Abstract
Previous findings suggest that medically underserved patients are prescribed medications with pharmacogenetic (PGx) guidelines at a high frequency. Thus, underserved patients may especially benefit from PGx testing, but little evidence exists regarding the effect of testing in this population. This pilot study aimed to generate key feasibility data and explore clinical outcomes of PGx implementation in underserved populations. Black and Latino patients were recruited from an outpatient clinic and underwent PGx testing. Feasibility measures included enrollment metrics and actionable genotype frequencies. The primary clinical outcome was patient medication treatment satisfaction 6 months after testing. Implementation outcomes included the number of healthcare provider encounters and medication changes within the 6‐month follow‐up. Effectiveness outcomes included medication adherence, patient‐perceived test value, and time spent discussing medications with providers. Ninety‐nine patients completed the study. Proton‐pump inhibitors were the most frequent PGx drug class prescribed at baseline (61%) followed by nonsteroidal anti‐inflammatory drugs (36%). Patients with an actionable genotype constituted 96% of the population, whereas 28% had an actionable genotype related to their PGx drug. Patient treatment satisfaction significantly increased over the 6 months after PGx testing. In addition, medication adherence and the number of provider encounters significantly increased over the study period. In a pilot study, preemptive PGx testing was feasible in primary care clinics, improved patient treatment satisfaction and adherence, and increased the number of provider encounters in medically underserved patients. Future clinical trials are warranted to assess the long‐term effects of PGx testing in a larger diverse patient population.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Medically underserved patients are prescribed pharmacogenetic (PGx) medications at a higher frequency and have fewer encounters with healthcare providers compared to patients with better access to health care.
WHAT QUESTION DID THIS STUDY ADDRESS?
Is clinical implementation of preemptive PGx testing feasible in a medically underserved patient population? Does clinical implementation of preemptive PGx testing in a medically underserved patient population improve treatment outcomes?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Clinical implementation of PGx is feasible in outpatient clinics serving medically underserved patients and may improve patient treatment satisfaction, medication adherence, and number of provider visits.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
This study warrants conducting additional research of preemptive PGx testing to include other underserved patient populations as well as to assess the long‐term effects of preemptive PGx testing on future prescribing and clinical outcomes because this population may especially benefit from this technology.
INTRODUCTION
The concept of clinical pharmacogenetic (PGx) testing was introduced in the early 1990s; yet, it is still primarily limited to large academic medical centers, minimizing the number of patients with access to this important component of precision medicine. 1 , 2 In addition, European or Asian ancestral groups have been the most extensively studied in genetic association analyses; meanwhile, patients of African descent have a higher frequency of genetic variants that could influence their drug response compared to those of European descent. 3 , 4 Therefore, clinical PGx implementation may exacerbate health disparities because its patterns are based on discovery work conducted mostly in European or Asian ancestral populations.
Although genotype‐guided drug therapy recommendations have been gaining more support, mainly due to increasing evidence regarding clinical utility and improvement of technologies, optimal timing for completing testing has not reached consensus. Preemptive panel‐based PGx testing allows for genotype information, for potentially dozens or hundreds of pharmacogenes, to be readily available in the patient's medical record prior to drug prescribing – greatly increasing clinical utility. 5 , 6 The benefits of preemptive PGx testing panels are that (1) the risk of adverse events and the prediction of effectiveness across multiple drugs can be addressed with a single test, (2) the genotype can be used and reused over the patient's lifetime, (3) reaching therapeutic effectiveness can potentially be achieved faster because results are already available for use, and (4) the incremental cost of multiple PGx tests can be prevented. 6 , 7 Therefore, with the necessary tools and guidance to support a preemptive model, implementation of pharmacogenomics can be more fully realized. 2 , 8
Drugs with PGx guidelines or recommendations available (referred to as PGx drugs) are often off‐patent and less expensive than newer counterparts. 9 We have previously found that medically underserved patients are prescribed PGx medications at a higher frequency and have fewer encounters with healthcare providers compared to patients with better access to health care. 10 Thus, medically underserved patients may especially benefit from minimizing the currently used trial‐and‐error process of drug selection and using a more personalized prescribing approach. In 2020, Medicare coverage included PGx testing for patients who were either already on a PGx drug or were being considered for a treatment with gene‐drug interactions, but it did not include preemptive PGx testing. 11 As for private insurance companies, coverage of PGx tests has been shown to vary widely, and the number of gene‐drug indication groups covered remains suboptimal across the board with only about 20% of gene‐drug indication groups covered on average. 12 The lack of complete information available as well as the suboptimal coverage found indicate a lack of transparency regarding PGx coverage and represent a financial burden on patients that would benefit from testing. 12 With some third‐party reimbursement patterns still not favoring PGx testing (especially when done preemptively), disparities in drug prescribing may continue to increase for patients unable to afford PGx testing costs out‐of‐pocket. 13 Moreover, racial differences contribute to this disparity with underserved Black patients having significantly fewer healthcare provider visits and being prescribed PGx drugs more frequently than underserved non‐Black patients. 10 Therefore, there is a crucial need to inform the clinical implementation of preemptive PGx testing in medically underserved populations to assure equitable distribution of this innovative healthcare technology. The objective of this pilot study was to develop key feasibility data for the clinical implementation of preemptive PGx testing within the University of Florida Health System (UF Health), and to generate important data to support future larger trials of clinical implementation of PGx in underserved populations.
