition, Lip-1 and Klotho administration significantly reduced the iron concentration, and the effect of Lip-1 was the most significant, while Nrf2-IN-1 treatment greatly reversed the effect of Klotho (Figure 4E).
Figure 3.
Effect of Klotho treatment on ferroptosis in CLP-induced SA-AKI. (A) Representative bands of WB and (B) quantification of GPX4 and Nrf2 in mouse kidneys in the sham and CLP groups. n=3. (C) Representative bands of WB and (D) quantification of GPX4 and Nrf2 in mouse kidneys among the four groups (sham, CLP, CLP + Lip-1, and CLP + Klotho groups). n=3. (E) Representative bands of WB and (F) quantification of GPX4 and Nrf2 among the four groups (sham, CLP, CLP + Klotho, and CLP + Klotho + Nrf2-IN-1 groups). β-actin was used as an internal control. The results are expressed as the means ± standard deviation. *, P<0.05; **, P<0.01. CLP, cecal ligation and puncture; SA-AKI, sepsis-associated acute kidney injury; WB, western blot; Nrf2, nuclear factor erythroid-2 related factor 2; Lip-1, liprostatin-1; Klotho, recombinant human Klotho protein.
Figure 4.
Effect of Klotho treatment on ferroptosis in CLP-induced SA-AKI. (A) Representative IF images and (B) quantification of GPX4 (red) in mouse kidneys from each group. n=3. (C) IF representative images and (D) quantification of Nrf2 (green) from mice in each group. n=3. (E) Levels of iron in tissue. n=6. The results are expressed as the means ± standard deviation. *, P<0.05; **, P<0.01. CLP, cecal ligation and puncture; Lip-1, liprostatin-1; Klotho, recombinant human Klotho protein; DAPI, 4,6-diamino-2-phenyl indole; Nrf2, nuclear factor erythroid-2 related factor 2; SA-AKI, sepsis-associated acute kidney injury; IF, immunofluorescence; ns, no significance.
The above findings suggest that the mechanism of action of Klotho is to nephroprotective by inhibiting the iron apoptosis signaling pathway through the activation of Nrf2 (Figures 3,4), which effectively reduced the iron content in the tissues (Figure 4E).
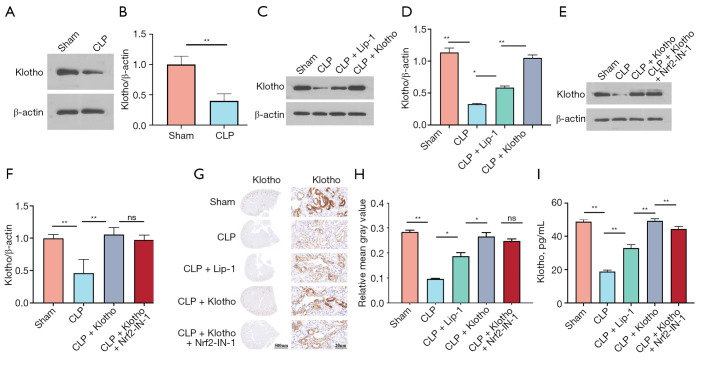
Klotho increases the expression level of Klotho in CLP mice
WB analysis is an antibody based protein analysis technique. WB results showed that Klotho levels were significantly reduced in the CLP group (Figure 5A,5B), increased after Lip-1 intervention, significantly increased after Klotho intervention (Figure 5C,5D), and reduced after Nrf2-IN-1 intervention, but not significantly different from the Klotho group (Figure 5E,5F). The WB results were again validated by immunohistochemical assay (Figure 5G,5H). The ELISA results for mouse serum Klotho levels were consistent with the histological assay results (Figure 5I).
Figure 5.
Klotho treatment increases the levels of Klotho in kidney tissue. (A) Representative bands and (B) quantification of Klotho in mouse kidneys in the sham and CLP groups. n=3. β-actin was used as an internal control. (C) Representative bands and (D) quantification of Klotho in mouse kidneys among the four groups (sham, CLP, CLP + Lip-1, and CLP + Klotho groups). n=3. (E) Representative bands and (F) quantification of Klotho among the four groups (sham, CLP, CLP + Klotho, and CLP + Klotho + Nrf2-IN-1 groups). n=3. β-actin was used as an internal control. (G) IHC staining and (H) quantification of Klotho. n=3. (I) Levels of Klotho in serum. n=6. The results are expressed as the means ± standard deviation. *, P<0.05; **, P<0.01. Klotho, recombinant human Klotho protein; CLP, cecal ligation and puncture; Lip-1, liprostatin-1; Nrf2, nuclear factor erythroid-2 related factor 2; IHC, immunohistochemical; ns, no significance.
