Abstract
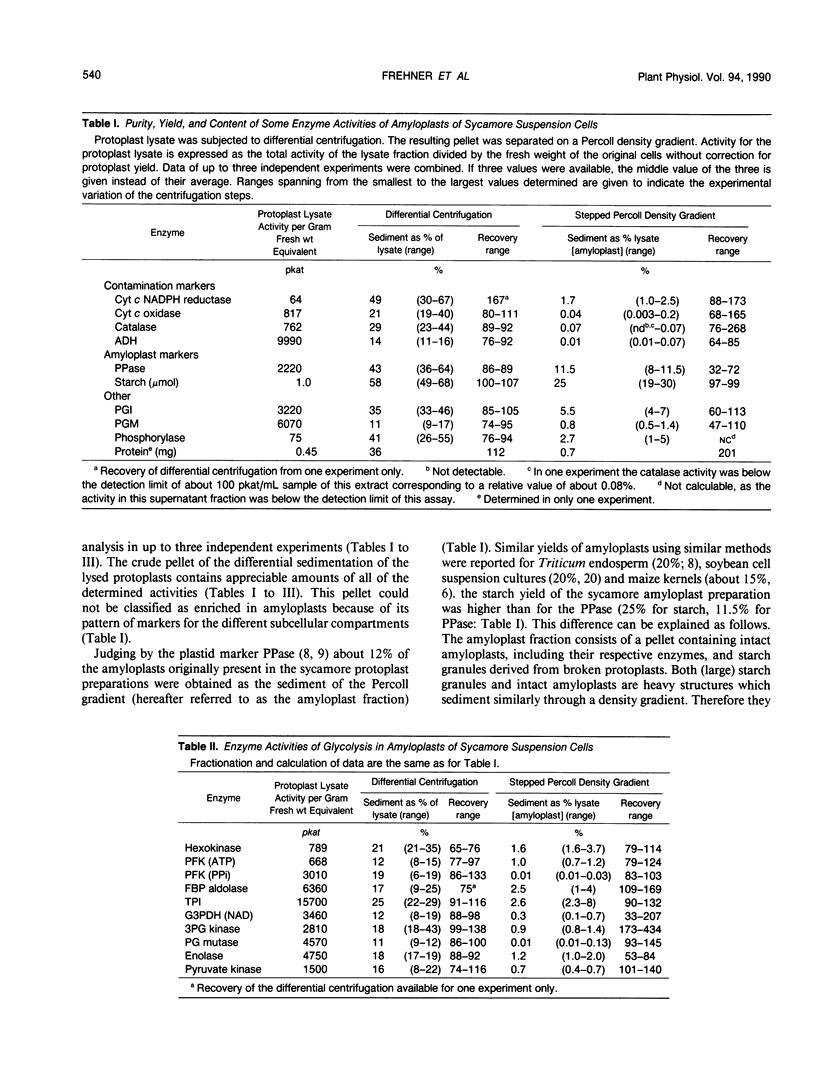
Differential centrifugation and Percoll-gradient centrifugation of protoplast lysates of suspension-cultured cells of sycamore (Acer pseudoplatanus L.) yielded pure amyloplasts. Contamination of the final amyloplast preparation by foreign compartments was assessed by measuring marker enzyme activities. The activity of alkaline pyrophosphatase was taken as a 100% plastid marker; relative to this marker, mitochondria (cytochrome c oxidase) averaged 0.34%, microbodies (catalase) 0.61%, and cytosol (alcohol dehydrogenase) 0.09%. Enzymatic activities of the glycolytic, gluconeogenic, pentose phosphate and the starch degradation pathways were found to be present in these amyloplast extracts in appreciable amounts. But the pyrophosphate-dependent phosphofructokinase and phosphoglyceromutase were judged to be essentially absent from amyloplasts because the activities of these enzymes were not enriched above the level of contaminating enzymatic activities in the amyloplast fractions. Additionally, the in vitro activities of starch phosphorylase, ATP dependent phosphofructokinase, NAD dependent glyceraldehyde-3 phosphate dehydrogenase, and glucose-6 phosphate dehydrogenase did not seem to support carbon fluxes from starch to triose phosphates as calculated from the rate of starch disappearance during carbon starvation of the cells. These results provide additional, indirect evidence for the recently emerged view that, in addition to the well known phosphate-triosephosphate translocator, another hexose phosphate and possibly also an ATP/ADP translocating system play major roles in nongreen plastids.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ap Rees T., Thomas S. M., Fuller W. A., Chapman B. Location of gluconeogenesis from phosphoenolpyruvate in cotyledons of Cucurbita pepo. Biochim Biophys Acta. 1975 Mar 14;385(1):145–156. doi: 10.1016/0304-4165(75)90082-3. [DOI] [PubMed] [Google Scholar]
- Doremus H. D., Jagendorf A. T. Subcellular localization of the pathway of de novo pyrimidine nucleotide biosynthesis in pea leaves. Plant Physiol. 1985 Nov;79(3):856–861. doi: 10.1104/pp.79.3.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverria E., Boyer C. D., Thomas P. A., Liu K. C., Shannon J. C. Enzyme activities associated with maize kernel amyloplasts. Plant Physiol. 1988 Mar;86(3):786–792. doi: 10.1104/pp.86.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entwistle G., Rees T. A. Enzymic capacities of amyloplasts from wheat (Triticum aestivum) endosperm. Biochem J. 1988 Oct 15;255(2):391–396. doi: 10.1042/bj2550391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt H. W., Rapley L. Unspecific permeation and specific uptake of substances in spinach chloroplasts. FEBS Lett. 1970 Apr 2;7(2):139–142. doi: 10.1016/0014-5793(70)80140-5. [DOI] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Journet E. P., Bligny R., Douce R. Biochemical changes during sucrose deprivation in higher plant cells. J Biol Chem. 1986 Mar 5;261(7):3193–3199. [PubMed] [Google Scholar]