METHODS
Study design
This was a pragmatic, clinical pilot study of patients self‐identifying as Black or Latino. Participants received clinical PGx testing as part of the study, with results placed in their medical records, and underwent assessments measuring both implementation and effectiveness outcomes at baseline, 3 months, and 6 months after testing. This study was approved by the University of Florida (UF) Institutional Review Board and was registered at ClinicalTrials.gov (NCT04630093).
Patient population
Patients were recruited between May 2021 and November 2021 from UF Health clinics within medically underserved areas serving a large number of low‐income, Black, and Latino patients. Patients were eligible if they (1) were 18 years of age or older, (2) self‐identified as Black or Latino, (3) had active prescriptions for at least three medications documented within their medical records (including at least 1 drug/drug class that could be informed by the UF PGx test panel described below), and (4) had a medication change within the past 6 months that was associated with a healthcare provider encounter. Patients were excluded if they already had a test result in their electronic health records (EHRs) for any of the following genes included on the UF PGx test panel: CYP2C19, CYP2D6, CYP3A5, SLCO1B1, CYP2C9, CYP2C, CYP4F2, and VKORC1.
Study procedures
Clinic schedules were screened by study staff to identify eligible patients. Study staff alerted the clinic team to eligible patients and requested that the provider inquire about the patient's interest in study participation and/or provide the patient with an approved flyer with study staff contact information for patients to use. Patients indicating interest were then approached by study staff either during the clinic visit or via phone call after the clinic visit if in‐person contact was not possible. All participants provided written informed consent prior to their participation in this research.
Upon enrollment, a DNA sample was obtained from each patient via buccal brush/swab. At baseline, and at 3‐month and 6‐month timepoints, subjects completed surveys regarding their demographics, socioeconomic status, medical history, and medication satisfaction. In addition to these measures, data regarding medical encounters and medication prescribing were collected from the patient's EHR. The surveys were either administered verbally by study staff or were completed electronically by the participants on a tablet available for the study.
PGx testing
PGx testing was completed using the UF PGx panel (Table 1) which is available for clinical use at UF Health, and is completed by the CAP/CLIA‐certified UF Health Pathology Laboratories' (UF Path Labs) Molecular Diagnostic Laboratory. The cost of the PGx testing was covered by the study. Once results were entered into the patient's EHR as discrete data fields through a laboratory data interface, Clinical Decision Support (CDS) Best Practice Advisories (BPAs) already built into the UF Health EHR system alerted providers if a PGx interaction (a drug being prescribed to a patient with a genetic variant that may affect response to that drug) might occur. In addition, a PGx clinical consult note was completed by a pharmacist within the UF Health Precision Medicine Program with test result interpretations and therapeutic recommendations. The consult note was forwarded to the patient's primary care provider(s), and all prescribing decisions were made at the discretion of the patient's healthcare provider.
TABLE 1.
Genes and variants included in the UF PGx panel.
| Gene | Variants |
|---|---|
| CYP2C19 | *2, *3, *4, *6, *8, *10, *17 |
| CYP2C9 | *2, *3, *5, *6, *8, *11 |
| CYP2D6 | *10, *2, *17, *41, *3, *4, *6, *9, *8, *7, *29, CYP2D6 copy number |
| CYP3A5 | *3, *6, *7 |
| CYP4F2 | *3 |
| CYP2C cluster | rs12777823 |
| SLCO1B1 | *5 |
| VKORC1 | 1639G>A |
Abbreviations: PGx, pharmacogenetic; UF, University of Florida.
Outcomes measured
As feasibility outcomes, the percent of patients agreeing to participate, from the total number approached, was recorded. In addition, the percent of patients with an actionable genotype as well as the percent of patients with an actionable genotype related to the PGx drug they were prescribed were also calculated. An actionable genotype was defined as a genotype that would lead to an institutionally approved CDS alert prompting a prescriber to alter dosing or medication selection. The alerts were based on Clinical Pharmacogenetic Implementation Consortium (CPIC) guidelines where prescribing changes are recommended for genotypes affecting response to treatment. 14 In addition, genotype turnaround time was calculated as (1) brain‐to‐brain turnaround time (time between when the UF PGx panel was ordered and when the genotype was entered into the EHR), (2) laboratory turnaround time (time between the sample being received by the UF Path Labs and the genotype being entered into the EHR), and (3) sample turnaround time (time between the DNA sample collection and the genotype result entered into Epic). The primary effectiveness outcome measured was the change in patient treatment satisfaction between baseline and 6 months after PGx testing. This was measured via the Treatment Satisfaction Questionnaire for Medication version 1.4 (TSQM). 15 Global satisfaction as well as scores from each individual domain (effectiveness, side effects, and convenience) were analyzed. The TSQM domain scores range from 0 to 100 with higher scores representing higher satisfaction for each domain. Other effectiveness outcomes measured via questionnaires included medication adherence, perceived value of the test, and time spent discussing medications with healthcare providers. Questionnaires were administered at baseline, at 3 months, and 6 months after PGx test results were entered in the EHR. Secondary implementation outcomes included the number of encounters with a healthcare provider as well as the number of medication changes within the 6‐month follow‐up period and within the 6‐month window prior to the baseline study visit, which were all collected from the EHRs.