The above study demonstrated that Klotho has good nephroprotective effects, and exogenous supplementation not only increased circulating Klotho levels but also increased tissue Klotho levels in mice.
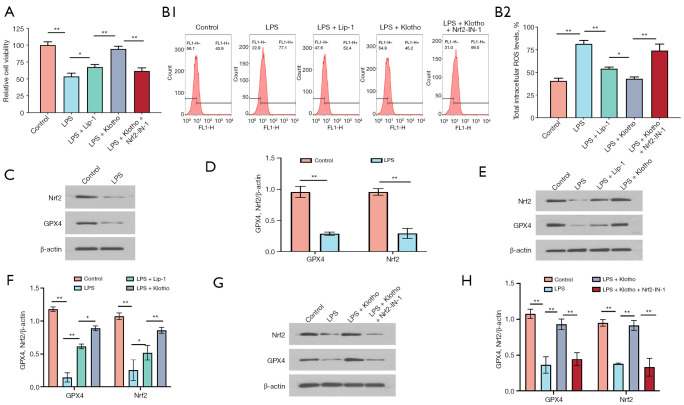
Klotho attenuates HK2 cell injury, increases viability and reduces ROS accumulation
The results of the cell viability assay of HK2 cells showed that LPS significantly decreased cell viability, while Lip-1 and Klotho treatment significantly increased the viability of HK2 cells, and the effect of Klotho was the most significant, while the effect of Lip-1 and Klotho was reduced when Nrf2-IN-1 was administered (Figure 6A).
Figure 6.
Effect of Klotho on LPS-induced HK2 cells. (A) Viability of HK2 cells in each group. n=3. (B1,B2) Levels of ROS in HK2 cells. n=3. (C) Representative bands and (D) quantification of GPX4 and Nrf2 in HK2 cells in the control and LPS groups. n=3. (E) Representative bands and (F) quantification of GPX4 and Nrf2 in HK2 cells in the control, LPS, LPS + Lip-1, and LPS + Klotho groups. n=3. (G) Representative bands and (H) quantification of GPX4 and Nrf2 in HK2 cells in the control, LPS, LPS + Klotho, and LPS + Klotho + Nrf2-IN-1 groups. n=3. β-actin was used as an internal control. *, P<0.05; **, P<0.01. LPS, lipopolysaccharide; Lip-1, liprostatin-1; Klotho, recombinant human Klotho protein; Nrf2, nuclear factor erythroid-2 related factor 2; ROS, reactive oxygen species; IF, immunofluorescence.
Intracellular ROS was significantly increased in the LPS group, while Lip-1 and Klotho significantly reduced the intracellular ROS content in HK2 cells, and the effect of Klotho was most significant when Nrf2-IN-1 was administered (Figure 6B).
Klotho ameliorates HK2 cell injury by activating Nrf-2 to inhibit the ferroptosis signaling pathway
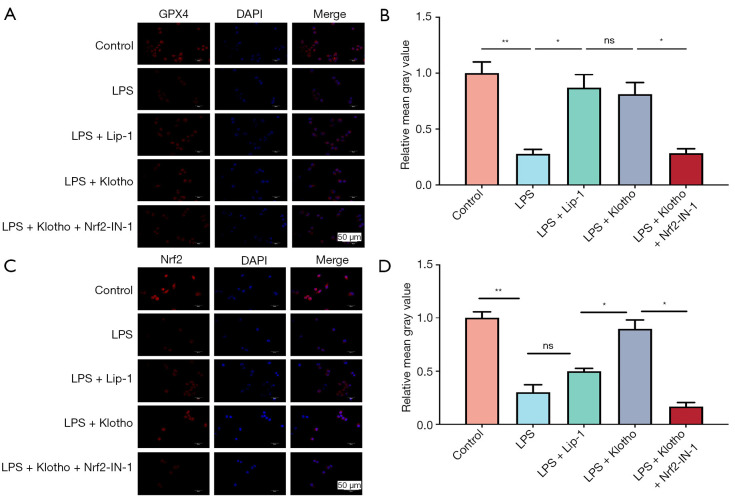
The results of this study showed that LPS significantly decreased the expression of GPX4 and Nrf2 in HK2 cells (Figure 6C,6D), and Lip-1 and Klotho interventions increased the expression of GPX4 and Nrf2 (Figure 6E,6F). In contrast, Nrf2-IN-1 intervention significantly decreased the expression of GPX4 and Nrf2 (Figure 6G,6H). Immunofluorescence detection of GPX4 and Nrf2 in HK2 cells was performed for verification (Figure 7A-7D).