- Journet E. P., Douce R. Enzymic capacities of purified cauliflower bud plastids for lipid synthesis and carbohydrate metabolism. Plant Physiol. 1985 Oct;79(2):458–467. doi: 10.1104/pp.79.2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling P. L., Wood J. R., Tyson R. H., Bridges I. G. Starch Biosynthesis in Developing Wheat Grain : Evidence against the Direct Involvement of Triose Phosphates in the Metabolic Pathway. Plant Physiol. 1988 Jun;87(2):311–319. doi: 10.1104/pp.87.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochetov G. A. Transketolase from yeast, rat liver, and pig liver. Methods Enzymol. 1982;90(Pt E):209–223. doi: 10.1016/s0076-6879(82)90128-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Macherel D., Kobayashi H., Akazawa T., Kawano S., Kuroiwa T. Amyloplast nucleoids in sycamore cells and presence in amyloplast DNA of homologous sequences to chloroplast genes. Biochem Biophys Res Commun. 1985 Nov 27;133(1):140–146. doi: 10.1016/0006-291x(85)91852-2. [DOI] [PubMed] [Google Scholar]
- Ngernprasirtsiri J., Takabe T., Akazawa T. Immunochemical Analysis Shows That an ATP/ADP-Translocator Is Associated with the Inner-Envelope Membranes of Amyloplasts from Acer pseudoplatanus L. Plant Physiol. 1989 Apr;89(4):1024–1027. doi: 10.1104/pp.89.4.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M., Beevers H. Subcellular distribution of gluconeogenetic enzymes in germinating castor bean endosperm. Plant Physiol. 1979 Jul;64(1):31–37. doi: 10.1104/pp.64.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlrogge J. B., Kuhn D. N., Stumpf P. K. Subcellular localization of acyl carrier protein in leaf protoplasts of Spinacia oleracea. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1194–1198. doi: 10.1073/pnas.76.3.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rébeillé F., Bligny R., Martin J. B., Douce R. Effect of sucrose starvation on sycamore (Acer pseudoplatanus) cell carbohydrate and Pi status. Biochem J. 1985 Mar 15;226(3):679–684. doi: 10.1042/bj2260679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura S., Nagai M., Fukui T. Comparative glucan specificities of two types of spinach leaf phosphorylase. J Biochem. 1982 Feb;91(2):703–717. doi: 10.1093/oxfordjournals.jbchem.a133743. [DOI] [PubMed] [Google Scholar]
- Tsolas O., Joris L. Transaldolase. Methods Enzymol. 1975;42:290–297. doi: 10.1016/0076-6879(75)42130-9. [DOI] [PubMed] [Google Scholar]
- WU R., RACKER E. Regulatory mechanisms in carbohydrate metabolism. III. Limiting factors in glycolysis of ascites tumor cells. J Biol Chem. 1959 May;234(5):1029–1035. [PubMed] [Google Scholar]
- Widholm J. M., Kishinami I. Allyl Alcohol Selection for Lower Alcohol Dehydrogenase Activity in Nicotiana plumbaginifolia Cultured Cells. Plant Physiol. 1988 Jan;86(1):266–269. doi: 10.1104/pp.86.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ap Rees T., Green J. H., Wilson P. M. Pyrophosphate:fructose 6-phosphate 1-phosphotransferase and glycolysis in non-photosynthetic tissues of higher plants. Biochem J. 1985 Apr 1;227(1):299–304. doi: 10.1042/bj2270299. [DOI] [PMC free article] [PubMed] [Google Scholar]