Statistical analyses
Patient baseline characteristics were computed as means and standard deviations for continuous variables and counts and percentages for categorical variables. Continuous outcome variables were compared in the same patients among baseline, 3‐month, and 6‐month visits using the paired Wilcoxon signed‐rank test. Binary outcome variables were compared between different visits using the McNemar's test. A p value ≤ 0.05 was considered statistically significant. To assess the magnitude of TSQM score changes between visits, effect sizes based on Cohen's d were calculated. 16 All analyses were conducted using R version 4.2.1.
RESULTS
Baseline patient characteristics
During the 6 months study period, 207 patients were approached, and 100 patients (48.3%) consented to be enrolled in the study. Ninety‐nine patients completed the study, with an average age of 54.4 (±14.8) years (Table 2). Women constituted 78.8% of the population, and 86.9% of patients self‐identified as Black. Most participants (62.6%) were not employed and over half (53.5%) did not possess a college degree. When asked about how comfortable they were understanding medical terminology and concepts, 12.1% were uncomfortable, 35.3% of patients felt neutral, and 52.6% were comfortable. As for insurance, 59.6% of patients were on Medicaid/Medicare/Medicare plus private or supplemental insurance, 37.4% had private insurance, and 3% were self‐paid.
TABLE 2.
Patient characteristics.
| Patient demographics and socioeconomic measures | N = 99 |
|---|---|
| Age, years | 54.4 (±14.8) |
| Female | 78 (78.8) |
| Race | |
| Black/non‐Latino | 85 (85.9) |
| Black/Latino | 1 (1.0) |
| White/Latino | 10 (10.10) |
| Other or Mixed Descent/non‐Latino a | 2 (2.0) |
| Other or Mixed Descent/Latino b | 1 (1.0) |
| Employment | |
| Full time | 32 (32.3) |
| Part time | 1 (1.0) |
| Disabled | 34 (34.3) |
| Seeking work | 6 (6.1) |
| Retired | 19 (19.2) |
| Homemaker | 1 (1.0) |
| Student | 2 (2.0) |
| Unknown | 3 (3.0) |
| Health literacy | |
| Extremely uncomfortable | 2 (2.0) |
| Very uncomfortable | 2 (2.0) |
| Somewhat uncomfortable | 8 (8.1) |
| Neutral | 35 (35.3) |
| Somewhat comfortable | 20 (20.2) |
| Very comfortable | 26 (26.3) |
| Extremely comfortable | 6 (6.1) |
| Income | |
| <$25,000 | 28 (28.2) |
| $25,001–50,000 | 20 (20.2) |
| $50,001–75,0000 | 3 (3.0) |
| $75,001–100,000 | 3 (3.0) |
| $100,001–150,000 | 1 (1.0) |
| >$150,000 | 0 (0.0) |
| Do not know | 35 (35.4) |
| Refuse to answer | 9 (9.1) |
| Education | |
| Less than high school (9th grade) | 3 (3.0) |
| Some high school | 14 (14.1) |
| High school/GED | 36 (36.4) |
| Some college or specialized training/technical school | 35 (35.4) |
| Bachelor's degree | 8 (8.1) |
| Master's degree | 2 (2.0) |
| Doctoral degree | 1 (1.0) |
| Insurance | |
| Medicaid/Medicare/Medicare plus private or supplemental insurance | 59 (59.6) |
| Private | 37 (37.4) |
| Self‐pay | 3 (3.0) |
Note: Values are expressed as mean (±SD) or N (%).
Abbreviation: GED, Government Education Development.
Asian/White/American Indian/Black (n = 1), Black/White (n = 1).
Haitian Puerto Rican/Dominican (n = 1).
At baseline, the most frequent health conditions were hypertension (25% of patients), followed by arthritis/chronic pain at 15%, and gastroesophageal reflux disease/ulcers, diabetes, and depression each at 14% of patients (Figure 1a). The PGx drug classes used most frequently were proton‐pump inhibitors (PPIs) being prescribed in 61%, followed by nonsteroidal anti‐inflammatory drugs in 36% of patients (Figure 1b).
FIGURE 1.
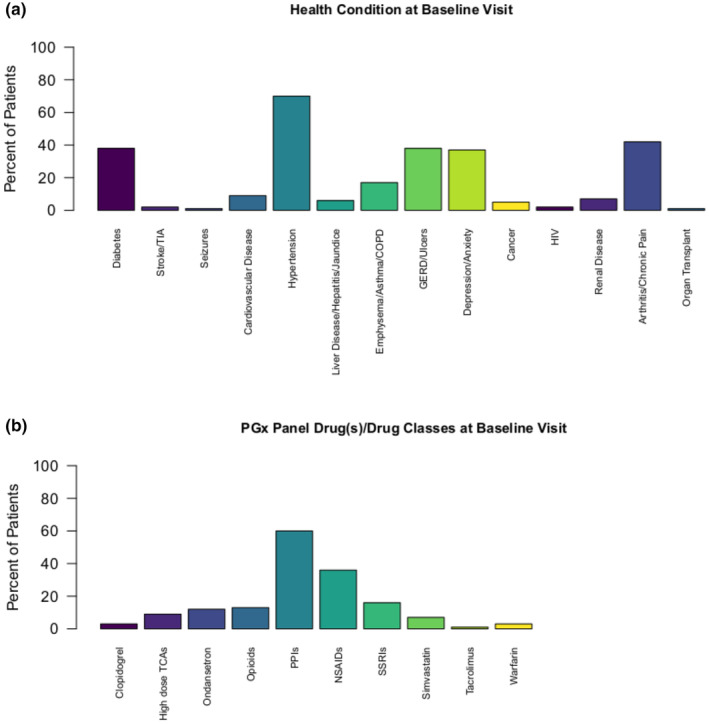
Frequency of patients by (a) health conditions at baseline and (b) PGx panel drug(s)/drug classes at baseline. COPD, chronic obstructive pulmonary disease; GERD, gastroesophageal reflux disease; HIV, human immunodeficiency virus; NSAIDs, non‐steroidal anti‐inflammatory drugs; PGx, pharmacogenetic; PPIs, proton pump inhibitors; SSRIs, selective serotonin reuptake inhibitors; TCAs, tricyclic antidepressants; TIA, transient ischemic attack.