Figure 7.
Effect of Klotho on LPS-induced HK2 cells. (A) Representative IF images and (B) quantification of GPX4 (red) in HK2 cells in each group. n=3. (C) Representative IF images and (D) quantification of Nrf2 (red) in HK2 cells in each group. n=3. The results are expressed as the means ± standard deviation. ns, no significance; *, P<0.05; **, P<0.01. LPS, lipopolysaccharide; Lip-1, liprostatin-1; Klotho, recombinant human Klotho protein; Nrf2, nuclear factor erythroid-2 related factor 2; ROS, reactive oxygen species; IF, immunofluorescence; ns, no significance.
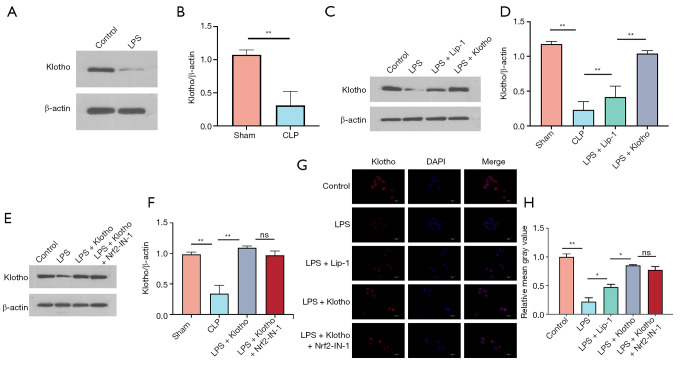
Klotho increases HK2 cell Klotho levels
WB results showed a significant decrease in Klotho content in the LPS group (Figure 8A,8B) and an increase in the Lip-1 group. The Klotho content was significantly higher in the Klotho group (Figure 8C-8F), and Nrf2-IN-1 intervention decreased the Klotho content of Klotho + Nrf2-IN-1 group but was not significantly different from that in the Klotho group. The immunohistochemical assay results were consistent with the WB results (Figure 8G,8H).
Figure 8.
Klotho treatment increases the levels of Klotho in HK2 cells. (A) Representative bands and (B) quantification of Klotho in HK2 cells in the control and LPS groups. n=3. (C) Representative bands and (D) quantification of Klotho in HK2 cells in the control, LPS, LPS + Lip-1, and LPS + Klotho groups. n=3. (E) Representative bands and (F) quantification of Klotho in HK2 cells in the control, LPS, LPS + Klotho, and LPS + Klotho + Nrf2-IN-1 groups. n=3. β-actin was used as an internal control. (G) Representative IF images and (H) quantification of Klotho (red) in HK2 cells in each group. n=3. The results are expressed as the means ± standard deviation. ns, no significance; *, P<0.05; **, P<0.01. Klotho, recombinant human Klotho protein; LPS, lipopolysaccharide; CLP, cecal ligation and puncture; Lip-1, liprostatin-1; Nrf2, nuclear factor erythroid-2 related factor 2; IF, immunofluorescence; ns, no significance.
Discussion
AKI has been of great concern because of its significant increase in mortality in septic patients, the complexity of disease management, and the lack of specific preventive and therapeutic approaches to date (3,4,18). Recently, researchers have started exploring new diagnostic and therapeutic approaches for SA-AKI based on relevant genetic biological characteristics (19). In this study, we centered around CLP- and LPS-induced sepsis in mice and human renal tubular epithelial cells to open new therapeutic avenues for SA-AKI around inflammation, oxidative stress, and iron death in anticipation of opening up new ideas for the treatment of SA-AKI and improving patient prognosis.
Ferroptosis is a form of programmed cell death closely related to amino acid, iron, and lipid metabolism and is caused by the accumulation of lethal lipid peroxidation products (20). GPX4 is a specific marker of the ferroptosis signaling pathway and plays a key role (21,22). There is a close association between ferroptosis and several diseases (digestive system tumors, neurodegenerative diseases, and cardiac and kidney ischemia neyrodegene), and the regulation of ferroptosis may have great potential for application in the treatment of diseases (23-26). In addition, ferroptosis can exacerbate the inflammatory response, further leading to organ functional damage and tissue cell death (27). A study suggests that modulation of the ferroptosis signaling pathway may be a new and effective therapeutic target (21). In this study, the presence and important role of ferroptosis in SA-AKI was confirmed by constructing in vivo and in vitro sepsis models, and inhibition of the ferroptosis signaling pathway reduced renal tissue and HK2 cell injury and improved renal function.