PGx test results and turnaround time
There were 96 patients with an actionable genotype for at least one of the genes included on the UF PGx panel (listed above). Of those, 28 had an actionable genotype related to the PGx drug they were prescribed (counts per drug/gene pair listed in Table S1). The turnaround time between the UF PGx panel being ordered and the genotype being entered into the EHR averaged 6.6 (±2.7) days. The time between the sample being received by the UF Path Labs and the genotype result being entered into the EHR averaged 5.9 (±2.6) days. Finally, the time between the DNA sample being collected and the genotype result being entered into the EHR averaged 7.3 (±2.7) days, as DNA can be collected at enrollment while awaiting a pharmacist to sign the PGx test order in the EHR.
Patient treatment satisfaction
We observed a significant increase in global treatment satisfaction scores between baseline and 3 months after PGx testing (59.9 ± 16.9 vs 62.2 ± 17.6, p = 0.023) which persisted through 6 months after PGx testing (59.9 ± 16.9 vs. 64.4 ± 13.6, p = 0.001; Figure 2). This increased satisfaction seemed to have been driven by significantly increased satisfaction within the effectiveness domain after both 3 months (59.6 ± 14.4 vs. 62.5 ± 15.7, p = 0.008) and 6 months (59.6 ± 14.4 vs. 63.3 ± 11.7, p = 0.003; Figure 2). The effect size, based on Cohen's d, for the mean differences in the TSQM global satisfaction was small between baseline and 3 months (0.13) and ranged from small to moderate between baseline and 6 months (0.29; Table S2). Similarly, the effect size for the mean differences in the TSQM effectiveness domain was small between baseline and 3 months (0.19) and ranged from small to moderate between baseline and 6 months (0.28; Table S1). Scores within the side effects and convenience domains also increased over time, but these differences were not statistically significant (Figure 2).
FIGURE 2.
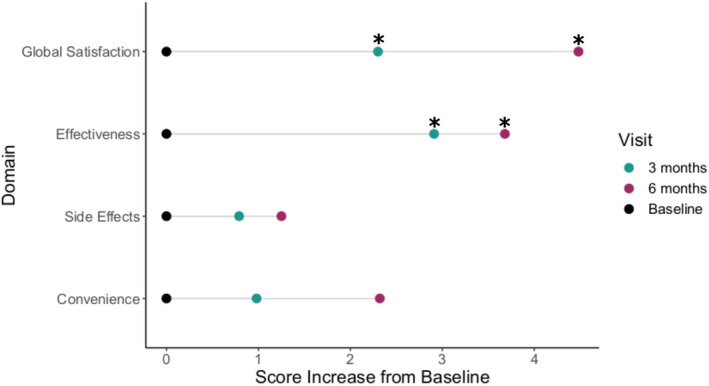
Score changes in TSQM global satisfaction, effectiveness, side effects, and convenience domains from baseline to 3 months and 6 months after PGx testing. *p ≤ 0.05 for baseline versus 3 months visit scores and baseline versus 6 months visit scores. PGx, pharmacogenetic; TSQM, Treatment Satisfaction Questionnaire for Medication.
Adherence
Overall, the majority of patients reported not having difficulty staying adherent to their treatment plan, and that behavior did not change during the study period. However, when specifically asked if patients took their prescribed medication the day before the interviews, there was a significant increase in the proportion of patients who answered “yes” at 6 months after PGx testing compared to baseline (p = 0.006; Figure 3). When asked if patients sometimes forget to take any of their prescribed pills, there was a trending numerical increase in the proportion of patients who answered “no” at the 3‐months visit and at the 6‐months visit compared to baseline, however, this increase was not statistically significant (Figure 3). When asked about average adherence over a 2‐week period, during travel, or when health conditions were controlled, behaviors did not seem to change between the study visits.
FIGURE 3.
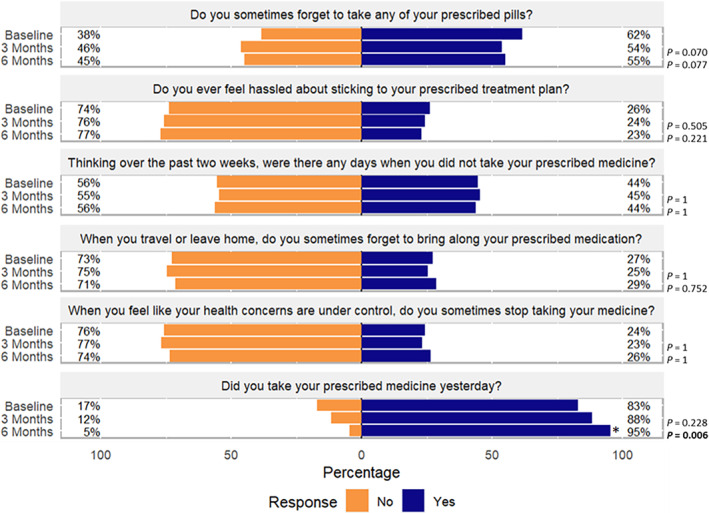
Adherence questionnaire responses for baseline, 3‐month, and 6‐month visits. *p ≤ 0.05 for baseline versus 3 months visit responses and baseline versus 6 months visit responses.