Klotho is a pleiotropic protein clearly associated with aging, as well as an excellent antioxidant that regulates multiple cellular signaling pathways and exerts different biological functions in organs and tissues (9). Klotho knockout mice are prone to blood-brain barrier damage and central nervous system disorders, and one study confirmed that cerebrospinal fluid levels of Klotho in patients with Alzheimer’s disease are reduced in patients with Alzheimer’s disease (10,14,28). Klotho overexpression protects cardiac function by regulating the Wnt signaling pathway and activating the AKT/Nrf2/HO-1 pathway to slow cellular senescence and antioxidant activity and reduce stem cell depletion (10,29). Studies have shown that Klotho promotes angiogenesis and maintains endothelial cell integrity by regulating apoptosis and autophagy of endothelial cells (16,30). Our study found that exogenous supplementation with Klotho increased Klotho levels in circulating blood, renal tissue and HK2 cells, which is of great significance in arresting disease progression and accelerating disease recovery.
Control of inflammation and oxidative stress is essential for the treatment and prevention of various diseases, especially sepsis (15). Pathogen invasion not only activates the inflammatory cascade signaling response but also accelerates the accumulation of proinflammatory factors and ROS, which directly leads to endothelial cell damage and increased leukocyte and platelet rolling and adhesion, resulting in microthrombosis. GSH, SOD and MDA are good markers of oxidative stress, and GSH and SOD can act as cofactors for a variety of enzymes to repair damaged DNA, reduce ROS accumulation and protect mitochondrial function (21,31). In this study, we found that Klotho significantly reduced the levels of the inflammatory factors TNF-α and IL-6 and decreased the oxidative stress response.
Renal tubular epithelial cell injury is a heterogeneous, focal and patchy injury and an extremely important aspect of SA-AKI (2,6,32). Its structural and functional separation is most likely due to the occurrence of metabolic adaptation, that is, cell survival at the expense of organ function, which is closely related to mitochondria-mediated energy depletion, substrate utilization, mitochondrial phagocytosis, biogenesis, and inhibition of apoptosis (6,32). When looking at the renal tissue structure of AKI rodent models and patients under electron microscopy, researchers found a decrease in the number of mitochondria, mitochondrial swelling, vesicle formation, mitochondrial cristae disorganization, lysis and even rupture in renal tubular epithelial cells, a phenomenon that was predominant in proximal tubular epithelial cells and appeared before the clinical manifestations (6,33). Mitochondria play an important role in the regulation of oxidative stress both by controlling the progression of inflammation and by acting as a source and target of oxidants (5,8,33,34). Our findings suggest that sepsis leads to reduced mitochondrial number and altered mitochondrial structure in HK2 cells. The application of ferroptosis inhibitors and exogenous supplementation with Klotho can improve mitochondrial number and structure in HK2 cells, and Klotho may reduce mitochondrial damage and improve mitochondrial function by inhibiting the ferroptosis signaling pathway.
An increasing number of studies have confirmed that Klotho plays a relevant role in disease processes by regulating Nrf2 signaling (16,35). Nrf2 is a suppressor of lipid peroxidation and ferroptosis and can exert its biological functions by regulating apoptosis and oxidative stress responses (15). Klotho attenuates the Nrf2/HO-1 signaling pathway by activating Klotho, ameliorates acute lung injury and protects human retinal pigment epithelial and endothelial cell functions by activating the Nrf2/HO-1 signaling pathway (35). Studies on the most critical anti-aging effects of Klotho have shown that a lack of inhibition of Nrf2 pathway activity by Klotho accelerates the process of cardiac aging, while increasing Nrf2 activity effectively reverses this phenomenon (29). In urological diseases, Klotho ameliorates diabetic nephropathy and slows the progression of chronic kidney disease by activating the Nrf2 signaling pathway (10,36). In this regard, we further explored the relationship between Klotho and Nrf2 and the ferroptosis signaling pathway by constructing in vivo and in vitro sepsis models and performing related functional rescue experiments, and the results showed that Klotho inhibited the ferroptosis signaling pathway in SA-AKI kidney tissues and HK2 cells by activating the Nrf2 pathway, exerting a renoprotective effect.
According to the results of this study, Klotho reduces the accumulation of lipid peroxides and iron in tissues and cells by inhibiting the release of inflammatory factors, reducing the degree of oxidative stress response, and improving the homeostasis of tissues and cells. This suppresses the discussion of ferroptosis signals and improves tissue and cell damage. The ferroptosis plays a crucial role in SA-AKI, and the relevant biological mechanisms of Klotho’s action can be verified. In future clinical management, drugs with ferroptosis inhibitory effects can be prioritized for use in sepsis patients to reduce kidney damage.
In this study, in vivo and in vitro models of SA-AKI animals were constructed by CLP and LPS, respectively, which can simulate the common patterns of sepsis under clinical conditions and fully elucidate its effects.
Conclusions
In summary, Klotho ameliorated renal tissue injury, increased HK2 cell viability and improved renal function by activating Nrf2 to inhibit the ferroptosis signaling pathway. Klotho also reduced inflammatory factor expression and oxidative stress in SA-AKI mice, thereby reducing mitochondrial damage in renal tubular epithelial cells. Exogenous supplementation with Klotho protein was able to increase the Klotho content in blood, renal tissue and HK2 cells in sepsis models.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by the Department of Science and Technology of Hebei Province of China (No. 20277707D).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (No. 2022006) granted by Animal Experiment Center of the Fourth Hospital of Hebei Medical University, in compliance with the National Institutes of Health (USA) Guidelines for the care and use of animals (NIH Publication No. 85-23, revised 1985: http://grants1.nih.gov/grants/olaw/references/phspol.htm).
Footnotes
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-23-573/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-573/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-573/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-23-573/coif). The authors have no conflicts of interest to declare.
References
- 1.Cecconi M, Evans L, Levy M, et al. Sepsis and septic shock. Lancet 2018;392:75-87. 10.1016/S0140-6736(18)30696-2 [DOI] [PubMed] [Google Scholar]
- 2.Bellomo R, Kellum JA, Ronco C, et al. Acute kidney injury in sepsis. Intensive Care Med 2017;43:816-28. 10.1007/s00134-017-4755-7 [DOI] [PubMed] [Google Scholar]
- 3.Peerapornratana S, Manrique-Caballero CL, Gómez H, et al. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int 2019;96:1083-99. 10.1016/j.kint.2019.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poston JT, Koyner JL. Sepsis associated acute kidney injury. BMJ 2019;364:k4891. 10.1136/bmj.k4891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet 2019;394:1949-64. 10.1016/S0140-6736(19)32563-2 [DOI] [PubMed] [Google Scholar]
- 6.Agarwal A, Dong Z, Harris R, et al. Cellular and Molecular Mechanisms of AKI. J Am Soc Nephrol 2016;27:1288-99. 10.1681/ASN.2015070740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hadian K, Stockwell BR. SnapShot: Ferroptosis. Cell 2020;181:1188-1188.e1. 10.1016/j.cell.2020.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Z, Wu J, Xu H, et al. XJB-5-131 inhibited ferroptosis in tubular epithelial cells after ischemia-reperfusion injury. Cell Death Dis 2020;11:629. 10.1038/s41419-020-02871-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchanan S, Combet E, Stenvinkel P, et al. Klotho, Aging, and the Failing Kidney. Front Endocrinol (Lausanne) 2020;11:560. 10.3389/fendo.2020.00560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Y, Sun Z. Molecular basis of Klotho: from gene to function in aging. Endocr Rev 2015;36:174-93. 10.1210/er.2013-1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landry T, Shookster D, Huang H. Circulating α-klotho regulates metabolism via distinct central and peripheral mechanisms. Metabolism 2021;121:154819. 10.1016/j.metabol.2021.154819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drew DA, Katz R, Kritchevsky S, et al. Soluble Klotho and Incident Hypertension. Clin J Am Soc Nephrol 2021;16:1502-11. 10.2215/CJN.05020421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li L, Liu W, Mao Q, et al. Klotho Ameliorates Vascular Calcification via Promoting Autophagy. Oxid Med Cell Longev 2022;2022:7192507. 10.1155/2022/7192507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neyra JA, Hu MC, Moe OW. Klotho in Clinical Nephrology: Diagnostic and Therapeutic Implications. Clin J Am Soc Nephrol 2020;16:162-76. 