Perceived value
The patient‐estimated average time spent with a provider discussing medications at their previous clinic visit was 11.5 (±10.7) min at baseline, 12.9 (±11.8) min at 3 months, and 12.2 (±8.6) min at 6 months post‐PGx testing, with no significant differences observed compared to baseline. When asked about what value (in dollars) they would assign to a preemptive PGx test, 21% of patients estimated it to be above $200. When asked about how much they would be willing to pay for a preemptive PGx test, 8% estimated it to be above $200. This trend did not seem to improve over the 6‐month study period, as the distribution of the answers were similar among all visits (Figure 4).
FIGURE 4.
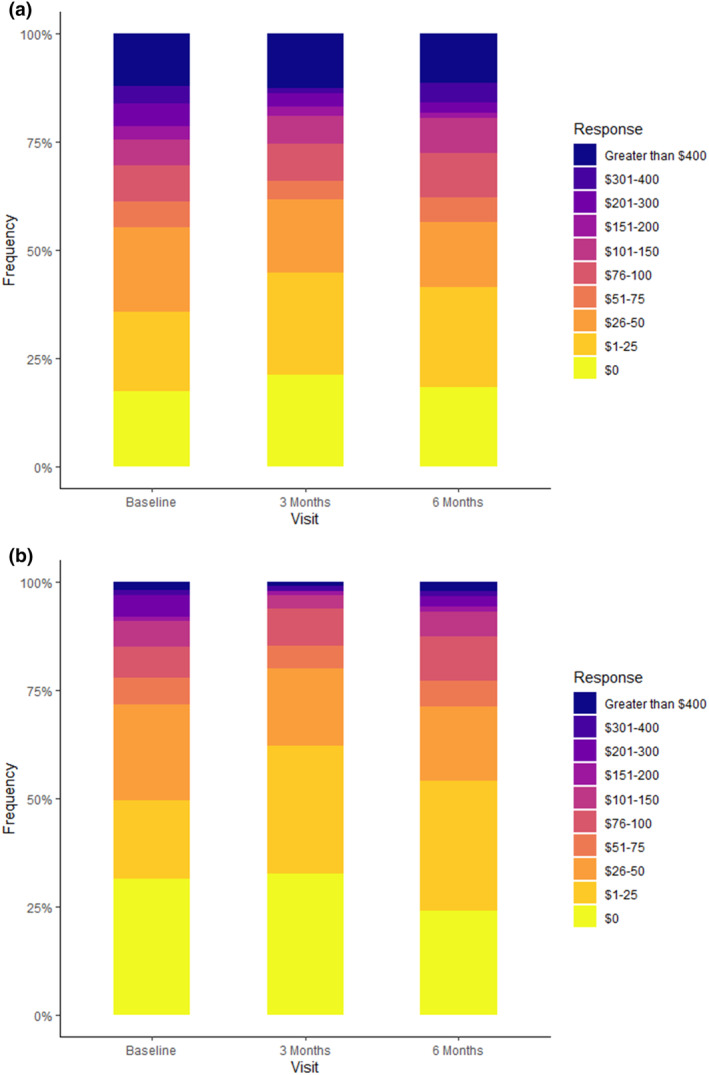
Perceived value of the preemptive PGx test. (a) The estimated value (in dollars) of the test as per patients at baseline, 3 months, and 6 months. (b) The amount (in dollars) the patients are willing to pay for the test. PGx, pharmacogenetic.
Secondary effectiveness outcomes
During the study period, 16 (2.4%) out of 664 total medications were changed. Of those, 10 (6.2%) were PGx medications out of a total of 161 PGx medications prescribed. The number of total healthcare provider encounters within the 6 months immediately prior to the baseline visit were significantly lower than within the 6 months after PGx testing (Figure 5). There were no significant differences in the number of hospitalizations and urgent care visits between the 6 months prior to, and the 6 months after, PGx testing.
FIGURE 5.
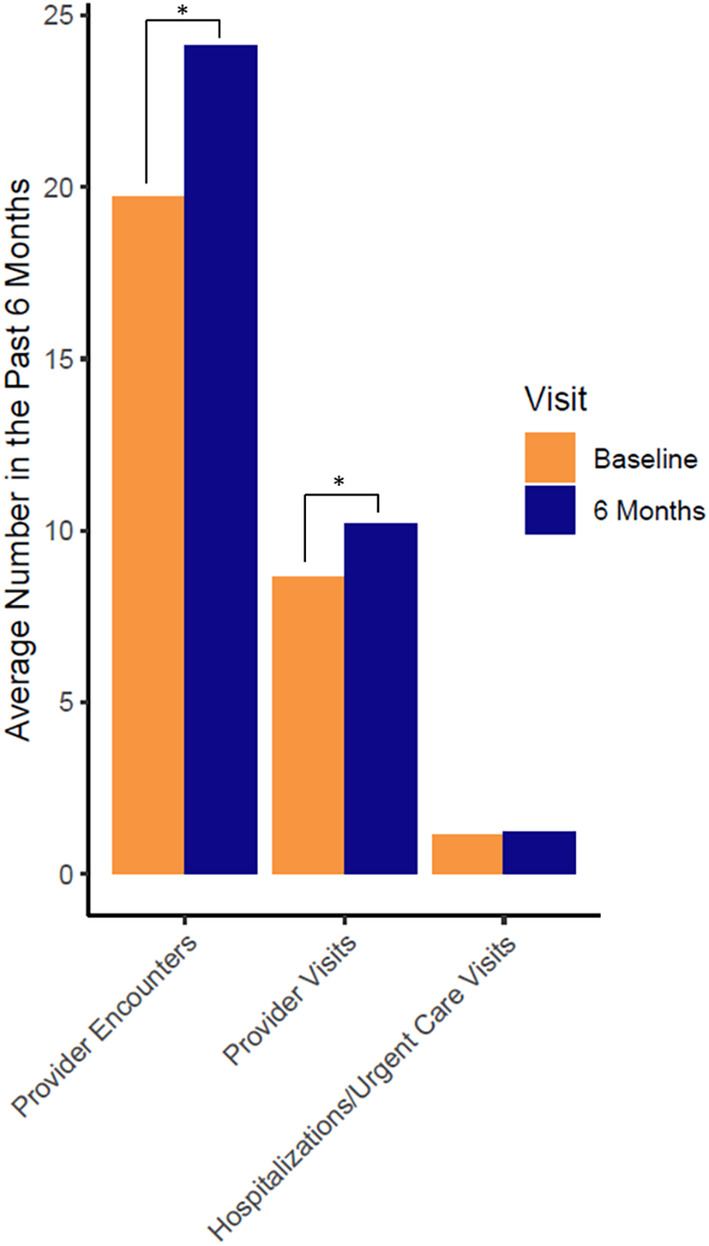
Number of provider encounters, visits, and hospitalizations within the past 6 months before baseline and within the 6 months‐study period. *p value < 0.05 compared to baseline.
DISCUSSION
A major goal of this pilot study was to evaluate the feasibility and clinical outcomes of clinically implementing preemptive PGx testing in underserved patient populations within the primary care setting. We found that the vast majority of our patients (96%) had an actionable genotype for at least one pharmacogene, and 28% of patients had an actionable genotype for the PGx drug that they were prescribed. We also found that global patient treatment satisfaction improved 3 months after PGx testing and persisted for at least 6 months, mostly driven by increased satisfaction with treatment effectiveness. This was paralleled by an increase in the number of provider encounters/visits as well as an improvement in medication adherence at 6 months. To our knowledge, this is the first study to report implementation data of preemptive PGx testing in medically underserved patients.
The percent of patients with at least one actionable genotype (96%) in our medically underserved population is similar to previous findings in other patient populations. 17 Moreover, 28% of our population had an actionable genotype related to a PGx drug they were already taking at baseline. Our study was a 6‐month pilot study representing only a small percentage of the patients' total lifetime drug exposure. Previous reports have shown that 40% of patients with actionable genotypes had evidence of lifetime exposure to the specific PGx drug related to that genotype. 17 This suggests that preemptive testing may benefit a substantial proportion of the population, particularly if they are already prescribed a PGx drug. Despite 28% of patients having an actionable genotype for the medication they were on, only 10 PGx medication changes were observed during the study period. Having an actionable genotype does not necessarily mean that patients were on an inappropriate drug or drug dose. In addition, because medication changes are at the discretion of the provider, they could refuse to follow the PGx recommendation for many reasons, such as lack of awareness of the impact of PGx, clinical factors outweighing the PGx recommendation, or the patient being responsive or stable on their current medication. However, these decisions could confound outcomes, such as patient treatment satisfaction or adherence, if they lead to a suboptimal treatment. In addition, the most common PGx drugs in our population were PPIs (61%) despite only 14% of patients having a diagnosis of gastroesophageal reflux disease. Because PPIs and other drugs covered by the PGx panel can also be obtained over the counter, our findings emphasize that exposure to PGx drugs may be higher than anticipated and future studies including these drug classes are warranted. The test turnaround time in this study was around 1 week, which would be challenging in clinical situations in which patients require immediate therapy. However, in the setting of preemptive testing where results are not needed urgently, this turnaround time is reasonable to enable future therapy recommendations and suggests that utilizing PGx testing in clinical practice would be feasible even if institutions were not able to receive results in a very short timeframe.
Known barriers to PGx implementation include limited evidence of cost‐effectiveness, availability of support tools for PGx integration, and acceptance of PGx testing among providers and patients. 18 , 19 Substantial progress has been made in educating healthcare professionals about the value of PGx testing, but the same is not true with patients, especially among medically underserved populations. 18 , 19 , 20 Patient and provider satisfaction with PGx implementation has been evaluated in the past, but the main focus has been about the program structure and its services rather than therapeutic outcomes after PGx testing. 21 One previous study reported a positive impact of PGx testing on patient experiences with their medications in a primary care setting, as measured by the Beliefs about Medicines Questionnaire (BMQ). 22 The study was conducted in an academic health center with the majority of patients self‐identifying as White, and the PGx tests were single‐gene based tests tailored to the medications each patient was on. In contrast, our patients were recruited from clinics in underserved areas and the majority self‐identified as Black, and our PGx test was a multi‐gene panel. Our primary outcome of measure in this study was the TSQM score, which is different from the BMQ because TSQM domains cover more specific aspects of therapeutic outcomes by including effectiveness, side effects, and convenience, whereas the BMQ measures patients' perceived overall necessity for medications and concerns with adverse effects.