10.2215/CJN.02840320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forman HJ, Zhang H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov 2021;20:689-709. 10.1038/s41573-021-00233-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen K, Wang S, Sun QW, et al. Klotho Deficiency Causes Heart Aging via Impairing the Nrf2-GR Pathway. Circ Res 2021;128:492-507. 10.1161/CIRCRESAHA.120.317348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li W, Yu W, Xu W, et al. Death-Associated Protein Kinase 1 Regulates Oxidative Stress in Cardiac Ischemia Reperfusion Injury. Cells Tissues Organs 2021;210:380-90. 10.1159/000518248 [DOI] [PubMed] [Google Scholar]
- 18.Gómez H, Kellum JA. Sepsis-induced acute kidney injury. Curr Opin Crit Care 2016;22:546-53. 10.1097/MCC.0000000000000356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu B, Ao S, Tan F, et al. Transcriptomic analysis and laboratory experiments reveal potential critical genes and regulatory mechanisms in sepsis-associated acute kidney injury. Ann Transl Med 2022;10:737. 10.21037/atm-22-845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 2012;149:1060-72. 10.1016/j.cell.2012.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xl L, Gy Z, R G, et al. Ferroptosis in sepsis: The mechanism, the role and the therapeutic potential. Front Immunol 2022;13:956361. 10.3389/fimmu.2022.956361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu H, Santo A, Jia Z, et al. GPx4 in Bacterial Infection and Polymicrobial Sepsis: Involvement of Ferroptosis and Pyroptosis. React Oxyg Species (Apex) 2019;7:154-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin-Sanchez D, Fontecha-Barriuso M, Martinez-Moreno JM, et al. Ferroptosis and kidney disease. Nefrologia (Engl Ed) 2020;40:384-94. 10.1016/j.nefro.2020.03.005 [DOI] [PubMed] [Google Scholar]
- 24.Weiland A, Wang Y, Wu W, et al. Ferroptosis and Its Role in Diverse Brain Diseases. Mol Neurobiol 2019;56:4880-93. 10.1007/s12035-018-1403-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang G, Dong D, Wan X, et al. Cardiomyocyte death in sepsis: Mechanisms and regulation (Review). Mol Med Rep 2022;26:257. 10.3892/mmr.2022.12773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu S, He Y, Lin L, et al. The emerging role of ferroptosis in intestinal disease. Cell Death Dis 2021;12:289. 10.1038/s41419-021-03559-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen X, Kang R, Kroemer G, et al. Ferroptosis in infection, inflammation, and immunity. J Exp Med 2021;218:e20210518. 10.1084/jem.20210518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao Y, Zeng CY, Li XH, et al. Klotho overexpression improves amyloid-β clearance and cognition in the APP/PS1 mouse model of Alzheimer's disease. Aging Cell 2020. [Epub ahead of print]. doi: . 10.1111/acel.13239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Z, Zheng S, Feng X, et al. Klotho gene improves oxidative stress injury after myocardial infarction. Exp Ther Med 2021;21:52. 10.3892/etm.2020.9484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou H, Pu S, Zhou H, et al. Klotho as Potential Autophagy Regulator and Therapeutic Target. Front Pharmacol 2021;12:755366. 10.3389/fphar.2021.755366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Q, Wu J, Zhang X, et al. Iron homeostasis and disorders revisited in the sepsis. Free Radic Biol Med 2021;165:1-13. 10.1016/j.freeradbiomed.2021.01.025 [DOI] [PubMed] [Google Scholar]
- 32.Pickkers P, Darmon M, Hoste E, et al. Acute kidney injury in the critically ill: an updated review on pathophysiology and management. Intensive Care Med 2021;47:835-50. 10.1007/s00134-021-06454-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zuo Z, Jing K, Wu H, et al. Mechanisms and Functions of Mitophagy and Potential Roles in Renal Disease. Front Physiol 2020;11:935. 10.3389/fphys.2020.00935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun J, Zhang J, Tian J, et al. Mitochondria in Sepsis-Induced AKI. J Am Soc Nephrol 2019;30:1151-61. 10.1681/ASN.2018111126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xing L, Guo H, Meng S, et al. Klotho ameliorates diabetic nephropathy by activating Nrf2 signaling pathway in podocytes. Biochem Biophys Res Commun 2021;534:450-6. 10.1016/j.bbrc.2020.11.061 [DOI] [PubMed] [Google Scholar]
- 36.Sanchez-Niño MD, Fernandez-Fernandez B, Ortiz A. Klotho, the elusive kidney-derived anti-ageing factor. Clin Kidney J 2019;13:125-7. 10.1093/ckj/sfz125 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as