Our results suggest that preemptive PGx testing might potentially improve medication adherence within an underserved patient population. Approximately 30%–50% of all US patients do not take their medications as prescribed, costing between 100 and 300 billion dollars each year in direct costs (such as poor clinical outcomes, unnecessary healthcare costs, and therapy intensification) and indirect costs (such as caregiver burden and loss of work productivity). 23 Evidence shows that medication nonadherence is even greater in low‐income underserved populations, which may lead to a higher rate of poor treatment outcomes, further increasing costs. 24 This was shown to be more pronounced in Black and Hispanic patients, widening existing racial/ethnic disparities in the healthcare system. 25 Other studies have previously shown that PGx testing has the potential to improve medication adherence and clinical outcomes in chronic therapies, but these either did not include a majority of low‐income or underserved populations or did not assess race in their analysis. 26 , 27
Although enrolled patients were already taking at least one PGx drug at baseline, only a minority of our patients valued the PGx test above $200, which approaches actual costs of PGx testing. That did not improve over the study period. Interestingly, perceived value of the test was always higher than the amount the patients were willing to pay for it. Cost is one of the major barriers of implementing PGx in the clinic, and medically underserved or low socioeconomic status patients are often vulnerable to limited health care access due to cost. 28 Approximately half of the population were low‐income patients, which could explain a lower willingness to pay for the test, given they likely have other financial priorities. Perceived value may also be low overall due to the fact that the benefits of a preemptive test are less obvious than those from a single test informing a current drug. Therefore, targeting low‐cost preemptive testing will be critical to improve this patient population's acceptance of PGx testing and their willingness to pay for it. This is especially true because a preemptive test would prevent incremental costs of multiple single‐gene tests and would inform more genes, potentially leading to broader clinical utility. In addition, we observed an increase in the total number of healthcare provider encounters within the 6 months after PGx testing. There is no evidence linking PGx testing with an increase in provider encounters, but this population may especially benefit from seeing their providers more often to reduce the trial‐and‐error process and optimize drug therapy because we have previously found that medically underserved patients are prescribed PGx medications at a higher frequency and have fewer encounters with healthcare providers. 10 In addition, there may be potential for improving and standardizing counseling for PGx as patients' understanding of the benefits of PGx testing remains low in the general population. 29 Previous reports have shown that patients are often disappointed when providers do not know how to interpret and implement their PGx results, which also highlights the need to educate healthcare professionals. 29
Our study has some limitations. First, all patients underwent PGx testing at enrollment, which did not allow the inclusion of a usual care comparator group. Paired statistical methods were used to account for the differences in the measurements before and after the test, but comparing changes in satisfaction to a usual care reference group would provide more robust and reliable results. In addition, the TSQM score was developed and validated for one medication at a time rather than an entire medication regimen. Although the questions in the tool were minimally adapted to include multiple medications, interpretation of the results must be made with caution in the context of aggregate treatment satisfaction. Additionally, because of our small sample size, the number of medication changes was not large enough to evaluate associations between PGx medication changes and outcomes, such as treatment satisfaction, adherence, and perceived value over the study period. Finally, this study recruited patients from two UF Health clinics serving underserved populations, thus the results may not necessarily be generalizable to other health systems serving different patient populations.
In conclusion, our findings indicate that clinical implementation of PGx may be feasible in outpatient clinics serving medically underserved patients. Moreover, treatment satisfaction, medication adherence, and number of provider visits may improve after receiving a preemptive genetic panel test in medically underserved patient populations. However, additional research including other underserved patient populations should assess the long‐term effects of preemptive PGx testing on future prescribing as well as clinical outcomes.
AUTHOR CONTRIBUTIONS
C.L. and J.D.D. wrote the manuscript. L.H.C. and J.D.D. designed the research. E.E., J.T., and J.M. performed the research. C.L. analyzed the data.
FUNDING INFORMATION
Research reported in this publication was supported by the University of Florida Clinical and Translational Science Institute, which is supported in part by the NIH National Center for Advancing Translational Sciences under award number UL1TR001427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Individual authors were supported by the NIH grant R01 HG011800.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Supporting information
Table S1.
Table S2.
Lteif C, Eddy E, Terrell J, Cavallari LH, Malaty J, Duarte JD. Feasibility of preemptive pharmacogenetic testing and improvement of medication treatment satisfaction among medically underserved patients. Clin Transl Sci. 2024;17:e13692. doi: 10.1111/cts.13692
REFERENCES
- 1. Kalow W. The Pennsylvania State University College of medicine 1990 Bernard B. Brodie lecture. Pharmacogenetics: past and future. Life Sci. 1990;47(16):1385‐1397. [DOI] [PubMed] [Google Scholar]
- 2. Dunnenberger HM, Crews KR, Hoffman JM, et al. Preemptive clinical pharmacogenetics implementation: current programs in five US medical centers. Annu Rev Pharmacol Toxicol. 2015;55:89‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Popejoy AB, Fullerton SM. Genomics is failing on diversity. Nature. 2016;538(7624):161‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Campbell MC, Tishkoff SA. African genetic diversity: implications for human demographic history, modern human origins, and complex disease mapping. Annu Rev Genomics Hum Genet. 2008;9:403‐433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Relling MV, Evans WE. Pharmacogenomics in the clinic. Nature. 2015;526(7573):343‐350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Swen JJ, van der Wouden CH, Manson LE, et al. A 12‐gene pharmacogenetic panel to prevent adverse drug reactions: an open‐label, multicentre, controlled, cluster‐randomised crossover implementation study. Lancet. 2023;401(10374):347‐356. [DOI] [PubMed] [Google Scholar]
- 7. Roden DM, Van Driest SL, Mosley JD, et al. Benefit of preemptive pharmacogenetic information on clinical outcome. Clin Pharmacol Ther. 2018;103(5):787‐794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weitzel KW, Cavallari LH, Lesko LJ. Preemptive panel‐based pharmacogenetic testing: the time is now. Pharm Res. 2017;34(8):1551‐1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duconge J, Ruano G. ‘Generic to genetic’ transition in cardiovascular and neuropsychiatric drugs: opportunity for personalized medicine. Pharmacogenomics. 2012;13(10):1097‐1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dalton R, Brown JD, Duarte JD. Patients with geographic barriers to health care access are prescribed a higher proportion of drugs with pharmacogenetic testing guidelines. Clin Transl Sci. 2021;14(5):1841‐1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. MolDX: Pharmacogenomics Testing . https://www.cms.gov/medicare‐coverage‐database/view/lcd.aspx?LCDId=38294&ver=16. 2020.
- 12. Park SK, Thigpen J, Lee IJ. Coverage of pharmacogenetic tests by private health insurance companies. J Am Pharm Assoc (2003). 2020;60(2):352‐356.e3. [DOI] [PubMed] [Google Scholar]
- 13. Keeling NJ, Rosenthal MM, West‐Strum D, Patel AS, Haidar CE, Hoffman JM. Preemptive pharmacogenetic testing: exploring the knowledge and perspectives of US payers. Genet Med. 2019;21(5):1224‐1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Relling MV, Klein TE. CPIC: clinical pharmacogenetics implementation consortium of the pharmacogenomics research network. Clin Pharmacol Ther. 2011;89(3):464‐467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Atkinson MJ, Sinha A, Hass SL, et al. Validation of a general measure of treatment satisfaction, the treatment satisfaction questionnaire for medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes. 2004;2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cohen J. A power primer. Psychol Bull. 1992;112(1):155‐159. [DOI] [PubMed] [Google Scholar]
- 17. Van Driest SL, Shi Y, Bowton EA, et al. Clinically actionable genotypes among 10,000 patients with preemptive pharmacogenomic testing. Clin Pharmacol Ther. 2014;95(4):423‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krebs K, Milani L. Translating pharmacogenomics into clinical decisions: do not let the perfect be the enemy of the good. Hum Genomics. 2019;13(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Klein ME, Parvez MM, Shin JG. Clinical implementation of pharmacogenomics for personalized precision medicine: barriers and solutions. J Pharm Sci. 2017;106(9):2368‐2379. [DOI] [PubMed] [Google Scholar]
- 20. Gawronski BE, Cicali EJ, McDonough CW, Cottler LB, Duarte JD. Exploring perceptions, knowledge, and attitudes regarding pharmacogenetic testing in the medically underserved. Front Genet. 2023;13:1085994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Borobia AM, Dapia I, Tong HY, et al. Clinical implementation of pharmacogenetic testing in a Hospital of the Spanish National Health System: strategy and experience over 3 years. Clin Transl Sci. 2018;11(2):189‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haga SB, Mills R, Moaddeb J, Allen Lapointe N, Cho A, Ginsburg GS. Patient experiences with pharmacogenetic testing in a primary care setting. Pharmacogenomics. 2016;17(15):1629‐1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bosworth HB, Granger BB, Mendys P, et al. Medication adherence: a call for action. Am Heart J. 2011;162(3):412‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bidwal M, Lor K, Yu J, Ip E. Evaluation of asthma medication adherence rates and strategies to improve adherence in the underserved population at a federally qualified health center. Res Social Adm Pharm. 2017;13(4):759‐766. [DOI] [PubMed] [Google Scholar]
- 25. Gellad WF, Haas JS, Safran DG. Race/ethnicity and nonadherence to prescription medications among seniors: results of a national study. J Gen Intern Med. 2007;22(11):1572‐1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grant RW, Hivert M, Pandiscio JC, Florez JC, Nathan DM, Meigs JB. The clinical application of genetic testing in type 2 diabetes: a patient and physician survey. Diabetologia. 2009;52(11):2299‐2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Charland SL, Agatep BC, Herrera V, et al. Providing patients with pharmacogenetic test results affects adherence to statin therapy: results of the additional KIF6 risk offers better adherence to statins (AKROBATS) trial. Pharmacogenomics J. 2014;14(3):272‐280. [DOI] [PubMed] [Google Scholar]
- 28. Virapongse A, Misky GJ. Self‐identified social determinants of health during transitions of care in the medically underserved: a narrative review. J Gen Intern Med. 2018;33(11):1959‐1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Allen JD, Pittenger AL, Bishop JR. A scoping review of attitudes and experiences with pharmacogenomic testing among patients and the general public: implications for patient counseling. J Pers Med. 2022;12(3):425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Table S2.


